Mutations producing single amino acid substitutions in neuron-specific β-tubulin isotype III cause congenital fibrosis of the extraocular muscles type 3 (CFEOM3), a variable and frequently asymmetrical congenital cranial dysinnervation disorder with ophthalmic findings that include blepharoptosis and strabismus. Magnetic resonance imaging demonstrates oculomotor and abducens nerve hypoplasia with misinnervation and secondary hypoplasia of multiple extraocular muscles and hypoplasia of the optic nerve.
Abstract
Purpose.
Orbital magnetic resonance imaging (MRI) was used to investigate the structural basis of motility abnormalities in congenital fibrosis of the extraocular muscles type 3 (CFEOM3), a disorder resulting from missense mutations in TUBB3, which encodes neuron-specific β-tubulin isotype III.
Methods.
Ophthalmic examinations in 13 volunteers from four CFEOM3 pedigrees and normal control subjects, were correlated with TUBB3 mutation and MRI findings that demonstrated extraocular muscle (EOM) size, location, contractility, and innervation.
Results.
Volunteers included clinically affected and clinically unaffected carriers of R262C and D417N TUBB3 amino acid substitutions and one unaffected, mutation-negative family member. Subjects with CFEOM3 frequently had asymmetrical blepharoptosis, limited vertical duction, variable ophthalmoplegia, exotropia, and paradoxical abduction in infraduction. MRI demonstrated variable, asymmetrical levator palpebrae superioris and superior rectus EOM atrophy that correlated with blepharoptosis, deficient supraduction, and small orbital motor nerves. Additional EOMs exhibited variable hypoplasia that correlated with duction deficit, but the superior oblique muscle was spared. Ophthalmoplegia occurred only when the subarachnoid width of CN3 was <1.9 mm. A-pattern exotropia was frequent, correlating with apparent lateral rectus (LR) muscle misinnervation by CN3. Optic nerve (ON) cross sections were subnormal, but rectus pulley locations were normal.
Conclusions.
CFEOM3 caused by TUBB3 R262C and D417N amino acid substitutions features abnormalities of EOM innervation and function that correlate with subarachnoid CN3 hypoplasia, occasional abducens nerve hypoplasia, and subclinical ON hypoplasia that can resemble CFEOM1. Clinical and MRI findings in CFEOM3 are more variable than those in CFEOM1 and are often asymmetrical. Apparent LR innervation by the inferior rectus motor nerve is an overlapping feature of Duane retraction syndrome and CFEOM1. These findings suggest that CFEOM3 is an asymmetrical, variably penetrant, congenital cranial dysinnervation disorder leading to secondary EOM atrophy.
Congenital fibrosis of the extraocular muscles (CFEOM) is typically a nonprogressive disorder of ocular dysmotility with accompanying blepharoptosis. CFEOM is classified, along with Duane syndrome, congenital ptosis, congenital facial palsy, and Moebius syndrome, as a congenital cranial dysinnervation disorder (CCDD), proposed to be caused by aberrant innervation of ocular and facial muscles.1 Three distinct phenotypes, CFEOM1, -2, and -3, are recognized. The classic form, CFEOM1 (MIM 135700; http://www.ncbi.nlm.nih.gov/Omim/, provided in the public domain by the National Center for Biotechnology Information [NCBI], Bethesda, MD), is typified by bilateral congenital blepharoptosis and ophthalmoplegia, with the eyes restricted to infraduction.2 Horizontal strabismus may coexist. CFEOM1 is autosomal dominant, maps to chromosome 12,3,4 and is caused by a small number of recurrent heterozygous missense mutations in the kinesin motor protein encoded by KIF21A.5 Engle et al.2 have suggested that CFEOM1 is the result of primary maldevelopment of cranial motor neurons and their axons, which causes secondary hypoplasia or atrophy of the EOMs that they innervate and contracture of their antagonists.
Persons with CFEOM2 (OMIM 602078) have congenitally bilateral exotropic ophthalmoplegia and blepharoptosis and may also have infra- or supraduction of the eyes. This form of CFEOM is a recessive one, identified in consanguineous pedigrees. It maps to the FEOM2 locus on the long arm of chromosome 11, region 136 and is caused by homozygous mutations in PHOX2A (ARIX),7 a homeodomain transcription factor necessary for development of oculomotor and trochlear motor neurons in mice and zebrafish.8,9 Affected individuals do not have detectable oculomotor or trochlear nerves by MRI,10 and these cranial nuclei most likely fail to form in CFEOM2.
The third CFEOM variant, CFEOM3, encompasses individuals from pedigrees with CFEOM that can be clinically indistinguishable from CFEOM1 or CFEOM2. Other pedigree members, however, have absent or unilateral ptosis, unilateral ophthalmoplegia, noninfraducted resting eye position, and/or the ability to supraduct one or both eyes above central position. CFEOM3 can be autosomal dominant, and large families that segregate CFEOM3 typically include affected individuals who meet CFEOM1 criteria.11 To avoid overlapping classifications, we defined a CFEOM1 pedigree as one in which all affected individuals meet CFEOM1 criteria and a CFEOM3 pedigree as one in which at least one affected individual failed to meet CFEOM1 criteria.12 Although in most large pedigrees, CFEOM3 maps to the FEOM3 locus on the long arm of chromosome 1611,13 (OMIM 600638), in rare families it maps to the FEOM1 locus,14 and affected members harbor KIF21A mutations15 (CFEOM3A; OMIM 607034).
We recently identified the FEOM3 gene as TUBB3 (GenBank 10381; http://www.ncbi.nlm.nih.gov/Genbank; provided in the public domain by NCBI), encoding neuron-specific β-tubulin isotype III.16 CFEOM3 can be caused by one of at least eight different heterozygous missense mutations in TUBB3. These TUBB3 mutations alter dynamic instability, and some also alter microtubule interactions with kinesins, including KIF21A. Moreover, although some TUBB3 mutations result in relatively isolated CFEOM, other mutations cause additional central and peripheral nervous system dysfunction.16
The clinical findings of blepharoptosis and strabismus are not highly specific, rendering them susceptible to phenocopy from a wide variety of causes, as well as to potential variation in presentation. The external phenotypic heterogeneity of CFEOM3 has particular potential to confound identification of the genotype or genotypes of this disorder. Magnetic resonance imaging (MRI) now affords the opportunity for detailed study of the endophenotype of EOMs and nerves in the orbits of living subjects,17 and the subarachnoid cranial nerves (CNs) can be imaged as they exit the brain stem.18 We used MRI to investigate the internal phenotype, or endophenotype, of a well-defined group of subjects with CFEOM3 caused by mutations in TUBB3. The purpose was to characterize orbital innervation and the structure and function of EOMs, to distinguish endophenotypes that may be unique to CFEOM3. Our findings support the hypothesis that CFEOM3 is a primary motor neuropathy that mainly affects the oculomotor nerve (CN3) and arises from the inability of motor axons to correctly target the cranial musculature.16 Moreover, although in some individuals MRI findings may be indistinguishable from CFEOM1, CFEOM3 is more often asymmetric and variable in its clinical and MRI presentation.
Methods
Subjects
Thirteen subjects from four unrelated and previously reported pedigrees participated in the study.16 The diagnosis of CFEOM3 was established by clinical criteria and was correlated with TUBB3 mutation status. Pedigrees AT, BT, and QX segregate the most common TUBB3 mutation that results in a R262C amino acid substitution. We established that most individuals harboring this mutation have CFEOM3 without other nervous system findings.16 Pedigree D segregates the mutation resulting in a D417N amino acid substitution. We established that most individuals harboring this mutation are born with CFEOM3 and then develop a later-onset axonal sensorimotor peripheral neuropathy.16 Subjects gave written informed consent to a protocol conforming to the Declaration of Helsinki and approved by the Institutional Review Boards of the University of California, Los Angeles (UCLA), and Children's Hospital Boston. At UCLA, subjects provided ophthalmic histories and underwent complete ophthalmic examination. They also underwent measurement of palpebral fissure height and levator function, with video recording of ocular versions and eyelid motility. Affected subjects also underwent an attempt to elicit Bell's phenomenon of involuntary supraduction on attempted eyelid closure; this maneuver was omitted in unaffected subjects who exhibited normal voluntary supraduction. Levator function was taken as the maximum vertical excursion of the upper eyelid associated with vertical duction.
