Summary
Most depressed patients don't respond to their first drug treatment, and the reasons for this treatment resistance remain enigmatic. Human studies implicate a polymorphism in the promoter of the serotonin-1A (5-HT1A) receptor gene in increased susceptibility to depression and decreased treatment response. Here we develop a new strategy to manipulate 5-HT1A autoreceptors in raphe nuclei without affecting 5-HT1A heteroreceptors, generating mice with higher (1A-High) or lower (1A-Low) autoreceptor levels. We show that this robustly affects raphe firing rates, but has no effect on either basal forebrain serotonin levels or conflict-anxiety measures. However, compared to 1A-Low mice, 1A-High mice show a blunted physiological response to acute stress, increased behavioral despair, and no behavioral response to antidepressant, modeling patients with the 5-HT1A risk allele. Furthermore, reducing 5-HT1A autoreceptor levels prior to antidepressant treatment is sufficient to convert non-responders into responders. These results establish a causal relationship between 5-HT1A autoreceptor levels, resilience under stress, and response to antidepressants.
Depression is one of the leading public health problems in the world today and antidepressants are amongst the most commonly prescribed medications(2007). Current evidence suggests that depressive disorders are precipitated by stressful life events, interacting with genetic and other predisposing factors (Caspi et al., 2003; Fava and Kendler, 2000; Leonardo and Hen, 2006). The response to antidepressants, like the response to external stressors, is variable, and fewer than half of depressed patients respond to their first drug treatment, leading to prolonged suffering and increased medical costs(Rush et al., 2006). Elucidating the exact nature of both the factors predisposing to depression and the mechanisms underlying treatment resistance remains an important and unmet need.
The serotonergic system modulates the acute stress response, and has been implicated in both the etiology of depression and anxiety as well as the response to treatment(Holmes, 2008; Lanfumey et al., 2008). Most drugs used for treating depression increase serotonin levels, including the most commonly used drugs, the selective serotonin reuptake inhibitors, (SSRIs) which are effective at treating both anxiety and depression(Schatzberg and Nemeroff, 2009). Serotonin is released from serotonergic neurons, which have cell bodies localized in the mid-brain raphe nuclei but send axonal projections throughout the brain, where released serotonin impacts a diverse group of serotonin receptors.
The serotonin-1A (5-HT1A) receptor is an inhibitory G-protein coupled receptor expressed both in serotonergic neurons (as an autoreceptor), where it controls serotonergic tone through feedback inhibition, and in target areas receiving serotonergic innervation (as a heteroreceptor)(Beck et al., 1992; Hamon et al., 1990; Riad et al., 2000). Thus, it has the dual ability to modulate both global serotonin levels and local responses to released serotonin. The role of 5-HT1A autoreceptors in controlling serotonergic tone has led to the hypothesis that these receptors delay the therapeutic action of SSRIs and other drugs that act by increasing serotonin levels (Gardier et al., 1996). Specifically, 5-HT1A autoreceptors exert negative feedback inhibition in response to increased serotonin; thus, progressive autoreceptor desensitization may be responsible for the delayed onset of action of these drugs(Blier et al., 1998).
Genetic and imaging studies in humans have suggested that differences in 5-HT1A receptor levels or regulation are also associated with depression, anxiety, and the response to antidepressants (Le Francois et al., 2008; Lesch and Gutknecht, 2004; Strobel et al., 2003). Most recently, an association has been reported between a C(-1019)G polymorphism in the promoter region of the Htr1a gene and a number of mood-related variables, including depression, the response to antidepressant treatment, and amygdala reactivity (Fakra et al., 2009; Le Francois et al., 2008). Although initial reports suggested that this polymorphism might control autoreceptor levels without impacting heteroreceptor levels(Lemonde et al., 2003), recent imaging findings suggest that 5-HT1A auto- and heteroreceptors are both affected(Parsey et al., 2006). Thus, despite significant attention and interest regarding the role of the 5-HT1A autoreceptors in the treatment and etiology of depression, a direct test of their involvement has remained beyond the reach of available techniques.
Studies in mice have suggested that 5-HT1A receptors are generally involved in modulating both anxiety and depression-related behavior (Heisler et al., 1998; Klemenhagen et al., 2006; Parks et al., 1998; Ramboz et al., 1998), but have not usually distinguished between auto- and heteroreceptors. 5-HT1A knockout (KO) mice (lacking the receptor everywhere, throughout life) display a robust anxiety-like phenotype in conflict-anxiety paradigms, while exhibiting decreased behavioral despair in response to stress(Heisler et al., 1998; Parks et al., 1998; Ramboz et al., 1998). Because behavioral despair in response to stress is decreased by acute treatment with a number of drugs used to treat depression, this phenotype has often been referred to as “anti-depressed.” However, anxiety and other stress-related disorders such as depression are often co-morbid in humans(Kendler et al., 1992), making the combination of an anxious phenotype with an “anti-depressed” phenotype in 5-HT1A KO mice difficult to interpret. Subsequently, the “anti-depressed” phenotype of mice lacking the 5-HT1A receptor has been largely ignored.
Overall, the role of 5-HT1A auto- versus heteroreceptors in determining the response to stress, the anxiety phenotype, or the response to treatment with antidepressants has not been adequately addressed. Both pharmacological approaches and genetic animal models have been hampered by the difficulty in separating effects on autoreceptors from effects on heteroreceptors. To directly test the role of 5-HT1A autoreceptors in anxiety, depression, and the response to antidepressants, we first developed a novel system capable of suppressing expression of 5-HT1A receptors in a tissue-specific and temporally specific manner. We used this system to examine the biological consequences of altering autoreceptor levels without affecting heteroreceptor levels. Specifically, we tested the hypothesis that altering autoreceptor levels may result in differences in anxiety, stress response, depression, or response to antidepressants.
Results
Conditional suppression of the 5-HT1A receptor
In order to generate mice in which we could conditionally suppress 5-HT1A receptors, we crossed mice containing two distinct engineered alleles. The first is a knock-in of the tetracycline operator (tetO) into the promoter region of the murine Htr1a gene, to create the tetO-1A allele. The second is a transgene expressing the tetracycline-dependent transcriptional suppressor (tTS) under the control of the β-actin promoter (Figure 1a)(Mallo et al., 2003). Insertion of the tetO element into the endogenous Htr1a locus does not interfere with normal 5-HT1A receptor expression patterns(Audero et al., 2008). tTS suppresses endogenous expression of the 5-HT1A receptor by binding to tetO in a doxycycline dependent manner (Figure 1a)(Mallo et al., 2003). Maintenance of mice on doxycycline prevents the tTS protein from binding the tetO sequence and results in unimpeded expression of the 5-HT1A receptor.
Figure 1. A transgenic system for suppression of 5-HT1Areceptors.
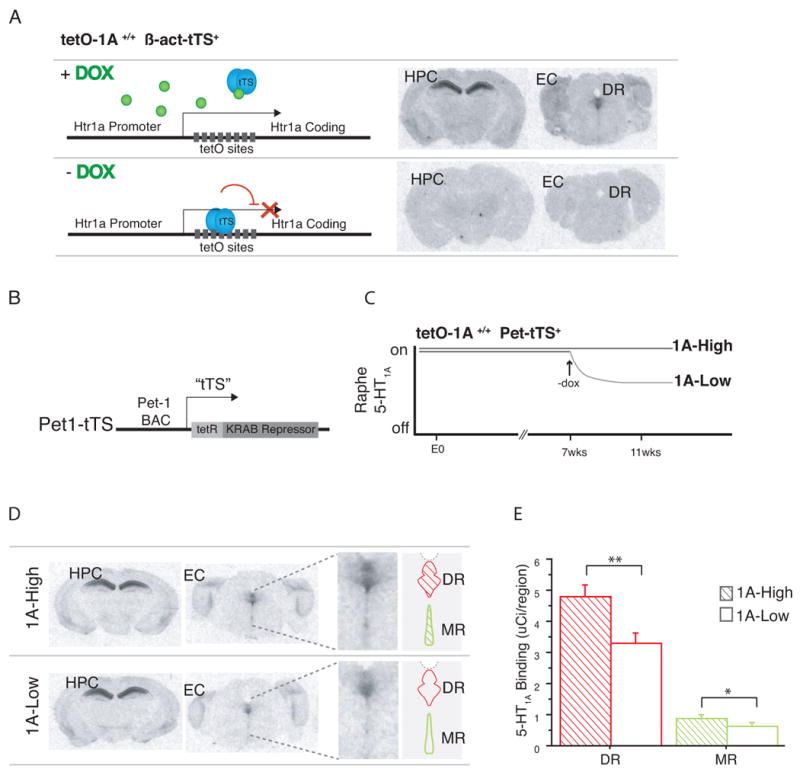
(a) Mice homozygous for the regulatable tetO-1A allele, with one copy of the β-act-tTS transgene (tetO-1A+/+ β-act-tTS+), express 5-HT1A receptors in normal patterns in the brain when maintained on doxycycline, assessed by 125I-labeled MPPI autoradiography. When maintained in the absence doxycycline, tetO-1A+/+ β-act-tTS+ display no detectable 5-HT1A receptor expression in the brain. (b) Tissue-specific expression of tTS in serotonergic raphe neurons was accomplished by placing tTS under the control of a 40kB Pet-1 mini-BAC (Pet1-tTS). (c) tetO-1A+/+ Pet1-tTS+ mice were maintained on dox either throughout life (1A-High), or only until postnatal day 50 (approximately 7 weeks of age) (1A-Low). (d), (e) 1A-High and 1A-Low mice express indistinguishable levels of 5-HT1A heteroreceptors in forebrain areas such as the hippocampus (HPC) and entorhinal cortex (EC), while 1A-Low mice display decreased 5-HT1A expression in the dorsal (DR) and median (MR) raphe nuclei, assessed by quantitative 125I-labeled MPPI autoradiography (N=4 mice; *** P<0.005 (DR), *P<0.05 (MR)). See also Fig S1
Since previous studies of the 5-HT1A receptor have suggested that the receptor is involved in the developmental establishment of anxiety-like behavior(Gross et al., 2002; Lo Iacono and Gross, 2008), a key goal of this system was achieving inducible suppression in adulthood, in order to distinguish between developmental and adult effects of lacking the receptor. We found that withdrawal of doxycycline allows binding of tTS to the tetO sequence and progressive suppression of 5-HT1A receptor levels. Four weeks after doxycycline removal, maximal suppression is achieved and 5-HT1A receptor levels are undetectable by I125 MPPI autoradiography, revealing a half-life of receptor disappearance of approximately 8 days (Figure S1a).
