Abstract
Tissue-specific innate-like γδ T cells are important components of the immune system critical for the first line of defense. But mechanisms underlying their tissue-specific development are poorly understood. Our study with prototypical skin-specific intraepithelial γδT lymphocytes (sIEL) found that among different thymic γδ T cell subsets, fetal thymic precursors of sIELs specifically acquire a unique skin-homing property after the positive selection, suggesting an important role of the TCR selection signaling in “programming” them for the tissue-specific development. Here we identified IL2-inducible T-cell kinase (ITK) as a critical signal molecule regulating the acquirement of the skin-homing property by the fetal thymic sIEL precursors. In ITK-knockout mice, the sIEL precursors could not undergo the positive selection-associated upregulation of thymus-exiting and skin-homing molecules S1PR1 and CCR10 and accumulated in the thymus. On the other hand, the survival and expansion of sIELs in the skin did not require the ITK-transduced TCR-signaling while its persistent activation impaired the sIEL development by inducing apoptosis. These findings provide insights into molecular mechanisms underlying differential requirements of the TCR signaling in peripheral localization and maintenance of the tissue specific T cells.
Introduction
Unlike conventional αβ T cells that primarily reside in secondary lymphoid organs for adaptive immune responses, various subsets of γδ T cells preferentially reside in epithelial tissues, such as the skin, reproductive tract, respiratory tracts and intestines where they function as the first line of defense (1). The different tissue-specific γδ T cells preferentially use different subsets of TCRs. In mice, skin intraepithelial γδ T lymphocytes (sIEL, also called dendritic epidermal T cells or DETCs), a prototype of the tissue-specific T cells, almost exclusively express canonical γδ TCRs composed of Vγ3–Jγ1Cγ1 and Vδ1–Dδ2–Jδ2Cδ chains while vaginal epithelial γδ T cells express Vγ4/Vδ1+ TCRs. By comparison, γδ T cells in secondary lymphoid organs express more diverse TCRs, predominantly of Vγ2 and Vγ1.1 associated with several Vδ chains. The preferential usage of specific TCRs by the different tissue-specific γδ T cells is suggested to be important for their tissue-specific functions. sIEL-specific Vγ3+γδ TCRs react with antigens upregulated on diseased skin cells and play an important role in tumor surveillance and wound healing among others, to maintain the integrity of the skin.
Precursors for the different tissue-specific γδ T cells are generated in the thymus at different stages of ontogeny. The Vγ3+ sIEL precursors are generated exclusively in the early fetal thymus where they are the first T cell population to arise during ontogeny (around day 15 of embryonic gestation, E15). Once out of the thymus, they take residence in the skin epithelium where they expand and sustain locally for the life span of mice (2–4). In contrast, fetal thymic Vγ4+γδ T cells localize to peripheral destinations such as the reproductive tract. In adults, the generation of Vγ3+ and Vγ4+γδ T cells is completely suppressed while Vγ2+ and Vγ1.1 γδ+ T cells are predominately generated and preferentially emigrate to secondary lymphoid organs, among other tissues. However, mechanisms regulating tissue-specific development of the various γδ T cell subsets are not well understood.
It has become clear recently that selection is involved in the development of tissue-specific γδ T cells, at least in the case of skin-specific sIELs (5–7). We reported that fetal thymic γδ T cell populations that display activated or memory phenotypes correlated with their development into sIELs (7). Compared to other γδ T cells, fetal thymic Vγ3+γδ T cells express a unique set of chemokine and cytokine receptors, including high levels of sphingosine 1-phosphate receptor 1 (S1PR1) and CCR10 (7), which are potentially important for their thymic egress and skin localization (8–11), and the cytokine receptor CD122 (IL-15 receptor β, IL-15Rβ), which is critical for their survival/expansion in the skin (12, 13). In absence of the positive selection, as observed in a sub-strain of FVB mice (FVB/Taconic) that express mutated Skint1, a selecting molecule for the Vγ3+ sIEL precursors, these cells could not develop into sIELs (14, 15). On the other hand, if transgenic fetal thymic γδ T cells are positively selected to express the proper chemokine and cytokine receptors, they could develop into sIELs (7, 16). These findings suggest that the TCR-dependent positive selection of fetal thymic γδ T cells is critical for their development into sIELs by promoting the expression of proper homing and cytokine receptors for epidermal localization and expansion.
Previous studies using various knockout mice found that multiple TCR signaling molecules, including Lck, Syk and ZAP-70, are important for the sIEL development (17–20). Although these molecules are involved in the TCR signaling in general, they may differentially affect the development of sIELs and other T cell populations. For example, mice deficient in Syk, a kinase down-stream of the TCR signaling, have normal development of conventional αβ T cells and splenic γδ T cells but impaired sIEL development, suggesting that there is a unique molecular signaling requirement for the sIEL development (17, 21). However, mechanisms by which TCR-signaling molecules affect the tissue-specific sIEL development are poorly understood.
ITK is a Tec family non-receptor tyrosine kinase that plays multiple roles downstream of the TCR signaling. During the TCR signaling, ITK forms a complex with the adaptor molecule Slp-76, and is involved in the phosphorylation of PLC-γ and intracellular Ca2+ mobilization. The ITK regulated signal is also involved in the activation of the ERK/MAPK pathway, and the activation of transcription factors AP-1 and NFAT. In addition, ITK mediated signals modulate the TCR-induced reorganization of actin cytoskeleton by interfacing with the guanine nucleotide exchange factor (GEF) Vav1, another important TCR signaling molecule (22–24). Besides its role in the TCR-signaling, ITK could transduce the integrin and chemokine receptor-initiated signaling (22, 25–29).
ITK-deficiency affects the development of various T cell populations differentially (30–34). While the absence of ITK impairs the development of conventional CD4+ and CD8+ T cells, the development of non-conventional or “innate memory phenotype” CD4+ and CD8+ T cells remains intact. The non-conventional CD8+ T cells in ITK-deficient mice exhibit activated/memory phenotypes including the expression of memory markers CD44, CD122 and NK1.1, rapid production of cytokines and dependency on IL-15, features shared by innate lymphocytes (35–37). ITK is also required for the development and function of iNKT cells (38, 39). More recently, we and others have reported that ITK-deficiency increased the generation of an IL-4 producing Vγ1.1+ T cell population (40, 41). These studies suggest that ITK is a key signal component that differentially regulates the development of various T cell populations.
In this study, we identified ITK as a critical signaling molecule specifically involved in the positive selection associated acquirement of the unique skin-homing property by the fetal thymic sIEL precursors for their specific peripheral location but dispensable for their maintenance, which provide molecular insights into differential requirements for the TCR signaling in peripheral localization and survival/expansion of the tissue-specific γδT cells.
Materials and Methods
Mice
ITK−/−, TCRδ−/−, Vav1−/− and β2M−/− and KN6 transgenic mice were previously described (32, 42, 43). CCR10-knockout/EGFP-knockin (CCR10EGFP/E/GPP) mice were generated in our lab (Jin, Xiong and et al). ITK−/−KN6 and ITK−/−CCR10+/EGPP mice were generated by crossing ITK−/− mice with KN6 and CCR10EGFP/EGFP mice respectively. All mice were kept in specific pathogen-free conditions and used for experiments at ages of 6–8 weeks unless indicated otherwise in the text or figure legends. Experiments were approved by the IACUC at The Pennsylvania State University.
