This study demonstrates that the mammalian retinal melanopsin system is strongly dependent on ambient light conditions and suggests that day/night cycles might have a decisive influence on its postnatal development.
Abstract
Purpose.
To study the melanopsin system of the albino CD1 mouse retina during postnatal development.
Methods.
Pups were kept under different ambient conditions: light/dark (LD) cycles, constant light (LL), constant darkness (DD), LL followed by LD, and DD followed by LL. Using immunohistochemistry, melanopsin-expressing cells were classified as M1 or M2 according to the location of their somata and dendritic processes and were counted.
Results.
Under LD cycles an increase in the number of immunoreactive cells was observed within the first week of postnatal development. When mice were maintained in DD, the increase in the number of immunopositive cells detected was significantly higher than that in LD. On the contrary, when mice were exposed to LL within the same period, no increase was detected. To determine whether the effect of LL during the early postnatal period was reversible, the authors studied animals born in LL and subsequently maintained under LD cycles. After 3 days in LD, these animals showed a significant increase in melanopsin cell number. However, after 1 month in LD, the number was similar to that of the LD controls. Surprisingly, when mice born in DD were exposed to LL, no decrease was detected, though the immunostaining was of low intensity.
Conclusions.
The amount of melanopsin protein per cell varies, depending on ambient light conditions. Periods of darkness or, more likely, the sequence of light and dark periods occurring under the daily cycles might be necessary for the normal development of the melanopsin system.
The vertebrate eye mediates both image-forming and non–image-forming photoreception. Image-forming photoreception (vision) enables the animal to detect and track objects in the environment, whereas non–image-forming photoreception is responsible for the measurement of ambient irradiance, so that, for example, the internal circadian biological clock can be synchronized with the astronomical day, a process called photoentrainment.1,2 The hypothalamic suprachiasmatic nucleus (SCN), which is considered the central circadian pacemaker of mammals, is adjusted on a daily basis to the environmental light/dark cycle1 by the detection of light by melanopsin-expressing, intrinsically photosensitive retinal ganglion cells (ipRGCs).3–6 Such ipRGCs transmit this light information to the SCN by way of the retinohypothalamic tract.7,8 These cells also project to other brain areas involved in pupil constriction, promotion of sleep, gaze control, image-forming vision, and other activities.9 Moreover, ipRGCs constitute the principal conduits for rod-cone input involved in non–image-forming responses, including circadian photoentrainment.10 In fact, the destruction of these cells altered the effects of light on circadian rhythms.10–12 Therefore, the rod and cone photoreceptors and the ipRGCs are complementary in providing signals for nonvisual photoreceptive functions.
In mice, at least 70% of the RGCs generated during retinal development die through programed cell death during the postnatal period13; however, as we previously demonstrated in pigmented mice, no diminution in the number of melanopsin-expressing cells occurs during postnatal development.14 ipRGCs are responsive to light from birth.15,16 Moreover, the SCN begins to function as a circadian pacemaker during late fetal development.17 Depending on the intensity of the stimulus, light was able to induce expression of the immediate early gene c-fos in the SCN at postnatal day (P) 0 to P118 or at P4.19 Taken together, these data indicate that the melanopsin-based system is functional as early as the day of birth.
Previous studies have demonstrated that melanopsin expression shows daily oscillation.20–22 Such rhythm was also demonstrated in neonatal albino rats and neonatal pigmented mice,22,23 when rods and cones are not yet fully developed. Hannibal et al.21 and Mathes et al.,24 using albino rats, also reported differential regulation of melanopsin expression in response to continuous darkness (DD) or continuous light (LL). Such changes in melanopsin expression were also detected in albino rat pups.23 This suggests that ipRGCs can adapt their responsiveness to the external illumination conditions by regulating their melanopsin content even in the absence of functional rod-cone photoreceptors.
Among the ipRGCs, two main morphologic types have been previously identified: M1 cells, with their dendritic arborization in the OFF sublayer of the inner plexiform layer (IPL), and M2 cells, with their dendrites forming a plexus in the ON sublayer of the IPL. Recently, two isoforms of melanopsin, Opn4S and Opn4L, have been identified. M1 cells express both melanopsin isoforms, whereas M2 cells express only the Opn4L isoform.25 Different electrophysiological responses,26 as well as different brain projections,27 were reported for these two cell subpopulations. In a previous study,22 we detected a different daily oscillation for M1 and M2 cells that was already present in the early postnatal period.
Albino animals are often used as models in numerous studies concerning the retina, despite the fact that most mutations causing albinism provoke anomalous retinal development, including lower numbers of rods, incomplete development of the central retina, and chiasmatic abnormalities.28 Therefore, it should be taken into account that results obtained in albino models are not fully comparable with those of pigmented animals.
To better understand the development of the ipRGCs, the present study analyzed for the first time in albino mice these cells and their main subpopulations within the postnatal period under standard 12-hour light/12-hour dark cycles. Furthermore, the effects of exposure to constant light or constant darkness on the postnatal development of these cells were also studied.
