Abstract
Matrix remodeling plays a fundamental role in physiological and pathological processes, as well as in tissue engineering applications. In this paper, optical coherence tomography (OCT), a nondestructive optical imaging technology, was used to image collagen gel remodeling by smooth muscle cells (SMCs). The optical scattering properties of collagen-SMC gels were characterized quantitatively by fitting OCT data to a theoretical model. Matrix remodeling over 5 days produced a 10-fold increase in the reflectivity of the collagen gels, corresponding to a decrease in scattering anisotropy from 0.91 to 0.46. The increase in reflectivity was corroborated in confocal mosaic images. Blocking matrix degradation in collagen-SMC gels with doxycycline, a non-specific matrix metalloproteinases (MMPs) inhibitor, impeded the decrease in scattering anisotropy and resulted in few macroscopic signs of remodeling. Causing matrix degradation in acellular gels with a 3 hr treatment of MMP-8 (collagenase 2) partially mimicked the decrease in anisotropy measured in collagen-SMC gels after 5 days. These results suggest that the decrease in scattering anisotropy in the collagen-SMC gels was due to MMP activity that degrades collagen fibrils into smaller fragments.
1. Introduction
Cellular remodeling of the extracellular matrix (ECM) plays an important role in developmental biology [1], cell motility [2], aging [3], wound healing [4], atherosclerosis [5], tumorigenesis [6], fibrosis [7], and tissue engineering applications [8-10]. ECM remodeling involves assembling, degrading, and reorganizing ECM structures [11]. Critical to ECM remodeling is the activity of the matrix metalloproteinases (MMPs) family of enzymes that degrade ECM structural proteins. MMP substrates include collagens, fibronectin, laminin, and proteoglycans [12]. Accordingly, real-time assessment of ECM remodeling would improve our understanding of these physiological and pathological processes.
One common in vitro model of ECM remodeling is a 3D collagen I matrix (collagen gel) populated by fibroblasts or SMCs [13]. Over time, these cells cause gel contraction that decreases the size of the gel matrix [14]. MMP activity has been implicated in this process of collagen gel remodeling [15-17].
Given the importance of ECM remodeling, there has been a search for methods that could monitor the process in real-time both quantitatively and non-destructively [18]. One promising technology is optical coherence tomography (OCT) [19]. OCT, which measures coherent light backscattered from tissues, has several advantages for imaging collagen gels and tissues, including an axial resolution <10 μm and imaging up to 2 mm depth at video-rate imaging speeds [18]. Importantly, contrast in OCT images is based on endogenous refractive index mismatches between the structures in the imaged sample, and thus no labels need to be added to the sample. OCT data also contain information on the scattering properties of structures within the imaged sample, and recently our group developed an algorithm that fits OCT signals to a theoretical model and determines the optical scattering properties of the imaged sample in local nanoliter volumes [20]. Measuring the scattering properties of collagen-SMC gels from OCT images determined that over a 5 day period, the reflectivity more than doubled, indicating a decrease in the scattering anisotropy [20]. We hypothesized that matrix remodeling, specifically MMP activity, was responsible for the reflectivity increase.
In the present report, OCT was used to show that collagen remodeling caused a 10-fold increase in reflectivity through a decrease in the scattering anisotropy. Specifically, collagen-SMC gels were treated with doxycycline (a non-specific MMP inhibitor), which impeded the decrease in scattering anisotropy over 5 days relative to that measured in untreated gels. Additionally, treatment of acellular collagen gels collagenase 2 (MMP-8) induced a partial decrease in scattering anisotropy. We propose that the mechanism by which reflectivity is increased is that MMPs break down local collagen fibrils into smaller fragments that scatter light more isotropically, thereby increasing the fraction of backscattered light and the measured reflectivity.
2. Theory
The theory of light propagation in low-coherence imaging systems [21] that was used in this study was derived from the inverse Monte Carlo method. For a homogeneous turbid medium characterized by scattering coefficient μs, anisotropy factor g, and absorption coefficient μa, the depth-dependent OCT signal R(z) can be described as an exponential decay:
| (1) |
with reflectivity ρ and attenuation μ. Specifically,
| (2) |
and
| (3) |
where Δz is the axial resolution and G is a geometry factor accounting for the extra pathlength caused by off-axis propagation, which can be approximated by 1/cos(sin-1(NA)). The parameter b(g) represents the fraction of light backscattered at the focus towards the objective lens. As the scattering particles decrease in size, the scattering anisotropy of the sample decreases, which results in increases in b(g) and hence increases in reflectivity. The term a(g) describes the fraction of light scattered at such small polar angles that it cannot be distinguished from the unscattered light. For highly anisotropic scattering media, a(g) has the effect of reducing the measured attenuation in the OCT signal. Note this model requires calibrating the OCT signal from arbitrary units into dimensionless units of reflectivity ρ. Moreover, this model requires implementation of focus-tracking (or quasi-focus-tracking) conditions during OCT imaging.
3. Materials and Methods
3.A. Cell culture
All cell culture materials were purchased from GIBCO unless otherwise stated. Experiments involving cells used primary SMCs (passage 4-5) isolated from a single baboon carotid artery by enzymatic digestion [22]. SMCs were fed with SMC growth medium (SGM), consisting of minimal essential medium (MEM) supplemented with 10 % fetal bovine serum, 1 % L-glutamine, and 1 % penicillin-streptomycin.