Cell preparations
Epidermal cells were prepared as previously described (44). Briefly, hair was removed from the skin with Nair. The treated skin was excised and trimmed of subcutaneous fat. Skin strips were digested with 0.3% Trypsin/GNK solution for 45 minutes at 37°C. Epidermal layers of skin strips were gently removed and incubated with the 0.3% Trypsin/GNK solution containing 0.0001% DNase for 10 minutes at 37°C. The epidermal cells were washed with medium and purified with Ficoll (GE Healthcare). Cells were cultured overnight in media containing IL-2 (20 units/ml) and used for analyses.
Antibodies and reagents
FITC-conjugated anti-Vγ2 (or Vγ3), PECy5-conjugated anti-CD3 and PE-conjugated anti-CD24 antibodies were purchased from BD Bioscience. FITC-conjugated anti-CD122, Biotin-conjugated anti-BrdU, CD122 and γδTCR antibodies were purchased from eBioscience. PE or FITC -conjugated Streptavidin was purchased from Invitrogen. S1P was purchased from VWR Inc and CCL27 from Pepro Tech.
Flow cytometry
Cells were incubated with fluorescent antibodies for 30 min at 4°C. For biotin-labeled antibodies, streptavidin–PE was added in the second step and incubated for 20 min at 4°C. All samples were analyzed using the flow cytometer FC500 (Beckman Counter).
Immunofluorescent microscopy of ear epidermal sheets to detect sIELs
The epidermal sheets were prepared as described (45). Briefly, 6-week-old mice were sacrificed; the ears were cut off, mechanically split into dorsal and ventral sides, and then placed in EDTA solution. After incubation, the epidermis was peeled off as a single sheet and stained with FITC-conjugated anti-Vγ2 or Vγ3 TCR antibodies and analyzed on a fluorescence microscope (Olympus BX61 or Nikon Eclipse TE300).
Semi-quantitative and real-time RT-PCR
Total RNA was extracted from sorted cells or skin tissue with Trizol reagent (Invitrogen) according to the manufacturer’s instructions. The first-strand cDNA was synthesized from the RNA using SuperScript III Reverse Transcriptase (Invitrogen). For semi-quantitative PCR, serial 5 fold dilutions of cDNA were subject to PCR with primer sets for rearranged Vγ3, CCL27 or β-actin. Quantitative real-time PCR was performed using SYBR Green Master Mix (Invitrogen). Primer sets for individual genes are as follows: CCR6F: AGAACTCCAAGAGGCACAGAGCAA, CCR6R: TGTTGTGAGGGATCTGACAA GCCA; CCR10F: TTCCTAGCCTGTATCAGCG, CCR10R: TAGAGCCAGAAAC AGCGAC; S1PR1F: GTGTAGACCCAGAGTCCT GCG, S1PR1R: AGCTTTTCCTTGGCTGGAGAG; KLF2F: TGTGAGAAATG CCTTTGAGTTTACTG, KLF2R: CCCTTATAGAAATACAATCGGTCATAGTC;β-ActinF: CCCATCTACGAGGGCTAT, β-ActinR: TGTCACGCACGATTTCC; L3 and J1 primers were previously described (46).
CFSE cell proliferation assay
Cells were loaded with CFSE by incubation at 1×107 cells/ml in PBS for 10 minutes with 2.5 μM CFSE (Molecular Probes) at 37°C, then washed with cold complete medium twice, and resuspended in culture medium. The labeled cells (5×106 cells/well, 2 ml) were cultured for 3 days in 12-well tissue culture plates (Becton Dickinson Labware) in the presence of IL-2 (10 units/ml) only, IL-15 (50 ng/ml) + IL-2 (10 units/ml) or anti-γδ TCR antibody (GL4, 1μg/ml) + IL-2 (10 units/ml). After the culture, the cells were stained with PE-anti-γδ TCR (GL3) and PECy5-CD3 antibodies and analyzed by flow cytometry.
BrdU incorporation assay
Mice were injected intraperitoneally with 100 μl BrdU dissolved in PBS (10 mg/ml) at the onset of experiments. At the same time, mice were fed water that contained 0.8 mg/ml BrdU and 5% glucose. For the incorporation of BrdU into two-week old mice, the mice were injected intraperitoneally with 50 μl of 10 mg/ml BrdU every other day. Nine days later, sIELs were isolated and assessed for the BrdU incorporation by staining with Biotin-conjugated anti-BrdU antibody/PE-streptavidin, FITC-anti-Vγ3 and PECy5-anti-CD3 antibodies and flow cytometric analysis.
Chemotaxis assay
The assay was performed using 24-well chemotaxis chambers (Corning Costar Corp.). E16 fetal thymocytes (2×105 cells/well, 100 μl) were added to the upper chamber, and 100 nM S1P, 100 nM CCL27 or fetal skin culture medium placed in the bottom chamber. Cells were then incubated for 4 hours at 37 °C, 5% CO2, and the cells in the upper and lower chambers were collected and analyzed. The percentage of migration was determined from the original cell input.
In situ TUNEL staining of ear epidermal sheets to detect apoptotic sIELs
These experiments were performed using the TMR red In Situ Cell Death Detection Kit (Roche Applied Science). Briefly, freshly isolated ear epidermal sheets were fixed with 4% paraformaldehyde for 20 min and permeablized with 0.1% Triton X-100 for 2 min on ice, followed by culture with the TUNEL reaction mixture for 30 min and FITC-conjugated anti-Vγ2 antibody overnight. The stained epidermal sheets were analyzed by Olympus FluoView TM FV300 laser scanning microscope.
Adoptive transfers
KN6 Vγ2+ transgenic γδ T cells were purified from E16 fetal thymus of ITK-sufficient or knockout KN6 mice by a cell sorter and injected intraperitoneally into 1-week-oldβ2m−/−TCRδ−/− recipients (5×105 cells/mouse). Eight weeks after the transfer, ear epidermal sheets of the recipients were analyzed for donor-derived sIELs by in situ immunofluorescent staining.
Statistical analyses
All data are expressed as means ± standard deviations. Statistical significance was determined by two-tail student T tests. P < 0.05 is considered significant.
Results
Defective development of epidermal γδ T cells in ITK-knockout mice
To evaluate the role of ITK-mediated signaling in the sIEL development, we first assessed the sIEL populations in ITK−/− and wild-type mice by flow cytometry (Fig. 1A). Compared to the wild type controls of same ages, 6–8 week old ITK−/− mice had reduced percentages of Vγ3+ sIEL (14.73±0.41% vs. 3.56±0.32%, p<0.0001). In situ examination of sIELs on skin epidermal sheets by immunofluorescent microscopy confirmed the impaired development of sIELs in ITK−/− mice [181.8±19.5 (WT) vs. 66.3±4.3 (ITK−/−) cells/field, p<0.05], demonstrating that ITK-mediated signaling is important in the sIEL development (Fig. 1B and C). In addition, although the remaining sIELs in ITK−/− mice displayed the normal dendritic morphology (Fig. 1B), they produced less IFN-γ when stimulated in vitro with anti-γδ TCR antibody (Supplementary Fig. 1), suggesting that the ITK-transduced TCR-signaling is also important for the function of sIELs.
Figure 1.
