This study demonstrates that γδ T cells play a major role in the generation and activation of uveitogenic T cells.
Abstract
Purpose.
To characterize the regulatory effect of γδ T cells in the activation of IL-17+ uveitogenic T cells.
Methods.
The authors administered the γδ TCR-specific antibody GL3 to B6 mice before or after antigen immunization and examined Th1- or Th17-polarized T-cell responses. The intensity of Th17 responses was also examined in responder T cells containing varying numbers of γδ T cells.
Results.
GL3 treatment resulted in varying degrees of depletion of circulating γδ T cells, depending on when the antibody was administered. The intensity of the αβTCR+IL-17+, but not the αβTCR+IFN-γ+, IRBP-specific T-cell responses was correlated to the percentage of γδ T cells in the responder T cells. Kinetic studies showed that early IL-17+ T cells were primarily γδ T cells, with a later gradual shift to αβ T cells. A close association was seen between the intensity of the IL-17+ autoreactive T-cell response and the percentage of γδ T cells in the responder T cells. Although a modest increase in γδ T cells among the responder T cells promoted the expansion of IL-17+ αβTCR+ T cells, a higher proportion of γδ T cells inhibited it.
Conclusions.
γδ T cells are actively involved in the generation of αβTCR+IL-17+ T cells. The number of γδ T cells and the αβ/γδ T-cell ratio in the responder T cells regulate the intensity of the Th17-type autoreactive T-cell response.
One of the many important immune functions attributed to γδ T cells is their ability to modulate adaptive immune responses.1 Although there is little doubt that γδ T cells have a regulatory effect on adaptive immune responses and can cause either upregulation or downregulation,2–5 the mechanisms involved remain largely unclear.
In our efforts to determine the role of γδ T cells in the generation of Th17 uveitogenic T cells in B6 mice susceptible to the induction of experimental autoimmune uveitis (EAU), we previously reported that αβ T cells purified from interphotoreceptor retinoid-binding protein (IRBP) peptide-immunized γδ TCR−/− mice generate only limited numbers of IL-17+ IRBP-specific T cells and that these numbers are increased when small numbers of γδ T cells are injected in vivo before immunization or are added to the responder T cells during in vitro stimulation.6 To further determine the role of γδ T cells in the generation of Th17 autoreactive T cells in EAU, we assessed the effect of in vivo administration of GL3, an antibody specific for mouse γδ TCR. We found that, though some treated mice showed significant amelioration of the subsequently induced EAU, in others the disease was unaffected or even exacerbated. To determine the underlying mechanisms, we carried out a systematic analysis of mice treated with antibody GL3 before and after immunization and assessed the kinetics of the generation of IFN-γ+ and IL-17+ IRBP-specific T cells in mice with or without GL3 treatment. Our results showed that mice that received a single dose of GL3 before immunization had almost undetectable levels of γδ T cells, whereas those that received the antibody after immunization showed partial retention of γδ T cells. The IL-17+ autoreactive T-cell response varied significantly between groups of responder T cells containing varying percentages of γδ T cells. Although modest increases in the number of γδ T cells significantly enhanced the response of αβTCR+IL-17+ T cells, a high percentage of γδ T cells among the responder T cells were associated with decreased activation of αβTCR+IL-17+ T cells. Our results support our previous observation that γδ T cells are an essential cellular component in the generation of αβTCR+IL-17+ T cells and show that the percentage of circulating γδ T cells and the αβ/γδ T-cell ratio in the responder T cells determine the intensity of the subsequent Th17 autoreactive T-cell response.
Materials and Methods
Animals and Reagents
Pathogen-free female C57BL/6 (B6) and γδ TCR−/− mice (age range, 12–14 weeks) were purchased from the Jackson Laboratory (Bar Harbor, ME) and were housed and maintained in the animal facilities of the University of Southern California. All animal studies conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Institutional approval was obtained, and institutional guidelines regarding animal experimentation followed. Recombinant murine IL-2 and IL-23 were purchased from R&D Systems (Minneapolis, MN). Peptide IRBP1–20 was synthesized by Sigma (St. Louis, MO), and complete Freund's adjuvant (CFA) was obtained from the same source. Fluorescein isothiocyanate (FITC)-conjugated anti–IL-17 antibodies were purchased from BioLegend (San Diego, CA), and antibodies against murine TCR-δ (GL3) were purchased from BD Biosciences (La Jolla, CA).
EAU Model
EAU was induced in B6 mice by subcutaneous injection of 200 μL emulsion containing 200 μg IRBP1–20 in CFA at six spots at the tail base and on the flank and by intraperitoneal injection with 300 ng pertussis toxin, as described previously.6–8 When used, the antibody GL3 was injected intraperitoneally at a dose of 300 μg at the indicated time before or after immunization with IRBP1–20.