3.B. Collagen gel preparation
Soluble calf skin collagen (MP Biomedicals, part # 150026) was dissolved in 0.02 N acetic acid at a concentration of 4 mg/ml. To prepare triplicate collagen gels, 1.625 ml collagen (pH = 2.5) was mixed with 0.406 ml 5X MEM and 0.260 ml 0.2 M NaOH (Sigma), with additional 10 μl drops of NaOH solution added until the pH was neutral. Immediately thereafter, 1 ml SGM containing 3.25×106 SMCs was added to the collagen suspension, resulting in a Day 0 cell concentration of 1×106 cells/ml. The collagen-SMC suspension was aliquotted as triplicate 1 ml samples in a 12-well plate (Corning) and thermally gelled for 1 hr at 37°; thereafter, 1 ml SGM was layered on top of each gel. After 24 hr, the gels were detached from the wells with a spatula and allowed to further compact. The gels were fed 1 ml SGM every 2 days. At each of the imaging times (days 1 and 5), 3 gels were removed from their wells, and imaged by OCT.
3.C. OCT imaging and image processing
The time-domain OCT setup used in this paper was previously described [23]. Briefly, a broadband light source operating at 1310 nm (B&W Tek) with a 3 dB bandwidth of 100 nm was coupled into a fiber-based Michelson interferometer. A rapid scanning optical delay line [24] varied the delay in the reference arm; a 10× microscope objective (Newport) mounted on a piezo-driven lens mount (Piezosystems Jena) focused the light on the sample. The interference signal between the backreflected light from the sample and reference arms was measured using an InGaAs photodiode (New Focus). The OCT system was controlled through a data acquisition card (National Instruments) using software written in LabVIEW (National Instruments).
Datasets of 175 × 150 × 1024 pixels (XYZ) representing 350 × 300 × 1000 μm were acquired from each collagen gel at a single site. For each collagen gel, image acquisition was performed 11 times at a single site, with the piezo-driven lens mount shifting the lens towards the gel by 40 μm in each successive dataset. Thus, in each of the eleven 3D datasets, a different depth range was in focus. The depth regions that were in focus from each of the (eleven) 3D datasets were stitched together to form one quasi-focus-tracked 3D dataset per sample. Acquisition of the 11 datasets took 50 minutes. During OCT imaging, the sample was mounted in a 35 mm Petri dish while immersed in phosphate buffered saline (PBS) at room temperature to maintain hydration.
3.D. OCT image processing algorithm
A single, Matlab-based algorithm, organized in separate modular steps, was used to evaluate optical properties from all the data. Details of this algorithm were described previously [20]. Briefly, the regions in focus from each of the eleven 3D datasets were stitched together. Next, the pixels of the 3D image of the gel were adjusted axially along Z to bring the gel surface to a constant Z position, i.e., the gel was flattened. The resulting 3D dataset was then subdivided into 10 × 10 regions of interest (ROIs) along X and Y. Along Z, the ROIs were truncated to cover depths spanning 150-400 μm. For each ROI, the signal was converted into reflectivity units by calibrating against the signal from a phantom (polystyrene microspheres suspended in an agarose gel) with known optical properties. This calibration dataset was imaged and processed before all other samples. The fitted reflectivity from each ROI in the calibration phantom was used to calibrate the signal from the corresponding (X, Y) position in the imaged collagen gels, to account for differences in power delivered to the sample between the center of the objective lens and the periphery that result from laterally scanning the beam. Finally, the natural log of the averaged calibrated depth-dependent signal [ln(R̄ROI(z))] was fit to Eq. 1, yielding one (μ, ρ) pair for each ROI. Statistical characterization of the algorithm showed that it overestimated μ and ρ by 10-20%. Fits from individual ROIs had an intra-ROI coefficient of variation of <5 % for μ and 10 % for ρ [20].
3.E. MMP inhibition in SMC gels
To determine whether MMP activity was necessary for the observed changes in optical properties, collagen-SMC gels were prepared according to the protocol in Section 3.B, with the addition of either 200 or 450 μM doxycycline [25]. Doxycycline is a tetracycline derivative that is also a non-specific MMP-inhibitor [26] shown to prevent remodeling of collagen gels populated with fibroblasts [27] as well as SMCs [25]. Macroscopic remodeling was monitored in these gels (details in the data supplement). The gels were imaged by OCT at day 5 and their optical properties were quantified.
3.F. Collagenase treatment of acellular gels
To show MMPs alone were sufficient to change the optical properties of collagen gels, acellular collagen gels were prepared and treated with collagenase 2 (MMP-8). MMP-8 has been previously shown to degrade collagen under cell-free conditions [28]. Specifically, after the acellular collagen gels were detached at 24 hr, the gels were washed 3 times in PBS and incubated with 0.5 ml MEM containing either 0.5 or 1 unit/ml collagenase 2 (Sigma) for 3 hr at 37°C. Thereafter, collagenase activity was blocked with 1 ml SGM and the gels were imaged by OCT and their optical properties quantified.