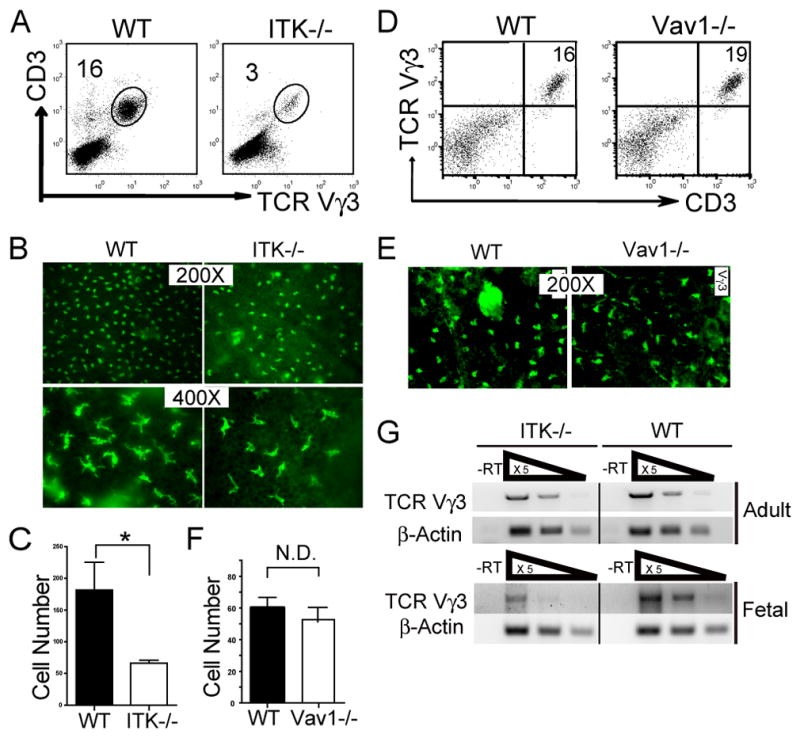
Impaired development of sIELs in ITK−/− but not Vav1−/− mice. A. Skin cell preparations from 6–8 week old ITK−/− and wild type mice were stained with anti-CD3 and Vγ3 antibodies, and analyzed for percentages of the CD3+Vγ3+ population by flow cytometry. One representative of three independent experiments is shown. B and C. Ear epidermal sheets from wild type and ITK−/− mice were stained with fluorescent anti-Vγ3 antibody and observed under a fluorescent microscope (Olympus BX61) for the Vγ3+ sIELs (B), average numbers of which per field at the 200X amplification were plotted (C). Data were obtained from three independent experiments. *P < 0.05. D-F. Flow cytometric and immunofluorescent microscopic analysis of skin Vγ3+ γδ T cells from Vav1−/− mice as performed in the panels A-C except that the Vγ3+ sIELs on the epidermal sheets was visualized under a different fluorescent microscope (Nikon Eclipse TE 300) that has a smaller field at the same 200X amplification (E). Experiments were repeated twice for both flow cytometric and immunofluorescent analyses. Average numbers of sIELs per field obtained in the panel E were plotted in the panel F. N.D: no difference. G. Total RNA from fetal and adult mouse skin was reverse transcribed to cDNA. Serially 5 fold-diluted cDNA were subject to semi-quantitative PCR to determine expression levels of rearranged TCRγ3 gene. β-actin was used as a control. Data shown were obtained from three independent experiments.
The Vav1 lies downstream of ITK and has been suggested to act directly with ITK to transduce TCR-activated signals (24). In addition, Vav1 has been previously found critical for γδTCR-mediated T cell proliferation (47), raising the possibility that it is also involved in the sIEL development. To test this, we analyzed the development of sIELs in Vav1-knockout mice. Surprisingly, unlike ITK−/− mice, 6–8 week old Vav1−/− mice had similar numbers of sIELs in the skin as wild type controls [60.5±6.3 (WT) vs. 54±7.1 (Vav1−/−) cells/field] (Fig. 1D-F), suggesting that Vav1-regulated signals are not involved in the ITK-mediated sIEL development.
Signals regulated by ITK could be potentially involved in multiple stages of the sIEL development, from the TCR-mediated positive selection of the fetal thymic sIEL precursors to their peripheral expansion in the skin. We therefore determined whether the seeding of fetal skin by fetal thymic Vγ3+ sIEL precursors was impaired in the ITK−/− mice. Compared to the wild type controls, transcripts of rearranged Vγ3 TCR in the fetal skin were dramatically decreased in ITK-knockout mice (> 10 fold reduction) (Fig. 1G), suggesting that the defective sIEL development originates at the fetal stage, likely due to impaired generation and/or selection of the fetal thymic sIEL precursors. As a control, the reduction of transcripts of rearranged Vγ3 TCR in the skin of 6–8 week old ITK−/− mice was about 3 fold and correlated with the reduction of the Vγ3+ sIEL numbers (Fig. 1G and Fig. 1A–C).
sIEL precursors undergo a seemingly normal positive selection process but accumulate in the fetal thymus of ITK−/− mice
To address how the ITK deficiency affects the development of sIEL precursors, we characterized the fetal thymic Vγ3+γδ T cells of ITK−/− mice. First, we determined whether ITK deficiency affects their positive selection and maturation. As previously reported, the positive selection and maturation of the fetal thymic Vγ3+ cells are associated with upregulation of CD122 and downregulation of CD24 in wild type mice (7, 48). Surprisingly, this was also the case in fetal thymic Vγ3+ cells of ITK−/− mice (Fig. 2A), indicating that ITK-deficiency does not affect their general selection and maturation processes.
Figure 2.
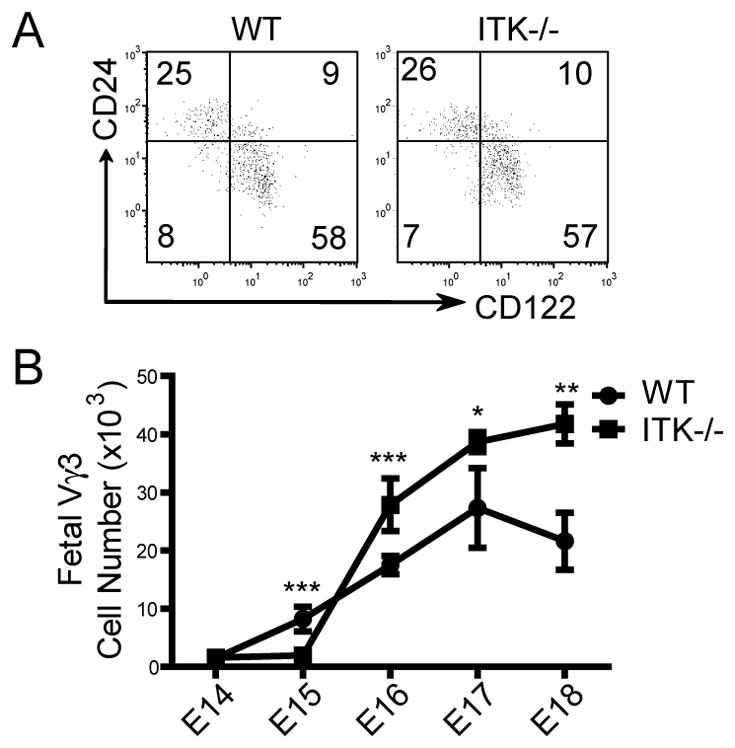
Vγ3+ sIEL precursors undergo a normal maturation process but accumulate in the fetal thymus of ITK−/− mice. A. Flow cytometric analysis of CD122 and CD24 expression on gated E16–17 fetal thymic Vγ3+ γδ cells. One representative of three independent experiments is shown. B. Numbers of Vγ3+ γδ T cells in wild type and ITK−/− fetal thymi of different gestation ages. The numbers were calculated based on total numbers of thymocytes and percentages of Vγ3+ cells per thymus. Data presented were means and standard deviations from three to five experiments. * P < 0.05, ** P <0.01, *** P < 0.001.