Quantitative MRI
Imaging was performed at UCLA by using published methods with a 1.5-T MRI scanner (General Electric Signa; Milwaukee, WI) supplemented with an array of surface coils embedded in a transparent face mask (Medical Advances, Milwaukee, WI). Illuminated fixation targets were used to minimize eye motion.19,20 Targets were secured as closely as possible to central position for each eye and, in selected cases, in secondary and tertiary gazes.
Imaging at and posterior to the orbital apex was performed with standard head coils. With surface coils, T1-21 or T2-weighted fast spin echo (FSE)22 quasicoronal images of 2 mm thickness in a matrix of 256 × 256 were obtained over an 8-cm field of view (FOV; resolution in the 312-μm plane, with two excitations) for imaging of extraocular muscles (EOMs), and 6 cm for imaging of intraorbital CNs (resolution in plane 234 μm). Quasisagittal images parallel to the orbital axis were obtained with an 8-cm FOV. Imaging of intracranial portions of CNs and the skull base region was performed in 0.8-mm thickness planes parallel to the optic chiasm and major cranial nerves to the orbit by using the heavily T2-weighted FIESTA (fast imaging with steady state acquisition) sequence.18 In-plane resolution was 195 μm over a 10-cm FOV (matrix 512 × 512) with 10 excitations.
Digital MRI images were analyzed with the program ImageJ (Wayne Rasband; National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/). The method of quantitative analysis is described elsewhere.23We computed rectus and SO EOM volumes in normal control subjects, affected subjects with CFEOM3 and unaffected family members, and strabismic subjects without CFEOM3 who had undergone surgical strabismus correction. Volumes were computed from a total of six contiguous image planes, beginning with the one that included the globe-ON junction and extending five image planes posteriorly. Thus, volume computations did not include the region of the orbital apex, which was generally deep in relation to the field of imaging. Even in the presence of prior strabismus surgery, orbital MRI is considered to reasonably reflect the sizes and positions of the rectus EOM bellies, since surgery is largely confined to the region of the insertional tendons, and because strabismus surgery does not alter measured EOM volumes in mid orbit.24 The exception may be the inferior rectus (IR) muscle, which had been freely tenotomized and allowed to retract posteriorly in several cases of CFEOM3. This prior surgery would cause the IR volume to shift posteriorly, potentially even posterior to the orbital region images. Confounding by this potential artifact may result in exaggeration of IR volumes. Volumes of the IO were computed from complete quasisagittal image sets from origin to insertion. Volume measurement was not attempted for the levator palpebrae superioris (LPS).
Results
Subjects
General characteristics are summarized in Table 1 for the seven male and six female subjects. Ten of them met clinical criteria for CFEOM3 and were TUBB3-mutation positive, whereas subjects 11 and 12 did not have CFEOM3 but were TUBB3-mutation positive, and subject 13 did not have CFEOM3 and was TUBB3-mutation negative. Subject 13 was included as an immediate family member of affected, mutation-positive subjects, to provide insight into the effects of family genetic background. The mean age of subjects with the CFEOM3 phenotype was 45 ± 6 years (mean ± SEM, range, 17–74), whereas clinically unaffected subjects had an average age of 18 ± 3.2 years (range 14 – 24). Although not tested formally, the subjects had generally normal intellectual function, although several reported having had developmental delay as children.
Table 1.
Characteristics of Subjects in Pedigrees with CFEOM3
| Affected Subject | Pedigree | TUBB3 Amino Acid Substitution | Age (y) | Sex | Bell's Phenom. |
Ptosis | Corrected Visual Acuity |
Alignment |
Limited Horizontal Duction |
Limited Vertical Duction |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R Eye |
L Eye |
R Eye |
L Eye |
||||||||||||||||
| R Eye | L Eye | R Eye (logMAR) | L Eye (logMAR) | Horizontal | Vertical | Ab | Ad | Ab | Ad | Up | Dn | Up | Dn | ||||||
| 1 | D | D417N | 26 | F | − | − | L | 0.01 | 0.04 | Primary ortho, λXT | Orthotropic | − | + | − | + | − | − | + | + |
| 2 | D | D417N | 57 | F | − | − | Both | 0.10 | 0.10 | A ET-XT | L HoT | − | + | − | + | + | + | + | + |
| 3 | AT | R262C | 17 | M | − | − | R | 0.20 | 0.20 | A ET-XT | R HoT | + | + | − | − | + | + | − | − |
| 4 | AT | R262C | 32 | M | − | − | Both | 0.80 | 0.05 | XT | Orthotropic | − | + | + | + | + | + | + | + |
| 5 | AT | R262C | 39 | F | + | − | L | 0.00 | 0.40 | XT | Orthotropic | − | − | + | − | − | − | + | + |
| 6 | BT | R262C | 48 | F | − | − | R | 0.40 | 0.10 | XT | R HoT | + | − | − | + | + | + | + | + |
| 7 | BT | R262C | 67 | F | − | − | Both | 0.25 | 0.40 | XT | Orthotropic down | − | + | + | − | + | + | + | + |
| 8 | QX | R262C | 33 | M | − | − | Both | 0.20 | 0.10 | XT | Orthotropic down | − | + | − | + | + | + | + | + |
| 9 | QX | R262C | 74 | F | − | − | Both | 1.6 | 0.20 | XT | Orthotropic down | − | + | + | + | + | + | + | + |
| 10 | QX | R262C | 53 | M | − | − | Both | 0.20 | 0.40 | XT | Orthotropic down | + | + | − | + | + | + | + | + |
| Mean | 45 | 0.46 | 0.23 | ||||||||||||||||
| SEM | 6 | 0.16 | 0.05 | ||||||||||||||||
| Mutation-Positive, Unaffected Subject | |||||||||||||||||||
| 11 | AT | R262C | 14 | M | ND | ND | None | −0.05 | 0.00 | Orthotropic | Orthotropic | − | − | − | − | − | − | − | − |
| 12 | D | D417N | 24 | M | ND | ND | None | −0.02 | −0.03 | Orthotropic | Orthotropic | − | − | − | − | − | − | − | − |
| Mean | 19 | −0.04 | −0.02 | ||||||||||||||||
| SEM | 5 | 0.02 | 0.02 | ||||||||||||||||
| Mutation-Negative, Unaffected Subject | |||||||||||||||||||
| 13 | AT | None | 15 | M | ND | ND | None | −0.10 | 0.00 | Orthotropic | Orthotropic | − | − | − | − | ||||
All subjects underwent MRI except for subject 10. ET, esotropia; XT, exotropia; λ-XT, lambda-pattern exotropia; CF, visual acuity of counting fingers only, assigned as 1.6 logMAR; HoT, hypotropia; L, left; R, right; ND, testing not done.
Comparisons are provided here with previously published rectus EOM data from 21 normal volunteers, 10 men and 11 women, who underwent MRI with the current technique with an 8-cm quasicoronal FOV.24 Six normal volunteers underwent MRI of the CNs in the skull base. Ten control subjects who contributed rectus and superior oblique (SO) EOM data had an average age of 20 ± 1 years (mean ± SEM; range, 14–28). An additional 13 control subjects of average age 23 ± 1 years (range, 14–33) underwent higher resolution MRI of the optic nerves (ONs) in quasicoronal planes with a 6-cm FOV, and 10 subjects underwent MRI of the IO muscles in the quasisagittal planes. All control subjects had normal ocular and lid motility and visual acuity in each eye correctable to 0 logarithm of the minimum angle resolvable in arcmin (logMAR, 20/20), or better.
Clinical Findings in CFEOM3
Overall, subjects with CFEOM3 had slightly subnormal visual acuities. The mean corrected visual acuity of the subjects exhibiting the CFEOM3 phenotype was 0.29 ± 0.08 averaged over the two eyes (Table 1). The mean corrected visual acuity of the two eyes of the three clinically unaffected subjects was normal at −0.03 ± 0.02 logMAR.
All subjects exhibiting the CFEOM3 phenotype had symmetrical pupils ranging in diameter from 5 to 8 mm and normal pupillary reactions, without afferent pupillary defect or light-near dissociation. The ophthalmoscopic appearance of the ON head was normal in all subjects with CFEOM3 except for subject 3, who had unilateral, and subject 4, who had bilateral ON hypoplasia. Unaffected, mutation-negative subject 13 had unilateral ON hypoplasia.