Assessment of the Uveitogenic Activity of IRBP1–20-Specific T Cells
Briefly, groups of B6 mice, with or without intraperitoneal injection of antibody GL3, were immunized as described. On day 13 after immunization, T cells were isolated from lymph node cells and spleen cells by passage through a nylon wool column, after which 1 × 107 cells in 2 mL RPMI 1640 medium (Mediatech, Herndon, VA) and 10% fetal calf serum (FCS; Thermo Scientific, Pittsburgh, PA) in a six-well plate (Costar, Corning, NY) were stimulated for 48 hours with 10 μg/mL IRBP1–20 in the presence of 1 × 107 irradiated syngeneic spleen cells as antigen-presenting cells (APCs) in the presence of either IL-2 or IL-23 (10 ng/mL). The activated T-cell blasts were separated by Ficoll gradient centrifugation and cultured for another 72 hours in RPMI 1640 medium containing 10% FCS and then were tested for pathogenic activity by transfer to syngeneic naive recipients.
Enrichment of γδ T Cells from IRBP1–20-Immunized B6 Mice
T cells prepared from the spleens and draining lymph nodes of IRBP1–20-immunized B6 mice were stimulated for 2 days in vitro with immunizing antigen, followed by culture in IL-23–containing (10 ng/mL) medium for 3 days. They were incubated for 10 minutes at 4°C with FITC-conjugated anti–mouse γδ TCR antibody and then for 15 minutes at 4°C with anti–FITC microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany).9 The cells were separated into bound and nonbound groups on an separator column (autoMACS; Miltenyi Biotec GmbH) and were washed with 15 mL medium according to the manufacturer's protocol, and the bound cells (γδ T cells) were collected. The purity of the isolated cell fraction was determined by flow cytometric analysis using FITC-conjugated anti–TCR antibodies and phycoerythrin-conjugated antibodies against γδ T cells or αβ T cells (BD Biosciences). Data collection and analysis were performed on a flow cytometer (FACSCalibur; BD Biosciences) using appropriate software (CellQuest; BD Biosciences). The γδ T cells had a purity of 95%. The αβ T cells were prepared similarly after incubation of the immunized T cells with bead-conjugated antibody specific for mouse αβ TCR (BioLegend).
Assessment of Th1- and Th17-Polarized Responses
Responder T cells were prepared from IRBP1–20-immunized TCR-δ−/− mice, after which 3 × 106 responder T cells were cocultured with IRBP1–20 (10 μg/mL) and APCs (irradiated spleen cells) in a 12-well plate, with or without the addition of 1% to 10% of purified γδ T cells from IRBP1–20-immmunized B6 mice under Th17-polarized conditions (culture medium supplemented with 10 ng/mL IL-23) or Th1-polarized conditions (culture medium supplemented with 10 ng/mL IL-2). After 48 hours of in vitro stimulation, cytokines in the culture medium were measured by ELISA, and the numbers of antigen-specific T cells expressing IL-17 or IFN-γ were determined by intracellular staining followed by FACS analysis.
Immunofluorescence Flow Cytometry
Aliquots of 2 × 105 cells were double stained with combinations of FITC- or phycoerythrin-conjugated monoclonal antibodies. Data collection and analysis were performed on a flow cytometer (FACSCalibur; BD Biosciences) using appropriate software (CellQuest; BD Biosciences). To assess intracellular cytokine expression, unfractionated IRBP1–20-specific T cells from immunized B6 mice were stimulated in vitro for 4 hours with 50 ng/mL phorbol myristate acetate, 1 μg/mL ionomycin, and 1 μg/mL Brefeldin A (Sigma, St. Louis, MO). Then they were washed, fixed, permeabilized overnight in buffer (Cytofix/Cytoperm; eBioscience, San Diego, CA), intracellularly stained with antibodies against IFN-γ or IL-17, and analyzed (FACSCalibur; BD Biosciences).
ELISA
IL-17 and IFN-γ were measured using commercially available ELISA kits (R&D Systems).
Scoring of EAU
The mice were examined twice a week for clinical signs of EAU by indirect funduscopy. Their pupils were dilated using 0.5% tropicamide and 1.25% phenylephrine hydrochloride ophthalmic solutions. Grading of disease was performed using the scoring system described previously.10
Statistical Analysis
Data are expressed as the mean ± SD for the results from at least three separate experiments.
Results
Treatment of B6 Mice with Antibody GL3 Inhibits the Generation of IL-17+ Uveitogenic T Cells, but the Effect Is Dependent on the Time of Antibody Administration
To determine the effect of GL3 (anti–mouse TCR-δ chain antibody) on the generation of IL-17+ uveitogenic T cells in vivo, we injected B6 mice with 300 μg GL3 either 3 days before or 3 days after immunization with the uveitogenic peptide IRBP1–20; control mice underwent only antigen immunization. The uveitogenic activity of IL-17+ IRBP-specific T cells isolated from the immunized mice, with or without antibody treatment, was assessed by adoptive transfer to naive B6 mice after in vitro stimulation with the immunizing antigen under Th17-polarized conditions (culture medium supplemented with 10 ng/mL IL-23). Development of EAU was monitored by funduscopy and histologic examination. Figure 1 shows that administration of GL3 antibody before immunization decreased the pathogenic activity of the IRBP-specific T cells, and the recipient mice showed delayed disease onset and early recovery (Fig. 1A) in addition to overall milder disease (Fig. 1C). If the antibody was administered after immunization, the uveitogenic activity of the generated IRBP-specific T cells was mostly unaffected, or, in some instances, enhanced (Fig. 1D).
Figure 1.