Generation of the fetal thymic Vγ3+ sIEL precursors was not impaired significantly in ITK−/− mice either. Although there was a slight delay in the appearance of Vγ3+γδ T cells in E15 fetal thymi of ITK−/− mice, this was no longer the case by E16 (Fig. 2B). In fact, as the fetuses aged, there was gradual accumulation of Vγ3+ cells in the ITK−/− fetal thymi. By E18 when the number of Vγ3+ cells was decreased in wild type fetal thymi due to the egress of mature Vγ3+γδ T cells and the reduced generation of new Vγ3+ cells, the number of Vγ3+ cells continued to increase in the ITK−/− fetal thymi, resulting in significantly more of these cells in ITK−/− than in wild type mice (Fig. 2B). These findings raise a possibility that the ITK deficiency might affect the proper migration of the sIEL precursors, which would correlate with their impaired seeding in the fetal skin (Fig. 1G).
ITK-deficient fetal thymic sIEL precursors cannot undergo a proper switch in the expression of thymus-exiting and skin-homing molecules
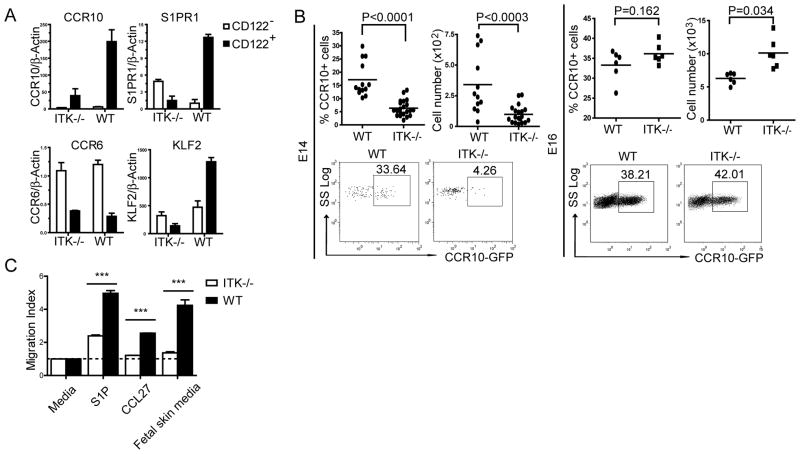
TCR-mediated positive selection of the fetal thymic Vγ3+ sIEL precursors promotes a coordinate switch in expression of multiple “migration” molecules, including the upregulation of S1PR1 and CCR10 and downregulation of CCR6, which are important for their thymic egress and skin-homing (7). To determine whether ITK-mediated signals regulate their expression, we sorted CD122− and CD122+ Vγ3+γδ T cells from ITK−/− and wild type fetal thymocytes and analyzed the expression of these migration molecules by real-time RT-PCR. Compared to the wild type controls, the upregulation of CCR10 and S1PR1 expression in the ITK−/− fetal thymic CD122+ Vγ3+ γδ T cells was significantly impaired (Fig. 3A). Therefore, although its deficiency did not affect the maturation of fetal thymic sIEL precursors, ITK is required for promoting the proper expression of the migration molecules in the mature fetal thymic sIEL precursors. The mature fetal thymic CD122+ Vγ3+ T cells of ITK−/− mice also expressed a lower level of KLF2 (Fig. 3A), a transcription factor critical in the regulation of chemokine receptor expression in positively selected αβ T cells (49, 50), suggesting that KLF2 might be involved in the ITK-mediated chemokine receptor expression. In contrast to the defect in upregulation of the migration molecules in the mature ITK−/− CD122+ Vγ3+ T cells, there was little difference in their expression on the immature CD122− Vγ3+ T cells of the ITK−/− and wild type mice (Fig. 3A), suggesting that the ITK-mediated signaling is mainly involved in regulation of the positive selection-associated acquisition of the skin homing property.
Figure 3.
ITK−/− fetal thymic sIEL precursors exhibit the altered migration molecule expression and defective migration capability. A. Real-time RT-PCR analysis of the expression of indicated molecules in purified CD122+ and CD122− CD3+Vγ3+ cells of E16 wild type and ITK−/− fetal thymi. Data shown were obtained from three independent experiments. B. E14 or16 fetal thymocytes of ITK−/−CCR10+/EGFP and ITK+/−CCR10+/EGFP mice were analyzed for CCR10 (EGFP) expression on Vγ3+ cells. Percentages and numbers of CCR10 (EGFP)+ Vγ3+ cells were shown. Data presented is one representative from at least 6 mice of each genotype. C. In vitro migration of wild type and ITK−/− E16 fetal thymic Vγ3+ γδ T cells to S1P, CCL27 and conditioned medium of fetal skin cultures. The migration index was calculated as a ratio of numbers of Vγ3+ cells migrating into the bottom chamber in presence of attractants vs. medium only. Data shown were obtained from two independent experiments. *** P < 0.001.
To further confirm that the ITK deficiency impaired the CCR10 upregulation in the positively selected sIEL precursors, we crossed ITK−/− mice with CCR10 knockout/GFP knockin mice that use the EGFP as a reporter for the CCR10 expression. The resultant ITK−/−CCR10+/EGFP mice had a significantly reduced percentage of CCR10(EGFP)+ Vγ3+ γδ T cells in the early fetal thymus (Fig. 3B, top group). However, as the fetus aged, this reduction disappeared (Fig. 3B, bottom group). Considering that the ITK−/− mature Vγ3+ γδ T cells T cells are defective in the upregulation of S1PR1, one plausible explanation for this is that although the ITK−/−Vγ3+ T cells have the impaired upregulation of CCR10 after the positive selection, the smaller number of CCR10+ Vγ3+ cells are unable to emigrate and accumulate in the thymus. By contrast, wild type mature CCR10+ Vγ3+ sIEL precursors constantly migrate out of the thymus.
To further dissect this, we assessed the in vitro migration capabilities of ITK−/− fetal thymic Vγ3+ γδ T cells towards sphingosine 1-phosphate (S1P), a ligand for S1PR1 involved in the mature thymic T cell emigration (9, 50–52). As shown in Fig. 3C, ITK−/− fetal thymic Vγ3+ γδ T cells migrated much less efficiently than wild type controls towards the S1P attraction. The ITK−/− Vγ3+ γδ T cells also had defects in migration towards CCL27 and culture media of the fetal skin (Fig. 3C), suggesting that they have impaired ability in the CCL27-mediated skin homing. Together, these results demonstrate that ITK-mediated signaling is important for the positive selection associated acquisition of the unique homing property in the fetal thymic Vγ3+ sIEL precursors for their egress from the thymus and localization in the skin.
ITK-regulated TCR signals are not required for the expansion of sIELs in the skin
We noted that even though the ITK−/− fetal thymic sIEL precursors exhibited severe defects in the skin-seeding at the fetal stage, the reduction in the number of sIELs in adult ITK−/− mice is not as much severe (Fig. 1A–C and G). This suggests that there might be a homeostatic compensation by which the few ITK-deficient sIEL precursors that make it to the skin are capable of the extensive expansion, which would result in the reduced difference in numbers of sIELs between ITK−/− and wild type mice when they grow older. Consistent with this, compared to wild type controls of same ages, 3–4 week old ITK−/− mice had on average 6 fold reduction in the number of sIELs [(8.5 ±1.5% (WT) vs. 1.5±0.7% (ITK−/−), p<0.01, n=5 each)] while the reduction was 3–4 fold in the 6–8 week old adults (Fig. 1A–C).
To directly assess whether the ITK-deficiency affected the proliferation of sIELs, we performed in vivo BrdU labeling experiments of wild type and ITK−/− mice and found that the ITK-deficient sIELs incorporated BrdU at the level similar as, if not higher than, wild type sIELs (Fig. 4A). Therefore, although ITK is critical for the regulated expression of multiple migratory molecules on the fetal thymic sIEL precursors for their epidermal localization, it is not required for their peripheral proliferation.
Figure 4.