3.G. Confocal mosaic imaging and analysis
Confocal mosaicing [29] is a new imaging technique in which a number of adjacent en face confocal images are stitched together laterally, resulting in an image that covers large areas (∼cm2) of tissue at sub-micron resolution. Recent work on confocal mosaics of excised tumors from Mohs surgery have shown that the reflectance and fluorescence confocal images from a tissue stained with a cellular dye represent the matrix and cells, respectively [30,31]. Four groups of collagen-SMC gels (5 gels per group) were imaged by confocal mosaicing – day 1 gels, day 5 untreated (dox-) gels, 200 μM dox+ day 5 gels, and 450 μM dox+ day 5 gels. Immediately following OCT imaging, the imaged collagen-SMC gels were washed in PBS and stained with eosin for 30 sec. Reflectance and fluorescence confocal mosaics were acquired using a modified VivaScope (Lucid, Inc.) at an imaging depth of approximately 20 μm from the (bottom) gel surface. Both reflectance and fluorescence images were captured at 8-bit resolution under the same detector setting to enable direct comparisons between the different gels. Each en face confocal image (“tile”) in the mosaic was composed of 1000 X 1000 pixels, which represented 500 X 500 μm. The number of tiles in each mosaic was approximately 20 X 20 (1 × 1 cm), though the exact numbers were tailored to the match the size of the imaged collagen gel.
The mosaic images were analyzed both visually and quantitatively. Specifically, the regions in each mosaic that corresponded to the gel were delineated with the mouse, creating a binary mask of the gel within the image. Using this mask, the probability density functions (PDFs) of reflectance pixel intensities inside the gel were determined within the 256 discrete intensity values. These pixel intensity values from the confocal mosaics are in arbitrary reflectance units.
3.H. Data analysis and statistics
All the OCT collagen gel experiments were repeated 3 times, with 3 gel samples imaged each time, resulting in n=9 samples imaged in total from each collagen gel condition. The data from each imaged gel resulted in ∼40-50 ROIs that met the optical property fitting algorithm's geometrical criteria. Each linear fit of ROI data resulted in a (μ, ρ) pair. The distribution of (μ, ρ) data from a single sample were grouped together. For each of these distributions, the PDFs f(μ) and f(ρ) were computed by binning log10(μ) and log10(ρ) values from an individual group into histograms made of Gaussian shaped kernels. Additionally, by treating attenuation μ and reflectivity ρ as statistically independent random variables, a joint PDF was computed by f(μ, ρ) = f(μ)f(ρ). The (μ, ρ) values from the peak of the joint PDFs were used as the representative values of the group and mapped back to the corresponding (μs, g). When calculating statistics, the (μs, g) values corresponding to the peak of the PDF of each collagen gel were used. A one-way ANOVA was used to calculate statistical significance in multiple distribution comparisons, using a Boneferroni post hoc to determine P values. P values less than 0.05 were considered significant. Additional statistics calculated using log10(ρ) and log10(μ) are also reported for completeness.
4. Results
4.A. Remodeling increased reflectance intensity in en face confocal mosaics
Confocal mosaics that imaged ∼1 cm2 of collagen gel area at a ∼20 μm depth were acquired to assess the spatial uniformity of the gels. The reflectance signal from each gel, representing the matrix, was false-colored in green, while the eosin fluorescence that represented the cytoplasm was false-colored in red. Fig. 1A-B shows a representative (en face) 0.6 × 0.6 cm confocal mosaic and a mosaic tile at higher magnification (500 × 500 μm) for the day 1 gels; Fig. 1C-D shows 0.6 × 0.6 cm mosaic and tile for the day 5 (dox-) gels. The tiles are highlighted in a dotted cyan line in the corresponding mosaic. To improve visualization of the weakly-reflecting confocal mosaics, the images in Fig. 1A-D were scaled up digitally by a factor of 3. Note that over a range of millimeters in X-Y, the gels had an irregular surface. Thus the variations in Z that measured >20 μm account for the patchy (out of focal plane) appearance of the mosaics in Figs. 1A, C.
Fig. 1.
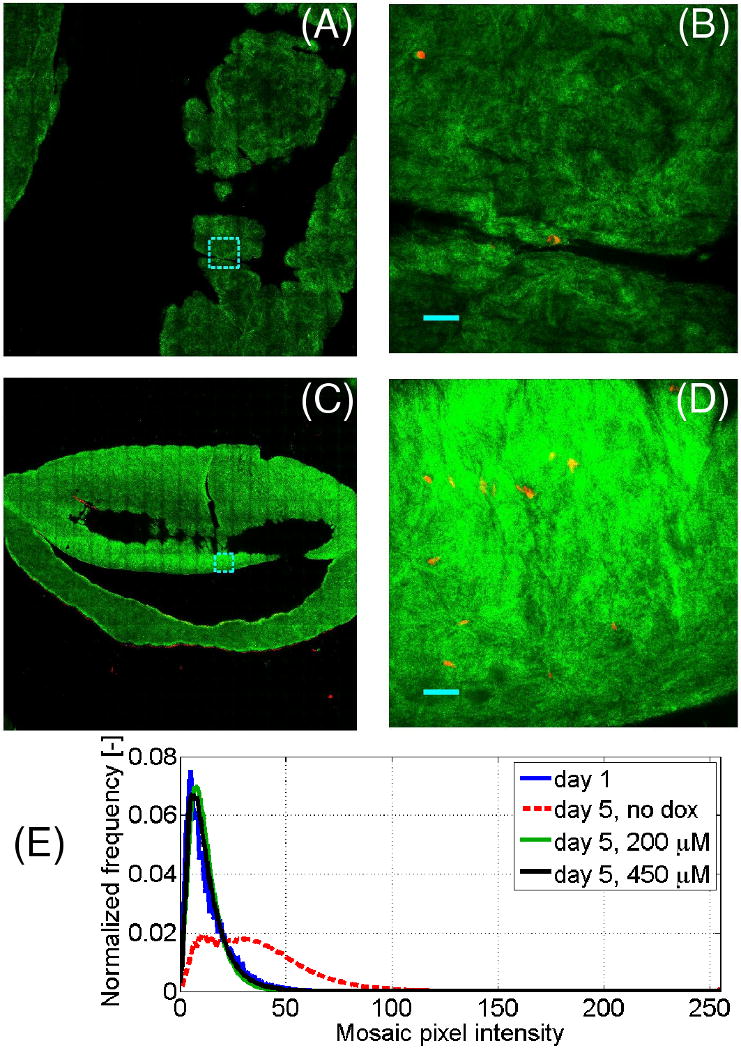
Representative confocal mosaics of collagen gels, with the reflectance signal (green) representing collagen and eosin fluorescence signal (red) representing cell cytoplasm. (A, C) Representative 0.6 × 0.6 cm mosaics of day 1 and day 5 collagen-SMC gels, respectively, with representative 500 × 500 μm tiles shown at higher magnification in (B, D), respectively. Note that in (A-D) the reflectance signal was digitally boosted 3-fold. (E) Statistical distribution of pixel intensities of the collagen-SMC gels outlined in the reflectance mosaics that quantify the reflectance increase in the day 5, dox- gels. Tile shown in (B) and (D) represent 500 × 500 μm, are outlined in cyan in (A) and (C), respectively. (B, D): Bar = 50 μm.