Subjects with CFEOM3 had unilateral or mild bilateral blepharoptosis. Four had unilateral blepharoptosis, and six had bilateral blepharoptosis. Seven subjects who exhibited the CFEOM3 phenotype had undergone one to two surgeries each for blepharoptosis.
All subjects who exhibited the CFEOM3 phenotype had undergone 1 to 15 strabismus surgeries. Even after strabismus surgeries, all subjects exhibiting the CFEOM3 phenotype had limited supraduction and were exotropic in some gaze positions. Although subjects 1, 4, 5, 7, 8, 9, and 10 had normal vertical binocular alignment, subjects 2, 3, and 6 had hypotropia due to asymmetric limitation of supraduction. Affected subjects 4, 5, 7, 9, and 10 exhibited abduction of one eye in deorsumversion (binocular downward gaze), and subjects 1 to 3 exhibited increased exotropia in deorsumversion, constituting a λ- or A-pattern incomitance. Bell's phenomenon was absent in all orbits of affected subjects, with the exception of subject 5, who exhibited normal Bell's phenomenon in her externally unaffected right eye.
Because of the heterogeneity of the CFEOM3 phenotype within pedigrees, informative features are described for each affected subject.
Ocular Motility in Subjects Manifesting the CFEOM3 Phenotype
D417N Amino Acid Substitution.
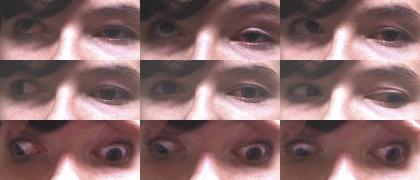
Subject 1 from pedigree D was a 26-year-old woman who harbored the D417N TUBB3 amino acid substitution and had CFEOM3 and a progressive sensorimotor axonal neuropathy that is common to this and several other specific TUBB3 mutations.16 She had undergone surgery for left-side blepharoptosis at 8 months of age and strabismus surgeries of the left eye at 9 and 11 years. Left eye supraduction and adduction were markedly limited, right eye adduction was mildly limited, and the both eyes abducted markedly during deorsumversion (binocular downward gaze, Fig. 1). Levator function was decreased to 4 mm on the left and was normal at 16 mm on the right.
Figure 1.
Subject 1 with the CFEOM3 phenotype, exhibiting left-side blepharoptosis, limited supraduction and adduction, and λ-pattern exotropia. Ductions were are normal for the right eye. The eyelids were manually elevated for photography in deorsumversion.
Subject 2, also from pedigree D, was a 57-year-old woman and the mother of patient 1. She also harbored the D417N amino acid substitution and had both CFEOM3 and later-onset upper and lower extremity weakness and sensory loss. She was born with exotropia, limited supraduction, and blepharoptosis greater on the right side than on the left. She underwent multiple eyelid and numerous strabismus surgeries in childhood and bilateral frontalis suspension surgery for eyelid elevation at 27 years of age. The left but not right eye exhibited high myopia and astigmatism, but vision in both eyes was correctable with spectacles to 0.1 logMAR. There was fine, horizontal, spontaneous nystagmus in central gaze and convergence nystagmus in levoversion. Neither eye supraducted above horizontal midposition. The left eye adducted markedly in attempted supraversion and abducted markedly in attempted deorsumversion. Levator function was absent on the right and mildly subnormal at 10 mm on the left.
Subject 12 from pedigree D was the 24-year-old son of subject 2 and was clinically unaffected, despite harboring the D417N amino acid substitution. Visual acuity in each eye was normal after correction of low myopia. Lid position and ocular motility were normal as was stereopsis when tested by the Titmus method at 40 arcsec. The ONs also appeared normal. The subject had no symptoms of peripheral neuropathy.
R262C Amino Acid Substitution.
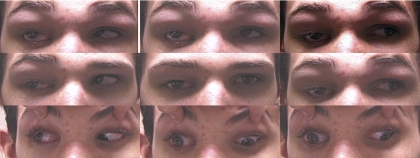
Subject 3 from pedigree AT was a 17-year-old boy who harbored the R262C substitution and had a mild intellectual deficit. He had A-pattern horizontal strabismus manifested by esotropia in supraversion and exotropia in infraversion (Fig. 2). In his right eye, he had blepharoptosis with limited adduction, supraduction, and infraduction. Lid position and ductions of the left eye were normal. The right ON was clinically hypoplastic, exhibiting the double-ring sign of a bare scleral annulus encircling an attenuated central ON head.
Figure 2.
Subject 3 with the CFEOM3 phenotype limited to the right eye, which exhibited blepharoptosis and limitation of supraduction, infraduction, and adduction. The subject had A-pattern strabismus, with esotropia in supraversion, and exotropia in infraversion.
Subject 4, also from AT and harboring the R262C substitution, was a 32-year-old man with bilateral congenital blepharoptosis, absence of vertical versions, exotropia, bilaterally limited adduction, and conjugate horizontal nystagmus. Although both ONs were ophthalmoscopically hypoplastic, visual acuity was 0.8 logMAR in the right eye and was essentially normal at 0.05 logMAR in the left eye. The right eye abducted in deorsumversion, whereas the left eye abducted poorly under all conditions.
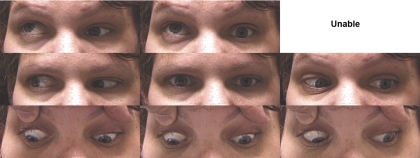
Subject 5, also from AT and harboring R262C, was a 39-year-old woman who had congenital left blepharoptosis and limitation of abduction, supraduction, and infraduction, with aberrant abduction during both infraversion and attempted lid closure during examination to evaluate Bell's phenomenon. Right eye motility was normal, and she was exotropic in central gaze (Fig. 3). Visual acuity was 0.4 logMAR in the affected left eye.
Figure 3.
Subject 5 from pedigree AT with the CFEOM3 phenotype exhibiting left-side congenital blepharoptosis and limitation of abduction, supraduction, and infraduction, with synkinetic abduction in both infraversion and lid closure. Right eye motility was normal. Unable, version position could not be attained.
Subject 11 from pedigree AT was a 14-year-old boy who harbored the R262C mutation and was clinically unaffected. Visual acuity in each eye was normal after correction of low myopia. Lid position and ocular motility were normal, but stereopsis determined by the Titmus method was slightly subnormal at 80 arcsec. The ONs appeared normal.
Subject 13 from pedigree AT was a 15-year-old boy who was mutation negative and clinically unaffected. Visual acuity in each eye was at least 0.0 logMAR after correction of low myopia. Lid position and ocular motility were normal, as well as stereopsis by the Titmus method at 40 arcsec. The left ON exhibited a double-ring sign suggestive of mild ON hypoplasia.
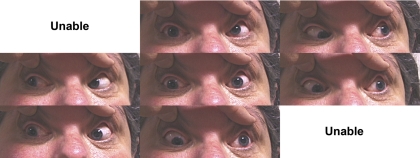
Subject 6 was a 48-year-old woman from pedigree BT, who harbored R262C (Fig. 4). She had right blepharoptosis that had not been treated surgically. Although both eyes had limited supraduction, the limitation was greater in the right eye, creating right hypotropia. The left eye abducted and, paradoxically, supraducted on attempted deorsumversion, whereas the left eye adducted in attempted supraduction.
Figure 4.
Subject 6 from pedigree BT with the CFEOM3 phenotype who had right blepharoptosis and right hypotropia, A-pattern strabismus, and paradoxical supraduction and abduction of the left eye on attempted deorsumversion. Both eyes had limited supraduction. Unable, version position could not be attained.
Subject 7, also from pedigree BT and R262C positive, was a 67-year-old woman who had bilateral blepharoptosis and complete ophthalmoplegia except for adduction of the left eye and abduction of the right eye on attempted infraduction. Convergence was absent. Visual acuity was 0.25 and 0.40 logMAR in the right and left eyes, respectively. The subject had undergone bilateral surgery for blepharoptosis and exotropia and bilateral IR tenotomy, to mitigate severe bilateral limitation of vertical gaze to deorsumversion only.
Subject 8 from pedigree QX harbors the 262C substitution and was a 33-year-old man with bilateral congenital blepharoptosis, large angle exotropia, and bilaterally fixed deorsumversion. He had nearly complete ophthalmoplegia, except for abduction of both eyes and for the transient ability to align the eyes during proximal convergence. Visual acuity was 0.2 and 0.1 logMAR for the right and left eyes, respectively. He exhibited disconjugate, jerky horizontal nystagmus. Both IR muscles had been surgically transected before examination.