Raphe specific suppression of 5-HT1A receptors
Having established the feasibility of inducible suppression of 5-HT1A receptors in the brain, we created a mouse in which we could specifically modulate 5-HT1A autoreceptor levels in serotonergic raphe neurons, without affecting heteroreceptor levels. We accomplished this by generating a mouse with raphe-specific expression of tTS under the control of the previously characterized 540Z Pet-1 promoter fragment(Fisher et al., 2006) (Pet1-tTS) (Figure 1b). We crossed these Pet1-tTs mice with the tetO-1A mice described above. In the presence of doxycycline, mice homozygous for the tetO-1A allele and possessing one copy of the Pet-tTS transgene display levels of 5-HT1A autoreceptor that are indistinguishable from littermates lacking the tTS transgene (1A-High) (Figure S1b). Removal of doxycycline at postnatal day 50 for four weeks creates a population of adult animals with lower expression of 5-HT1A autoreceptors (1A-Low) (Figure 1c).
Quantitative autoradiography in the raphe and selected forebrain structures (entorhinal cortex, amygdala, ventral dentate gyrus) demonstrates that, compared to 1A-High mice, 1A-Low mice have indistinguishable levels of 5-HT1A heteroreceptor expression (Figure S1c), but display about 30% less autoreceptor expression than 1A-High mice (Fig 1d). Similar differences are seen in both the dorsal and median raphe (Dorsal Raphe one tailed t-test, t14=2.965, P=0.005; Median Raphe one tailed t-test, t14=1.967, P=0.041) (Fig 1e). An overall difference of 30% in autoreceptor levels is consistent with the range of receptor levels that are seen within human populations(Drevets et al., 2007).
Decreased response to agonist after adult suppression of 5-HT1A autoreceptors
To directly confirm that the differences in 5-HT1A autoreceptor levels revealed by autoradiography had functional consequences, we performed whole cell recordings in the dorsal raphe and measured the response to the 5-HT1,7 agonist 5-carboxyamidotryptamine (5-CT)(Figure 2a). After recording, we confirmed that neurons were serotonergic by filling recorded neurons with biocytin and performing immunohistochemistry for biocytin and TPH (Figure 2c). We observed a significantly higher average current elicited by agonist challenge in the serotonergic neurons of 1A-High mice versus 1A-Low mice (two tailed Mann-Whitney test, U=104.0; P=0.0008) (Figure 2b). Much of this difference resulted from a significant proportion of neurons in the 1A-Low mice that fail to respond to the agonist challenge (defined by current < 5pA) (c2=15.914; P<0.0001) (Table S1). These data suggest that the tTS-mediated transcriptional suppression in the 1A-Low mice results in a mosaic population of serotonergic neurons, some of which retain full responsiveness to 5-HT1A agonists while others are no longer responsive. The reasons for this mosaicism are unclear; it may represent all-or-nothing genetic silencing as a result of variable transgene expression. Alternately, it may arise secondarily as a result of further autoreceptor desensitization in some neurons with low levels of gene expression.
Figure 2. Decreased 5-HT1Aautoreceptor response to agonist in 1A-Low mice.
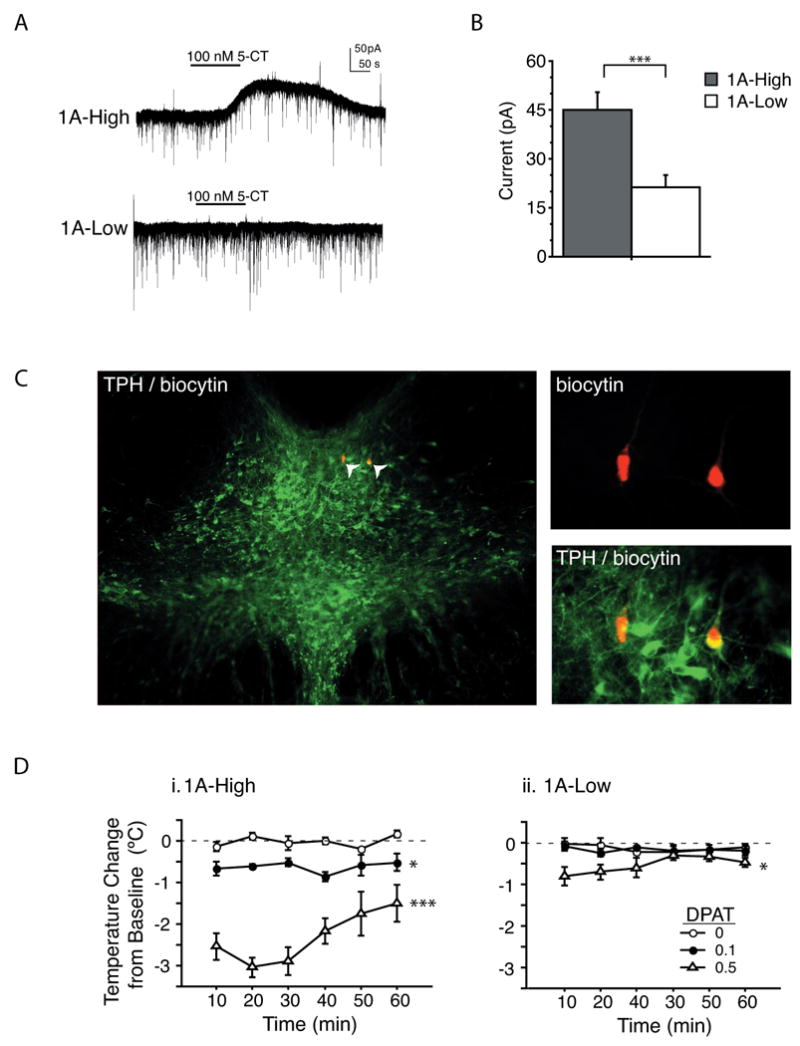
(a) Representative current traces from whole cell recordings in the dorsal raphe of 1A-High and 1A-Low mice in response to the 5-HT1A agonist 5-CT. (b) Mean outward current amplitude in response to 100nm 5-CT was decreased in 1A-Low mice (N=43 1A-High and 57 1A-Low neurons, ***P<0.001). (c) Recorded neurons were filled with biocytin and processed for TPH. Photomicrographs of the dorsal raphe are shown. (d) Hypothermic response to the 5-HT1A agonist 8-OH DPAT. In 1A-Low mice, only the 0.5 mg/kg dose caused a significant temperature change relative to the saline control. In 1A-High mice, both the 0.1 mg/kg and the 0.5 mg/kg doses elicited significantly larger temperature changes relative to control (N=4-5/dose/group; *P<0.05 and ***P<0.001 for a main effect of dose). See also Table S1
To independently assess the in vivo functional status of the 5-HT1A autoreceptors in 1A-High and 1A-Low mice, we examined their hypothermic response to 5-HT1A agonist challenge. While 1A-High mice display the expected dose-dependent hypothermic response to the 5-HT1A agonist, 8-OH-DPAT (repeated measures two-way ANOVA with time as a within-subject factor and dose as a between-subject factor; main effect of dose F2,12=61.689; P<0.0001; post hoc between vehicle and 0.1 mg/kg, P=0.0155; between vehicle and 0.5 mg/kg, P=0.0001), 1A-Low mice displayed a markedly attenuated response, which was detected only at the higher dose (repeated measures two-way ANOVA; main effect of dose F2,11=6.109; P=0.0164; post-hoc between vehicle and 0.5 mg/kg, P=0.0113) (Fig 2d i, ii). These findings are consistent with previous literature indicating that the 5-HT1A autoreceptors are responsible for the hypothermic effect of 8-OH DPAT in the mouse(Martin et al., 1992). In summary, our results demonstrate that a modest difference in autoreceptor expression between 1A-High and 1A-Low mice results in robust differences in their response to agonist treatment both in vitro and in vivo.
Increased spontaneous activity of serotonergic neurons following adult autoreceptor suppression
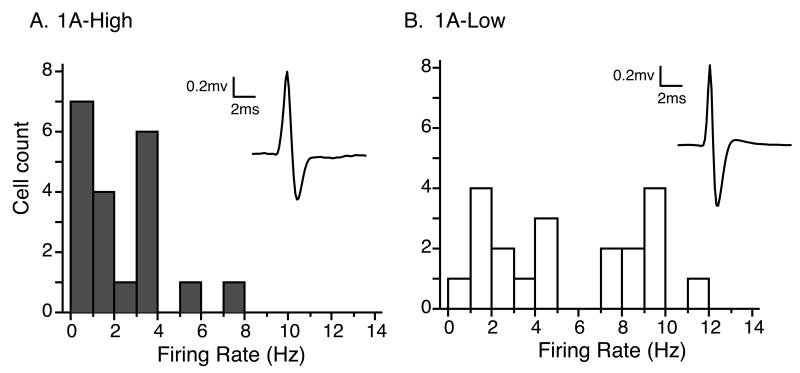
To determine whether the functional differences in autoreceptor levels had an effect on overall serotonergic tone, we measured the firing rates of serotonergic dorsal raphe neurons in an in vivo anesthetized preparation. Neurons were included in the analysis based on the characteristics of their action potentials, and averaged traces of these action potentials are shown as insets (Figure 3)(Vandermaelen and Aghajanian, 1983). We observed significantly different distributions of firing rates between the groups (two-tailed Mann Whitney test, U=104; P=0.0057), with raphe neurons from 1A-Low mice more likely to fire at higher rates (5.5 ± 0.8 Hz) than the 1A-High mice (2.6 ± 0.6 Hz) (two-tailed T-test for group, T39=2.874; P=0.0065). This overall firing rate increase demonstrates higher serotonergic tone in 1A-Low mice, consistent with decreased autoinhibition.