In comparing the mosaics from the day 1 gels and the day 5 (dox-) gels, the overall reflectance of the day 1 gels (Fig. 1A-B) was very weak, while the reflectance from the day 5 gels was much stronger. To quantify these differences, the areas of each mosaic that represented the gels were delineated, creating a binary mask that represented the gels. The pixel intensity distributions in the (raw) reflectance images were binned, and are plotted in Fig. 1E. It can be seen that most of the pixels from the day 1 gels were found between 0 and 30 a.u. on an 8-bit 0-255 scale, while most of the pixels in the day 5 (dox-) gels were found between 0 and 80 a.u.
The differences between the day 1 and day 5 gels (Fig. 1), illustrate how collagen gel development and remodeling change the optical properties and thus increase the reflectance. But while the images in Fig. 1 were acquired at a single plane, the OCT data presented below were acquired over entire 3D volumes. The remainder of the paper shows a more detailed, depth-resolved, analysis of the reflectivity of collagen gels at a different wavelength (1310 vs. 532 nm).
4.B. OCT measures decrease in scattering anisotropy of collagen-SMC gels over time
OCT non-destructively measured the optical properties of collagen-SMC and acellular collagen gels at day 1 and day 5 (Fig. 2A). The optical data is displayed in its entirety as a scatter plot on a grid of iso-μs and iso-g lines that represent theoretical values [21]. Each datum point represents the (μ, ρ) values from the peak of the joint PDF of a single gel. As can be seen in Fig. 2A, the reflectivity of the day 5 collagen-SMC gels is roughly 10-fold greater than that of the other 3 groups, with little change in attenuation. These changes correspond to a decrease in scattering anisotropy from 0.91 to 0.46 with little change in the scattering coefficient. Mapping the PDF peaks back to (μs, g) space and comparing the 4 gel groups statistically, there was a significant difference in both scattering anisotropy and reflectivity between the day 5 collagen-SMC gels and the 3 other gel conditions (P<0.001). In contrast, both attenuation and scattering coefficients of the gel groups did not significantly differ. In addition, the joint PDFs of the 4 collagen gel groups in Fig. 2A are plotted on a grid in Fig. 2B, showing there was slightly more statistical variation in the acellular gels than in the SMC gels.
Fig. 2.
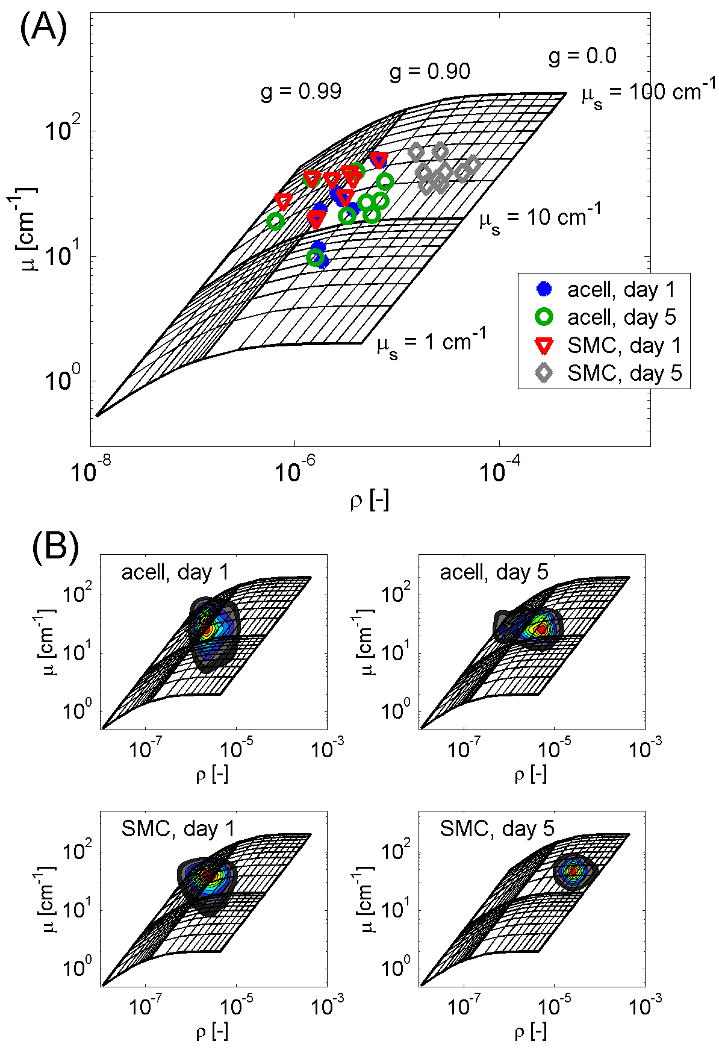
Optical properties of acellular and collagen–SMC gels. (A) Scatter plot of the (μ, ρ) values from the peak of the joint PDF of each imaged sample, displayed on a log-log scale and superimposed on a grid that maps theoretical iso-μs and iso-g lines to the experimental conditions. (B) Joint probability density functions of the optical properties of the collagen-SMC gels and calibration spheres, displayed as a color map and superimposed on the grid.