Subject 9 from pedigree QX harbored the R262C substitution and was a 74-year-old woman with bilateral congenital blepharoptosis, large angle exotropia, and bilaterally fixed deorsumversion. She had undergone surgeries for blepharoptosis and strabismus. Visual acuity was counting fingers in the right eye and 0.2 logMAR in the left eye. She had nearly complete ophthalmoplegia, except for abduction of the right eye in attempted infraduction. Both eyes incycloducted in attempted infraduction, suggesting bilateral SO function. Both IR muscles had been surgically transected before her examination.
Subject 10 from pedigree QX harbored the R262C substitution and was a 53-year-old man with bilateral congenital blepharoptosis, large angle exotropia, and bilaterally fixed deorsumversion. He had undergone surgeries for blepharoptosis and strabismus. Visual acuity was 0.2 in the right eye and 0.4 logMAR in the left eye. He had nearly complete ophthalmoplegia, except for abduction of the left eye in attempted infraduction, and limited adduction with slow adducting saccades bilaterally. Both eyes incycloducted in attempted infraduction, suggesting bilateral SO function. Claustrophobia precluded MRI in this subject.
MRI Findings in CFEOM3
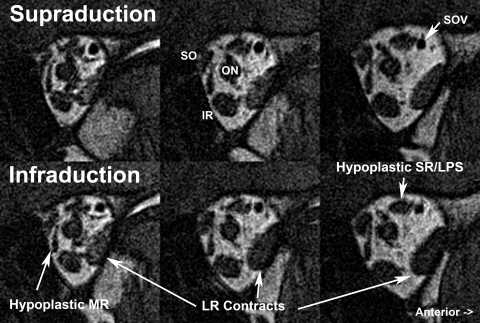
All except subject 10 underwent MRI. The three affected subjects (8, 9, and 10) in pedigree QX harbored the R262C substitution and exhibited the most severe ocular findings, with exotropia and infraduction and almost total external ophthalmoplegia, except for residual abduction and limited incycloduction on attempted infraduction. Subject 8 could intermittently achieve binocular alignment by proximal convergence. MRI findings in severely affected subjects 8 and 9 revealed profound bilateral hypoplasia of the SR, LPS, and MR muscles (Figs. 5 and 6). Both subjects also exhibited highly aspheric globes (Fig. 5), a feature that had been observed in members of a large CFEOM3 pedigree25 now known to harbor the R262C substitution.16
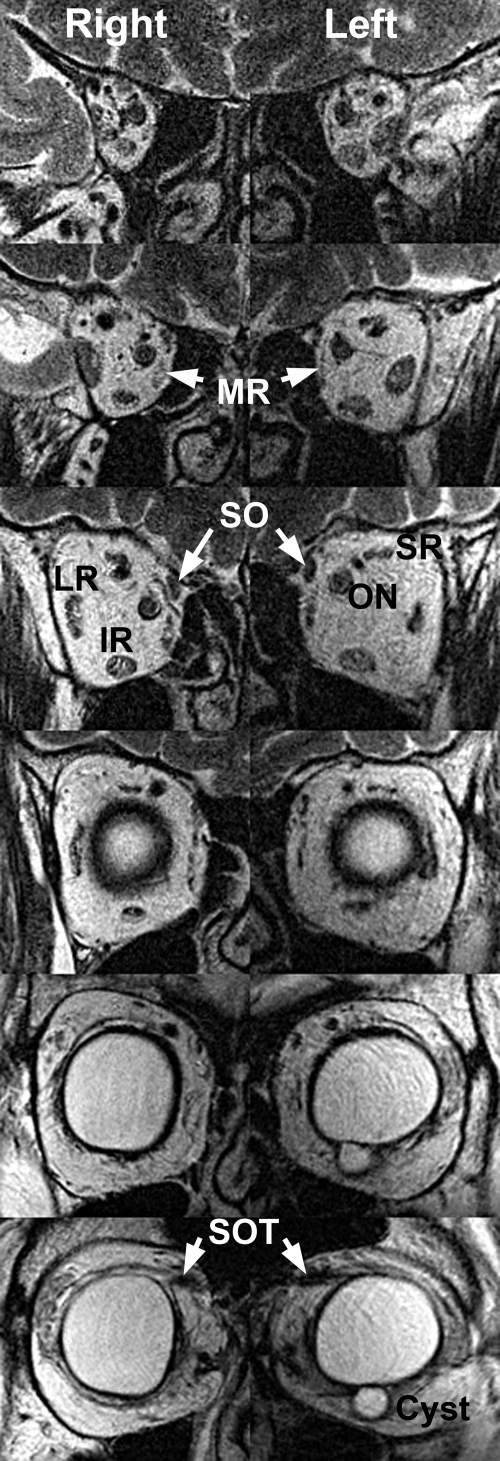
Figure 5.
Quasicoronal T2 FSE MRI in subject 9 with a severe CFEOM3 phenotype. Image planes are 2 mm thick with a 4-mm gap between them, arranged from posterior at top to anterior at bottom. There was marked bilateral hypoplasia of the LPS, SR, and MR. The IR was mildly hypoplastic bilaterally. The SO muscle was preserved. An epithelial inclusion cyst present near the IR insertion was a probable effect of prior IR surgery. The globe cross section was bilaterally irregular and nonspherical. SOT, reflected SO tendon.
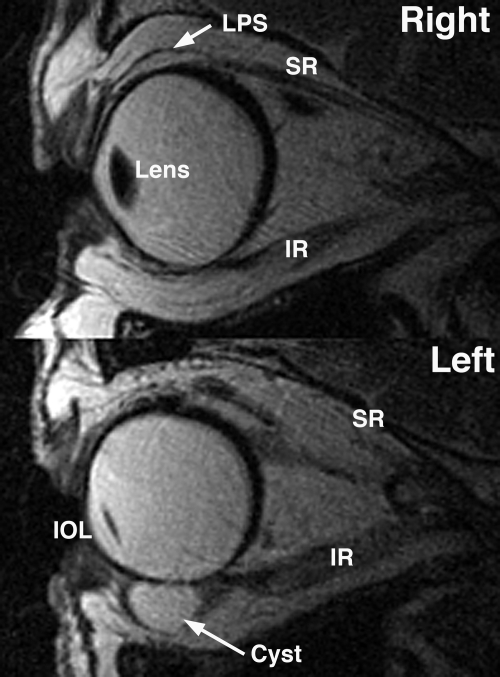
Figure 6.
Quasi-sagittal T2 FSE MRI in subject 9 with a severe CFEOM3 phenotype, exhibiting severe bilateral hypoplasia of the LPS and SR muscles, as well as bilateral absence of the IO muscle. An epithelial inclusion cyst and artificial IOL were present in the surgically treated left orbit.
Other subjects exhibited a less severe, asymmetrical phenotype. For example, EOMs in the right orbit of subject 1 from pedigree D appeared normal on quasicoronal (Fig. 7) and quasisagittal (Fig. 8) MRIs, yet in the left obit, there was marked hypoplasia of the LPS and the deep portions of the SR, MR, and IO muscles. Thus, MRI findings in the left but not right orbit of subject 1 were compatible with the CFEOM1 phenotype.
