Abstract
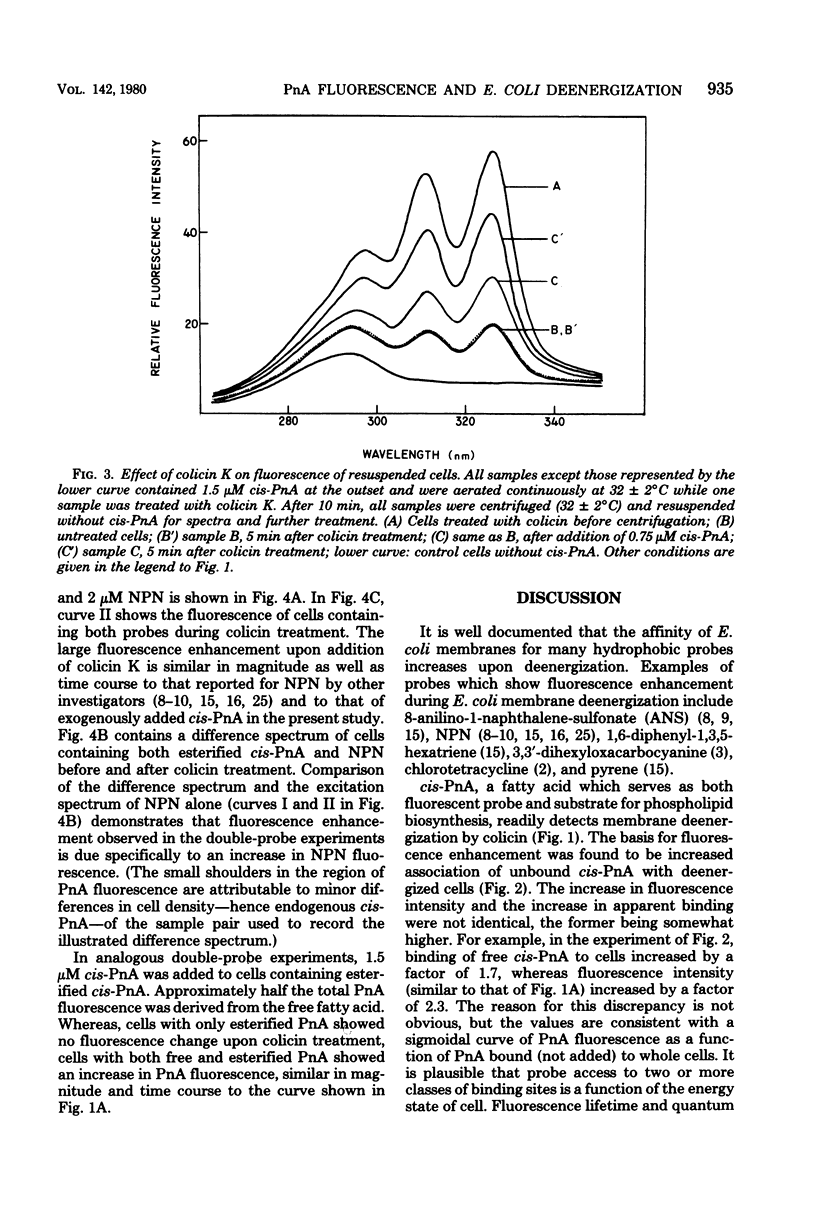
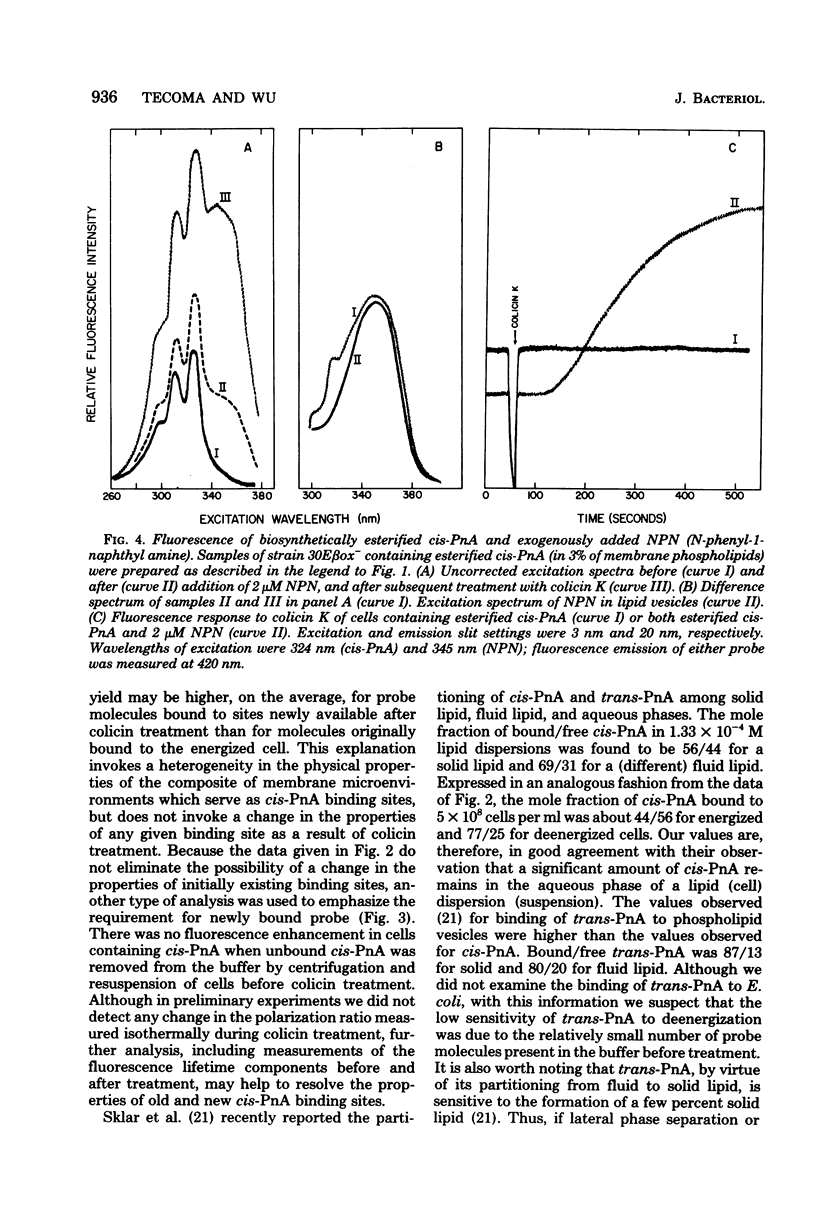
Fluorescence of the conjugated polyene fatty acid, parinaric acid (PnA), was studied in membranes of Escherichia coli during deenergization by colicin K. The free fatty acid and biosynthetically esterified forms of cis-PnA (9,11,13,15-cis,trans,trans,cis-octadecatetraenoic acid), both of which are sensitive to E. coli lipid-phase transitions, were compared. When free cis-PnA was added exogenously to respiring bacteria, dissipation of the energized state of the membrane resulted in a dramatic increase in cis-PnA fluorescence; all-trans-PnA was much less sensitive. Neither spectral shifts nor a change in cis-PnA fluorescence polarization were observed. Analysis of the PnA content of extracellular fractions of deenergized and control cells revealed a difference in probe distribution: the membranes of energy-poisoned E. coli bound about 77% of exogenously added cis-PnA, whereas membranes of actively respiring controls bound only about 44%. No fluorescence enhancement was observed in cells centrifuged to remove unbound cis-PnA before colicin treatment. When cis-PnA was biosynthetically esterified to phospholipids of an unsaturated fatty acid auxotroph of E. coli, the fluorescence did not change during membrane deenergization. In double-probe experiments, membrane deenergization resulted in fluorescence enhancement of exogenously added N-phenyl-1-naphthylamine, without change in esterified PnA fluorescence. We conclude that deenergization of E. coli membranes leads to increased binding and fluorescence of exogenously added PnA and cannot be detected from within the inner and outer membranes by PnA esterified in vivo.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brewer G. J. Chlorotetracycline as a fluorescent probe for membrane events in the action of colicin K on Escherichia coli. Biochemistry. 1974 Nov 19;13(24):5038–5045. doi: 10.1021/bi00721a027. [DOI] [PubMed] [Google Scholar]