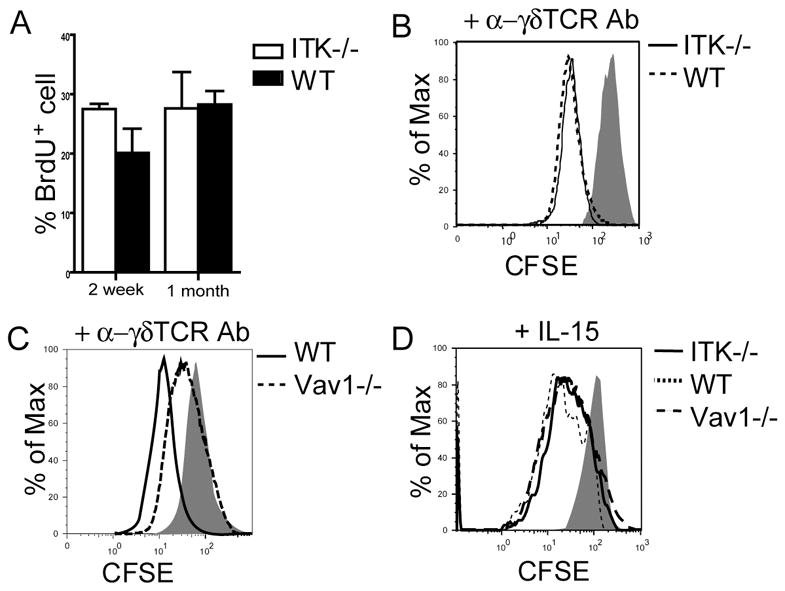
ITK−/− sIELs and their fetal thymic precursors have normal proliferation capacities. A. Similar in vivo proliferation rates of wild type and ITK−/− sIELs. Two week or one-month old mice were treated with BrdU for 9 days and then sIELs were isolated and analyzed for BrdU incorporation by flow cytometry. Data presented were means and standard deviations from three to five experiments. B-D. CFSE-labeled E16 fetal thymocytes from ITK−/−, Vav1−/− or wild type mice were stimulated with anti-γδ TCR antibody (1μg/ml, GL4) or IL-15 (50 ng/ml) for 3 days, and analyzed by flow cytometry for the proliferation of CD3+γδ T cells. One representative of three independent experiments was shown.
Correlating with their normal in vivo proliferation, there was no significant difference in the in vitro proliferation of the ITK−/− and wild-type fetal thymic sIEL precursors in response to the anti-γδ TCR antibody stimulation (Fig. 4B), which was in contrast with the requirement of Vav1 in the TCR-signaling mediated proliferation of γδ T cells (Fig. 4C) (47). Together with the fact that the Vav1 knockouts did not have any defects in the sIEL development (Fig. 1D–F), these results support a notion that the TCR-mediated signaling is not required for the peripheral expansion and maintenance of sIELs. Likely, the expansion of sIELs in the skin is driven by the IL-15/receptor signaling (12, 13). Consistent with this, both ITK−/− and Vav1−/− fetal thymic γδ T cells proliferated normally in response to IL-15 (Fig. 4D).
Continuous ITK-transduced, TCR/ligand initiated signals in sIELs impairs their maintenance in the skin due to activation-induced apoptosis
To directly characterize the role of ITK-regulated TCR signaling in the development of sIELs, we crossed ITK−/− mice with KN6 γδTCR transgenic (Tg) mice (42). The Vγ2+ KN6 γδTCR recognizes ligands T10/T22, two non-classical MHC class I molecules whose expression is high in C57BL/6 (B6), low in Balb/c, but absent inβ2M−/− mice (53, 54).
As observed in ITK−/− Vγ3+ sIEL precursors, the fetal thymic transgenic Vγ2+ T cells of wild type and ITK−/− KN6 mice on the ligand-high B6 background could be positively selected to undergo the Vγ3-like maturation process but the ITK−/− transgenic Vγ2+ cells had defects in seeding the fetal skin (Supplementary Fig. 2). Surprisingly, the absence of ITK had opposite effects on development of the transgenic Vγ2+ and natural Vγ3+ sIELs in adult mice. Compared to the ITK-sufficient KN6 mice, ITK−/− KN6 mice had significantly increased numbers of transgenic Vγ2+ sIELs (Fig. 5A–C), while ITK-deficiency impaired the Vγ3+ sIEL development (Fig. 1A–C).
Figure 5.
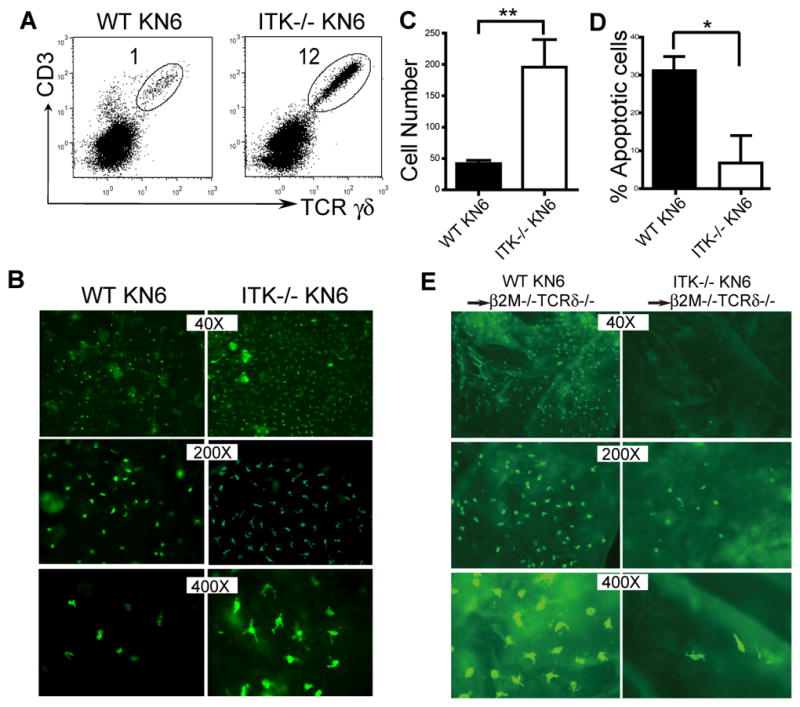
ITK-mediated TCR/ligand induced signaling in the skin impairs the development of sIELs by promoting their apoptosis. A. Skin cell preparations of KN6 transgenic mice of wild type and ITK−/− backgrounds were analyzed for transgenic Vγ2+ sIELs by flow cytometry. Percentages of the transgenic sIELs were indicated. One representative of three independent experiments is shown. B and C. Ear epidermal sheets of ITK-sufficient and knockout KN6 mice were stained and observed under a fluorescent microscope (Olympus BX61) for transgenic Vγ2+ sIELs (B), average numbers of which per field at the 200x amplification were plotted (C). Data were obtained from three experiments. ** P < 0.01. D. Lower percentages of apoptotic KN6 transgenic sIELs on ITK−/− than wild type background. The percentages of apoptotic sIELs were calculated based on ratios of numbers of apoptotic vs. total sIELs from in situ TUNEL analyses of ear epidermal sheets, as shown in the Supplementary Fig. 3. Data shown were obtained from at least four mice of each genotype in two independent experiments. * P < 0.05. E. The development of KN6 transgenic sIELs in β2m−/−TCRδ −/− recipients from adoptively transferred fetal thymic ITK-sufficient or knockout fetal thymic KN6 transgenic γδ T cells. Ear epidermal sheets of the recipients were analyzed for donor-derived sIELs by in situ immunofluorescent staining (Olympus BX61). Data presented is one representative from three mice of each genotype.