Figure 3. Increased spontaneous neuronal activity in the dorsal raphe of 1A-Low mice.
Histograms depicting distribution of spontaneous firing rates for individual neurons in an in vivo anesthetized preparation of 1A-High and 1A-Low animals, with averaged action potential traces inset. The distributions are significantly different (N= 20 and 21 neurons respectively; two-tailed Mann Whitney test; P=0.0057).
Decreasing autoinhibition in adult animals does not change baseline anxiety measures
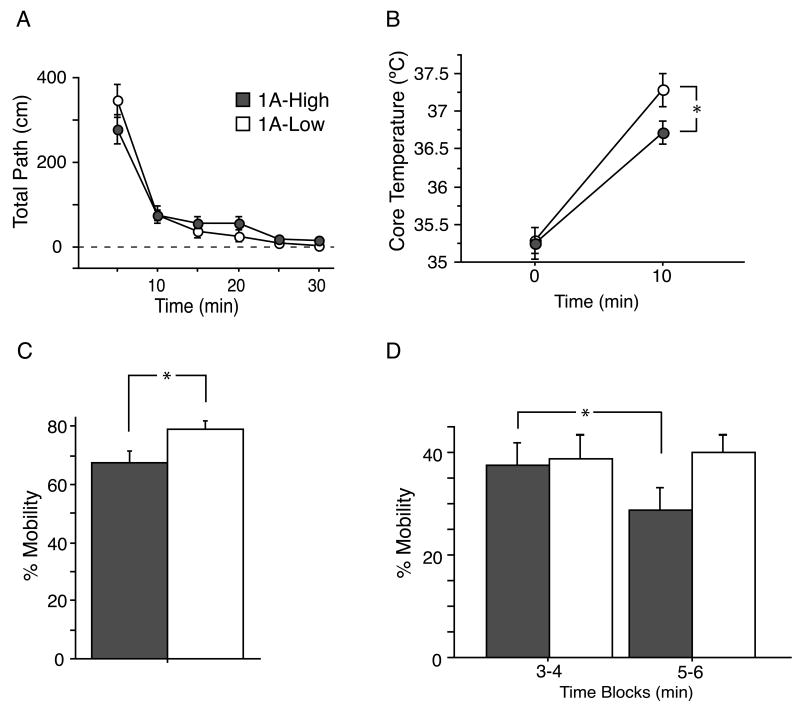
Complete 5-HT1A KO mice, lacking both auto- and heteroreceptors throughout life, have consistently shown increased anxiety in conflict-based tasks(Heisler et al., 1998; Klemenhagen et al., 2006; Parks et al., 1998; Ramboz et al., 1998). To test whether specifically modulating 5-HT1A autoreceptors in adulthood impacts anxiety-like behavior, we tested our mice in two conflict-based tests: the open field paradigm, and the light/dark choice test. 1A-High and 1A-Low mice displayed no difference in either total exploration (two-way repeated measures ANOVA with time as a within-subject factor and genotype as a between-subject factor; F1,40=0.583; P=0.45) or exploration in the center of the open field (two-way repeated measures ANOVA F1,40=0.225; P=0.64) (Figure 4a). Similarly, in the light/dark test, we detected no difference between the groups in total exploration (ANOVA F1,38=1.105; P=0.2998) or in the amount of time spent in the light compartment (ANOVA F1,38=0.249; P=0.521) (Figure 4b). These data directly demonstrate that changes in adult levels of 5-HT1A autoreceptors do not alter anxiety-like behavior, consistent with previous findings suggesting a developmental role for 5-HT1A receptors in the establishment of anxiety-related circuitry (Gross et al., 2002; Lo Iacono and Gross, 2008).
Figure 4. No change in anxiety-like behavior, but altered response in stress/depression-related tests in 1A-Low mice.
(a) No group differences were detected in the total exploration (i) or time spent in the center (ii) of the open field (N=21/group). (b) No group differences were detected in percent time spent in the light (i) or total path (ii) in the light/dark choice test (N=19 and 21/group). (c) 1A-High mice displayed an attenuated Stress-Induced Hyperthermic response to novel cage stress, compared to 1A-Low mice (N=11/group; ***P<0.0001). Although no differences were detected between the groups in mobility in the Tail Suspension Test (d) (N=25-26 mice/group), 1A-Low mice displayed increased mobility compared to 1A-High mice across a two-day Forced Swim Test (e) (repeated measures ANOVA across all time points, group by time interaction F3,43=4.535, P=0.0047). Only 1A-High mice displayed decreased mobility over time on the second day of testing, and 1A-Low mice were more mobile in the final testing block (N=21, 22/group; ANOVA, between group minutes 5-6, F1,41 = 3.953, #P= 0.0535, *P<0.05). See also Fig. S2.
Decreased autoinhibition in adulthood alters response to stress
Studies in humans suggest that 5-HT1A receptor levels might influence behavioral resilience to stressful situations, with high-expressers being more susceptible to depression than low-expressers(Anttila et al., 2007; Kraus et al., 2007; Lemonde et al., 2003; Neff et al., 2008). Moreover, 5-HT1A KO mice display increased physiological responses to acute stress(Van Bogaert et al., 2006). To assess whether altering serotonergic autoinhibition is sufficient to alter stress responsivity, we examined the response of 1A-High and 1A-Low mice in the stress induced hyperthermia paradigm(Adriaan Bouwknecht et al., 2007). This paradigm measures one of the acute physiological responses to stress, namely that body temperature is increased as a result of autonomic system arousal. Hyperthermia in this paradigm correlates with measures of HPA axis reactivity, such as corticosterone, ACTH and glucose plasma levels, and other measures of autonomic reactivity, such as heart rate(Groenink et al., 1994). In this test, the 1A-Low mice displayed a more robust autonomic response to an acute stressor compared to 1A-High mice (ANOVA F1,20=43.201, P<0.0001) (Figure 4c).
Having observed a difference in a physiological response to acute stress, we next examined the behavioral response of these animals in two distinct stress-related paradigms: the tail suspension test and the forced swim test. In both tests, immobility is scored as a measure of behavioral despair (Lucki, 1997). No difference between groups was detected in the tail suspension test (F1,49=0.001, P=0.9735) (Figure 4d). In the forced swim test, animals were exposed to the stressor twice over a 24-hour period and the last 4 minutes of a six-minute session was scored on each day. Unlike the tail suspension test where periods of immobility appear early and occur in brief bouts throughout the duration of the test, in the forced swim test, animals are initially fairly active with immobility generally emerging in the third minute of the test (Buccafusco, 2009; Cryan et al., 2005; Porsolt et al., 1977). 1A-High and 1A-Low mice responded indistinguishably to the initial stressor on Day 1, and both groups showed the expected decrease in mobility on Day 2. However, 1A-High, but not 1A-Low mice displayed progressively less mobility, or more behavioral despair, upon re-exposure the second day (Figure 4e) (repeated measures ANOVA, group by time interaction F3,43=4.535, P=0.0047), consistent with prior results demonstrating the need for repeated exposure to uncover effects of serotonergic manipulations(Ramboz et al., 1998; Wellman et al., 2007). Moreover, the mobility of the 1A-Low mice appears to be higher than 1A-High mice during the final two minutes of the test, suggesting a different adaptation to stress over time in the two groups (ANOVA, between group minutes 5-6, F1,41 = 3.953, P=0.0535) (Figure 4e). Thus, while decreasing adult levels of 5-HT1A autoreceptors does not alter either conflict-based anxiety (Figure 4a and b), or the behavioral response to an acute stressor (Figure 4d and e), decreasing adult autoreceptor levels results in increased physiological reactivity to stress (Figure 4c) and appears to elicit a more active response to a repeated stress in a depression-related task (Figure 4e).
To further test the possibility that 1A-High and 1A-Low mice differed in their behavioral sensitivity to repeated stress, we subjected animals to a repeated daily mild stressor, oral gavage, for 4 weeks (28 days). This manipulation has been shown to increase stress-response measures in rodents, such as circulating corticosterone, body temperature, and heart rate (Dalm et al., 2008). Following four weeks of repeated stress, 1A-High and 1A-Low mice remained indistinguishable in their total exploration in the open field (two-way repeated measures ANOVA, F1,25=0.003, P= 0.9586) (Figure 5a) and in time spent in the center of the open field (ANOVA, F1,25= 1.587, P= 0.2195) (data not shown), and retained their distinct physiological reactivity to stress as assessed by the SIH test (1-tailed T-test, t10=2.057, P=0.0334) (Figure 5b). However, following repeated mild stress, 1A-High, but not 1A-Low mice, displayed decreased mobility over time on the first day (Day 1) of the forced swim test (Paired T-test for 1A-High group over time t13=3.492, P=0.004 Paired t-Test for 1A-Low group over time t13=.-.276, P=0.7872) (Figure 5d), a result that had only been observed after repeated (two-day) swim stress previously (Figure 4c). Moreover, following 4 weeks of repeated stress, 1A-High mice displayed significantly less mobility in the tail-suspension test, compared to 1A-Low mice (F1,25 = 4.478, P= 0.0445) (Figure 5c). This difference emerged only after a repeated mild stressor (Figure 4d). Overall, these results are consistent with a differential susceptibility to stress between the two groups of animals, as measured by behavioral responses in depression-related stress paradigms.