Materials and Methods
Animals and Experimental Design
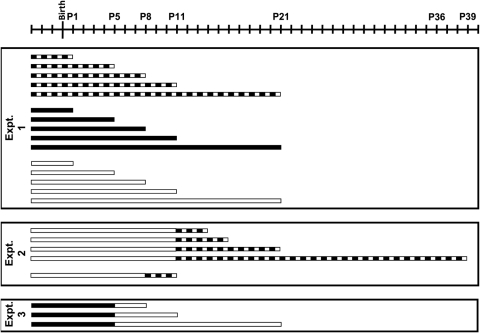
Pregnant female albino mice (CD1; Charles River Laboratories, Malvern, PA) were used in the present study. All animals were maintained in the central animal care facilities under constant temperature conditions (20°C ± 2°C), fed with standard food and tap water ad libitum. Pregnant females were subjected to a 12-hour light/12-hour dark cycle (LD). Three days before the estimated date of delivery, a group of females continued under LD conditions, whereas others were kept under constant light (LL) or constant darkness (DD). The illumination source for LD or LL conditions was a white light fluorescent lamp, so that the animals were exposed to an intensity of 200 ± 60 lux at cage level. This intensity of light was sufficient to stimulate the melanopsin system and prevent possible damage of the retina by prolonged exposure to light. Pups were maintained with their mothers under the quoted ambient conditions (LD, LL, and DD) within the postnatal period. Five ages were analyzed: 1, 5, 8, 11, and 21 days (Fig. 1, experiment 1). In addition, we analyzed the retinas of pigmented C3H/He pups exposed to LL to compare the postnatal development of both strains under such conditions. The experiment carried out was identical with that in albino pups (Fig. 1, experiment 1, LL). In a previous paper we studied the postnatal development of the melanopsin ganglion cells of C3H/He mice under LD conditions.14 Four animals per group (age and ambient condition) were analyzed.
Figure 1.
Representation of the experiments and the different light treatments performed. Experiment 1 under LL conditions was also carried out in pigmented mice. The periods of light and darkness were drawn in white and dark colors, respectively. The days of birth and sample collection are indicated above. Note that pregnant females were subjected to different ambient conditions 3 days before the estimated day of delivery.
To study whether the effect of constant light was or not reversible in the albino pups, they were maintained under LL until P11 and then were subjected to several LD cycles: 3, 5, 10, and 28. Another albino group was under LL until P8 and then was exposed to 3 LD cycles (Fig. 1, experiment 2). To analyze the effect of constant light in the developed melanopsin system, albino animals were exposed to DD until P5 and then were maintained under LL conditions for 3, 6, and 16 days (Fig. 1, experiment 3).
Tissue Preparation
All the animals were killed by decapitation 3 hours after the lights were on. To minimize pain, animals were anesthetized before euthanatization. All the experimental and animal handling procedures complied with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Tissue preparation and melanopsin immunohistochemistry were performed using the UF006 anti-mouse melanopsin polyclonal antibody (Advanced Targeting Systems, San Diego, CA) and the avidin-biotin-peroxidase method (Elite ABC kit; Vector Laboratories, Burlingame, CA), as described in detail previously.14,22
Cell Classification and Counting
Retinal sections were observed in a bright-field microscope (Eclipse E400; Nikon, Tokyo, Japan). Immunopositive cells were classified as M1 or M2 according to the location of their soma and dendritic processes. Somata were counted across the entire length of each retinal section of the series (1 of 6 parallel series per retina was used).
Statistical Analysis
Statistical software (SPSS 15; SPSS, Chicago, IL) was used for all the statistical analyses. The Kolmogorov-Smirnov test was used to confirm the normality of the data. The homogeneity of the variances was assessed with Levene's test. Student's t-tests were performed when comparing two groups. To detect possible differences between groups throughout the postnatal period, factorial ANOVA tests were performed. One-way ANOVA tests were used to analyze the total number of melanopsin-immunopositive cells throughout postnatal development, the recovery detected after LL, and the effect of LL after DD. To detect differences between specific time points, post hoc tests were performed. To detect possible differences between M1 and M2 cell subpopulations, factorial ANOVA tests were performed. Additional one-way ANOVA tests were performed separately for M1 and M2 cells. Post hoc tests were also performed for each cell subpopulation. The number of melanopsin-expressing cells per retinal sample was presented as mean ± SEM. P < 0.05 was considered statistically significant.