4.C. Doxycycline mitigates increase in reflectivity in remodeling collagen gels
To assess the role MMPs play in the optical properties of collagen gels, collagen-SMC gels were treated with 2 different doses of the non-specific MMP inhibitor doxycycline. These collagen-SMC gels were imaged at 5 days by both confocal mosaics (n=5) and OCT (n=9), and the optical properties were quantified from the OCT data. Images of the collagen-SMC gels treated with (dox+) and without (dox-) doxycycline indicate the dramatic differences in appearance (Fig. 3A). The 450 μM dox+ gels appeared flat with uniform thickness, had a large diameter, and were more transparent, resembling unremodeled day 1 gels. However, the dox- gels were shaped as a bi-concave disc, with a smaller diameter relative to day 0, and a cloudier (i.e., more reflective) appearance. The 200 μM dox+ gels appear to be partially remodeled, in between the dox- and 450 μM dox+ gels. Macroscopic measurements of remodeling, as well as confocal mosaic images from the dox+ gels corresponding to data in Fig. 1E, are provided in the data supplement.
Fig. 3.
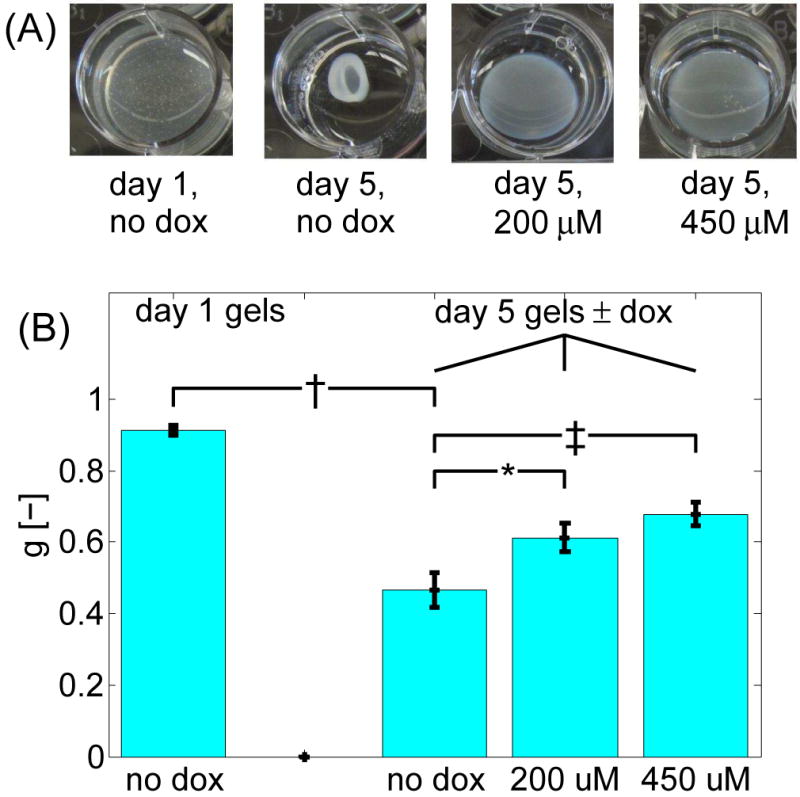
Effect of doxycycline dosage on collagen-SMC gels. (A) Photos of collagen-SMC gels in 12-well plates with and without doxycycline treatment. (B) Scattering anisotropy of collagen gels. All P-values were calculated against the day 5 dox- gels. Error bars represent the standard error of the means. †: P<0.001; *: P<0.05; ‡: P<0.01.
Reflectivity measurements of day 5 dox+ and dox- gels are shown in Fig. 3B. Since no significant differences in μs were observed between the various gel groups, only information on g is presented. For comparison, the g values from the day 1 gels are also plotted. The anisotropy of the dox- gels was in between the optical properties of the day 1 and day 5 dox- gels. At 5 days, the differences in the anisotropy between the dox- and dox+ were significant for both the 450 μM and 200 μM doses (P<0.01 and P<0.05, respectively). Similarly, the differences in log10(ρ) between the day 5 dox- and 450 μM and 200 μM dox+ gels were P<0.001 and P<0.01, respectively.