Figure 5. 1A-High mice display a less active behavioral response in stressful paradigms following a repeated mild stressor.
Following four weeks of a daily mild stressor, 1A-High and 1A-Low mice displayed indistinguishable behavior in the Open Field Paradigm (a) (N=13-14 mice/group), and 1A-Low mice retained a more robust temperature increase in response to novel cage stress (b) (N=6/group, *P<.05), similar to naive mice. However, after repeated stress, 1A-High mice displayed less mobility than 1A-Low mice in the Tail Suspension Test (c) (N=13-14 mice/group; *P=0.0445), and less mobility over time in a single exposure to the Forced Swim Test (d) (N=13-14 mice/group, **P=.004).
Decreased autoinhibition alters behavioral response to fluoxetine
Having demonstrated that decreased serotonergic autoinhibition yielded a consistent difference in responsiveness to stress, we asked whether such a change might also be sufficient to impact responsiveness to antidepressant drugs. To directly test whether the behavioral response to antidepressant treatment is affected by autoreceptor levels, we chose the novelty-suppressed feeding (NSF) paradigm (Bodnoff et al., 1988; Gross et al., 2000; Santarelli et al., 2003). This paradigm has two features which make it useful to model the variable human response to antidepressants: 1) like many behavioral tests, the response is affected by the genetic background of the mice (Lucki et al., 2001), with some strains not responding to SSRIs in this paradigm (Ibarguen-Vargas et al., 2008); and 2) unlike other commonly used tests of antidepressant response, such as the tail suspension test or the forced swim test, the NSF is sensitive to chronic (>3 weeks) but not acute or sub-chronic (<10 days) treatment with antidepressant drugs (Dulawa and Hen, 2005; Lira et al., 2003; Wang et al., 2008). Thus, by testing the response to fluoxetine in this paradigm we can model both the time frame required for response to treatment, and the factors that mediate treatment response.
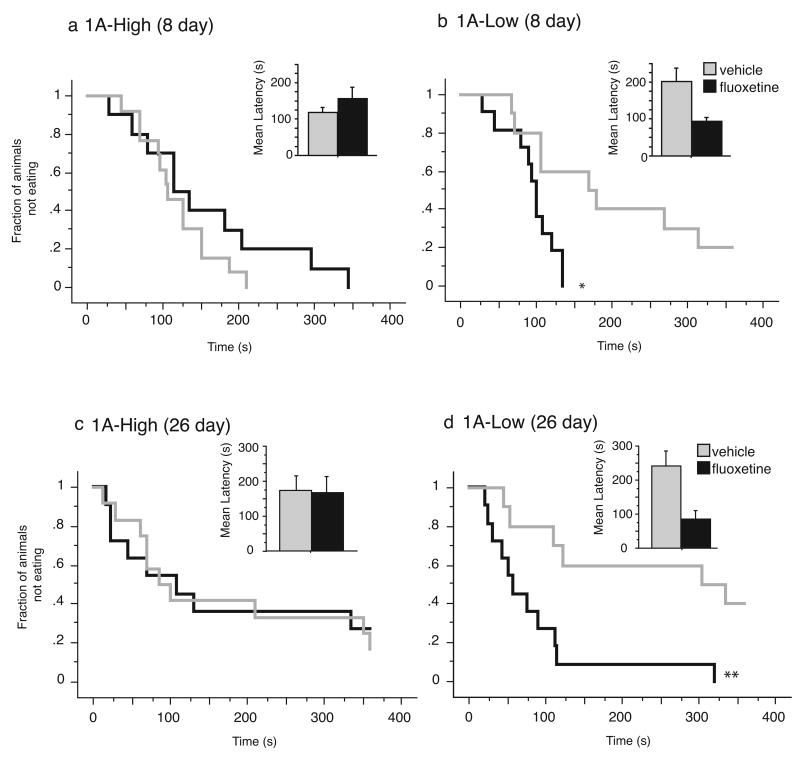
We administered fluoxetine or vehicle to 1A-High and 1A-Low mice and tested them in the NSF paradigm, a test of hyponeophagia that measures the latency of a mouse to consume food placed in the middle of a brightly lit, aversive arena (Bodnoff et al., 1988; Gross et al., 2000; Santarelli et al., 2003). Following a chronic, twenty-six day treatment with fluoxetine, we observed that 1A-Low mice respond robustly, as evidenced by their lower latency to feed relative to their vehicle treated controls (P=0.0031 by Mantel-Cox log rank test) (Fig. 6d). However, no response to fluoxetine was observed in the 1A-High mice (P=0.8475 by Mantel-Cox log rank test) (Figure 6c). Thus, like many mouse strains, the 1A-High mice do not respond to fluoxetine in this paradigm. Furthermore, this experiment establishes a causal relationship between 5-HT1A autoreceptor levels and response to antidepressants; namely, a decrease in 5-HT1A autoreceptor levels in adulthood, prior to antidepressant treatment, is sufficient to confer responsiveness to fluoxetine in an otherwise treatment-resistant population.
Figure 6. Robust response to fluoxetine treatment in the novelty-suppressed feeding test in 1A-Low, but not 1A-High mice.
(a) and (b) 1A-High mice treated for 8 days with fluoxetine (18mg/kg/day p.o.) display no difference in latency to consume a food pellet in the middle of an aversive arena as compared to 1A-High mice treated with vehicle, while 1A-Low mice treated with fluoxetine for 8 days display a shorter latency than their vehicle controls. (c), (d) Continuation of fluoxetine treatment for 26 days failed to decrease the latency to feed in 1A-High mice, while 1A-Low mice still displayed a shorter latency to feed than their vehicle controls (N=11-13/treatment/group; *P<0.05, **P<0.01 by Mantel-Cox log-rank test). See also Fig. S3.
To determine whether autoreceptors might determine time to response, we also examined the response of both 1A-High and 1A-Low mice to sub-chronic (8 day) treatment with fluoxetine. Under these conditions, 1A-Low mice show a robust response to fluoxetine (P= 0.011 by Mantel-Cox log rank test), while no such response is seen in the 1A-High mice (P=0.2343 by Mantel-Cox log rank test) (Figure 6a and b). This result suggests that decreased autoreceptor function may permit an early response to treatment, consistent with the hypothesis that feedback inhibition by 5-HT1A autoreceptors delays the onset of response by limiting the initial increase in serotonin (Artigas et al., 1996).
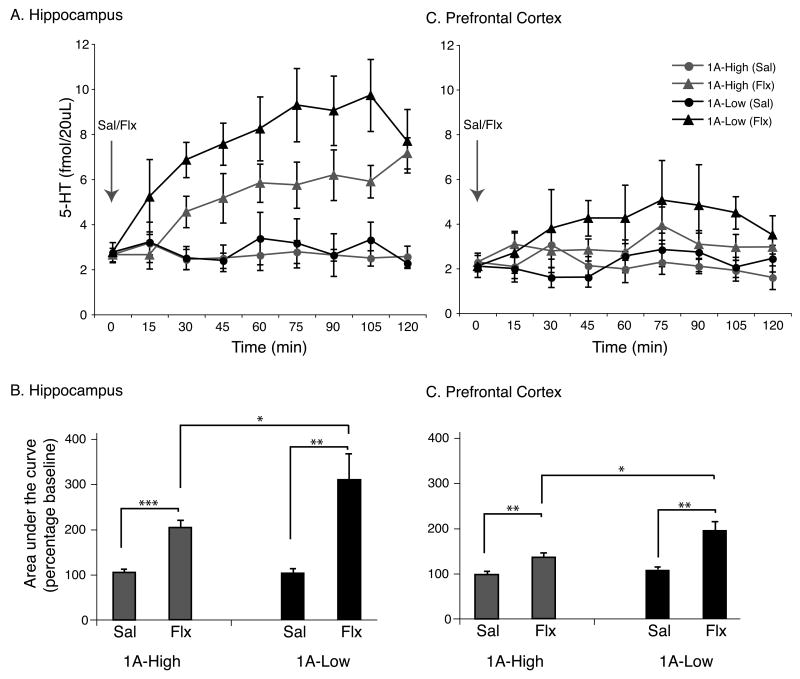
Serotonin levels in 1A-High and 1A-Low mice are indistinguishable at baseline, but differ significantly in response to fluoxetine challenge
Having observed behavioral differences between 1A-High and 1A-Low mice in response to challenge with both repeated stress and serotonin transporter blockade, we next asked how these differences were reflected at the neurochemical level. We performed in-vivo microdialysis in two representative forebrain areas: the ventral hippocampus (vHPC) and the pre-frontal cortex (PFC). Despite the differences in basal raphe firing, no difference was detected in serotonin levels at baseline between the groups in either the vHPC or PFC (two-way ANOVA for brain region and group, main effect of group, F1,26=0.006, P=0.937) (Table 1). Following 8 days of fluoxetine treatment, we observed a difference in serotonin levels in the vHPC, with higher levels of serotonin in the 1A-Low animals (two-way ANOVA for brain region and group, main effect of group, F1,22=9.705; P=0.005; region by group interaction, F1,22=8.977; P=0.0067; post hoc for group in the vHPC, P=0.003). Interestingly, serotonin levels continued to increase in both groups with chronic fluoxetine treatment. Differences in extracellular serotonin levels were normalized between the groups by 26 days of fluoxetine treatment, in both forebrain areas measured (two-way ANOVA for brain region and group, main effect of group, F1,24=0.202, P=0.657).