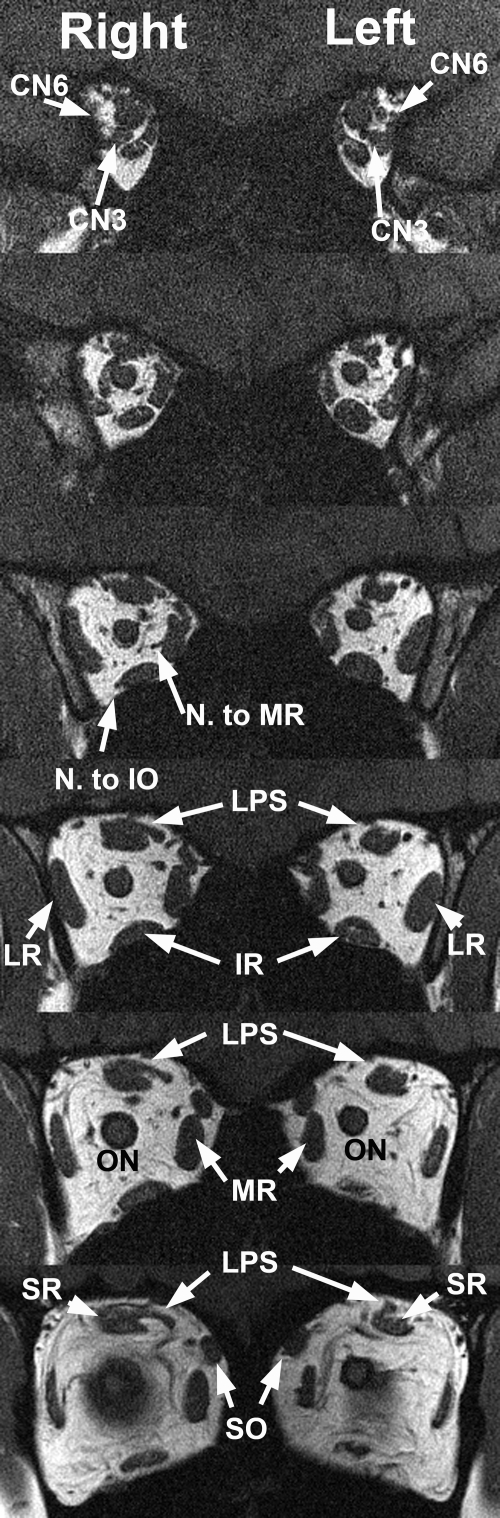
Figure 7.
Quasicoronal T1 MRI in phenotypically less affected subject 1, with asymmetric hypoplasia of the left LPS and milder hypoplasia of the deep portion of the left MR and SR muscles. The inferior division of the left oculomotor nerve (CN3) was in contact with the inferior portion of the left LR muscle (top right). The motor nerve branch to the right but not left MR was visualized. CN6, abducens nerve; N to IO, motor nerve to inferior oblique muscle.
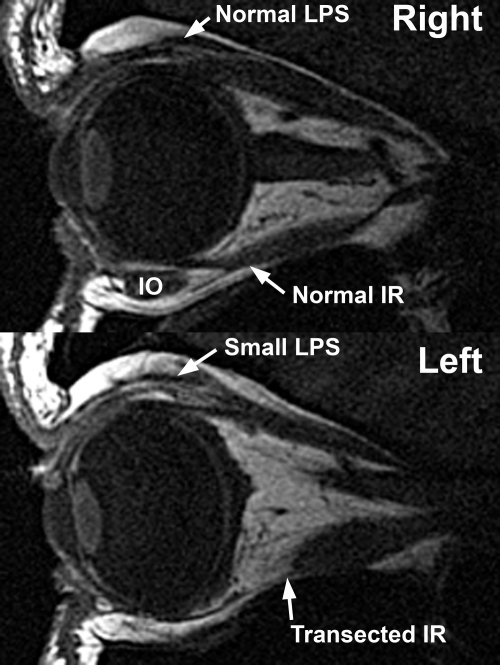
Figure 8.
Quasisagittal T1 MRI in less affected subject 1, in whom only the left eye was phenotypically involved. The subject exhibited hypoplasia of the involved left LPS and SR muscles, as well as severe hypoplasia of the left IO muscle in this image plane. The left IR muscle had been surgically transected, displacing the IO nasally.
Quantitative Features of EOMs
EOM volumes in affected subjects with CFEOM3 were subnormal for the SR, MR, and IR (Table 2). This effect was significant at the 0.0002 level for the SR and at the 0.02 level for the MR and IR. IO volume was slightly but not significantly subnormal, and LR volume was slightly but not significantly greater than normal. Subjects with CFEOM3 who had evidence of surgical IR disinsertion had displacement of the IO belly without significant change in IO volume. Such an IO path change would not have been expected to change apparent IO volume. On average, externally unaffected members of families with CFEOM3 had normal EOM volumes. However, the total number of unaffected family members who were studied was small, and externally unaffected subject 12, who carried the D417N substitution, exhibited in his left orbit volume reductions of the SR (220 mm3), MR (278 mm3), and IR (246 mm3) that were below the 2.5 percentile for normal EOM volumes. Subject 12 had no other subnormal EOM volumes, and subjects 11 and 13 did not have any subnormal EOM volumes. Subject 12 was thus regarded as carrying the endophenotype of CFEOM3.
Table 2.
Muscle Volumes in Control Subjects and Subjects with the CFEOM3 Phenotype
| Muscle | Normal Control Muscles (n = 21) | Strabismic Control Muscles, Postoperative (n = 12) | Clinically Affected Muscles (n = 18) | Unaffected, Mutation-Positive Muscles (n = 4) | Unaffected, Mutation-Negative Muscles (n = 2) |
|---|---|---|---|---|---|
| MR | 395 ± 16 | 369 ± 14 | 314 ± 31* | 402 ± 45 | 450 ± 2 |
| SR | 370 ± 13 | 336 ± 14 | 251 ± 21† | 396 ± 60 | 429 ± 2 |
| LR | 428 ± 15 | 374 ± 17 | 461 ± 18 | 547 ± 50 | 588 ± 50 |
| IR | 386 ± 12 | 327 ± 15 | 342 ± 16* | 414 ± 58 | 403 ± 28 |
| SO | 202 ± 7 | — | 212 ± 9 | 263 ± 6 | 263 ± 10 |
| IO | 263 ± 10 | — | 192 ± 24 | 332 ± 48 | 244 ± 13 |
Data are expressed as the mean volume (mm3) ± SEM.
P < 0.02.
P < 0.0002.
Extraocular Muscle Dysplasia
In externally affected subjects 4, 5, 7, 8, and 9, the LR exhibited a longitudinal fissure separating it into distinct superior and inferior zones. Such fissuring was also evident in externally unaffected subjects 11 and 12, who carried the R262C and D417N substitutions, respectively. In addition, in the left orbit of subject 12, CN3 was in continuity with the inferior zone of the LR, whereas CN6 entered the superior zone. All other EOMs were free of dysplasia in the remaining subjects with CFEOM3 who underwent MRI.
Globe Position and EOM Paths
Coordinates of the LR, MR, and SR pulleys in subjects exhibiting the CFEOM3 phenotype were not significantly different from normal (P > 0.05), and the lateral coordinate of the IR pulley was minimally abnormal, as a likely result of IR disinsertion surgeries that had been performed in nearly all affected orbits.
Imaging of Intraorbital Motor Nerves
Orbits affected by CFEOM3 exhibited abnormal motor innervation, in approximate proportion to the degree of clinical motility restrictions. It was possible to visualize the inferior but not the superior division of CN3 in the subjects with CFEOM3 who were imaged. The superior division was seen, however, only in a subset of normal subjects. In some subjects with CFEOM3, the inferior division of CN3 was too small to trace its branches.
Image resolution was insufficient for quantitative analysis of intraorbital motor nerve size.24 When the nerve was of sufficient size to trace, however, MRI demonstrated misinnervation of EOMs in some subjects with CFEOM3. The inferior division of CN3 failed to innervate the MR in subjects 2, 3, 6, and 7 and was in intimate continuity with the inferior zone of the LR as well its normal target EOMs in subjects 2, 6, 7, and 9 (Fig. 7). In subjects 1, 2, 4, 8, 9, and 10, CN6 entered the superior zone of the LR, whereas a branch of CN3 was in continuity with the inferior zone (Fig. 7). Contraction of the LR in infraduction would explain exotropia in that gaze position, with relaxation in supraduction accounting for the associated esotropia in that gaze position.
Functional imaging in subject 1 was also consistent with LR misinnervation. In subjects 1 and 2 from pedigree D, one LR exhibited contractile thickening in attempted infraduction (Fig. 9). Although subjects 4, 5, 7, 9, and 10 also exhibited abduction on attempted infraduction, MRI could not be performed in this situation because infraduction to a target could not be maintained in the scanner. Misinnervation of the LR by a CN3 branch normally destined for the IR appears to account for the A- and λ-pattern strabismus in pedigrees D and AT and for the anomalous abduction in attempted infraduction in pedigrees BT and QX.
Figure 9.
Coronal MRI of left orbit of subject 2 with CFEOM3, illustrating anomalous contractile thickening of LR with the fellow right eye fixating in infraduction. The subject had hypoplasia of the MR and superior rectus/levator (SR/LPS) muscles. SOV, superior ophthalmic vein.