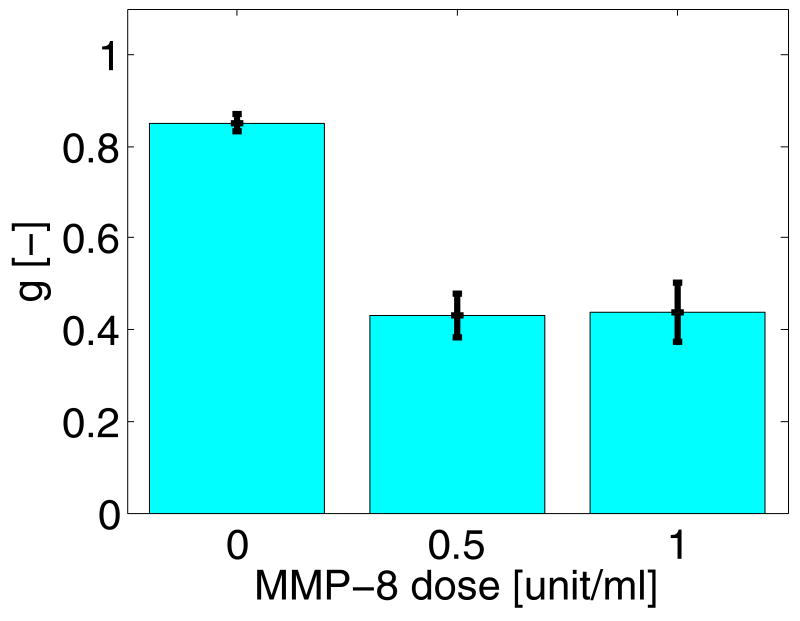
4.D. Collagenase activity increases reflectivity of acellular gels
Finally, to show that MMP activity is sufficient to cause an increase in reflectivity, multiple acellular collagen gels were treated with MMP-8 for 3 hr, imaged by OCT, and had their optical properties determined. Macroscopically, no signs of remodeling were evident as the treated gel and untreated gels looked very similar. The mean anisotropies from the treated and untreated day 1 acellular gels are plotted in Fig. 4. These data show that in 3 hours, collagenase activity alone induced a significant decrease in the scattering anisotropy of acellular gels, with P< 0.001 between the untreated and either treated groups. The increase in reflectivity of the treated gels was significantly higher (P<0.05) than the untreated group.
Fig. 4.
Effect of treating day 1 acellular gels for 3 hr with MMP-8 on the reflectivity measured from the OCT data. Error bars represent the standard error of the means. Both collagenase treated gels differed significantly from untreated gels (P<0.001).
5. Discussion
In this paper, we showed that OCT is capable of measuring and quantifying changes in optical scattering properties caused by SMC remodeling of collagen fibrils. By measuring these optical properties from OCT data, we showed that from day 1 to day 5, there was a 10-fold increase in reflectivity ρ with no change in attenuation μ, which corresponded to a decrease in anisotropy g from 0.91 to 0.46, with little change to scattering coefficient μs (Fig. 2). Blocking MMP activity in the gels by treating them with doxycycline for 5 days impeded both collagen gel remodeling macroscopically (Fig. 3A, supplementary Fig. 1) and the corresponding decrease in scattering anisotropy (Fig. 3B). The effect that the presence (or absence) of MMPs had on collagen reflectance could be seen in the confocal mosaics (Fig. 1, supplementary Fig. 2), where the collagen matrix in the day 5 dox- gels had a much higher reflectance signal than the day 1 gels and both doses of the day 5 dox+ gels. Treating acellular collagen gels with MMP-8 for 3 hr partially mimicked the increase in reflectivity that occurs over 5 days (Fig. 4). Taken together, the data presented here demonstrate that: (A) a relationship exists between MMP activity and the optical properties of collagen gels, and (B) that measuring optical properties from OCT data provides an non-destructive assessment of remodeling in local nanoliter volumes.
Using OCT to measure optical properties is advantageous for several reasons. The optical scattering properties of a sample, particularly the scattering phase function P(θ) and scattering anisotropy g, are very sensitive to the molecular microstructure of the sample [32]. The decrease in scattering anisotropy between the day 1 and day 5 gels illustrates that the optical properties are sensitive to ECM degradation (among other things). Moreover, measuring optical properties is non-destructive, allowing for repeated measurements on the same sites and on the same sample. This OCT technique is based on endogenous contrast between the collagen, the cells, and the surrounding medium, making it label-free, which reduces both the costs and complexity associated with adding exogenous fluorescent labels. As a fiber-based technology, OCT probes can be readily integrated into the incubator for continuous, time-lapse monitoring of construct uniformity. Finally, since the sample arm of the OCT system is essentially a confocal microscope, an OCT system can be designed to acquire mosaics of data (analogous to confocal mosaics in Fig. 1, supplementary Fig. 2), that cover centimeters of area and millimeters of depth, compared to the ∼100 μm depth limit on confocal mosaic imaging. Thus, such OCT-based techniques that continuously and non-destructively monitor milliliter volumes of a sample at micron resolution, and detect changes in the ECM in nanoliter volumes without any exogenous labels, could prove to be powerful tools for surveying engineered tissues.