Table 1. Serotonin Levels Measured by In Vivo Microdialysis in 1A-High and 1A-Low Mice Treated with Fluoxetine.
| Naive | 8 Days Fluoxetine | 26 Days Fluoxetine | ||||
|---|---|---|---|---|---|---|
| HPC | PFC | HPC | PFC | HPC | PFC | |
| 1A-High | 2.6 ± 0.2 | 2.2 ± 0.4 | 3.1 ± 0.3 | 2.4 ± 0.5 | 10.8 ± 1.2 | 3.2 ± 0.4 |
| 1A-Low | 2.7 ± 0.4 | 2.1 ± 0.4 | 6.8 ± 0.9** | 2.5 ± 0.4 | 10.5 ± 0.8 | 3.3 ± 0.3 |
Mean basal serotonin levels (fmol/20 μl dialysate) ± SEM in ventral hippocampus (HPC) and prefrontal cortex (PFC).
p < 0.01 compared to 1A-High in HPC.
To further dissect the neurochemical effects of fluoxetine on mice with different levels of serotonergic autoinhibition, we assessed changes in serotonin levels in response to an acute challenge with fluoxetine or saline. Both groups of mice displayed significant increases in serotonin in response to acute fluoxetine treatment compared to saline in both the vHPC (Figure 8a, b) (two-way ANOVA, main effect of group, F1, 26=4.352, P=0.0469; main effect of treatment, F1, 26=37.822, P<0.0001; group by treatment interaction, F1, 26=4.512, P=0.0433; posthoc for treatment in 1A-High mice, P<0.0001; posthoc for treatment in 1A-Low mice, P=0.0042), and the PFC (Figure 8c, d) (two-way ANOVA, main effect of group, F1, 26=6.769, P=0.0151; main effect of treatment, F1, 26=23.080, P<0.0001; posthoc for treatment in 1A-High mice, P<0.0065; posthoc for treatment in 1A-Low mice, P=0.0039). However, 1A-Low mice displayed a larger increase in 5-HT in response to fluoxetine in both brain regions (posthoc for group in fluoxetine-treated animals in the vHPC, P=0.0474; posthoc for group in fluoxetine-treated animals in the PFC, P=0.0251), consistent with increased serotonergic tone in these animals.
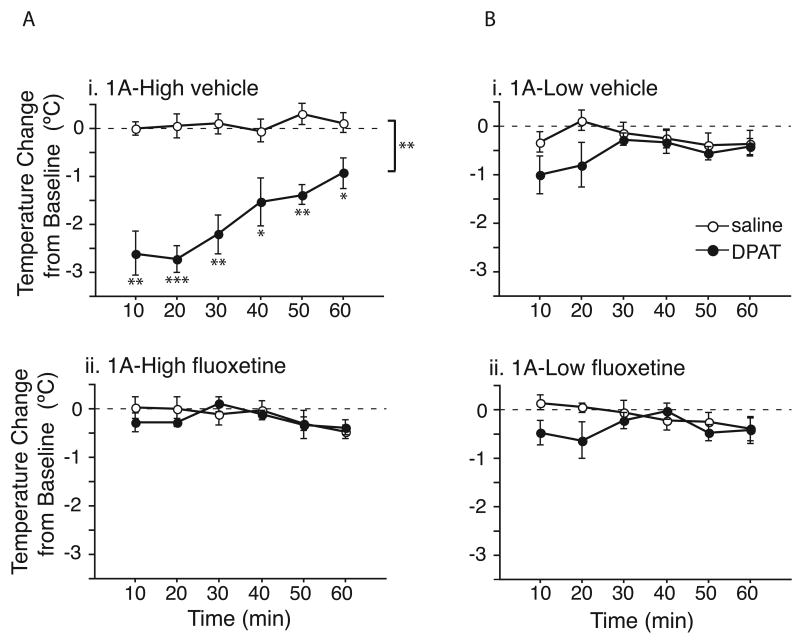
Figure 8. Loss of 5-HT1A agonist response in 1A-High mice treated with chronic fluoxetine.
(a, i and ii) 1A-High mice treated with vehicle for 35 days display a robust hypothermic response to 0.5mg/kg 8-OH DPAT challenge, while those treated with fluoxetine for 35 days do not, demonstrating full desensitization of 5-HT1A autoreceptors in 1A-High mice after fluoxetine treatment (N=3/group/treatment/dose; **P<0.01, main effect of dose; P=0.002, dose by time interaction; *P<0.05, **P<0.01, ***P<0.001 for between dose comparisons at each timepoint). (b, i and ii) 1A-Low mice treated with vehicle display a similarly attenuated response to those treated with fluoxetine, consistent with decreased 5-HT1A autoreceptor levels and function (N=3/group/treatment/dose; P<0.05, dose by time interaction) in these mice.
5-HT1A receptors desensitize after chronic treatment with fluoxetine
One long-standing hypothesis states that 5-HT1A autoreceptor desensitization both delays response and is necessary for behavioral response to occur(Blier et al., 1998). Thus, to ensure that the lack of behavioral response to fluoxetine in 1A-High mice was not due to a failure of autoreceptor desensitization, we assessed the animals' hypothermic response to 8-OH DPAT after chronic fluoxetine treatment. While 1A-High mice treated chronically with vehicle displayed a robust hypothermic response to 8-OH DPAT challenge (repeated measures two-way ANOVA, main effect of dose F1,6 = 35.477, P=0.001; dose by time interaction F1,5 = 5.080, P= 0.0017), 1A-High mice treated chronically with fluoxetine no longer responded to 8-OH-DPAT challenge (repeated measures ANOVA, main effect of dose F1,6 =0.085, P=0.781; dose by time interaction F1,5 = 1.479, P= 0.226) (Figure 8a i and ii). This result is in agreement with previous reports showing desensitization of 5-HT1A autoreceptors following chronic treatment with a number of drugs used to treat depression, including SSRIs(Blier et al., 1998). A similarly attenuated response to 8-OH-DPAT challenge is seen in 1A-Low mice treated chronically with both vehicle (two-way repeated measures ANOVA, main effect of dose F1,6= 1.252, P=0.306; dose by time interaction F1,5 = 2.831, P= 0.0328) and fluoxetine (repeated measures ANOVA, main effect of dose F1,6= 0.922, P=0.374; dose by time interaction F1,5 = 3.537, P= 0.0124) (Figure 8b i and ii), consistent with the blunted response we observed previously in these animals (Figure 2d). Therefore these results suggest that desensitization of 5-HT1A autoreceptors alone is not sufficient for the behavioral response to fluoxetine, but rather that 5-HT1A -mediated serotonergic tone prior to treatment is critical for establishing treatment response.
Discussion
tetO based gene suppression
Conditional knockout and transgenic mice are powerful tools for probing the behavioral roles of genes expressed in the brain. In practice, however, most approaches have been limited by ectopic expression, lack of temporal control, or irreversibility. These weaknesses are largely overcome in the system presented here. We use an adaptation of the tetO inducible strategy that relies on insertion of tetO sites into the endogenous promoter of a gene of interest. In the case of the tetO-1A mice used here, this insertion is largely silent (i.e. does not noticeably alter the pattern of 5-HT1A receptor expression) in the absence of tTS. We have now successfully generated silent tetO insertions in several other genes (data not shown), suggesting that this strategy is broadly generalizable.
Expression of the 5-HT1A receptor in this system is tightly suppressed by a ubiquitously expressed tetracycline-sensitive transcriptional suppressor (tTS) binding to tetO sequences that are knocked-in to the endogenous Htr1a locus. Importantly, suppression can be achieved at any point in the life of the animal by withdrawing doxycycline. Furthermore, specificity of gene suppression is dictated by an overlap between transgenic tTS expression patterns and endogenous expression of the gene. This ensures that tTS-mediated suppression only occurs in cells that normally express the gene of interest, eliminating the possibility for ectopic gene expression. Finally, another advantage of this system is that, unlike systems that rely on genetic recombination, suppression can be reversed in the presence of doxycycline (data not shown).
Modeling the human Htr1a C(-1019)G polymorphism
Our 1A-High and 1A-Low mice provide a mechanistic model of one of the predicted consequences of the recently identified human Htr1a C(-1019)G polymorphism: namely, that it results in differential transcriptional suppression of the Htr1a gene in serotonergic neurons and creates populations of individuals with higher and lower expression of 5-HT1A autoreceptors. Initial in vitro characterization of expression driven off this polymorphic allele revealed preferential suppression of the C-allele by several transcription factors in a raphe-derived cell line, but not in cell lines derived from other brain areas. This suggested that C carriers might express lower levels of 5-HT1A autoreceptor than G-carriers(Lemonde et al., 2003). However, the only subsequent binding study to report an association between the G-allele and increased 5-HT1A receptor binding reported increases in both the raphe and other brain regions(Parsey et al., 2006). It remains unclear whether the human polymorphism directly affects 5-HT1A gene expression throughout the brain, or whether the changes in forebrain levels are a secondary consequence of a primary change in autoreceptors.