Results
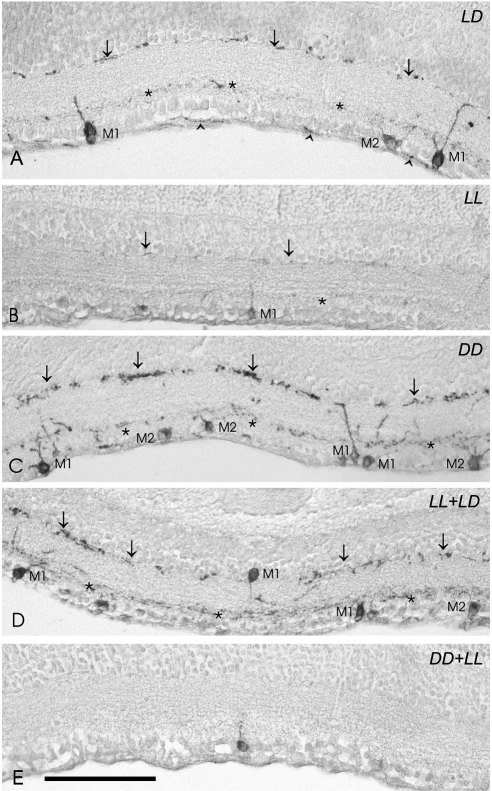
Melanopsin immunoreactivity could be detected in somata, dendrites, and proximal segments of axons. M1 and M2 cell subpopulations (Figs. 2A–E) were identified according to the location of the somata and dendritic processes. M1 cells showed robust dendritic arborization in the outer margin of the IPL (OFF sublayer). Most of the M1 cells, which frequently were heavily stained, had somata located in the ganglion cell layer (GCL), whereas other somata were seen in the inner margin of the inner nuclear layer (INL). M2 cells usually were weakly stained and had somata located in the GCL. Their dendritic processes were finer and more numerous that those of M1 cells and were placed in the inner margin of the IPL (ON sublayer).
Figure 2.
Representative micrographs of retinas immunostained with the UF006 anti–melanopsin antibody corresponding to mice (P21) maintained under five different conditions of illumination and darkness. (A) Retina of a mouse kept under LD conditions. Three immunostained cells can be seen: two M1 cells (with dendritic arborization in the OFF sublayer) and one M2 cell (with dendritic arborization in the ON sublayer). Some axons also showed melanopsin immunostaining (arrowheads). (B) The retina of a mouse kept under LL conditions shows a low number of melanopsin-expressing cells; only one M1 cell soma and diminished dendritic processes can be observed. Note that the OFF plexus shows a low number of dendrites, and the ON plexus is barely detectable. (C) Under DD conditions, a higher number of immunopositive cells can be seen in the retina. This micrograph shows four M1 cells and three M2 cells. In addition, an increase of dendritic immunostaining is observed. (D) Although melanopsin immunostaining was low after exposure to LL, a recovery in the number of immunopositive somata and dendritic processes was detected when the LD conditions were restored. The micrograph shows three M1 cells and one M2 cell. (E) Progressive diminution in staining intensity of the dendritic network and somata was detected in pups kept 5 days under DD and then subjected to LL. This micrograph shows an immunopositive soma. The dendritic plexuses are not observed and, therefore, M1 and M2 cells cannot be identified. Asterisks and arrows: inner and outer immunostained plexuses of the IPL, respectively. Scale bar, 100 μm.
Postnatal Development of Albino Mice Retinas
Factorial ANOVA revealed an interaction between “ambient conditions” and “postnatal day” (P < 0.001), which indicates that the number of immunostained cells differed significantly within the postnatal period, depending on the ambient light conditions to which the animals were subjected.
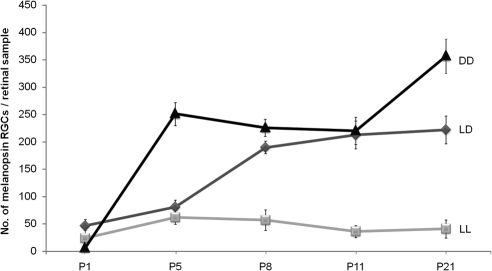
Under the standard LD cycle, melanopsin immunostaining was present since P1. Although at this stage the number of melanopsin immunoreactive cells was small and the staining seemed relatively weak compared with later stages, some melanopsin-containing cells had already formed dendritic processes in the IPL. From P8 onward, two plexuses could be observed (Fig. 2A). One-way ANOVA revealed that the number of melanopsin-expressing cells varied significantly (P < 0.001) throughout the postnatal period. Post hoc tests detected a progressive increase in the number of these cells from P5 to P8 (P < 0.01; Fig. 3). Later, the number of immunostained cells reached the maximum values, ranging in a narrow interval (200–250 cells per retinal sample) in the remaining ages analyzed.
Figure 3.
Number of immunopositive cells during postnatal development under LD, LL, and DD cycles. One-way ANOVA for LD revealed a significant increase in the number of melanopsin-expressing cells throughout the postnatal period (P < 0.001). No significant differences were detected under constant light conditions (P > 0.05). However, in animals maintained under DD conditions, the number of immunostained cells increased significantly in two intervals: P1 to P5 and P11 to P21 (one-way ANOVA; P < 0.001). n = 4 animals per group.
Under LL no variation in the number of immunoreactive cells was detected in the ages studied (one-way ANOVA, P > 0.05). Under these LL conditions, the count remained around 30 to 50 cells/retinal sample during the whole postnatal development (Fig. 3). Both ON and OFF plexuses could be observed under LL, but the dendritic network was much less extensive (Fig. 2B) than under LD conditions.