Administration of GL3 to B6 mice inhibits the generation of uveitogenic T cells, but the effect is dependent on the timing of antibody administration. B6 mice were randomly divided into 3 groups (n = 4) and immunized with uveitogenic peptide IRBP1–20. Control mice received antigen alone; the other two groups were also injected intraperitoneally with a single dose of GL3 (300 μg/mouse) either 3 days before (GL3–3) (A, C) or 3 days after (GL3+3) (A, D) antigen immunization. The immunized T cells were stimulated for 2 days in vitro with immunizing peptide and APCs. Separated activated T cells were adoptively transferred to the (n = 4) naive B6 mice group. EAU was scored by funduscopy (A) and histologic examination (B–D). Results shown are representative of those from five experiments.
Effect of GL3 Administration on the Generation of Th1 and Th17 Uveitogenic T Cells in Immunized B6 Mice
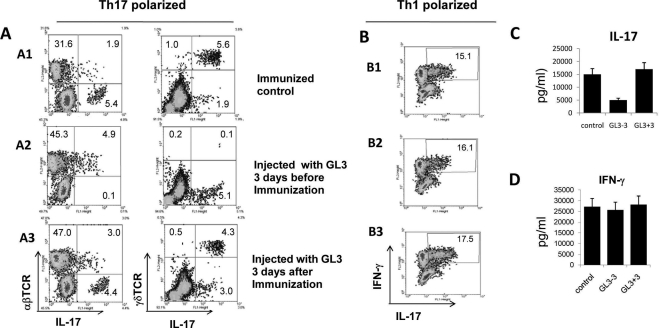
To compare the effect of GL3 treatment on the generation of Th1 and Th17 IRBP-specific T cells, in vivo primed IRBP-specific T cells were stimulated with the immunizing antigen under Th17-polarized (culture medium supplemented with IL-23) or Th1-polarized (culture medium supplemented with IL-2) conditions, and the activated T cells were examined for kinetics of expression of IL-17 or IFN-γ. The percentages of γδ and αβ T cells in the IL-17+ T cells were determined by separate staining of IL-17+ T cells with antibodies specific for αβTCR and γδTCR. The results in Figure 2A show that, at 8 days after in vitro stimulation of the IRBP-specific T cells of immunized mice treated with GL3 at the indicated time points under Th17-polarized conditions, the percentage of generated αβTCR+IL-17+ IRBP-specific T cells was significantly higher in mice treated 3 days before (4.9%, A2) or after (3.0%, A3) immunization than in untreated mice (1.9%, A1).
Figure 2.
Effect of GL3 administration on Th1 and Th17 T-cell generation. (A) γδ T cells are more resistant to depletion by GL3 if the antibody is administered after antigen immunization. B6 mice were randomly divided into three groups (n = 5) and were immunized with IRBP1–20. One group was not treated with GL3, but the other two groups received single doses of GL3 (300 μg/mouse) intraperitoneally 3 days before or after antigen immunization. Responder T cells were stimulated with immunizing antigen for 2 days under Th17-polarized conditions and were cultured in IL-23–conditioned medium for an additional 3 days. Then the proliferating T cells were stained for γδ TCR or αβTCR and IL-17. The results shown are representative of those from five experiments. (B) GL3 has little effect on the Th1-polarized, IRBP-specific T-cell response. In vivo primed T cells, prepared from immunized B6 mice with or without GL3 treatment, were stimulated with immunizing antigen under Th1-polarized conditions (culture medium contains 10 ng/mL IL-2). Then IFN-γ+ T cells among the IRBP-specific T cells were assessed by intracellular staining. (C, D) In vivo primed IRBP-specific T cells from the three groups of mice were stimulated with immunizing antigen under Th17 (C) or Th1 polarization (D) conditions for 48 hours, and the culture supernatants were assayed for IFN-γ and IL-17 by ELISA.
Under Th1-polarized conditions, the percentage of IFN-γ+ T cells among the IRBP-specific T cells differed only slightly between the group of mice that were treated (B2 and B3) or not treated (B1) with GL3 antibody (Fig. 2B). ELISA showed that GL3 had a significant inhibitory effect on IL-17 production if administered before immunization (Fig. 2C), whereas the effect on IFN-γ production was marginal (Fig. 2D).
γδ T Cells Are More Resistant to Depletion by GL3 if the Antibody Is Administered after Antigen Immunization
The results shown in Figure 2 also demonstrated that GL3 administration at different time points relative to immunization had different effects on αβTCR+ IL-17+ IRBP-specific T cells. As shown, very few (0.3%) γδ T cells were detected among the responder T cells in mice that received GL3 before immunization (Fig. 2A2). However, in mice given GL3 treatment 3 days after immunization (Fig. 2A3), γδ T cells were only incompletely depleted (4.8%) compared with mice not injected with GL3 (6.6%), suggesting that, after immunization, some of the γδ T cells became more resistant to antibody depletion in vivo.