Closer microscopic examination found a morphological difference in the transgenic sIELs of ITK−/− and wild type background. While the transgenic sIELs in ITK−/− KN6 mice displayed the normal dendritic morphology, those of ITK-sufficient KN6 mice looked more rounded (Fig. 5C, at the 400X magnification), resembling activated sIELs (19). Since ligands for the KN6 γδTCR, unlike those for the natural sIEL-specific Vγ3+γδTCR, are highly expressed in the skin of B6 mice (53), this suggests that the continuous TCR/ligand mediated signaling in transgenic sIELs, transduced via ITK, may lead to their reduction, likely due to the persistent activation-induced apoptosis, while reduction of such signaling in the absence of ITK reverses the effect. Supporting this idea, the in situ TUNEL analysis of epidermal sheets found significantly lower percentages of apoptotic transgenic sIELs in ITK−/−KN6 than in ITK-sufficient KN6 mice (Fig. 5D and Supplementary Fig. 3).
These data suggest the increased number of transgenic sIELs in adult ITK−/− KN6 mice to be a result of improved peripheral maintenance, which overcomes the reduced initial skin-seeding by the ITK−/− fetal thymic transgenic γδ T cells. To further test this, we eliminated the effect of peripheral TCR/ligand interaction-induced signaling by transferring ITK−/− or ITK+/+ fetal thymic KN6Tg γδ T cells into ligand-negativeβ2m−/−TCRδ −/− recipients, which lack endogenous γδ T cells. Eight weeks after the transfer, the recipients were analyzed for donor-derived sIELs. As shown in Figure 5E, the adoptively transferred ITK−/− fetal thymic KN6Tg γδ T cells gave rise to fewer sIELs in the ligand-negative recipients than the ITK+/+ donor cells, a difference that is a reversal from that seen in the ITK−/− KN6 mice. In addition, the sIELs that developed in the recipients also displayed normal dendritic morphology (Fig. 5E). Together, these results demonstrate that the continuous peripheral TCR/ligand interaction, signaling through ITK, impairs the maintenance of transgenic sIELs by promoting their apoptosis, which could be corrected by removing ITK-mediated signals.
Discussion
While it is increasingly realized that the various subsets of tissue-specific γδ T cells are important components of immune system critical for the first line of defense, mechanisms regulating their development are poorly understood. Our recent studies found that thymic “educational” processes of different γδ T cell subsets promote their acquirement of unique homing properties (7, 48)(Jin, Xiong and et al), suggesting a critical role of the TCR-mediated selection signaling in programming thymic γδ T cells for their tissue-specific development. Here we investigated molecular mechanisms underlying the involvement of the TCR signaling in tissue specific development of the skin-specific γδ sIELs and identified ITK as a critical signaling molecule that specifically controls the skin-homing property of fetal thymic sIEL precursors and their seeding into the skin. The fetal thymic sIEL precursors from ITK−/− mice could not undergo the coordinate switch in expression of S1PR1 and CCR10 after the positive selection and had impaired migration abilities towards their ligand attraction, suggesting that the ITK transduced selection signaling is critical to upregulate the expression of these migration molecules for their exit from thymus and migration into the skin (9, 50–52). In addition, considering previous studies that ITK is also involved in the chemokine receptor-mediated signaling (22, 26–29), ITK-deficiency might potentially impair the migration of the sIEL precursors by directly affecting the chemokine receptor-signaling.
The TCR-mediated selection signaling in the fetal thymic sIEL precursors not only promotes their acquirement of the unique skin-homing property but also endows them capacities to survive and expand in the skin, such as the upregulation of CD122 (IL15Rβ) that is critical for the survival/expansion of sIELs (12, 13). Interestingly, the absence of ITK does not affect the CD122 upregulation and normal maturation of the fetal thymic sIEL precursors. In addition, although there were fewer sIELs in the ITK knockout mice, their in vivo proliferation rates were same as, if not higher than those of wild type mice, indicating that the ITK-mediating signaling is not involved in controlling the survival/proliferation capacity of the fetal thymic sIELs in the skin. These suggest that different TCR-signaling molecules are responsible for promoting the skin-homing and survival/proliferation properties of the selected fetal thymic sIEL precursors. In this regard, it is likely that although other TCR-downstream signaling molecules, such as Lck, Zap-70 and Syk, are all involved in the sIEL development (17–20), they could affect different aspects of the development. Consistent with this, sIELs in ZAP-70−/− mice displayed significantly morphological changes while sIELs in ITK−/− mice maintain the normal dendritic shape (17–19). This difference might reflect the fact that ZAP-70, which is located at the upstream of ITK signal pathway, may regulate a larger subset of TCR signals than ITK, with correspondingly greater effect (55).
ITK−/− fetal thymic sIELs proliferate normally in response to the TCR stimulation in vitro, consistent with the normal peripheral survival/expansion of sIELs in vivo. In addition, even in Vav1−/− mice whose Vγ3+ fetal thymic sIELs are defective in the TCR-mediated proliferation, they still had normal sIEL development, suggesting that the TCR signaling mediated proliferation is not required for the maintenance of sIELs in the skin. This agrees with the notion that the TCR-specific ligand(s) of sIELs are not expressed in the normal skin, but are upregulated on “stressed” or “diseased” keratinocytes that would activate the sIELs for proper functions (56, 57). Therefore, there is a close interplay between the establishment of sIELs and their subsequent function. Considering that positively selected fetal thymic sIEL precursors display an activated “memory”-like phenotype and are independent of the TCR signaling for survival and proliferation in the periphery, this suggests that their development is intrathymically programmed through the TCR signaling molecules mediated selection for their specific function.
Not only is the sIEL development independent of the peripheral TCR signaling, but also the continuous stimulation of TCRs in the sIELs impairs their development by promoting apoptosis. Such enhanced cell death was reduced by the ITK deficiency, suggesting the involvement of ITK in the TCR-induced activation of sIELs that would result in the apoptosis if persisting. Consistent with this, ITK−/− sIELs are defective in producing IFN-γ in response to the TCR stimulation. Therefore, although the ITK-transduced TCR signaling in the peripheral sIELs is not required for their normal maintenance, it is important for their activation, suggesting that the ITK-transduced thymic and peripheral TCR signals are differentially involved in the development and function of sIELs. However, to fully understand these, how the ITK signaling is involved in the in vivo functions of sIELs needs to be addressed.
There are increasing types of tissue-specific lymphocytes that function in various roles to provide the first lines of defense (1, 58–60). In humans, the preferential distribution of specific T cell subsets in the skin was also reported (61–63). Although the human and murime skin T cells use different TCR compositions, they seem to perform the similar functions. It was reported that like the murine sIELs, the human skin γδ T cells could lyse skin tumor cells and produce similar cytokines in response to stimulation in vitro (61). In addition, human epidermal γδ T cells, as well as epidermal αβ T cells, were shown to contribute to the wound healing(63), suggesting that even though they have different TCR usages, the human and murine epidermal T cells share the similar functional properties and might develop similarly. Our findings with the murine skin-specific sIELs would aid in understanding how ITK and other TCR-associated signaling are involved in the development of the human skin T cells, as well as other different tissue-specific lymphocytes. In addition, in light of the role of ITK in regulating CCR10 expression in the sIEL precursors, whether ITK is involved in its expression in other cells of the skin, such as melanocytes and melanoma tumor cells, under physiological and pathological conditions would be also interesting questions.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (N.X) and, in part, under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds (N.X.). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
We thank Joonsoo Kang for critical comments and Christina Saylor for technical support. The experiments with Vav1−/− mice were initiated in David Raulet Lab (University of California, Berkeley).