When animals were exposed to DD, a significant increase in immunopositive cells was detected between P1 and P5 (P < 0.001) and between P11 and P21 (P < 0.05) (Fig. 3). At the end of the postnatal period (P21), these DD mice showed a higher number of immunostained cells than the LD controls (Student's t-test; P < 0.05). One-way ANOVA revealed significant differences during postnatal development (P < 0.001). Melanopsin expression seemed to be stimulated by exposure to DD. In addition, the number of dendrites detected in the immunolabeled plexuses was much higher than that observed in the LD controls (Fig. 2C).
Postnatal Development of M1 and M2 Cells under LD, LL, or DD Conditions
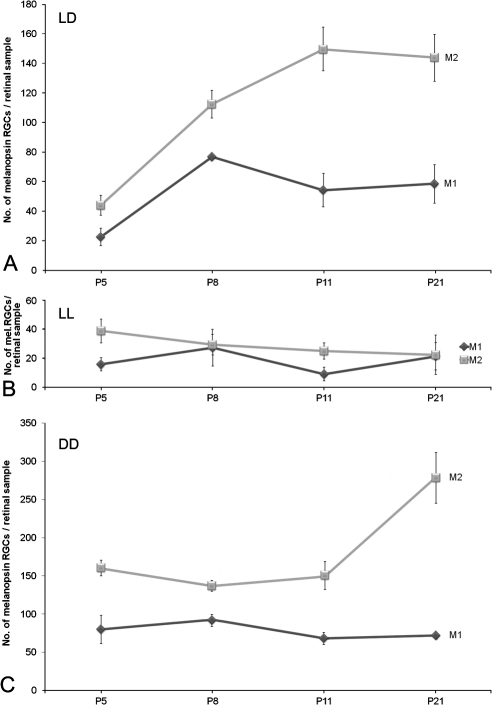
When M1 cells under LD, LL, and DD conditions were compared, factorial ANOVA did not reveal significant differences among groups (P > 0.05). That is, M1 cells behaved similarly under different ambient conditions. Factorial ANOVA did detect significant differences (P < 0.001) for the M2 cell subpopulation, which showed different response patterns depending on the light treatment. Indeed, the M2 cells seemed to be the only ones affected by the different ambient conditions.
When M1 and M2 cell subpopulations were analyzed separately under LD conditions, one-way ANOVA tests performed revealed significant increases for M1 (P < 0.05) and for M2 (P < 0.001) cells. Post hoc tests detected an increase between P5 and P8 in both subpopulations (P < 0.01; Fig. 4A). M2 cells were more abundant than M1 cells, as revealed by Student's t-tests performed to compare them in parallel at the same postnatal day (P5, P < 0.05; P8, P < 0.01; P11 and P21, P < 0.001).
Figure 4.
(A) Development of M1 and M2 cells under LD conditions. No global differences were detected (factorial ANOVA; P > 0.05). One-way ANOVA performed separately for each subpopulation revealed significant increases in number for M1 (P < 0.05) and for M2 (P < 0.001) cells. Both subpopulations showed an initial parallel increase between P5 and P8 (P < 0.01). (B) Postnatal development of M1 and M2 cells under LL conditions. No global differences were detected (factorial ANOVA: P > 0.05). (C) M1 and M2 cells under DD. M1 cell number did not change during development, whereas M2 cell number showed a significant increase at the late postnatal period (P < 0.01). n = 4 animals per group.
M1 and M2 cells were also analyzed in animals subjected to LL. One-way ANOVA tests did not detect any variation in the number of each cell type during the postnatal period (P > 0.05; Fig. 4B).
When animals were subjected to DD, one-way ANOVA detected that the number of M2 cells increased at the end of this period (P < 0.01), whereas the number of M1 remained constant (Fig. 4C).
Postnatal Development of Pigmented Mouse Retinas
In a previous report we demonstrated that under LD conditions, no change in the number of melanopsin-expressing cells was detected during postnatal development.14 The number of melanopsin-positive cells ranged between 160 and 250 per retinal sample.
Under LL no variation in the number of immunoreactive cells was detected (one-way ANOVA, P > 0.05) from P1 to P21. Also, the number of melanopsin-positive cells of this LL group, which ranged between 215 and 266 per retinal sample, was similar to that of the LD controls of the previous study (Student's t-test, P > 0.05 at all the comparisons between LL and LD groups).
Factorial ANOVA test did not reveal interaction between ambient condition and postnatal day, which means that both groups (LL and LD) behaved similarly throughout the postnatal period analyzed.
Exposure of Albino Mice to LL Followed by LD Cycles
To examine whether the melanopsin system could be restored after being exposed to LL, albino animals were subjected to LL from the day of birth to P11 (when rods and cones start to be functional) followed by 3, 5, 10, or 28 LD cycles.