Imaging of Intracranial Motor Nerves
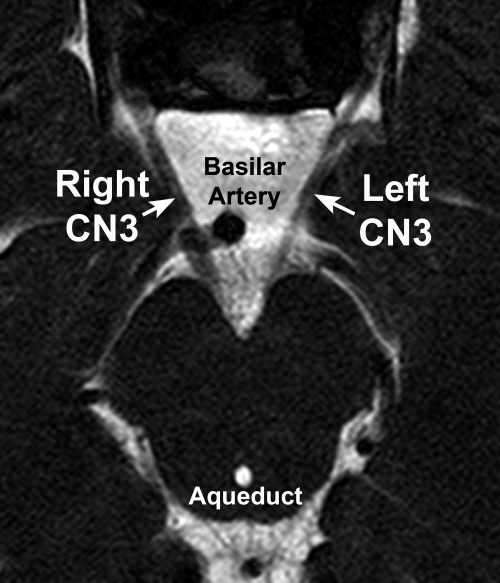
Heavily T2-weighted imaging of the skull base region has sufficient resolution to demonstrate easily and consistently the course of CN3 of normal subjects.26 CN3s were strikingly hypoplastic ipsilateral to all affected orbits, except for the nearly normal left CN3 of subject 5. CN3 was of normal size in subject 1's unaffected right orbit and was of normal size bilaterally in the clinically unaffected family members, regardless of mutation status (Table 3). No orbit was affected by the clinical CFEOM3 phenotype when the subarachnoid oculomotor nerve ipsilateral to that orbit had a width of at least 1.9 mm. For comparison, normal CN3 width measured using this technique is 2.01 ± 0.36 mm.26 Asymmetry of subarachnoid CN3 size is illustrated in Figure 10 for subject 1. The left CN3 was affected with a width of 1.05 mm, whereas on the right side, CN3 was normal, with a width of 2.01 mm.
Table 3.
Duction Abnormalities and Oculomotor Nerve Width in Members of Families Affected by CFEOM3
| Subject | Duction Abnormality |
Subarachnoid Oculomotor Nerve Width (mm) |
||
|---|---|---|---|---|
| Right Eye | Left Eye | Right | Left | |
| 1 | − | + | 2.01 | 1.05 |
| 2 | + | + | 0.87 | 0.70 |
| 3 | + | − | 1.18 | 2.90 |
| 4 | + | + | 1.09 | 1.04 |
| 5 | − | + | 1.90 | 1.79 |
| 6 | + | + | 0.82 | 0.97 |
| 7 | + | + | 0.35 | 0.69 |
| 8 | + | + | 0.62 | 0.92 |
| 9 | + | + | 0.42 | 0.73 |
| 10 | + | + | N.D. | ND |
| 11 | − | − | 2.14 | 2.46 |
| 12 | − | − | 2.01 | 2.01 |
| 13 | − | − | 2.27 | 1.98 |
| Normal mean | 2.01 | |||
| Normal SD | 0.36 | |||
Figure 10.
Heavily T2-weighted MRI of the subarachnoid oculomotor nerve (CN3) in subject 1 with a unilateral left CFEOM3 phenotype. The right CN3 was normal at 2.0 mm wide, whereas the left CN3 was subnormal at 1.0 mm wide.
In normal subjects, the heavily T2-weighted imaging technique also demonstrates the course of CN6 after it exits the pons.27,28 The CN6 was unilaterally or bilaterally hypoplastic in subjects 7, 8, and 9 with the CFEOM3 phenotype; abnormalities of CN6 were not evident in the remaining subjects.
Optic Nerve
ON cross sections normally decrease anteriorly to posteriorly, because of the reduction of connective tissues surrounding axon bundles.29 Thus, ON cross section was analyzed as closely as possible posterior to the globe-ON junction. Subjects with the CFEOM3 phenotype exhibited mean ON cross sections of 9.19 ± 0.60 mm2, which was a 35% reduction and significantly smaller than the mean of 14.1 ± 0.77 mm2 for 17 control ONs (P < 0.00005). All ophthalmoscopically hypoplastic ONs exhibited reduced MRI cross sections, yet not all ONs with reduced MRI cross sections were ophthalmoscopically hypoplastic. The ophthalmoscopic finding of ON hypoplasia was concordant with MRI in subject 4, whose right ON had a cross section of 4.3 and left ON had cross section of 4.8 mm2. The unilateral ophthalmoscopic finding of the double-ring sign indicative of right ON hypoplasia in subject 3 also correlated with reduction in MRI cross section to 7.5 mm2, compared with 13.1 mm2 on the ophthalmoscopically left side. Subject 3 also had a dysplastic corpus callosum on sagittal MRI. The ophthalmoscopically hypoplastic left ON of CFEOM3-unaffected, mutation-negative subject 13 had a subnormal cross section of 8.0 mm2, compared with 10.0 for his ophthalmoscopically normal right ON. However, ONs were ophthalmoscopically normal in on the right-affected subject 8, whose right ON cross section was 7.0 mm2, and on the left in affected subject 1, whose left ON cross section was 6.7 mm2.
Facial Nerve
The facial nerve was bilaterally normal in all subjects imaged. Consistent with this, no subject exhibited clinical signs of facial weakness, and we have not found facial weakness to cosegregate with either the R262C or D417N substitutions.16
Discussion
Employing high-resolution MRI, we demonstrated variable and asymmetric affection of CNs and EOMs correlating closely with the external findings of CFEOM3. This correlation confirms and extends imaging findings in other CCDDs that manifest ocular motility disorders. The most severely affected phenotypes in CFEOM3 may be indistinguishable from CFEOM1, both externally and in the internal involvement of EOMs and associated nerves by orbital MRI. These phenotypes include severe bilateral hypoplasia of the SR, LPS, MR, and occasionally the IO, with anatomic contiguity suggestive of misinnervation of the LR by CN3 and marked bilateral hypoplasia of the subarachnoid CN3. Unlike individuals with CFEOM1, some with CFEOM3 who are less severely affected may exhibit only unilateral, relatively mild hypoplasia of a few EOMs, with less severe hypoplasia of the subarachnoid portion of CN3.
External Ocular Phenotype of CFEOM3
Affected subjects had a range of presentations, typically with exotropia in at least one gaze position and limited supraduction in at least one eye. Most, but not all, subjects also had either vertical gaze restricted to deorsumversion or unilateral hypotropia. None had pupillary abnormalities. Corrected visual acuities averaged approximately 0.3 logMAR (20/40 Snellen), despite strabismus and occasional clinically evident ON hypoplasia. The mild nystagmus present in some subjects was similar to that observed in association in early-onset strabismus. No affected subject had vision binocularly impaired to the degree that nystagmus might be attributable to sensory loss. If presented as isolated cases, subject 1 from pedigree D and subject 5 from pedigree AT might be considered to have unilateral partial CN3 palsies. At the other end of the spectrum, subject 7 from pedigree BT and subjects 8, 9, and 10 from pedigree QX exhibited profound bilateral hypotropia and blepharoptosis with A- or λ-pattern exotropia similar to the presentation of a subset of subjects with CFEOM1. Bell's phenomenon was uniformly absent in affected eyes. Thus, the external phenotype of CFEOM3 can vary widely among affected individuals within CFEOM3 families. However, severe cases of CFEOM3 always present with exotropia and vertical gaze limited to deorsumversion.
Structural Correlations in CFEOM3
In subjects with CFEOM3, blepharoptosis and limited supraduction correlate with variable hypoplasia of the LPS and SR muscles, both of which are innervated by the superior division of CN3. Other EOMs exhibiting clinically deficient function, such as the MR and IR, also exhibit hypoplasia that correlates in severity with the corresponding functional deficit. This EOM hypoplasia is also found in typical cases of CFEOM124 and occasionally in cases of DURS2,28 which are CCDD disorders due to mutation in KIF21A5 and CHN1,30 respectively. Curiously, EOM hypoplasia is not a feature of Duane radial ray syndrome,27 another form of Duane syndrome caused by mutation in the zinc finger transcription factor SALL4.31
While his visual function and ocular motility were entirely normal, externally unaffected subject 12 with the D417N substitution exhibited the endophenotype of significant unilateral hypoplasia of multiple EOMs innervated by CN3. Subjects 1 and 2 with this same substitution exhibited unilateral blepharoptosis, bilateral ocular motility abnormalities, and A- or λ-pattern strabismus. These different presentations highlight the variability in CFEOM3 phenotype resulting from the same mutation, even within the same pedigree.