A Significant Proportion of the γδ T Cells in Immunized Mice Express Intracellular, but Not Surface, γδ TCR
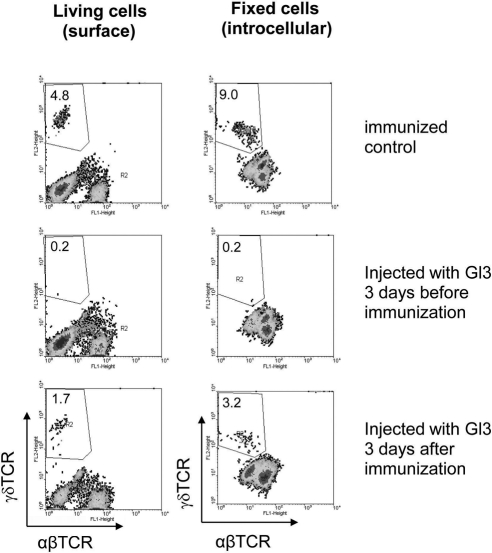
On the basis of our previous observation that γδ cells stop expressing surface TCR when they become activated,11 we examined whether the percentage of γδ T cells in injected mice administered with GL3 antibody at different time points of immunization was caused by the immunization process, leading to γδ T cell activation in vivo and activated γδ T cells expressing decreased levels of surface TCR. γδ T cells from immunized mice were assessed by FACS analysis after surface and intracellular staining. As shown in Figure 3, approximately half the γδ T cells in immunized mice expressed intracellular, but not surface, TCR.
Figure 3.
A significant proportion of the γδ T cells in immunized mice express intracellular, but not surface, γδ TCR. One group of B6 mice was untreated, and the other received a single dose of GL3 (300 μg/mouse) intraperitoneally. Three days later, both were immunized with IRBP1–20. Enriched T cells prepared from draining lymph nodes and spleens 13 days after immunization were stimulated in vitro with immunizing antigen in the presence of IL-23, and the separated T cells were cultured in IL-23–conditioned medium for another 6 days (8 days of stimulation). Living cells (left) or fixed cells (right) were stained for expression of γδ TCR. Results shown are representative of those from three experiments.
The Early IL-17+ T Cell Response Is Dominated by γδ T Cells, Which Are Gradually Replaced by αβ T Cells
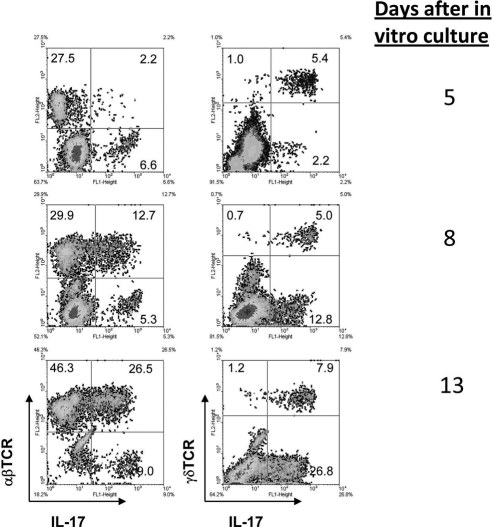
We previously reported that both γδ T cells and αβ T cells are able to express IL-17 once activated.7,11 To determine the percentages of γδ and αβ T cells in the IL-17+ T cells, IRBP1–20-specific T cells from the spleens and the draining lymph nodes of immunized B6 mice were enriched by passage through nylon wool at day 13 after immunization and were subjected to antigenic stimulation in vitro by exposure for 2 days to immunizing antigen and APCs in medium containing IL-23 (Th17 polarized). After that, the activated T-cell blasts were separated by Ficoll gradient centrifugation, cultured in IL-23–conditioned medium, and subjected to intracellular staining with FITC-anti–IL-17 antibodies. These kinetic studies showed that, under these conditions, the percentage of IL-17+ T cells increased gradually during in vitro expansion of the antigen-specific T cells. As shown in Figure 4, over the 13-day in vitro observation period, most of the IL-17+ T cells during the first 5 days were γδ T cells; αβ T cells became the dominant IL-17+ T cells at days 8 to 13. Thus, T cells cultured from control mice immunized with antigen alone (no GL3 treatment) contained 5.4% of IL-17+ γδ T cells at 5 days of in vitro culture, and this increased to 7.9% by day 13. IL-17+ αβT cells, on the other hand, accounted for only 2.2% of the total responder T cells on day 5 but 26.5% after 13 days of culture.
Figure 4.
The early Th17+ T-cell response is dominated by γδ T cells, which are gradually replaced by αβ T cells. B6 mice were immunized with IRBP1–20, and T cells were prepared and stimulated in vitro with the immunizing antigen under Th17-polarized conditions. On days 5 (A), 8 (B), and 13 (C) of in vitro culture, the proliferating T cells were sampled for staining and FACS analysis.