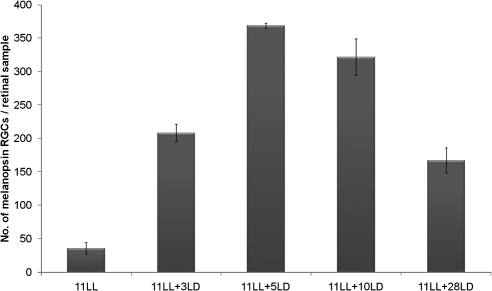
After keeping animals 11 days in LL plus 3 days in LD cycles, the number of immunopositive cells increased significantly (fourfold increase; P < 0.001; Fig. 5). Immunoreactive processes were more numerous and showed more intense staining. After 5 LD cycles the number of immunopositive somata was even higher (P < 0.001), which suggests that the increase was time dependent. Dense dendritic plexuses comparable to those seen in mice exposed to DD could be observed in the IPL (Fig. 2D). After 10 LD cycles (post-LL), the values did not increase further (P > 0.05). Finally, after 28 LD cycles (post-LL), the number of melanopsin cells decreased (P > 0.001), and the values were comparable to those in untreated animals (LD controls).
Figure 5.
Increase in the number of melanopsin-immunopositive cells in animals maintained in LD conditions after a period of exposure to LL. One-way ANOVA showed significant differences after the LD periods (P < 0.001). n = 4 animals per group.
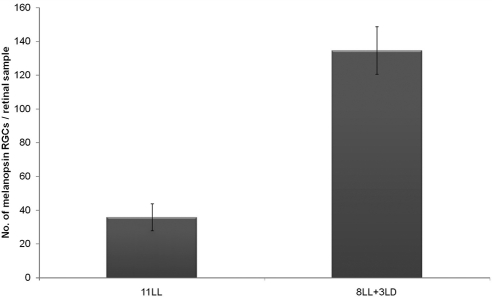
Therefore, changes induced by continuous light on the melanopsin system in the early postnatal period seemed to be reversible. Assuming that until P11 rods and cones were not fully developed, we intended to examine the recovery of the melanopsin system independently of rod-cone inputs.29 Animals were exposed to LL from the day of birth to P8, followed by 3 LD cycles. In this group an increase in the number of immunopositive cells (P < 0.001; Fig. 6) was detected in response to the LD cycles. Within this period, the ipRGCs are the only functional photoreceptors in the retina, which means that these cells are capable in the absence of rods and cones. However, the cell number increase observed under 8 days in LL + 3 LD cycles was less than that observed after 11 days in LL + 3 LD cycles (P < 0.01), suggesting that in the presence of functional rods and cones, recovery is somewhat faster.
Figure 6.
Effect of exposure to three LD cycles after 8 days in LL on the melanopsin-expressing cell number in the early postnatal period, when pathways of rod-cone photoreceptors are not functional. Student's t-test detected significant differences (P < 0.001) between the two ambient lighting conditions. n = 4 animals per group.
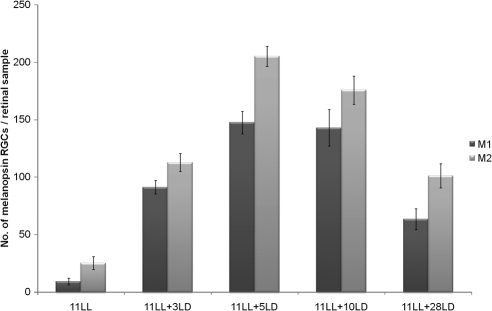
In addition, we analyzed the recovery of this non–image-forming vision mediated by the M1 and M2 cell subtypes. After 11 days under LL conditions and 3, 5, 10, and 28 LD cycles, factorial ANOVA showed that M1 and M2 cells behaved similarly (P > 0.05). Post hoc tests detected that both subtypes increased gradually until 11 days under LL plus 5 days in LD cycles (P < 0.001; Fig. 7). Then a significant diminution of both subtypes after 28 LD cycles could be observed (P < 0.001). At this time, no differences in M1 and M2 cells were detected between 11 days LL + 28 LD cycles and animals that have been maintained only under LD conditions. M1 and M2 cells were also analyzed after 8 days LL + 3 LD cycles, and post hoc tests detected a similar increase of M1 and M2 cells.
Figure 7.
Increase in the number of M1 and M2 cells under LD after the severe decrease induced by LL. Both cell subpopulations showed an increase in numbers during the first days of the LD period (P < 0.001). Afterward a decrease (P < 0.001) was observed, and the values reached the standard ones observed in the LD controls. n = 4 animals per group.
Exposure of Albino Mice to DD Followed by LL
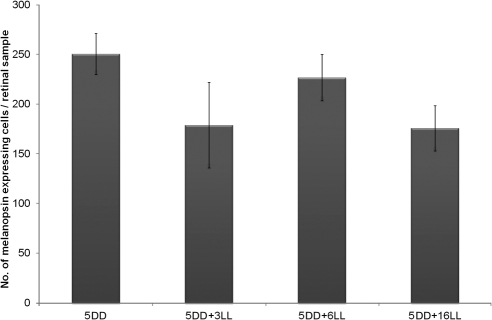
At P5, mice kept under DD conditions showed a high number of positive cells. To determine whether LL was able to inhibit melanopsin expression during postnatal development, mice were kept under DD until P5 and then were exposed to LL for 3, 6, and 16 days. Surprisingly, after 16 LL cycles, we did not detect a diminution in the number of immunostained cells (P > 0.05; Fig. 8). However, the intensity of staining was very weak, particularly in the dendrites, which were barely visible (Fig. 2E).