- Brewer G. J. The state of energization of the membrane of Escherichia coli as affected by physiological conditions and colicin K. Biochemistry. 1976 Apr 6;15(7):1387–1392. doi: 10.1021/bi00652a006. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Epstein W., Fox C. F. Mapping of a locus for unsaturated fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1970 Jul;103(1):273–274. doi: 10.1128/jb.103.1.273-274.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A. Changes in E. coli cell envelope structure caused by uncouplers of active transport and colicin E1. J Supramol Struct. 1976;5(3):291–308. doi: 10.1002/jss.400050304. [DOI] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A. Changes in Escherichia coli cell envelope structure and the sites of fluorescence probe binding caused by carbonyl cyanide p-trifluoromethoxyphenylhydrazone. Biochemistry. 1977 Sep 6;16(18):4109–4117. doi: 10.1021/bi00637a026. [DOI] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A., Harris J. M., Lytle F. E. Evidence for a microviscosity increase in the Escherichia coli cell envelope caused by colicin E1. Biochemistry. 1974 Jul 16;13(15):3057–3061. doi: 10.1021/bi00712a010. [DOI] [PubMed] [Google Scholar]
- Kunugita K., Matsuhashi M. Purification and properties of colicin K. J Bacteriol. 1970 Nov;104(2):1017–1019. doi: 10.1128/jb.104.2.1017-1019.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Nieva-Gomez D., Gennis R. B. Affinity of intact Escherichia coli for hydrophobic membrane probes is a function of the physiological state of the cells. Proc Natl Acad Sci U S A. 1977 May;74(5):1811–1815. doi: 10.1073/pnas.74.5.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieva-Gomez D., Konisky J. Membrane changes in Escherichia coli induced by colicin Ia and agents known to disrupt energy transduction. Biochemistry. 1976 Jun 29;15(13):2747–2753. doi: 10.1021/bi00658a006. [DOI] [PubMed] [Google Scholar]