Consequences of decreased 5-HT1A autoreceptor levels in adulthood
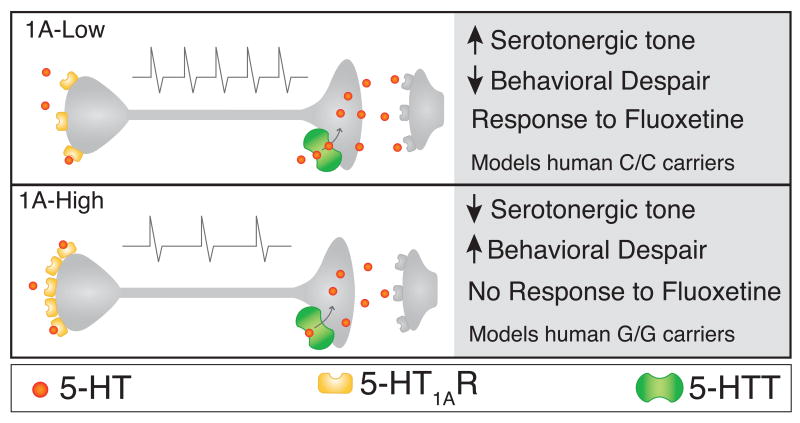
Our data from 1A-High and 1A-Low mice provides the first direct evidence for a functional model incorporating the predictions generated from both pre-clinical and clinical studies, including the recent human Htr1a C(-1019)G polymorphism studies(Albert and Lemonde, 2004; Lesch and Gutknecht, 2004). In this model, 5-HT1A autoreceptor-modulated intrinsic raphe firing rates are directly related to resilience under stress and to the response to antidepressant treatment, demonstrated here with the prototypical SSRI fluoxetine (Figure 9). In such a model, when the serotonergic system is activated, higher intrinsic 5-HT1A autoreceptor levels (either in 1A-High mice or G/G individuals) results in lower raphe firing rate, and lower intrinsic 5-HT1A autoreceptor (in 1A-Low mice or C/C individuals) results in higher raphe firing rate. The increased raphe firing rate (in 1A-Low mice or C/C individuals) would increase resilience to chronic stress by increasing serotonin release throughout the brain upon challenge, as seen by the decreased behavioral despair of 1A-Low mice following stress. Interestingly, our data suggests that at baseline (i.e., non-stressful conditions), levels of serotonin do not differ between the 1A-High and 1A-Low mice.
Figure 9. Model of 5-HT1A autoreceptor effects on the serotonergic raphe.
Diagram depicts representative raphe neurons in 1A-High and 1A-Low animals, emphasizing the differences between the two groups. 1A-High mice have lower basal firing rate (indicated above the cell) and high levels of somatodendritic 5-HT1A autoreceptor, which exert robust inhibitory effects on raphe firing. This results in increased behavioral despair in response to stress, compared to 1A-Low mice. Conversely, 1A-Low mice have higher basal firing rate and low levels of somatodendritic 5-HT1A autoreceptors, which exert less inhibitory control over raphe firing rates. This results in less behavioral despair in response to stress, compared to 1A-High mice. While 1A-High mice do not respond behaviorally to treatment with the antidepressant fluoxetine, 1A-Low mice display a robust behavioral response. 1A-High and 1A-Low mice provide a mechanistic model for humans carrying, respectively, the G/G and C/C alleles of the Htr1aI C(-1019)G polymorphism.
Studies in rats treated chronically with SSRIs have shown an initial decrease of raphe firing at the beginning of treatment, with firing rates recovering to baseline following chronic treatment and 5-HT1A autoreceptor desensitization(Blier et al., 1998). Thus, in the presence of an SSRI, we expect 5-HT1A autoreceptor-mediated inhibition of raphe firing to occur in both 1A-High and 1A-Low animals, albeit to different extents. Indeed, 1A-Low animals display faster increases in extracellular serotonin in the hippocampus upon repeated (8 day) fluoxetine treatment, directly reflecting differential autoinhibition in response to reuptake blockade. Interestingly, extracellular serotonin levels reach a similar plateau in both 1A-High and 1A-Low animals following chronic (26 day) treatment and autoreceptor desensitization, demonstrating that the behavioral differences between the groups cannot be fully explained by extracellular serotonin levels. Because our behavioral groups differ only by the levels of their 5-HT1A autoreceptors at the start of treatment, the differences in behavioral response to fluoxetine must be mediated by either differential downstream changes or subtler differences in serotonergic tone.
In summary, two of the main associations from studies of the C(-1019)G polymorphism in humans are recapitulated in our model: susceptibility to stress and response to antidepressant treatment. In addition, our data suggest that the effects of the polymorphism may be easier to detect under conditions of chronic stress or pharmacological intervention.
Behavioral dissociation and treatment implications
Together with previous work, this study also establishes a double dissociation of 5-HT1A receptor function in baseline anxiety- and depression-related behavior between development and adulthood. Previous work has shown that transgenic developmental over-expression of 5-HT1A in the forebrain is sufficient to establish normal anxiety-like behavior, regardless of 5-HT1A receptor status at the time of testing(Gross et al., 2002). Furthermore, pharmacological blockade of 5-HT1A receptors in development but not adulthood is sufficient to increase anxiety-like behavior in WT mice (Lo Iacono and Gross, 2008). The data presented here demonstrate the complementary point: specific manipulation of 5-HT1A autoreceptors in adulthood is sufficient to impact reactivity to stress and depression-related behavior without affecting conflict-anxiety measures.
Finally, this study underscores the difference between decreased intrinsic 5-HT1A-mediated autoinhibition and desensitization of 5-HT1A autoreceptors. Specifically, one canonical hypothesis postulates that 5-HT1A autoreceptor desensitization determines the behavioral response to antidepressant treatment. Our data does not support this hypothesis, as 1A-High mice displayed desensitized autoreceptors (in terms of both 8-OH DPAT hypothermia and extracellular serotonin levels), yet do not respond behaviorally to fluoxetine treatment. Conversely, mice that differed only by possessing lower autoreceptor levels before treatment—1A-Low mice—displayed a robust behavioral response to fluoxetine after both chronic (26 day) and subchronic (8 day) treatment.
Indeed, we conclude that 5-HT1A autoreceptor desensitization alone is not sufficient for the response to fluoxetine to occur, as 1A-High mice display a desensitized 8-OH DPAT response but do not respond behaviorally to chronic fluoxetine treatment. Rather, our data suggest that serotonergic tone—governed by intrinsic autoreceptor levels—prior to the onset of treatment is critical for establishing responsiveness and time to response. Thus, we predict that treatments aimed at increasing serotonergic tone prior to beginning SSRI administration might prove to be efficacious and even faster-acting antidepressant therapies, particularly for individuals with higher autoreceptor levels, such as those carrying the G/G alleles of the C(-1019)G polymorphism.
Experimental Procedures
Materials and Methods
Transgenic Mice
tetO-1A mice were generated by removing a loxP-flanked pGK-neo transcriptional stop cassette from Htr1aSTOP-teto knockout mice by crossing to an HSP70-cre line that deletes in the germline (Dietrich et al., 2000; Gross et al., 2002). The resulting tetO-1A mice contain a tetO-CMV promoter inserted 5′ of the Htr1a coding region and express the 5-HT1A receptor in a pattern that is indistinguishable from the wild-type. β-actin tTS+ tetO-1A+/+ mice were created by breeding mice with tTS expressed under control of a human β-globin transgene (Mallo et al., 2003) onto a background homozygous for the tetO-1A allele. Tg(Pet-1-tTS) was produced by cloning the coding sequence of tetracycline trans-suppressor (tTS) protein followed by an SV40 polyadenylation signal(Deuschle et al., 1995) into the T3 polylinker region of the NarI/BgzA modification 5 plasmid, placing the coding sequence downstream of a β-globin promoter(Scott et al., 2005). The β-globin promoter and tTS coding sequence were then released with an RsrII digest and the resulting fragment was cloned into the EPet-1 Mini-BAC Mod#1 containing a 40 kb genomic DNA fragment (540z) that lies upstream of the Pet-1 coding sequence(Scott et al., 2005). The resulting BAC EPet-1-tTs was linearized and subjected to pronuclear injection into B6CBA/F2 Hybrid zygotes. Founders were identified by PCR using the primers (5′-TTGATCACCAAGGTGCAGAG-3′ and 5′-CAGGGCTCTTCTCCCTTCTC-3′). Pet-1-tTS+ tetO-1A+/+ mice were generated for behavioral experiments by breeding the Pet-1-tTS mice onto tetO-1A+/+ background. Pet-1-tTS+ tetO-1A+/+ males were then bred to tetO-1A+/+ females. As a result, the Pet-1-tTS transgene was transmitted through the male germline, ensuring that all pups were raised by mothers of the same genotype, regardless of doxycycline status. All experiments were conducted on male offspring. Animals were maintained on a mixed 129S6/Sv; C57B6; CBA background.
Pet-1-tTS+ tetO-1A+/+ mice and their Pet-1-tTS- tetO-1A+/+ littermates were fed chow containing 40mg/kg doxycycline (Product # F5545, Bioserv, Frenchtown, NJ) throughout development to prevent tTS-mediated transcriptional suppression of the 5-HT1A receptor. This chow was otherwise identical in composition to standard laboratory chow (described below). At 50 days postnatal, animals were randomly split into two groups; one continued receiving doxycycline chow (1A-High), and the other began receiving doxycycline-free standard laboratory chow (1A-Low) (Prolab Isopro RMH 3000, PMI Nutrition International, Brentwood, MO). To control for the possible effects of doxycycline on behavior, littermate controls lacking the tTS transgene, in which doxycycline had no effect on 5-HT1A receptor expression, were also tested in baseline behavioral experiments (Figure S2).
Animal Husbandry
Animals were housed in groups of between 3 and 5 per cage and had access to food and water, ad libitum. Animals were maintained on a 12:12 light/dark schedule, and all testing was conducted during the light period. Animal protocols were approved by the Institutional Animal Care and Use Committee and were conducted in accordance to the NIH Guide for the Care and Use of Laboratory Animals.
Receptor autoradiography
Mice of the ages indicated were sacrificed by cervical dislocation and decapitation. Extracted brains were frozen immediately on crushed dry ice (-75°C) and maintained at -80°C until sectioning. Brains were cryosectioned at a thickness of 18μm, and sections were thaw-mounted on Superfrost slides (Fisher, Fairlawn, NJ, USA). Sections were maintained at -80°C until processing.