Figure 8.
Effect of LL exposure after 5 days in DD. No decrease in the number of immunopositive cells was detected (one-way ANOVA test; P > 0.05). n = 4 animals per group.
M1 and M2 cells were also analyzed after 5 days under DD plus 3–6 days under LL, and factorial ANOVA revealed that both cell subpopulations behaved similarly (P > 0.05). When one-way ANOVA was performed separately for M1 and M2 cells, no changes in the number of cell subtypes were detected (P > 0.05). M1 and M2 cell numbers could not be analyzed after 5 days under DD plus 16 days under LL because of the absence of two well-defined plexuses. In this group, only the somata were clearly immunolabeled.
Discussion
Within the past decade, the role of melanopsin-expressing RGCs in image-forming and non–image-forming photoreceptive processes has been a matter of thorough study. The ipRGCs contribute to circadian photoentrainment but have also other important functional roles, such as the promotion of sleep and the control of pupil diameter.2
Classically, it was believed that pups were indirectly synchronized to external cues through melatonin present in maternal milk30,31; however, there are many findings suggesting that the melanopsin system is indeed active at very early stages. Tarttelin et al.32 found evidence of melanopsin transcripts in the mouse retina as early as embryonic day 10.5. This means that melanopsin is expressed much earlier than rod and cone opsins, which appear in the postnatal period.33 In addition, it has been shown that as early as P1, melanopsin immunoreactivity was present in retinal ganglion cells.14,15,29,34 Additionally, it is known that melanopsin-expressing cells respond to light at birth15 and it has been reported that the SCN is photically responsive in an intensity-dependent manner from the day of birth18 or from P4 on.19 This means that functional connections between the retina and the SCN are already established on the day of birth, when classical photoreceptors are not functional. In a previous study we detected that the number of melanopsin-expressing cells in pigmented mice does not change significantly during the postnatal period.14 Taken together, these data indicate that melanopsin-expressing cells, which are principal elements of the non–image-forming photoreceptive system, are functional during early postnatal development.
Under LD conditions, we detected an increase in the number of melanopsin-expressing cells during the early postnatal period of albino CD1 mice. Fahrenkrug et al.34 showed that the immunostaining intensity and the number of melanopsin-positive cells increased during the postnatal development of the albino Wistar rat, which is in agreement with our findings on a different albino rodent model.
When pups were maintained under LL conditions, a lower number of melanopsin-immunoreactive RGCs was detected relative to LD controls, which showed an increase. On the contrary, when newborn mice were kept under DD, the number of immunopositive cells increased significantly compared with the LD controls. Moreover, differences in the immunolabeling of the dendrites were also detected. During DD melanopsin immunoreactive dendrites formed an extensive network that could fulfill the requirements for a broad-capture photoreceptive system, whereas during LL melanopsin immunoreactive dendrites were limited to the somata and proximal dendrites. These results agree with those of Hannibal et al.21,23 and Mathes et al.24 in albino rats, who reported a diminution of melanopsin protein and mRNA under LL and an increase under DD. Changes in the number of melanopsin-immunoreactive cells and dendritic network that we observed in LL and DD conditions suggest a functional adaptation of the retina to the external illumination conditions. This regulation at such early developmental stages must necessarily be due to melanopsin-expressing RGCs. Schmidt et al.29 detected synaptically driven light responses in the ipRGCs that were evoked by cone/rod pathways by the second postnatal week (P11–P14). Some of our observations were made at ages when rod-cone pathways are not yet functional (P1, P5, P8), indicating that melanopsin-expressing RGCs are capable of adapting their responsiveness to ambient lighting conditions even in the absence of the classical photoreceptors, likely through their inherent photosensitivity. Differences in the number of immunopositive cells among LD, LL, and DD groups could be attributed to the amount of melanopsin protein per cell. However, pigmented mice (C3H/He) raised under LL conditions from birth did not show a decrease in the number of immunopositive cells. Therefore, we hypothesized that such melanopsin suppression might be associated with albinism. However, both strains have genetically different backgrounds (i.e., the mutation causing albinism is not the only difference between them); thus, additional data on other strains are needed to elucidate this issue.