The Intensity of the αβTCR+ IL-17+ IRBP-Specific T Cell Response in Immunized Mice Changes with the Percentage of γδ T Cells in the Responder T Cells
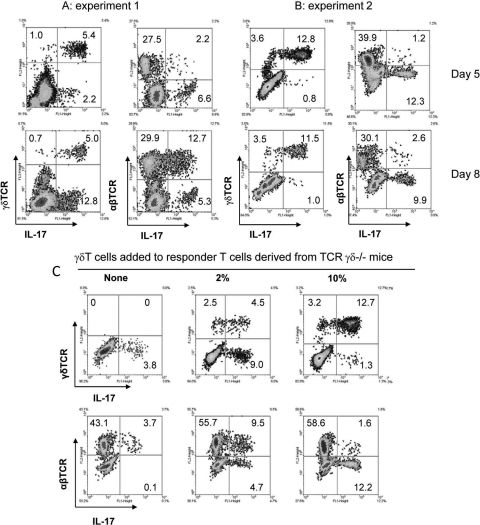
We repeatedly observed that not only the absolute number, but also the IL-17+γδTCR+/IL-17+αβTCR+ T-cell ratio, varies significantly between experiments. The percentage of γδ T cells in the responder T cells from immunized mice varied between 5% and 15% after antigenic stimulation in vitro under Th17-polarized conditions. Interestingly, a modest increase in the number of γδ T cells (2%–5%) among the responder T cells was always followed by a strong expansion of IL-17+ αβ T cells. However, the appearance of a higher proportion of γδ T cells among the responder T cells was always followed by significantly weaker expansion of IL-17+ αβ T cells, as illustrated in the two representative experiments shown in Figure 5. In experiment 1 (Fig. 5A), γδ T cells accounted for 5% to 7% of the total responder T cells (5.4% γδTCR+IL-17+ and 1.0% γδTCR+IL-17−) when assessed on day 5 of in vitro expansion under Th17-polarized conditions, whereas IL-17+ αβ T cells accounted for only 2.2% of the total responder T cells on day 5, but this rapidly increased to 12.7% by day 8. In experiment 2, γδ T cells made up 16.4% of the total responder T cells on day 5 (Fig. 5B), whereas the percentage of IL-17+ αβ T cells was 1.2% and only marginally increased to 2.6% on day 8, five times lower than the corresponding percentage (12.7%) of IL-17+ γδ T cells on the same day.
Figure 5.
The intensity of the αβTCR+ IL-17+ IRBP-specific T-cell response is markedly influenced by the percentage of γδ T cells in the responder T cells. (A, B) Two representative assays showed that a modest percentage of γδ T cells in the responder T cells was associated with high numbers of αβTCR+ IL-17+ T cells, while a higher percentage was associated with a weaker expansion of IL-17+ αβTCR+ T cells. (C) The addition of a small percentage (2%) of γδ T cells to γδ-deficient responder T cells promotes the generation of αβTCR+ IL-17+ T cells, whereas the addition of a higher percentage (10%) reverses the promoting effect of γδ T cells. Responder αβTCR+ IRBP-specific T cells were prepared from IRBP-immunized γδTCRδ−/− mice, after which 3 × 106 total responder T cells were stimulated in 12-well plates with immunizing antigen (10 ng/mL) and syngeneic APCs, with or without the addition of 2% or 10% of γδ T cells isolated from immunized B6 mice. Intracellular staining and enumeration of IL17+ T cells were performed as described. Results shown are representative of those from three experiments.
To further determine whether a high percentage of γδ T cells has a suppressive effect on the generation of αβ IL-17+ IRBP-specific T cells, we prepared in vivo–primed αβ T cells from immunized TCR-δ−/− mice and stimulated them with the immunizing antigen in vitro, with or without the addition of purified γδ T cells isolated from immunized B6 mice. As shown in Figure 5C, the αβ T cells from TCR-δ−/− mice contained only a small percentage of IL-17+ T cells (Fig. 5C). However, when 2% of γδ T cells was added, the percentage of αβ (i.e., γδ-negative) IL-17+ IRBP-specific T cells increased significantly to 9.5%. As the percentage of γδ T cells was increased to 10% of the total responder T cells, however, the percentage of γδ-negative IL-17+ IRBP-specific T cells declined significantly.
Discussion
γδ T cells have been shown to be major infiltrating cells in the virally infected lung12–14 and in inflamed autoimmune organs in diseases such as encephalomyelitis,15–17 arthritis,18 colitis,19,20 and myositis.21 The role of these T cells in tissue inflammation and in the pathogenesis of autoimmune diseases remains largely unknown. Studies have shown that γδ T cells act to “bridge the gap” between innate and adaptive immunity22–26 or to regulate the intensity of adaptive immune response.4,27–29 There is also evidence that different γδ T-cell subsets have different immunoregulatory roles.30–36
In a previous report, we demonstrated that the number of generated IL-17+ αβTCR+ IRBP-specific T cells in γδTCR−/− mice was increased if a small number of γδ T cells was administered to the γδTCR−/− mice before antigen immunization.6 Because knockout mice often behave differently than wild-type mice,37 we wanted to verify this observation by testing whether the administration of antibody GL3 to B6 mice affected EAU susceptibility and the generation of uveitogenic T cells. We found that GL3 administration has a different effect on EAU development, depending on when GL3 is administered relative to immunization (Fig. 1). In fact, such a variable effect has been previously observed in a study in which injection of the anti–γδTCR antibody before type II collagen injection significantly delayed the onset and severity of the induced arthritis, whereas antibody injection after collagen injection resulted in rapid onset of severe arthritis.18