References
- 1.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annual review of immunology. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 2.Raulet DH, Spencer DM, Hsiang YH, Goldman JP, Bix M, Liao NS, Zijstra M, Jaenisch R, Correa I. Control of gamma delta T-cell development. Immunological reviews. 1991;120:185–204. doi: 10.1111/j.1600-065x.1991.tb00592.x. [DOI] [PubMed] [Google Scholar]
- 3.Elbe A, Tschachler E, Steiner G, Binder A, Wolff K, Stingl G. Maturational steps of bone marrow-derived dendritic murine epidermal cells. Phenotypic and functional studies on Langerhans cells and Thy-1+ dendritic epidermal cells in the perinatal period. J Immunol. 1989;143:2431–2438. [PubMed] [Google Scholar]
- 4.Havran WL, Allison JP. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature. 1990;344:68–70. doi: 10.1038/344068a0. [DOI] [PubMed] [Google Scholar]
- 5.Mallick-Wood CA, Lewis JM, Richie LI, Owen MJ, Tigelaar RE, Hayday AC. Conservation of T cell receptor conformation in epidermal gammadelta cells with disrupted primary Vgamma gene usage. Science (New York, NY) 1998;279:1729–1733. doi: 10.1126/science.279.5357.1729. [DOI] [PubMed] [Google Scholar]
- 6.Ferrero I, Wilson A, Beermann F, Held W, MacDonald HR. T cell receptor specificity is critical for the development of epidermal gammadelta T cells. The Journal of experimental medicine. 2001;194:1473–1483. doi: 10.1084/jem.194.10.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong N, Kang C, Raulet DH. Positive selection of dendritic epidermal gammadelta T cell precursors in the fetal thymus determines expression of skin-homing receptors. Immunity. 2004;21:121–131. doi: 10.1016/j.immuni.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bunemann E, Lehto M, Wolff H, Yen D, Marxhausen H, To W, Sedgwick J, Ruzicka T, Lehmann P, Zlotnik A. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nature medicine. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- 9.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 10.Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, Orozco R, Copeland NG, Jenkins NA, McEvoy LM, Zlotnik A. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:14470–14475. doi: 10.1073/pnas.96.25.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarmin DI, Rits M, Bota D, Gerard NP, Graham GJ, Clark-Lewis I, Gerard C. Cutting edge: identification of the orphan receptor G-protein-coupled receptor 2 as CCR10, a specific receptor for the chemokine ESkine. J Immunol. 2000;164:3460–3464. doi: 10.4049/jimmunol.164.7.3460. [DOI] [PubMed] [Google Scholar]
- 12.Kawai K, Suzuki H, Tomiyama K, Minagawa M, Mak TW, Ohashi PS. Requirement of the IL-2 receptor beta chain for the development of Vgamma3 dendritic epidermal T cells. The Journal of investigative dermatology. 1998;110:961–965. doi: 10.1046/j.1523-1747.1998.00214.x. [DOI] [PubMed] [Google Scholar]
- 13.De Creus A, Van Beneden K, Stevenaert F, Debacker V, Plum J, Leclercq G. Developmental and functional defects of thymic and epidermal V gamma 3 cells in IL-15-deficient and IFN regulatory factor-1-deficient mice. J Immunol. 2002;168:6486–6493. doi: 10.4049/jimmunol.168.12.6486. [DOI] [PubMed] [Google Scholar]
- 14.Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, Tigelaar RE, Lifton RP. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nature genetics. 2008;40:656–662. doi: 10.1038/ng.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis JM, Girardi M, Roberts SJ, Barbee SD, Hayday AC, Tigelaar RE. Selection of the cutaneous intraepithelial gammadelta+ T cell repertoire by a thymic stromal determinant. Nat Immunol. 2006;7:843–850. doi: 10.1038/ni1363. [DOI] [PubMed] [Google Scholar]
- 16.Uche UN, Huber CR, Raulet DH, Xiong N. Recombination signal sequence-associated restriction on TCRdelta gene rearrangement affects the development of tissue-specific gammadelta T cells. J Immunol. 2009;183:4931–4939. doi: 10.4049/jimmunol.0901859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallick-Wood CA, Pao W, Cheng AM, Lewis JM, Kulkarni S, Bolen JB, Rowley B, Tigelaar RE, Pawson T, Hayday AC. Disruption of epithelial gamma delta T cell repertoires by mutation of the Syk tyrosine kinase. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9704–9709. doi: 10.1073/pnas.93.18.9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colucci F, Guy-Grand D, Wilson A, Turner M, Schweighoffer E, Tybulewicz VL, Di Santo JP. A new look at Syk in alpha beta and gamma delta T cell development using chimeric mice with a low competitive hematopoietic environment. J Immunol. 2000;164:5140–5145. doi: 10.4049/jimmunol.164.10.5140. [DOI] [PubMed] [Google Scholar]
- 19.Endo Y, Ishikawa O, Negishi I. Zeta-chain-associated protein-70 molecule is essential for the proliferation and the final maturation of dendritic epidermal T cells. Exp Dermatol. 2005;14:188–193. doi: 10.1111/j.0906-6705.2005.00264.x. [DOI] [PubMed] [Google Scholar]
- 20.Kawai K, Kishihara K, Molina TJ, Wallace VA, Mak TW, Ohashi PS. Impaired development of V gamma 3 dendritic epidermal T cells in p56lck protein tyrosine kinase-deficient and CD45 protein tyrosine phosphatase-deficient mice. The Journal of experimental medicine. 1995;181:345–349. doi: 10.1084/jem.181.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VL. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 22.Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL. Tec family kinases in T lymphocyte development and function. Annual review of immunology. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- 23.Finkelstein LD, Schwartzberg PL. Tec kinases: shaping T-cell activation through actin. Trends Cell Biol. 2004;14:443–451. doi: 10.1016/j.tcb.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Finkelstein LD, Shimizu Y, Schwartzberg PL. Tec kinases regulate TCR-mediated recruitment of signaling molecules and integrin-dependent cell adhesion. J Immunol. 2005;175:5923–5930. doi: 10.4049/jimmunol.175.9.5923. [DOI] [PubMed] [Google Scholar]
- 25.Woods ML, Kivens WJ, Adelsman MA, Qiu Y, August A, Shimizu Y. A novel function for the Tec family tyrosine kinase Itk in activation of beta 1 integrins by the T-cell receptor. The EMBO journal. 2001;20:1232–1244. doi: 10.1093/emboj/20.6.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takesono A, Horai R, Mandai M, Dombroski D, Schwartzberg PL. Requirement for Tec kinases in chemokine-induced migration and activation of Cdc42 and Rac. Curr Biol. 2004;14:917–922. doi: 10.1016/j.cub.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Sahu N, Mueller C, Fischer A, August A. Differential sensitivity to Itk kinase signals for T helper 2 cytokine production and chemokine-mediated migration. J Immunol. 2008;180:3833–3838. doi: 10.4049/jimmunol.180.6.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Rodriguez J, Readinger JA, Viorritto IC, Mueller KL, Houghtling RA, Schwartzberg PL. Tec kinases, actin, and cell adhesion. Immunological reviews. 2007;218:45–64. doi: 10.1111/j.1600-065X.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 29.Fischer AM, Mercer JC, Iyer A, Ragin MJ, August A. Regulation of CXC chemokine receptor 4-mediated migration by the Tec family tyrosine kinase ITK. The Journal of biological chemistry. 2004;279:29816–29820. doi: 10.1074/jbc.M312848200. [DOI] [PubMed] [Google Scholar]