The present work analyzes for the first time the postnatal development of M1 and M2 cells in albino mice, which are the main subpopulations recognized among the melanopsin- expressing RGCs.26,27 All previous studies in albino models were conducted in rats,20,21,23,34 in which only one of the two cell subpopulations mentioned has been reported to date, corresponding to the mouse M1 cells. Under LD conditions, we analyzed the development of M1 and M2 cells from P5 through P21, and the ratio of the two cell types changed through the postnatal period. Under LL conditions, we did not detect any change in the number of M1 and M2 cells. Under DD conditions, an increase in both cell types was observed; however, M2 cells showed a higher number than M1 cells. Such differences in the M1/M2 ratio might be attributed to daily fluctuations given the fact that in a previous study in pigmented mice, we detected a daily oscillation of both cell types from P5 on.22
In the present study we demonstrated that the melanopsin system can be restored by simply reestablishing standard LD conditions after inhibition by continuous light. Therefore, as we mentioned previously, the low number of melanopsin-expressing cells detected under LL seems to have resulted from an inhibition of the melanopsin system during early development or from a decrease of melanopsin protein per cell, perhaps because of a light-dependent degradation of the protein or a light-dependent downregulation of melanopsin transcription or translation. It should be noted that our results, which agree with those of Hannibal et al.21 in Wistar adult rats were obtained in mouse pups at very early postnatal stages, when rods and cones were not yet functional. This led to the conclusion that there is a light-mediated intrinsic response from these melanopsin-expressing cells. However, this recovery is somewhat faster when rod-cone photoreceptors are fully developed (at P11, they start to be functional). Therefore, rod-cone input might also contribute to the response detected in the melanopsin-expressing cells. Interactions of the ipRGCs with the rod-cone pathways were reported previously. Melanopsin-expressing RGCs were shown to synapse with both bipolar and amacrine cells,35,36 which indicates interactions with the rod-cone pathway. Moreover, Güler et al.10 have demonstrated that melanopsin-expressing RGCs are the principal conduits for rod-cone input to the non–image-forming vision. Our data reinforce the idea that classical photoreceptors and melanopsin interact actively.
Finally, the duration of the LD period (after LL exposure) also seems important. We found that 2 weeks are required to reach the standard levels observed in the LD controls. At the end of the LD period, the values of both M1 and M2 cell subpopulations in the LL+LD group were similar to those of the LD group. Our experiments demonstrate that the effects of constant light exposure on the ipRGC postnatal development can be reverted if the animals are maintained in a period of LD cycles. However, Canal-Corretger et al.37 reported that albino mice raised in an environment of continuous light during the first days of life showed normal functioning of the suprachiasmatic nucleus but that these mice manifest a significant decline in photic visual sensitivity (i.e., continuous light during postnatal development affects visual perception of mice).
Surprisingly, when newborn mice were kept in constant darkness and maintained under LL conditions afterward, the expected decrease in the number of melanopsin-immunostained cells was not observed. However, the intensity of immunolabeling was lower, and dendritic arborization was not seen.
As mentioned, differences observed between albino and pigmented mice under LL might be related to albinism. It is known that albinism provokes a delay in retinal development,38 and, in fact, we have observed that the development of the melanopsin system of albino mice is delayed with regard to C3H/He mice. However, as shown in Results, LL did not provoke a decrease in the number of melanopsin-expressing cells in P5 albino mice that were previously exposed to DD. Hence, we suggest that the inhibition of melanopsin expression detected might be an effect of LL on a yet immature retina. If this is the case, delayed development makes this strain a very interesting model in which to study the influence of ambient illumination on the melanopsin photoreceptive ganglion cells at the early postnatal period.
Albino mice kept in LL since P1 showed a low number of melanopsin-immunostained cells compared with those exposed to LD/DD. These data suggest that darkness is somehow necessary for the development of the melanopsin system. As we have shown, exposure to LD cycles also restored the melanopsin expression previously inhibited by LL, which again points to the periods of darkness as responsible for the recovery. Therefore, periods of darkness or, more likely, the sequence of light/dark periods taking place under LD conditions may be necessary for the proper development of the melanopsin system.
The complex physiology of the photosensitive RGCs and the attendant signal pathways undoubtedly require further investigation with a multidisciplinary approach. Here we provide an initial analysis of the development and responsiveness of these cells in an albino mouse strain. New experiments will be carried out in the short term to understand more deeply the effects of ambient illumination on the murine melanopsin system during perinatal life.
Footnotes
Supported by Spanish Ministry of Science and Innovation Grant BFU2006-15576 (JMGF), National Institutes of Health Grant R01 NS052112 (IP), and Spanish FICYT Fellowships BP07-088 (IGM) and BP09-081 (FC).