Recently, we demonstrated that over 5 days SMC remodeling of collagen gels decreased both anisotropy g and scattering coefficient μs, thereby causing the reflectivity to double and the attenuation to remain the same [20]. Moreover, the measured reflectivity statistically followed a bimodal distribution, with a sizeable subpopulation of data points with 10-fold reflectivity relative to that from the first 24 hr. Although the trends are similar, this earlier data contrasts with Fig. 2, where nearly all the day 5 reflectivity data was 10-fold higher than the day 1 reflectivity data. Subsequent experiments showed that this discrepancy was caused both by the cell source (i.e., from different baboons) and the serum lot. Moreover, the gel compaction time dynamics from the previous study also contrasted with those in the present study: Earlier, the gel volume reduced in incremental steps every day, while in this study most of the compaction happened between days 1 and 2.
In trying to dissect the optical property data and identify what caused these observed decreases in scattering anisotropy (increases in reflectivity), 4 possibilities were hypothesized: increased collagen density, increased cell density, SMC contraction, and ECM remodeling. In the SMC gels, there was no correlation between anisotropy and either collagen density or cell density [20]. To assess whether SMC contraction, i.e., a change in cell morphology, caused the measured increase in reflectivity, collagen gels were prepared and seeded with endothelial cells (ECs) instead of SMCs. The ECs and SMCs were determined to have different morphologies in 3D collagen gels by F-actin staining (data not shown). At day 1, the EC gels and SMC gels differed in μs (P<0.05), but not in g (P>0.05) [33]. Since the primary changes in the remodeling of SMC gels were in g, the EC data suggests such changes were not caused by a change in cell shape, which further supports the hypothesis that ECM remodeling caused the changes in the optical properties of the collagen gels.
Mie theory [32], which calculates the angular distribution of light energy scattered off a sphere, can be used to interpret the optical properties of the collagen gels. Mie theory states that in general, as the sphere diameter increases, light is scattered more anisotropically, that is, mostly in the forward direction (g→1); conversely, as the sphere diameter decreases, light is scattered more isotropically, that is, relatively equally in all directions (g→0). The OCT signal depends on light backscattered from the sample, specifically, the fraction of light scattered back towards the lens relative to the total scattered energy. The fraction of light scattered in the backwards direction increases as the scattering anisotropy decreases, forming an inverse relationship between scattering anisotropy and reflectivity, as has been described in our theoretical model [21]. Applying these principles to collagen-SMC gels, scattering by the collagen fibril network is intrinsically very forward-directed, as shown by the high scattering anisotropy/low reflectivity measured from these samples (Figs. 2, 4). Proteolytic degradation of the collagen matrix by local MMP activity breaks down the fibrillar collagen network into small fibril fragments. These fibril fragments constitute a dense collection of local isotropic scatterers that would scatter more light back to the objective lens, thereby increasing the reflectivity.
Collagen gels prepared in our lab and seeded with fibroblasts, cells that contract collagen fibrils as well as express and activate MMPs, showed both similar macroscopic remodeling and a similar decrease in scattering anisotropy/increase in reflectivity over 5 days (data not shown). However, collagen gels seeded with ECs, a cell type that generates little secreted MMP activity in 3D collagen matrices [34], did not produce comparable remodeling at 5 days, neither macroscopically nor microscopically [33]. Considering the fibroblast gels as positive controls and the EC gels as negative controls further confirms our hypothesis that ECM remodeling is causing the measured changes in optical properties.
In general, there are 2 types of remodeling – mechanical remodeling, in which the collagen matrix is pressed down and fluid is expelled, and biochemical remodeling, which involves proteolytic enzymes such as MMPs breaking down collagen [15,35]. Several studies measuring MMP activity in collagen-SMC gels using gelatin zymography showed that secreted MMPs, specifically MMP-2 (gelatinase A), play a critical role in matrix remodeling [8,25]. In the present study, blocking MMP activity with doxycycline impeded the decrease in scattering anisotropy (Fig. 3B) and impeded gel compaction (supplementary Fig. 1, [25]), but did not eliminate it. Similarly, treating acellular gels with collagenase partially mimicked the increase in reflectivity, but did not reproduce it in full. In both the doxycycline and collagenase experiments, the dose response of anisotropy was nonlinear. Thus, it is likely that other mechanisms contribute to the measured changes in optical properties. More research is needed to identify such mechanisms.
6. Conclusion
In this study, OCT quantitatively monitored ECM remodeling in SMC-seeded collagen gels from day 1 to day 5 by measuring the optical properties. Over 5 days, the scattering anisotropy of the collagen-SMC gels decreased from 0.91 to 0.46, while the scattering coefficient stayed the same, corresponding to a 10-fold increase in reflectivity with no change in attenuation. Treatment of the collagen-SMC gels with doxycycline, a non-specific MMP inhibitor, impeded remodeling macroscopically over 5 days and impeded the decrease in anisotropy that was measured in the dox- gels. Confocal mosaicing visually confirmed the presence or absence of remodeling in the collagen gels, as well as increase in reflectivity that was measured with OCT. Additionally, treatment of day 1 acellular gels with MMP-8 caused a decrease in anisotropy that partially mimicked the decrease measured in collagen-SMC gels after 5 days of remodeling. Together these data suggest that the activity of MMPs in collagen-SMC gels degrades collagen fibrils into smaller fragments, and that OCT can visualize and quantify the process.
Supplementary Material
acroscopic compaction in collagen gels calculated as a percent reduction in volume (B) and diameter (C). At day 0, the gel volume was 1 ml and the gel area was 4 cm2. Error bars represent the standard deviation. *: P<0.05; ‡: P<0.001.