125I-MPPI—4-(2′-Methoxyphenyl)-1-[2′-(n-2″-pyridinyl)-p-[125I]iodobenzamido]ethylpiperazine) binding
Mounted sections were processed for 125I-MPPI autoradiography using a previously described method (Kung et al., 1995; Ramboz et al., 1998). Sections were exposed to BioMax MR film (Kodak, Rochester, NY) for a period of between 6 and 72, hours, depending on the activity of the ligand at time of binding. All experimental and control brains within a group were processed and exposed to film as a batch. Films were digitized at a resolution of 1200dpi using an Epson 3200 Photo Scanner, and signal density was measured using the mean luminosity function in Adobe Photoshop, as described(Rattiner et al., 2004; Ressler et al., 2002). Levels of 5-HT1A binding were determined by analyzing the region of interest (ROI) and subtracting from a same-size adjacent “background” region of tissue lacking specific binding, to obtain a normalized luminosity value for each ROI. Signals were determined to be within the linear range of the film by comparison to a standard curve constructed from an ARC146-F 14C standard (ARC, St. Louis, MO).
Electrophysiology
Whole cell recordings
Whole cell recordings of dorsal raphe neurons were made as previously described(Beck et al., 2004). Briefly, 200μm coronal slices were taken throughout the rostro-caudal extent of the dorsal raphe and maintained in oxygenated ACSF for a one-hour recovery period. Dorsal raphe neurons were visualized and the membrane was patched and then ruptured as described(Beck et al., 2004). Following collection of passive membrane characteristics, cells were voltage-clamped at -60mV and the current response to application of 100nM 5-CT was recorded. If cells showed no response to 5-CT, the GABAB antagonist baclofen (30 mM) was added and the cell response was measured. Cells that did not respond to 5-CT or baclofen were excluded from the analysis. After recording, cells were biocytin filled and identified as serotonergic by co-labeling with TPH.
In vivo recordings
Single-unit potentials were collected with an Axoclamp 2A amplifier, Digitdata 1440A/D converter (Molecular Devices, Downingtown, PA), and amplified (100 ×) and filtered (3 kHz to 30 Hz) with a LPF 200 DC Amplifier/Filter (Warner Instruments, Hamden, CT) and collected on-line using Clampex 10.1 software (Molecular Devices, Downingtown, PA). A serotonin neuron was characterized by a biphasic action potential that was approximately 2 ms in duration. A stable baseline of spontaneous activity was recorded for at least 3 min. Multiple neurons per mouse were recorded through multiple descents. Only firing rates within two standard deviations of the mean were included in the analysis.
Intracerebral in vivo microdialysis
Mice were treated with fluoxetine for 0, 8, or 26 days, as indicated (18mg/kg, p.o.). Extracellular 5-HT levels were measured by in vivo microdialysis as previously described (Guiard et al., 2008). Briefly, after the last dose of fluoxetine, two concentric dialysis probes were implanted in the vHPC and PFC (outer diameter × active length: 0.3 × 1.6 and 0.3 mm × 2 mm, respectively) of anesthetized mice (chloral hydrate, 400mg/kg, i.p.). Stereotaxic coordinates (in mm) were, PCF: A = 1.6, L = 1.3, V = 1.6; vHPC: A = -2.8, L = 3.0, V = 3.0 (Franklin and Paxinos, 1997). Animals were allowed to recover for a period of 24 hours. Following recovery, probes were continuously perfused with aCSF, and dialysate was collected every 15 minutes for analysis by HPLC-amperometry(Guiard et al., 2008). Baseline 5-HT levels were calculated as the average of the first four samples, ±SEM. Freely moving mice were treated (t=0) with either a challenge dose of fluoxetine (18 mg/kg; i.p.) or its vehicle, and dialysate samples were collected for a 0–120 min post-treatment period. The limit of sensitivity for 5-HT was 0.5 fmol/sample (signal-to-noise ratio 2). Following sample collection, brains were removed and sectioned to ensure proper probe placement.
Behavioral and Physiological Testing
All animals used for behavioral testing were age matched within two weeks. Animals were initially tested at 11-13 weeks of age, at least four weeks after the cessation of doxycycline in 1A-low animals. Baseline anxiety tests were completed before other behavioral tests. Fluoxetine was given after baseline behavioral and physiological measures were assessed, at 18 mg/kg/day p.o. for up to 28 days. Testing in the novelty-suppressed feeding paradigm occurred on day 26 of treatment.
8-OH DPAT Induced Hypothermia
Body temperature was assessed intra-rectally, using a lubricated probe inserted approximately 2 cm and a Thermalert TH-5 thermal monitor (Physitemp, Clifton, NJ). Mice were singly housed in clean cages for 10 minutes, and three baseline body temperature measurements were taken. Ten minutes after the third baseline measurement, animals received 8-OH DPAT i.p. at the doses indicated and body temperature was monitored every ten minutes for a total of 60 minutes. Temperatures are represented as a change from the final baseline measurement.
Stress-Induced Hyperthermia
Stress induced hyperthermia paradigm measures a physiologic response to a stressful stimuli(Adriaan Bouwknecht et al., 2007). Briefly, animals in their home cages were moved to a testing room and allowed to acclimate for 1 hour. One animal per cage was removed and a baseline body temperature was measured intrarectally. Each animal was then placed in a novel, clean cage for ten minutes, after which a second body temperature was recorded.
Open Field Test
Exploration in response in response to a novel open field was measured as described(Weisstaub et al., 2006), with the following modifications: 1) animals were singly housed for at least 30 minutes prior to testing to minimize order effects within a cage, 2) light levels in the open field chambers were maintained at 10-20 lux to encourage exploration of the full environment, 3) animals were placed in a corner of the maze and allowed to explore the center at will, and 4) the test was conducted for a total of thirty minutes. Dependent measures were total path length (cm), number of entries into the center, time in the center, percent distance in the center (distance travelled in the center divided by the total distance travelled).
Light/Dark Choice Test
Exploration of the light/dark chamber was measured as described(Klemenhagen et al., 2006). Dependent measures were total distance and percent time spent in the light compartment.
Modified Forced Swim Test
Behavioral response to forced swimming was assayed as described previously(David et al., 2007). Briefly, mice were placed into clear plastic buckets 20 cm in diameter and 23 cm deep filled 2/3 of the way with 26°C water and videotaped from the side for 6 min. Only the last 4 minutes were scored. All animals were exposed to the swim test on two consecutive days. Scoring was done using an automated Viewpoint Videotrack software package (Montreal, Canada). Dependent variables were immobility, swimming and climbing.
Tail suspension test
Mice were suspended by the tail using tape to secure them to a horizontal bar. The animals were suspended for 5 minutes and immobility during this period was assessed using an automated Viewpoint Videotrack software package (Montreal, Canada).
Repeated mild stressor
Animals were gavaged daily with 10ml/kg/day of drinking water for 28 days prior to testing.
Novelty suppressed Feeding
Testing was performed as previously described(David et al., 2007). Briefly, animals were food restricted for 24 hours and were place in a 40 × 60 cm brightly lit arena (800-900 lux) with a food pellet placed in the center. Latency of the animals to begin chewing food was recorded. Immediately after the latency was recorded, the food pellet was removed from the arena. The animals were then placed in their home cage and the amount of food consumed in 5 minutes was measured (home cage consumption), followed by an assessment of post-restriction weight. Percentage body weight lost and home cage consumption were assessed as relative measures of animal hunger. No effect of fluoxetine was observed in home cage measures (Figure S3).
Statistical Analysis
In general, the effect of treatment or dose was analyzed using an analysis of variance (ANOVA), using repeated measures where appropriate. Significant ANOVAs were followed up with Fisher PLSD test for behavioral and physiological measures, and with Student-Neuman-Keuls t-test for electrophysiological characterization. In the case of the NSF paradigm, survival analysis was performed and statistical differences were determined using the Kaplan-Meier product-limit method.
Supplementary Material
Figure 7. Increased serotonin levels in response to fluoxetine in 1A-Low mice.
Extracellular serotonin levels measured by in vivo microdialysis in the (a) ventral hippocampus, and (c) prefrontal cortex of naïve 1A-High and 1A-Low mice, following acute challenge with fluoxetine (18mg/kg, i.p.) or saline. Total extracellular 5-HT, measured by area-under-the-curve analysis, increases in the (b) ventral hippocampus, and (d) prefrontal cortex of both 1A-High and 1A-Low mice in response to acute fluoxetine treatment. 1A-Low mice display a larger increase in extracellular 5-HT than 1A-High mice in both brain areas (N=6-9 mice/group; *P<0.05, **P<0.01, ***P<0.001).