Analysis of the effect of GL3 injection on Th1 and Th17 uveitogenic T cells revealed that manipulation of γδ T cells readily altered the generation of αβTCR+IL-17+ IRBP-specific T cells. In mice that received GL3 treatment, when modestly increased numbers of γδ T cells were left in the responder T cells (compared with the naive mouse), the percentage of generated IL-17+ αβTCR+ T cells was significantly higher than in those immunized mice, which contained a higher percentage of γδ T cells. A negative correlation between the percentage of γδ T cells and that of the generated αβTCR+IL-17+ T cells was repeatedly observed, suggesting that a high percentage of γδ T cells disfavors the expansion of IL-17+αβ TCR+ T cells (Fig. 5). Such an observation implies that variations in the number of the γδ T cells may regulate the generation of autoreactive T cells in autoimmune diseases. It was interesting to note that a previous report showed that γδ T cells constitute 0.5% to 16% of CD3+ cells in peripheral human lymphoid organs and adult peripheral blood, even though physiologic and pathologic relevance of such cellular alteration to immune response remained unclear.38
At first, the observation that partial depletion of expanded γδ T cells in the immunized T cells enhances the generation of Th17 IRBP-specific T cells appeared not to be compatible with our previous observation that injection of a small number of γδ T cells into TCR-δ−/− mice enhances the generation of Th-17 IRBP-specific T cells.6 We previously reported that the relative frequency of γδ T cells increases greatly in the draining lymph nodes and spleens of immunized mice and increases further during in vitro culture under Th17-polarized conditions.6 We therefore postulated that, in the early phase of the immune response, the activation and expansion of γδ T cells favor a faster and stronger Th-17 response, but, once the percentage of γδ T cells reaches an optimum, any further increase may suppress the immune response. As a result, the addition to γδTCR−/− mice of γδ T cells promotes the response, and partial depletion of an excessive number of γδ T cells from immunized mice promotes the generation of IL-17+ IRBP-specific T cells. Such a prediction was supported by our in vitro study, in which the addition of a low percentage of γδ T cells to responder αβ T cells enhanced, and the addition of a high percentage of γδ T cells suppressed, the response of αβTCR+IL-17+ IRBP-specific T cells. Our studies show that γδ T cells have a dual effect on the generation of IL-17+ αβTCR+ IRBP-specific T cells. Modestly increased numbers of γδ T cells promote, but excessive numbers inhibit, the generation of IL-17+ αβTCR+ IRBP-specific T cells. In 10 separate experiments, approximately half the results were similar to those seen in Figure 5A, and the rest of the results were similar to those seen in Figure 5B. As shown, the percentage of γδ T cells among the total responder T cells varied significantly between experiments, and the appearance of a higher proportion of γδ T cells was followed by significantly weaker expansion of IL-17+ αβTCR+ T cells. At this point, we are unable to determine the exact factors causing the variation in γδ T-cell numbers between experiments. However, previous studies have shown that components in the mycobacteria and pertussis toxin play a major role regulating γδ T-cell activation in immunized animals.11,39 It remains to be determined whether minimal change in the use of mycobacteria between experiments has led to the observed variation. These studies demonstrate the complex regulatory effect of γδ T cells on the adaptive immune response. Further studies should allow us to determine the underlying mechanism.
Results shown in Figure 2 demonstrated that though GL3 antibody administration enhanced the generation of IRBP-specific, IL-17+ αβ T cells, IL-17 production assessed by ELISA was significantly decreased. Given that both γδ and αβ T cells are able to produce IL-17 and that early IL-17 production is dominated by γδ T cells (Fig. 4), it is likely that the γδ T-cell depletion caused the decrease in IL-17 production, which was not compensated by the increased activation of IL-17+ αβ T cells. It remains to be determined whether the effect on EAU development of GL3 treatment is affected by the balance of increased activation of the IL-17+ αβ T cells, the production of inflammatory cytokine such as IL-17, and other unidentified cellular or molecular changes.
Whether in vivo administration of GL3, a monoclonal antibody specific for the mouse γδ TCR,40–43 has a depleting or a modulating effect on γδ T cells has been debated.34,40,44 A recent study has reported that treatment with GL3 mAb did not deplete γδ T cells in vivo but rather led to TCR internalization and thereby generated “invisible” γδ T cells.45 Our study showed that GL3 antibody did deplete γδ T cells in vivo when applied to naive mice but failed to do so effectively in immunized mice. Given that activated γδ T cells may downregulate surface TCR,11 we analyzed γδ T cells using both intracellular and surface staining. Our results showed that intracellular staining detected a higher percentage of γδ T cells than surface staining in immunized mice; in addition, the administration of GL3 after antigen immunization left more residual γδ T cells. These observations suggest that a portion of the γδ T cells in immunized mice lost their surface-expressed TCR, which might explain why GL3 administration after immunization left more residual γδ T cells. Given our previous observation that γδ T cells can be activated by exposure to proinflammatory cytokines or TLR ligands,11 we predict that a preexisting immune response may result in the generation of proinflammatory cytokines that cause γδ T-cell activation and thus thwart attempts at γδ T-cell depletion in vivo. However, our results do not formally exclude the possibility that residual γδ T cells are activated by GL3 to a different activation status and thus become functionally altered. We are investigating whether γδ T cells at different activation states also differ in their functions.