Aspheric globes were observed in subjects 8 and 9, neither of whom was highly myopic. Little is known about globe asphericity in the absence of high myopia.32 Hemiretinal deprivation33 and regional defocus34 can also create aspheric globes by induction of local eye shape changes in primates. Conceivably, both of these factors could have been present in subjects 8 and 9. It is also possible that abnormal EOM forces in CFEOM altered globe shape or that unknown factors contribute.
Oculomotor Nerve Hypoplasia
Although the number of subjects was small, evidence suggests a threshold effect of subarachnoid CN3 width in CFEOM3 on manifestation of EOM hypoplasia. The external phenotype of blepharoptosis and impaired motility is most commonly evident when CN3 width is less than 1.0 mm. Subarachnoid CN3 width in the internally affected left orbit of subject 12, who had no external phenotype was 2.0 mm, similar to the width of CN3 in the unaffected right orbit and to that in the unaffected left orbit of mutation-negative subject 13. The current, variable MRI findings of small subarachnoid CN3 supports previous autopsy findings showing a decrease in axon number and overall size in the proximal common trunk of CN3 in CFEOM1.2 Since the CN3 cross-sectional area and its maximum possible number of axons varies with the square of the CN3 radius, it may be estimated that the CFEOM phenotype is likely to be manifested when the total number of axons in CN3 is reduced to less than approximately 25% of normal. Of course, this reduction need not be homogeneous and, for example, may be more severe in the superior than in the inferior division of CN3. The separate divisions were not distinguishable in this study by overall measurements of subarachnoid CN3 width. Like CFEOM1, the SR and LPS EOMs innervated by the superior division of CN3 were most severely affected by CFEOM3. The oblique EOMs were more likely to be spared in CFEOM3, but might be involved.
LR Muscle
The present findings confirm widespread LR misinnervation in CFEOM3. Particularly common was A-pattern exotropia, increasing in deorsumversion. MRI confirmed that this phenomenon is due to LR contraction in infraduction, presumably because a branch of CN3 normally destined to innervate the IR muscle also innervates the LR. This finding is typical of both CFEOM1 resulting from KIF21A mutations,24 and chromosome 2-linked Duane's syndrome (DURS2),28 which we recently demonstrated to result from gain-of-function missense mutations in the signaling protein α-2-chimerin, which is responsible for ocular motor axon pathfinding.30 Similarly, CFEOM3 caused by TUBB3 mutations results in axon guidance defects in mouse.16 Overall, these findings further support a primary defect in cranial motor neuron axon guidance in CFEOM3 and other closely related strabismus disorders.
In several CCDDs, particularly CFEOM124 and DURS2,28 the deep LR muscle is commonly dysplastic, typically split longitudinally into superior and inferior portions or otherwise structurally disorganized. Dysplasia of the LR was observed in subjects 2, 4, 7, 8, 9, and 12, but this finding was less prominent than in the other CCDDs. We proposed in earlier work that normal CN6 innervation is essential for normal structure of the deep LR.28 We proposed that variability in severity and pattern of ophthalmoplegia in DURS2 depends on the degree to which residual axons of the hypoplastic inferior division of CN3 reach their normal target EOMs or are misdirected to innervate the LR.28 It seems reasonable to suppose that CFEOM3 is due to one or several cranial nerve targeting abnormalities that are relatively specific for CN3.
SO Muscle
The SO is hypoplastic in acquired and congenital SO palsy,17,35 commonly hypoplastic in CFEOM1,24 occasionally hypoplastic in DURS2,28 but not hypoplastic in DRRS.27 Mean SO volume is preserved in subjects exhibiting the CFEOM3 phenotype and in externally unaffected carriers of the R262C and D417N substitutions (subjects 11 and 12). No subject in the present study had SO volume less than the 5th percentile of normal.
Optic Nerve Involvement in CFEOM3
We have found quantitative MRI useful for ON analysis.29 Size of the ON is normal in DRRS.27 However, subjects with CFEOM1 consistently exhibit a subclinical reduction from normal of approximately 30% to 40% in ON cross section,24 and subjects with dominant Duane syndrome linked to chromosome 2 (DURS2) average a 25% reduction in ON cross section.28 Two of the subjects with CFEOM3 and unaffected, mutation-negative subject 13 exhibited clinically evident ON hypoplasia on ophthalmoscopy, but typical subjects affected with CFEOM3 manifest only subclinical ON hypoplasia. The present finding of a significant but usually subclinical 35% reduction in ON cross section in CFEOM3 is similar. Notably, ON hypoplasia can cosegregate with polymicrogyria resulting from homozygous truncating mutations in TUBA8, suggesting that this phenotype is commonly associated with tubulin mutations in general. No subject with CFEOM3 has a gross visual field deficit or afferent pupillary defect. The only marked visual loss in the subjects with CFEOM3 is due to amblyopia associated with anisometropia and strabismus.
Absence of Widespread Pulley Abnormalities
Pulley disorders can cause strabismus.17,36 Markedly abnormal rectus EOM paths due to misplaced pulleys are associated with craniosynostosis syndromes that are caused by mutations in FGFR37 that spare orbital nerves and EOM volumes. In CFEOM1,24 DRRS,27 and DURS2,28 EOM paths are normal or only minimally abnormal. Overall, rectus pulley positions in the coronal plane remain remarkably normal in CFEOM3, despite severe congenital dysinnervation. This finding further buttresses the accumulating genetic evidence that normal EOM innervation is not requisite for establishment and maintenance of normal rectus pulley positions.24,27,28 It provides additional evidence favoring the idea that pulley abnormalities are primary in cases of incomitant strabismus with which they are associated38–40 and that pulley heterotopy is unlikely to be secondary to acquired EOM denervation.39
Fundamental Implications
We previously reported that subjects harboring the TUBB3 mutations R262C and D417N can have thinning of the anterior commissure and corpus callosum and, as found in subjects 1 and 2, D417N can result in a peripheral axonal neuropathy.16 Subject 3 exhibited localized corpus callosum thinning and dysplasia, as well as in intellectual deficit. TUBB3 mutations not represented in this study can be associated with more severe structural brain abnormalities and fairly consistent and severe external ophthalmoplegia.16 Thus, it is possible that the ocular motor endophenotype associated with other mutations differs from the present cases of CFEOM3, but we anticipate that it would be similar to the more severely affected subjects included in this study.
Overall, these findings suggest that CFEOM3 is an asymmetric, variably penetrant, congenital cranial dysinnervation disorder characterized mainly by oculomotor nerve hypoplasia that affects the superior more than the inferior division and leads to secondary atrophy of EOMs, with associated occasional CN6 and mild ON hypoplasia. The CFEOM3 ocular phenotype presenting asymmetrically may closely resemble incomitant strabismus due to a variety of etiologies, including trauma17 and anomalous EOM bands.41 High-resolution imaging would be useful in recognition of the MRI features of CFEOM3 in cases presenting outside of well-established dominant pedigrees and may reveal clinically unsuspected structural abnormalities of EOMs and their innervation.
Footnotes
Supported by National Eye Institute Grants EY13583, EY12498, EY08313, and EY00331. JLD received an award from Research to Prevent Blindness and is Leonard Apt Professor of Ophthalmology. ECE is a Howard Hughes Medical Institute Investigator.