- Rintoul D. A., Sklar L. A., Simoni R. D. Membrane lipid modification of chinese hamster ovary cells. Thermal properties of membrane phospholipids. J Biol Chem. 1978 Oct 25;253(20):7447–7452. [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Petersen M., Diamond J. Conjugated polyene fatty acids on fluorescent probes: spectroscopic characterization. Biochemistry. 1977 Mar 8;16(5):813–819. doi: 10.1021/bi00624a001. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Simoni R. D. Conjugated polyene fatty acids as fluorescent probes: synthetic phospholipid membrane studies. Biochemistry. 1977 Mar 8;16(5):819–828. doi: 10.1021/bi00624a002. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Simoni R. D. Conjugated polyene fatty acids as membrane probes: preliminary characterization. Proc Natl Acad Sci U S A. 1975 May;72(5):1649–1653. doi: 10.1073/pnas.72.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Miljanich G. P., Dratz E. A. Phospholipid lateral phase separation and the partition of cis-parinaric acid and trans-parinaric acid among aqueous, solid lipid, and fluid lipid phases. Biochemistry. 1979 May 1;18(9):1707–1716. doi: 10.1021/bi00576a012. [DOI] [PubMed] [Google Scholar]
- Tecoma E. S., Sklar L. A., Simoni R. D., Hudson B. S. Conjugated polyene fatty acids as fluorescent probes: biosynthetic incorporation of parinaric acid by Escherichia coli and studies of phase transitions. Biochemistry. 1977 Mar 8;16(5):829–835. doi: 10.1021/bi00624a003. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Konisky J. Mode of action of colicin Ia: effect of colicin on the Escherichia coli proton electrochemical gradient. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2579–2583. doi: 10.1073/pnas.75.6.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weber G., Helgerson S. L., Cramer W. A., Mitchell G. W. Changes in rotational motion of a cell-bound fluorophore caused by colicin E1: a study by fluorescence polarization and differential polarized phase fluorometry. Biochemistry. 1976 Oct 5;15(20):4429–4432. doi: 10.1021/bi00665a014. [DOI] [PubMed] [Google Scholar]
- Weiss M. J., Luria S. E. Reduction of membrane potential, an immediate effect of colicin K. Proc Natl Acad Sci U S A. 1978 May;75(5):2483–2487. doi: 10.1073/pnas.75.5.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]