Although the Th17-polarized autoreactive T-cell response was closely regulated by the percentage of γδ T cells, the Th1-polarized T-cell response was less affected. It remains to be determined whether such a dissociated regulatory effect on Th1 and Th17 T-cell activation might have implications for therapeutic interventions aimed at individual autoreactive T-cell subsets. Together, our results illustrate the complex regulatory interaction between γδ and αβ T cells during the pathogenesis of autoimmune disease.
Footnotes
Supported in part by National Institutes of Health Grants EY018827, EY017373, and EY003040 (Core).
Disclosure: H. Nian, None; H. Shao, None; G. Zhang, None; W.K. Born, None; R.L. O'Brien, None; H.J. Kaplan, None; D. Sun, None
References
- 1.Moser B, Eberl M. γδ T cells: novel initiators of adaptive immunity. Immunol Rev. 2007;215:89–102 [DOI] [PubMed] [Google Scholar]
- 2.Odyniec A, Szczepanik M, Mycko MP, et al. γδ T cells enhance the expression of experimental autoimmune encephalomyelitis by promoting antigen presentation and IL-12 production. J Immunol. 2004;173:682–694 [DOI] [PubMed] [Google Scholar]
- 3.Tagawa T, Nishimura H, Yajima T, et al. Vδ1+ γδ T cells producing CC chemokines may bridge a gap between neutrophils and macrophages in innate immunity during Escherichia coli infection in mice. J Immunol. 2004;173:5156–5164 [DOI] [PubMed] [Google Scholar]
- 4.D'Souza CD, Cooper AM, Frank AA, et al. An anti-inflammatory role for γδ T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–1221 [PubMed] [Google Scholar]
- 5.Stinissen P, Zhang J, Vandevyver C, et al. γδ T-cell responses to activated T cells in multiple sclerosis patients induced by T cell vaccination. J Neuroimmunol. 1998;87:94–104 [DOI] [PubMed] [Google Scholar]
- 6.Cui Y, Shao H, Lan C, et al. Major role of γδ T cells in the generation of IL-17+ uveitogenic T cells. J Immunol. 2009;183:560–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng Y, Han G, Shao H, et al. Characterization of IL-17+ interphotoreceptor retinoid-binding protein-specific T cells in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2007;48:4153–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng Y, Shao H, Ke Y, et al. Minimally activated CD8 autoreactive T cells specific for IRBP express a high level of Foxp3 and are functionally suppressive. Invest Ophthalmol Vis Sci. 2007;48:2178–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng Y, Shao H, Ke Y, et al. In vitro activation of CD8 interphotoreceptor retinoid-binding protein-specific T cells requires not only antigenic stimulation but also exogenous growth factors. J Immunol. 2006;176:5006–5014 [DOI] [PubMed] [Google Scholar]
- 10.Thurau SR, Chan CC, Nussenblatt RB, Caspi RR. Oral tolerance in a murine model of relapsing experimental autoimmune uveoretinitis (EAU): induction of protective tolerance in primed animals. Clin Exp Immunol. 1997;109:370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng L, Cui Y, Shao H, et al. Mouse γδ T cells are capable of expressing MHC class II molecules, and of functioning as antigen-presenting cells. J Neuroimmunol. 2008;203:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carding SR, Allan W, Kyes S, et al. Late dominance of the inflammatory process in murine influenza by γδ T cells. J Exp Med. 1990;172:1225–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukasa A, Lahn M, Pflum EK, et al. Evidence that the same γδ T cells respond during infection-induced and autoimmune inflammation. J Immunol. 1997;159:5787–5794 [PubMed] [Google Scholar]
- 14.Uezu K, Kawakami K, Miyagi K, et al. Accumulation of γδ T cells in the lungs and their regulatory roles in Th1 response and host defense against pulmonary infection with Cryptococcus neoformans. J Immunol. 2004;172:7629–7634 [DOI] [PubMed] [Google Scholar]
- 15.Wucherpfennig KW, Newcombe J, Li H, et al. γδ T-cell receptor repertoire in acute multiple sclerosis lesions. Proc Natl Acad Sci USA. 1992;89:4588–4592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selmaj K, Brosnan CF, Raine CS. Colocalization of lymphocytes bearing γδ T-cell receptor and heat shock protein hsp65+ oligodendrocytes in multiple sclerosis. Proc Natl Acad Sci USA. 1991;88:6452–6456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimonkevitz R, Colburn C, Burnham JA, et al. Clonal expansions of activated gamma/δ T cells in recent- onset multiple sclerosis. Proc Natl Acad Sci USA. 1993;90:923–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterman GM, Spencer C, Sperling AI, Bluestone JA. Role of γδ T cells in murine collagen-induced arthritis. J Immunol. 1993;151:6546–6558 [PubMed] [Google Scholar]
- 19.Fukushima K, Masuda T, Ohtani H, et al. Immunohistochemical characterization, distribution, and ultrastructure of lymphocytes bearing T-cell receptor γδ in inflammatory bowel disease. Gastroenterology. 1991;101:670–678 [DOI] [PubMed] [Google Scholar]
- 20.Tsuchiya T, Fukuda S, Hamada H, et al. Role of γδ T cells in the inflammatory response of experimental colitis mice. J Immunol. 2003;171:5507–5513 [DOI] [PubMed] [Google Scholar]