- 30.Schaeffer EM, Broussard C, Debnath J, Anderson S, McVicar DW, Schwartzberg PL. Tec family kinases modulate thresholds for thymocyte development and selection. The Journal of experimental medicine. 2000;192:987–1000. doi: 10.1084/jem.192.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaeffer EM, Debnath J, Yap G, McVicar D, Liao XC, Littman DR, Sher A, Varmus HE, Lenardo MJ, Schwartzberg PL. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science (New York, NY) 1999;284:638–641. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 32.Liao XC, Littman DR. Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity. 1995;3:757–769. doi: 10.1016/1074-7613(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 33.Prince AL, Yin CC, Enos ME, Felices M, Berg LJ. The Tec kinases Itk and Rlk regulate conventional versus innate T-cell development. Immunological reviews. 2009;228:115–131. doi: 10.1111/j.1600-065X.2008.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Readinger JA, Mueller KL, Venegas AM, Horai R, Schwartzberg PL. Tec kinases regulate T-lymphocyte development and function: new insights into the roles of Itk and Rlk/Txk. Immunological reviews. 2009;228:93–114. doi: 10.1111/j.1600-065X.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Berg LJ. Signalling through TEC kinases regulates conventional versus innate CD8(+) T-cell development. Nature reviews. 2007;7:479–485. doi: 10.1038/nri2091. [DOI] [PubMed] [Google Scholar]
- 37.Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, Schwartzberg PL. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Felices M, Berg LJ. The Tec kinases Itk and Rlk regulate NKT cell maturation, cytokine production, and survival. J Immunol. 2008;180:3007–3018. doi: 10.4049/jimmunol.180.5.3007. [DOI] [PubMed] [Google Scholar]
- 39.Au-Yeung BB, Fowell DJ. A key role for Itk in both IFN gamma and IL-4 production by NKT cells. J Immunol. 2007;179:111–119. doi: 10.4049/jimmunol.179.1.111. [DOI] [PubMed] [Google Scholar]
- 40.Qi Q, Xia M, Hu J, Hicks E, Iyer A, Xiong N, August A. Enhanced development of CD4+ {gamma}{delta} T cells in the absence of Itk results in elevated IgE production. Blood. 2009 doi: 10.1182/blood-2008-12-196345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ. Tec kinase Itk in gammadeltaT cells is pivotal for controlling IgE production in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8308–8313. doi: 10.1073/pnas.0808459106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonneville M, Ishida I, Itohara S, Verbeek S, Berns A, Kanagawa O, Haas W, Tonegawa S. Self-tolerance to transgenic gamma delta T cells by intrathymic inactivation. Nature. 1990;344:163–165. doi: 10.1038/344163a0. [DOI] [PubMed] [Google Scholar]
- 43.Ishida I, Verbeek S, Bonneville M, Itohara S, Berns A, Tonegawa S. T-cell receptor gamma delta and gamma transgenic mice suggest a role of a gamma gene silencer in the generation of alpha beta T cells. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:3067–3071. doi: 10.1073/pnas.87.8.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan S, Bergstresser PR, Streilein JW. Intravenously injected, TNP-derivatized, Langerhans cell-enriched epidermal cells induce contact hypersensitivity in Syrian hamsters. The Journal of investigative dermatology. 1985;84:249–252. doi: 10.1111/1523-1747.ep12265316. [DOI] [PubMed] [Google Scholar]
- 45.Miyauchi S, Hashimoto K. Epidermal Langerhans cells undergo mitosis during the early recovery phase after ultraviolet-B irradiation. The Journal of investigative dermatology. 1987;88:703–708. doi: 10.1111/1523-1747.ep12470379. [DOI] [PubMed] [Google Scholar]
- 46.Goldman JP, Spencer DM, Raulet DH. Ordered rearrangement of variable region genes of the T cell receptor gamma locus correlates with transcription of the unrearranged genes. The Journal of experimental medicine. 1993;177:729–739. doi: 10.1084/jem.177.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swat W, Xavier R, Mizoguchi A, Mizoguchi E, Fredericks J, Fujikawa K, Bhan AK, Alt FW. Essential role for Vav1 in activation, but not development, of gammadelta T cells. International immunology. 2003;15:215–221. doi: 10.1093/intimm/dxg021. [DOI] [PubMed] [Google Scholar]
- 48.Van Beneden K, De Creus A, Stevenaert F, Debacker V, Plum J, Leclercq G. Expression of inhibitory receptors Ly49E and CD94/NKG2 on fetal thymic and adult epidermal TCR V gamma 3 lymphocytes. J Immunol. 2002;168:3295–3302. doi: 10.4049/jimmunol.168.7.3295. [DOI] [PubMed] [Google Scholar]
- 49.Sebzda E, Zou Z, Lee JS, Wang T, Kahn ML. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat Immunol. 2008;9:292–300. doi: 10.1038/ni1565. [DOI] [PubMed] [Google Scholar]
- 50.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 51.Matsuyuki H, Maeda Y, Yano K, Sugahara K, Chiba K, Kohno T, Igarashi Y. Involvement of sphingosine 1-phosphate (S1P) receptor type 1 and type 4 in migratory response of mouse T cells toward S1P. Cellular & molecular immunology. 2006;3:429–437. [PubMed] [Google Scholar]
- 52.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. The Journal of biological chemistry. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 53.Ito K, Van Kaer L, Bonneville M, Hsu S, Murphy DB, Tonegawa S. Recognition of the product of a novel MHC TL region gene (27b) by a mouse gamma delta T cell receptor. Cell. 1990;62:549–561. doi: 10.1016/0092-8674(90)90019-b. [DOI] [PubMed] [Google Scholar]
- 54.Schild H, Mavaddat N, Litzenberger C, Ehrich EW, Davis MM, Bluestone JA, Matis L, Draper RK, Chien YH. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 55.Au-Yeung BB, Deindl S, Hsu LY, Palacios EH, Levin SE, Kuriyan J, Weiss A. The structure, regulation, and function of ZAP-70. Immunological reviews. 2009;228:41–57. doi: 10.1111/j.1600-065X.2008.00753.x. [DOI] [PubMed] [Google Scholar]
- 56.Havran WL. A role for epithelial gammadelta T cells in tissue repair. Immunologic research. 2000;21:63–69. doi: 10.1385/IR:21:2-3:63. [DOI] [PubMed] [Google Scholar]
- 57.Havran WL, Chien YH, Allison JP. Recognition of self antigens by skin-derived T cells with invariant gamma delta antigen receptors. Science (New York, NY) 1991;252:1430–1432. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- 58.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 59.Hardy RR. B-1 B cell development. J Immunol. 2006;177:2749–2754. doi: 10.4049/jimmunol.177.5.2749. [DOI] [PubMed] [Google Scholar]
- 60.Lambolez F, Kronenberg M, Cheroutre H. Thymic differentiation of TCR alpha beta(+) CD8 alpha alpha(+) IELs. Immunological reviews. 2007;215:178–188. doi: 10.1111/j.1600-065X.2006.00488.x. [DOI] [PubMed] [Google Scholar]
- 61.Ebert LM, Meuter S, Moser B. Homing and function of human skin gammadelta T cells and NK cells: relevance for tumor surveillance. J Immunol. 2006;176:4331–4336. doi: 10.4049/jimmunol.176.7.4331. [DOI] [PubMed] [Google Scholar]
- 62.Bos JD, Teunissen MB, Cairo I, Krieg SR, Kapsenberg ML, Das PK, Borst J. T-cell receptor gamma delta bearing cells in normal human skin. J Invest Dermatol. 1990;94:37–42. doi: 10.1111/1523-1747.ep12873333. [DOI] [PubMed] [Google Scholar]
- 63.Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, Rudolph R, Jameson J, Havran WL. A role for human skin-resident T cells in wound healing. J Exp Med. 2009;206:743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.