Disclosure: I. González-Menéndez, None; F. Contreras, None; R. Cernuda-Cernuda, None; I. Provencio, None; J.M. García- Fernández, None
References
- 1.Foster RG, Hankins MW. Non-rod, non-cone photoreception in the vertebrates. Prog Retin Eye Res. 2002;21:507–527 [DOI] [PubMed] [Google Scholar]
- 2.Fu Y, Liao HW, Do MT, Yau KW. Non-image-forming ocular photoreception in vertebrates. Curr Opin Neurobiol. 2005;15:415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073 [DOI] [PubMed] [Google Scholar]
- 4.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells. Science. 2002;295:1065–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panda S, Sato TK, Castrucci AM, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216 [DOI] [PubMed] [Google Scholar]
- 6.Ruby NF, Brennan TJ, Xie X, et al. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213 [DOI] [PubMed] [Google Scholar]
- 7.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;12:1165. [DOI] [PubMed] [Google Scholar]
- 8.Hannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J Neurosci. 2002;22:191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hattar S, Kumar M, Park A, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Güler AD, Ecker JL, Lall GS, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Göz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS ONE. 2008;3:e3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatori M, Le H, Vollmers C, et al. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3:e2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strom RC, Williams RW. Cell production and cell death in the generation of variation in neuron number. J Neurosci. 1998;18:9948–9953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Menendez I, Contreras F, Cernuda-Cernuda R, Garcia-Fernandez JM. No loss of melanopsin-expressing ganglion cells detected during postnatal development of the mouse retina. Histol Histopathol. 2010;25:73–82 [DOI] [PubMed] [Google Scholar]
- 15.Sekaran S, Lupi D, Jones SL, et al. Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Curr Biol. 2005;15:1099–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tu DC, Zhang D, Demas J, et al. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999 [DOI] [PubMed] [Google Scholar]
- 17.Reppert SM, Schwartz WJ. The maternal suprachiasmatic nuclei are necessary for maternal coordination of the developing circadian system. J Neurosci. 1986;6:2724–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lupi D, Sekaran S, Jones SL, Hankins MW, Foster RG. Light-evoked FOS induction within the suprachiasmatic nuclei (SCN) of melanopsin knockout (Opn4−/−) mice: a developmental study. Chronobiol Int. 2006;23:167–179 [DOI] [PubMed] [Google Scholar]
- 19.Muñoz-Llamosas M, Huerta JJ, Cernuda-Cernuda R, García-Fernández JM. Ontogeny of a photic response in the retina and suprachiasmatic nucleus in the mouse. Brain Res Dev Brain Res. 2000;15:1–6 [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto K, Liu C, Tosini C. Classical photoreceptors regulate melanopsin MRNA levels in the rat retina. J Neurosci. 2004;24:9693–9697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannibal J, Georg B, Hindersson P, Fahrenkrug J. Light and darkness regulate melanopsin in the retinal ganglion cells of the albino Wistar rat. J Mol Neurosci. 2005;27:147–155 [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Menendez I, Contreras F, Cernuda-Cernuda R, Garcia-Fernandez JM. Daily rhythm of melanopsin-expressing cells in the mouse retina. Front Cell Neurosci. 2009;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannibal J, Georg B, Fahrenkrug J. Melanopsin changes in neonatal albino rat independent of rods and cones. Neuroreport. 2007;18:81–85 [DOI] [PubMed] [Google Scholar]
- 24.Mathes A, Engel L, Holthues H, Wolloscheck T, Spessert R. Daily profile in melanopsin transcripts depends on seasonal lighting conditions in the rat retina. J Neuroendocrinol. 2007;19:952–957 [DOI] [PubMed] [Google Scholar]
- 25.Pires SS, Hughes S, Turton M, et al. Differential expression of two distinct functional isoforms of melanopsin (Opn4) in the mammalian retina. J Neurosci. 2009;29:12332–12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29:476–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27:1763–1770 [DOI] [PubMed] [Google Scholar]
- 28.Jeffery G. The albino retina: an abnormality that provides insight into retinal development. Trends Neurosci. 1997;20:165–169 [DOI] [PubMed] [Google Scholar]
- 29.Schmidt TM, Taniguchi K, Kofuji P. Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. J Neurophysiol. 2008;100:371–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naitoh N, Watanabe Y, Matsumura K, et al. Alteration by maternal pinealectomy of fetal and neonatal melatonin and dopamine D1 receptor binding in the suprachiasmatic nuclei. Biochem Biophys Res Commun. 1998;253:850–854 [DOI] [PubMed] [Google Scholar]
- 31.Rowe SA, Kennaway DJ. Melatonin in rat milk and the likelihood of its role in postnatal maternal entrainment of rhythms. Am J Physiol Regul Integr Comp Physiol. 2002;282:797–804 [DOI] [PubMed] [Google Scholar]
- 32.Tarttelin EE, Bellingham J, Bibb LC, et al. Expression of opsin genes early in ocular development of humans and mice. Exp Eye Res. 2003;76:393–396 [DOI] [PubMed] [Google Scholar]
- 33.Morrow EM, Furukawa T, Cepko CL. Vertebrate photoreceptor cell development and disease. Trends Cell Biol. 1998;8:353–358 [DOI] [PubMed] [Google Scholar]
- 34.Fahrenkrug J, Nielsen HS, Hannibal J. Expression of melanopsin during development of the rat retina. Neuroreport. 2004;15:781–784 [DOI] [PubMed] [Google Scholar]
- 35.Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393 [DOI] [PubMed] [Google Scholar]
- 36.Østergaard J, Hannibal J, Fahrenkrug J. Synaptic contact between melanopsin-containing retinal ganglion cells and rod bipolar cells. Invest Ophthalmol Vis Sci. 2007;48:3812–3820 [DOI] [PubMed] [Google Scholar]
- 37.Canal-Corretger MM, Vilaplana J, Cambras T, Díez-Noguera A. Effect of light on the development of the circadian rhythm of motor activity in the mouse. Chronobiol Int. 2001;18:683–696 [DOI] [PubMed] [Google Scholar]
- 38.Ilia M, Jeffery G. Delayed neurogenesis in the albino retina: evidence of a role for melanin in regulating the pace of cell generation. Brain Res Dev Brain Res. 1996;95:176–186 [DOI] [PubMed] [Google Scholar]