- 21.Hohlfeld R, Engel AG, Ii K, Harper MC. Polymyositis mediated by T lymphocytes that express the γδ receptor. N Engl J Med. 1991;324:877–881 [DOI] [PubMed] [Google Scholar]
- 22.Fu YX, Roark CE, Kelly K, et al. Immune protection and control of inflammatory tissue necrosis by γδ T cells. J Immunol. 1994;153:3101–3115 [PubMed] [Google Scholar]
- 23.Sciammas R, Kodukula P, Tang Q, et al. T cell receptor γδ cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J Exp Med. 1997;185:1969–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore TA, Moore BB, Newstead MW, Standiford TJ. γδ T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J Immunol. 2000;165:2643–2650 [DOI] [PubMed] [Google Scholar]
- 25.Tam S, King DP, Beaman BL. Increase of γδ T lymphocytes in murine lungs occurs during recovery from pulmonary infection by Nocardia asteroides. Infect Immun. 2001;69:6165–6171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakasone C, Yamamoto N, Nakamatsu M, et al. Accumulation of γδ T cells in the lungs and their roles in neutrophil-mediated host defense against pneumococcal infection. Microbes Infect. 2007;9:251–258 [DOI] [PubMed] [Google Scholar]
- 27.Mombaerts P, Arnoldi J, Russ F, et al. Different roles of αβ and γδ T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–56 [DOI] [PubMed] [Google Scholar]
- 28.Girardi M, Lewis J, Glusac E, et al. Resident skin-specific γδ T cells provide local, nonredundant regulation of cutaneous inflammation. J Exp Med. 2002;195:855–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braun R, Ferrick C, Neubauer P, et al. IL-17 producing γδ T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation. 2008;31:167–179 [DOI] [PubMed] [Google Scholar]
- 30.Dalton JE, Pearson J, Scott P, Carding SR. The interaction of γδ T cells with activated macrophages is a property of the Vγ1 subset. J Immunol. 2003;171:6488–6494 [DOI] [PubMed] [Google Scholar]
- 31.Glatzel A, Wesch D, Schiemann F, et al. Patterns of chemokine receptor expression on peripheral blood γδ T lymphocytes: strong expression of CCR5 is a selective feature of Vδ2/Vγ9 γδ T cells. J Immunol. 2002;168:4920–4929 [DOI] [PubMed] [Google Scholar]
- 32.Hahn YS, Taube C, Jin N, et al. Different potentials of γδ T cell subsets in regulating airway responsiveness: Vγ1+ cells, but not Vγ4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J Immunol. 2004;172:2894–2902 [DOI] [PubMed] [Google Scholar]
- 33.Huber SA, Graveline D, Newell MK, et al. Vγ1+ T cells suppress and Vγ4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J Immunol. 2000;165:4174–4181 [DOI] [PubMed] [Google Scholar]
- 34.Meissner N, Radke J, Hedges JF, et al. Serial analysis of gene expression in circulating γδ T cell subsets defines distinct immunoregulatory phenotypes and unexpected gene expression profiles. J Immunol. 2003;170:356–364 [DOI] [PubMed] [Google Scholar]
- 35.Spencer CT, Abate G, Blazevic A, Hoft DF. Only a subset of phosphoantigen-responsive γ9δ2 T cells mediate protective tuberculosis immunity. J Immunol. 2008;181:4471–4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Heyde HC, Elloso MM, Chang WL, et al. Expansion of the γδ T cell subset in vivo during bloodstage malaria in B cell-deficient mice. J Leuk Biol. 1996;60:221–229 [DOI] [PubMed] [Google Scholar]
- 37.Ward PA. Adhesion molecule knockouts: one step forward and one step backward. J Clin Invest. 1995;95:1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groh V, Porcelli S, Fabbi M, et al. Human lymphocytes bearing T cell receptor γ/δ are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989;169:1277–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669 [DOI] [PubMed] [Google Scholar]
- 40.Kaufmann SHE, Blum C, Yamamoto S. Crosstalk between αβ T cells and γδ T cells in vivo: activation of αβ T-cell responses after γδ T-cell modulation with the monoclonal antibody GL3. Proc Natl Acad Sci USA. 1993;90:9620–9624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ke Y, Pearce K, Lake JP, et al. γδ T lymphocytes regulate the induction and maintenance of oral tolerance. J Immunol. 1997;158:3610–3618 [PubMed] [Google Scholar]
- 42.Maeda Y, Reddy P, Lowler KP, et al. Critical role of host γδ T cells in experimental acute graft-versus-host disease. Blood. 2005;106:749–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajan AJ, Klein JD, Brosnan CF. The effect of γδ T cell depletion on cytokine gene expression in experimental allergic encephalomyelitis. J Immunol. 1998;160:5955–5962 [PubMed] [Google Scholar]
- 44.O'Brien RL, Yin X, Huber SA, et al. Depletion of a γδ T cell subset can increase host resistance to a bacterial infection. J Immunol. 2000;165:6472–6479 [DOI] [PubMed] [Google Scholar]
- 45.Koenecke C, Chennupati V, Schmitz S, et al. In vivo application of mAb directed against the γδ TCR does not deplete but generates ‘invisible’ γδ T cells. Eur J Immunol. 2009;39:372–379 [DOI] [PubMed] [Google Scholar]