Disclosure: J.L. Demer, None; R.A. Clark, None; M.A. Tischfield, None; E.C. Engle, None
References
- 1.Gutowski NJ, Bosley TM, Engle EC. The congenital cranial dysinnervation disorders (CCDDs). 110th ENMCC International Workshop, Naarden, The Netherlands, October, 25–27, 2002. Neuromusc Dis. 2003;13:573–578 [DOI] [PubMed] [Google Scholar]
- 2.Engle EC, Goumnerov BC, McKeown CA, et al. Oculomotor nerve and muscle abnormalities in congenital fibrosis of the extraocular muscles. Ann Neurol. 1997;41:314–325 [DOI] [PubMed] [Google Scholar]
- 3.Engle EC, Kunkel LM, Specht LA, Beggs AH. Mapping a gene for congenital fibrosis of the extraocular muscles to the centromeric region of chromosome 12. Nat Genet. 1994;7:69–73 [DOI] [PubMed] [Google Scholar]
- 4.Engle EC, Marondel I, Houtman WA, et al. Congenital fibrosis of the extraocular muscles (autosomal dominant congenital external ophthalmoplegia): genetic homogeneity, linkage refinement, and physical mapping on chromosome 12. Am J Hum Genet. 1995;58:1086–1094 [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada K, Andrews C, Chan W-M, et al. Heterozygous mutations of the kinesin KIF21A in congenital fibrosis of the extraocular muscles type 1 (CFEOM1). Nat Genet. 2003;35:318–321 [DOI] [PubMed] [Google Scholar]
- 6.Wang SM, Zwann J, Mullaney PB, et al. Congenital fibrosis of the extraocular muscles type 2, an inherited exotropic strabismus fixus, maps to distal 11q13. Am J Hum Genet. 1998;63:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakano M, Yamada K, Fain J, et al. Homozygous mutations in ARIX(PHOX2A) result in congenital fibrosis of the extraocular muscles type 2. Nat Genet. 2001;29:315–320 [DOI] [PubMed] [Google Scholar]
- 8.Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expression and interactions of the two closely related homeobox genes Phox2A and Phox2b during neurogenesis. Development. 1997;124:4065–4075 [DOI] [PubMed] [Google Scholar]
- 9.Guo S, Brush J, Teraoka H, et al. Development of a noradrenergic neurons in the zebrafish hindbrain requires BMP, FGF8, and the homeodomain protein soulless/Phox2a. Neuron. 1999;24:555–566 [DOI] [PubMed] [Google Scholar]
- 10.Bosley TM, Oystreck DT, Robertson RL, al Awad A, Abu-Amero K, Engle EC. Neurological features of congenital fibrosis of the extraocular muscles type 2 with mutations in PHOX2A. Brain. 2006;129:2363–2374 [DOI] [PubMed] [Google Scholar]
- 11.Doherty EJ, Macy ME, Wang SM, Dykman CP, Melanson MT, Engle EC. CFEOM3: A new extraocular congenital fibrosis syndrome that maps to 16q24.2-q24.3. Invest Ophthalmol Vis Sci. 1999;40:1687–1694 [PubMed] [Google Scholar]
- 12.Engle EC, McIntosh N, Yamada K, et al. CFEOM1, the classic familial form of congenital fibrosis of the extraocular muscles, is genetically heterogeneous but does not result from mutations in ARIX. BMC Genet. 2002;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackey DA, Chan WM, Chan C, et al. Congenital fibrosis of the vertically acting extraocular muscles maps to the FEOM3 locus. Hum Genet. 2002;110:510–512 [DOI] [PubMed] [Google Scholar]
- 14.Sener EC, Lee BA, Turgut B, Akarsu AN, Engle EC. A clinically variant fibrosis syndrome in a Turkish family maps to the CFEOM1 locus on chromosome 12. Arch Ophthalmol. 2000;118:1090–1097 [DOI] [PubMed] [Google Scholar]
- 15.Yamada K, Chan W-W, Andrews C, et al. Identification of KIF21A mutations as a rare cause of congenital fibrosis of the extraocular muscles type 3 (CFEOM3). Invest Ophthalmol Vis Sci. 2004;45:2218–2223 [DOI] [PubMed] [Google Scholar]
- 16.Tischfield MA, Baris HN, Gupta ML, et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and neuronal circuitry. Cell. 2010;140:74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demer JL. A 12 year, prospective study of extraocular muscle imaging in complex strabismus. J AAPOS. 2003;6:337–347 [DOI] [PubMed] [Google Scholar]
- 18.Seitz J, Held P, Strotzer M, et al. MR imaging of cranial nerve lesions using six different high-resolution T1 and T2(*)-weighted 3D and 2D sequences. Acta Radiol. 2002;43:349–353 [DOI] [PubMed] [Google Scholar]
- 19.Demer JL, Miller JM. Orbital imaging in strabismus surgery. In: Rosenbaum AL, Santiago AP. eds. Clinical Strabismus Management: Principles and Techniques. Philadelphia: WB Saunders; 1999:84–98 [Google Scholar]
- 20.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–3797 [PubMed] [Google Scholar]
- 21.Demer JL, Ortube MC, Engle EC, Thacker N. High resolution magnetic resonance imaging demonstrates abnormalities of motor nerves and extraocular muscles in patients with neuropathic strabismus. J AAPOS. 2006;10:135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demer JL, Narasimhan A. T2-weighted magnetic resonance imaging (MRI) of extraoccular muscles (EOMs). Abstracts of 35th Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus 2009:Abstract 56 [Google Scholar]
- 23.Demer JL, Oh SY, Clark RA, Poukens V. Evidence for a pulley of the inferior oblique muscle. Invest Ophthalmol Vis Sci. 2003;44:3856–3865 [DOI] [PubMed] [Google Scholar]
- 24.Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci. 2005;46:530–539 [DOI] [PubMed] [Google Scholar]
- 25.Gillies WE, Harris AJ, Brooks AM, Rivers MR, Wolfe RJ. Congenital fibrosis of the vertically acting extraocular muscles: a new group of dominantly inherited ocular fibrosis with radiologic findings. Ophthalmology. 1995;102:607–612 [DOI] [PubMed] [Google Scholar]
- 26.Lim KH, Engle EC, Demer JL. Abnormalities of the oculomotor nerve in congenital fibrosis of the extraocular muscles and congenital oculomotor palsy. Invest Ophthalmol Vis Sci. 2007;48:1601–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demer JL, Clark RA, Lim K-H, Engle EC. Magnetic resonance imaging of innervational and extraocular muscle abnormalities in Duane-radial ray syndrome. Invest Ophthalmol Vis Sci. 2007;48:5505–5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demer JL, Clark RA, Lim KH, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in dominant Duane's retraction syndrome linked to the DURS2 locus. Invest Ophthalmol Vis Sci. 2007;48:194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karim S, Clark RA, Poukens V, Demer JL. Quantitative magnetic resonance imaging and histology demonstrates systematic variation in human intraorbital optic nerve size. Invest Ophthalmol Vis Sci. 2004;45:1047–1051 [DOI] [PubMed] [Google Scholar]
- 30.Miyake N, Chilton J, Psatha M, et al. Human CHN1 mutations hyperactivate alpha2-chimaerin and cause Duane's retraction syndrome. Science. 2008;321:839–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Baradie R, Yamada K, St Hilaire C, et al. Duane radial ray syndrome (Okihiro syndrome) maps to 20q13 and results from mutations in SALL4, a new member of the SAL family. Am J Hum Genet. 2002;71:1195–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atchison DA, Jones CE, Schmid KL, et al. Eye shape in emmetropia and myopia. Invest Ophthalmol Vis Sci. 2004;45:3380–3386 [DOI] [PubMed] [Google Scholar]
- 33.Smith EL, Huang J, Hung LF, et al. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2009;50:5057–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith EL, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Optical defocus influences refractive development in monkeys via local, regionally selective mechanisms. Invest Ophthalmol Vis Sci. Published online March 10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demer JL, Miller MJ, Koo EY, Rosenbaum AL, Bateman JB. True versus masquerading superior oblique palsies: muscle mechanisms revealed by magnetic resonance imaging. In: Lennerstrand G. ed. Update on Strabismus and Pediatric Ophthalmology. Boca Raton, FL: CRC Press; 1995:303–306 [Google Scholar]
- 36.Oh SY, Clark RA, Velez F, Rosenbaum AL, Demer JL. Incomitant strabismus associated with instability of rectus pulleys. Invest Ophthalmol Vis Sci. 2002;43:2169–2178 [PubMed] [Google Scholar]
- 37.Muller U, Steinberger D, Kunze S. Molecular genetics of craniosynostotic syndromes. Graefes Arch Clin Exp Ophthalmol. 1997;235:545–550 [DOI] [PubMed] [Google Scholar]
- 38.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25 [DOI] [PubMed] [Google Scholar]
- 39.Clark RA, Miller JM, Demer JL. Displacement of the medial rectus pulley in superior oblique palsy. Invest Ophthalmol Vis Sci. 1998;39:207–212 [PubMed] [Google Scholar]
- 40.Demer JL, Clark RA, Miller JM. Heterotopy of extraocular muscle pulleys causes incomitant strabismus. In: Lennerstrand G. ed. Advances in Strabismology. Buren, The Netherlands: Aeolus Press; 1999:91–94 [Google Scholar]
- 41.Khitri MR, Demer JL. Magnetic resonance imaging of tissues compatible with supernumerary extraocular muscles. Am J Ophthalmol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]