Acknowledgments
The authors gratefully acknowledge Moises Mallo for providing the β-actin tTS mouse line and the tTS construct and thank Evan Deneris for providing the Pet-1 mini-BAC. We thank Randall Sewell for technical assistance. Adaure Akanwa is acknowledged for performing the immunohistochemistry on the brain slices used for electrophysiology. Finally, we are grateful to Joshua Gordon, Susanne Ahmari, and Jay Gingrich for helpful discussions and critical reading of the manuscript. This work was supported by NIH grant K08 MH076083 to E.D.L., R01 MH075047 to S.G.B, R01 MH068542 to R.H. and AstraZeneca grant CU08-8439 to R.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Health, United States. NCfH Statistics, ed. Hyattsville, MD: 2007. 2007 With Chartbook on Trends in the Health of Americans. [PubMed] [Google Scholar]
- 2.Adriaan Bouwknecht J, Olivier B, Paylor RE. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev. 2007;31:41–59. doi: 10.1016/j.neubiorev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10:575–593. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- 4.Anttila S, Huuhka K, Huuhka M, Rontu R, Hurme M, Leinonen E, Lehtimaki T. Interaction between 5-HT1A and BDNF genotypes increases the risk of treatment-resistant depression. J Neural Transm. 2007;114:1065–1068. doi: 10.1007/s00702-007-0705-9. [DOI] [PubMed] [Google Scholar]
- 5.Artigas F, Romero L, de Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- 6.Audero E, Coppi E, Mlinar B, Rossetti T, Caprioli A, Banchaabouchi MA, Corradetti R, Gross C. Sporadic autonomic dysregulation and death associated with excessive serotonin autoinhibition. Science. 2008;321:130–133. doi: 10.1126/science.1157871. [DOI] [PubMed] [Google Scholar]
- 7.Beck SG, Choi KC, List TJ. Comparison of 5-hydroxytryptamine1A-mediated hyperpolarization in CA1 and CA3 hippocampal pyramidal cells. J Pharmacol Exp Ther. 1992;263:350–359. [PubMed] [Google Scholar]
- 8.Beck SG, Pan YZ, Akanwa AC, Kirby LG. Median and dorsal raphe neurons are not electrophysiologically identical. J Neurophysiol. 2004;91:994–1005. doi: 10.1152/jn.00744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blier P, Pineyro G, el Mansari M, Bergeron R, de Montigny C. Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Ann N Y Acad Sci. 1998;861:204–216. doi: 10.1111/j.1749-6632.1998.tb10192.x. [DOI] [PubMed] [Google Scholar]
- 10.Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- 11.Buccafusco JJ. Methods of behavioral analysis in neuroscience. 2nd. Boca Raton: CRC Press; 2009. [PubMed] [Google Scholar]
- 12.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 13.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Dalm S, Brinks V, van der Mark MH, de Kloet ER, Oitzl MS. Non-invasive stress-free application of glucocorticoid ligands in mice. J Neurosci Methods. 2008;170:77–84. doi: 10.1016/j.jneumeth.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 15.David DJ, Klemenhagen KC, Holick KA, Saxe MD, Mendez I, Santarelli L, Craig DA, Zhong H, Swanson CJ, Hegde LG, et al. Efficacy of the MCHR1 antagonist N-[3-(1-{[4-(3,4-difluorophenoxy)phenyl]methyl}(4-piperidyl))-4-methylphen yl]-2-methylpropanamide (SNAP 94847) in mouse models of anxiety and depression following acute and chronic administration is independent of hippocampal neurogenesis. J Pharmacol Exp Ther. 2007;321:237–248. doi: 10.1124/jpet.106.109678. [DOI] [PubMed] [Google Scholar]
- 16.Deuschle U, Meyer WK, Thiesen HJ. Tetracycline-reversible silencing of eukaryotic promoters. Mol Cell Biol. 1995;15:1907–1914. doi: 10.1128/mcb.15.4.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietrich P, Dragatsis I, Xuan S, Zeitlin S, Efstratiadis A. Conditional mutagenesis in mice with heat shock promoter-driven cre transgenes. Mamm Genome. 2000;11:196–205. doi: 10.1007/s003350010037. [DOI] [PubMed] [Google Scholar]
- 18.Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, Mathis C. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Fakra E, Hyde LW, Gorka A, Fisher PM, Munoz KE, Kimak M, Halder I, Ferrell RE, Manuck SB, Hariri AR. Effects of HTR1A C(-1019)G on amygdala reactivity and trait anxiety. Arch Gen Psychiatry. 2009;66:33–40. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fava M, Kendler KS. Major depressive disorder. Neuron. 2000;28:335–341. doi: 10.1016/s0896-6273(00)00112-4. [DOI] [PubMed] [Google Scholar]
- 22.Fisher PM, Meltzer CC, Ziolko SK, Price JC, Moses-Kolko EL, Berga SL, Hariri AR. Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nat Neurosci. 2006;9:1362–1363. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- 23.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 24.Gardier AM, Malagie I, Trillat AC, Jacquot C, Artigas F. Role of 5-HT1A autoreceptors in the mechanism of action of serotoninergic antidepressant drugs: recent findings from in vivo microdialysis studies. Fundam Clin Pharmacol. 1996;10:16–27. doi: 10.1111/j.1472-8206.1996.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 25.Groenink L, van der Gugten J, Zethof T, van der Heyden J, Olivier B. Stress-induced hyperthermia in mice: hormonal correlates. Physiol Behav. 1994;56:747–749. doi: 10.1016/0031-9384(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 26.Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Altered fear circuits in 5-HT(1A) receptor KO mice. Biol Psychiatry. 2000;48:1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- 27.Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- 28.Guiard BP, David DJ, Deltheil T, Chenu F, Le Maitre E, Renoir T, Leroux-Nicollet I, Sokoloff P, Lanfumey L, Hamon M, et al. Brain-derived neurotrophic factor-deficient mice exhibit a hippocampal hyperserotonergic phenotype. Int J Neuropsychopharmacol. 2008;11:79–92. doi: 10.1017/S1461145707007857. [DOI] [PubMed] [Google Scholar]
- 29.Hamon M, Lanfumey L, el Mestikawy S, Boni C, Miquel MC, Bolanos F, Schechter L, Gozlan H. The main features of central 5-HT1 receptors. Neuropsychopharmacology. 1990;3:349–360. [PubMed] [Google Scholar]
- 30.Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibarguen-Vargas Y, Surget A, Touma C, Palme R, Belzung C. Multifaceted strain-specific effects in a mouse model of depression and of antidepressant reversal. Psychoneuroendocrinology. 2008;33:1357–1368. doi: 10.1016/j.psyneuen.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Major depression and generalized anxiety disorder. Same genes, (partly) different environments? Arch Gen Psychiatry. 1992;49:716–722. doi: 10.1001/archpsyc.1992.01820090044008. [DOI] [PubMed] [Google Scholar]
- 34.Klemenhagen KC, Gordon JA, David DJ, Hen R, Gross CT. Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. Neuropsychopharmacology. 2006;31:101–111. doi: 10.1038/sj.npp.1300774. [DOI] [PubMed] [Google Scholar]
- 35.Kraus MR, Al-Taie O, Schafer A, Pfersdorff M, Lesch KP, Scheurlen M. Serotonin-1A receptor gene HTR1A variation predicts interferon-induced depression in chronic hepatitis C. Gastroenterology. 2007;132:1279–1286. doi: 10.1053/j.gastro.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 36.Kung MP, Frederick D, Mu M, Zhuang ZP, Kung HF. 4-(2′-Methoxyphenyl)-1-[2′-(n-2″-pyridinyl)-p-iodobenzamido]-ethyl- piperazine ([125I]p-MPPI) as a new selective radioligand of serotonin-1A sites in rat brain: in vitro binding and autoradiographic studies. J Pharmacol Exp Ther. 1995;272:429–437. [PubMed] [Google Scholar]
- 37.Lanfumey L, Mongeau R, Cohen-Salmon C, Hamon M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci Biobehav Rev. 2008;32:1174–1184. doi: 10.1016/j.neubiorev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Le Francois B, Czesak M, Steubl D, Albert PR. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 39.Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leonardo ED, Hen R. Genetics of affective and anxiety disorders. Annu Rev Psychol. 2006;57:117–137. doi: 10.1146/annurev.psych.57.102904.190118. [DOI] [PubMed] [Google Scholar]
- 41.Lesch KP, Gutknecht L. Focus on The 5-HT1A receptor: emerging role of a gene regulatory variant in psychopathology and pharmacogenetics. Int J Neuropsychopharmacol. 2004;7:381–385. doi: 10.1017/S1461145704004845. [DOI] [PubMed] [Google Scholar]
- 42.Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, et al. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- 43.Lo Iacono L, Gross C. Alpha-Ca2+/calmodulin-dependent protein kinase II contributes to the developmental programming of anxiety in serotonin receptor 1A knock-out mice. J Neurosci. 2008;28:6250–6257. doi: 10.1523/JNEUROSCI.5219-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- 46.Mallo M, Kanzler B, Ohnemus S. Reversible gene inactivation in the mouse. Genomics. 2003;81:356–360. doi: 10.1016/s0888-7543(03)00032-6. [DOI] [PubMed] [Google Scholar]
- 47.Martin KF, Phillips I, Hearson M, Prow MR, Heal DJ. Characterization of 8-OH-DPAT-induced hypothermia in mice as a 5-HT1A autoreceptor response and its evaluation as a model to selectively identify antidepressants. Br J Pharmacol. 1992;107:15–21. doi: 10.1111/j.1476-5381.1992.tb14457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neff CD, Abkevich V, Packer JC, Chen Y, Potter J, Riley R, Davenport C, Degrado Warren J, Jammulapati S, Bhathena A, et al. Evidence for HTR1A and LHPP as interacting genetic risk factors in major depression. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.8. [DOI] [PubMed] [Google Scholar]
- 49.Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci U S A. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ. Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology. 2006;31:1745–1749. doi: 10.1038/sj.npp.1300992. [DOI] [PubMed] [Google Scholar]
- 51.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 52.Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rattiner LM, Davis M, Ressler KJ. Differential regulation of brain-derived neurotrophic factor transcripts during the consolidation of fear learning. Learn Mem. 2004;11:727–731. doi: 10.1101/lm.83304. [DOI] [PubMed] [Google Scholar]
- 54.Ressler KJ, Paschall G, Zhou XL, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. J Neurosci. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- 56.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 57.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 58.Schatzberg AF, Nemeroff CB. The American Psychiatric Publishing textbook of psychopharmacology. 4th. Washington, DC: American Psychiatric Pub.; 2009. [Google Scholar]
- 59.Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci U S A. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strobel A, Gutknecht L, Rothe C, Reif A, Mossner R, Zeng Y, Brocke B, Lesch KP. Allelic variation in 5-HT1A receptor expression is associated with anxiety- and depression-related personality traits. J Neural Transm. 2003;110:1445–1453. doi: 10.1007/s00702-003-0072-0. [DOI] [PubMed] [Google Scholar]
- 61.Van Bogaert M, Oosting R, Toth M, Groenink L, van Oorschot R, Olivier B. Effects of genetic background and null mutation of 5-HT1A receptors on basal and stress-induced body temperature: modulation by serotonergic and GABAA-ergic drugs. Eur J Pharmacol. 2006;550:84–90. doi: 10.1016/j.ejphar.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 62.Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289:109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- 63.Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- 65.Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.