Confocal mosaics of day 5, dox+ gels, with the reflectance signal (matrix) false-colored green and the cells false-colored red. (A, C) Representative 1 × 1 cm mosaics of 200 μM and 450 μM dox+ gels, respectively, with representative 500 × 500 μm tiles shown at higher magnification in (B, D), respectively. Similar to Fig. 1, the reflectance signal was digitally boosted 3-fold. Note that for both doxycycline doses the reflectance signal from the matrix is weak, similar to day 1 gels (Fig. 1A and B).
Acknowledgments
The authors would like to thank Dan Gareau of the Department of Biomedical Engineering at OHSU for help aligning the VivaScope. Additionally, the authors would like to thank Fred Grinnell, Kate Phelps, and Miguel Miron of the Cell Biology Department at University of Texas-Southwestern Medical Center for helpful discussions on collagen gel remodeling. This study was funded in part by the National Institutes of Health grant R01-HL084013 (Jacques) and R01-HL095474 (Hanson).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lemaitre V, D'Armiento J. Matrix metalloproteinases in development and disease. Birth Defects Res C Embryo Today. 2006;78(1):1–10. doi: 10.1002/bdrc.20065. [DOI] [PubMed] [Google Scholar]
- 2.Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2000;149(6):1309–23. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144(5):666–72. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13(1):7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 5.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94(6):2493–503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27(31):5287–97. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardo A, Selman M. Matrix metalloproteases in aberrant fibrotic tissue remodeling. Proc Am Thorac Soc. 2006;3(4):383–8. doi: 10.1513/pats.200601-012TK. [DOI] [PubMed] [Google Scholar]
- 8.Seliktar D, Nerem RM, Galis ZS. The role of matrix metalloproteinase-2 in the remodeling of cell-seeded vascular constructs subjected to cyclic strain. Ann Biomed Eng. 2001;29(11):923–34. doi: 10.1114/1.1415522. [DOI] [PubMed] [Google Scholar]
- 9.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 10.Abraham LC, Zuena E, Perez-Ramirez B, Kaplan DL. Guide to collagen characterization for biomaterial studies. J Biomed Mater Res B Appl Biomater. 2008;87(1):264–85. doi: 10.1002/jbm.b.31078. [DOI] [PubMed] [Google Scholar]
- 11.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91(4):439–42. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 12.Sternlicht M, Werb Z. Matrix Metalloproteinases (MMPs) In: Kreis T, Vale R, editors. Guidebook to the Extracellular Matrix and Adhesion Proteins. 2nd. 1999. pp. 519–523. [Google Scholar]
- 13.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979;76(3):1274–8. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummings CL, Gawlitta D, Nerem RM, Stegemann JP. Properties of engineered vascular constructs made from collagen, fibrin, and collagen-fibrin mixtures. Biomaterials. 2004;25(17):3699–706. doi: 10.1016/j.biomaterials.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 15.Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13(5):264–9. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 16.Sabeh F, Li XY, Saunders TL, Rowe RG, Weiss SJ. Secreted versus membrane-anchored collagenases: relative roles in fibroblast-dependent collagenolysis and invasion. J Biol Chem. 2009;284(34):23001–11. doi: 10.1074/jbc.M109.002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 18.Mason C, Markusen JF, Town MA, Dunnill P, Wang RK. The potential of optical coherence tomography in the engineering of living tissue. Phys Med Biol. 2004;49(7):1097–115. doi: 10.1088/0031-9155/49/7/002. [DOI] [PubMed] [Google Scholar]
- 19.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254(5035):1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levitz D, Hinds MT, Choudhury N, Tran NT, Hanson SR, Jacques SL. Quantitative characterization of developing collagen gels using optical coherence tomography. J Biomed Opt. 15(2):026019. doi: 10.1117/1.3377961. in press. [DOI] [PubMed] [Google Scholar]
- 21.Jacques SL, Levitz D, Samatham R, Gareau DS, Choudhury N, Truffer F. Light scattering in confocal reflectance microscopy. In: Wax A, Backman V, editors. Biomedical Applications of Light Scattering. p. 026019. in press. [Google Scholar]
- 22.Stegemann JP, Nerem RM. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann Biomed Eng. 2003;31(4):391–402. doi: 10.1114/1.1558031. [DOI] [PubMed] [Google Scholar]
- 23.Choudhury N, Song G, Chen F, Matthews S, Tschinkel T, Zheng J, et al. Low coherence interferometry of the cochlear partition. Hear Res. 2006;220(1-2):1–9. doi: 10.1016/j.heares.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Rollins A, Yazdanfar S, Kulkarni M, Ung-Arunyawee R, Izatt J. In vivo video rate optical coherence tomography. Opt Express. 1998;3(6):219–29. doi: 10.1364/oe.3.000219. [DOI] [PubMed] [Google Scholar]
- 25.Franco C, Ho B, Mulholland D, Hou G, Islam M, Donaldson K, et al. Doxycycline alters vascular smooth muscle cell adhesion, migration, and reorganization of fibrillar collagen matrices. Am J Pathol. 2006;168(5):1697–709. doi: 10.2353/ajpath.2006.050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golub LM, Lee HM, Lehrer G, Nemiroff A, McNamara TF, Kaplan R, et al. Minocycline reduces gingival collagenolytic activity during diabetes. Preliminary observations and a proposed new mechanism of action. J Periodontal Res. 1983;18(5):516–26. doi: 10.1111/j.1600-0765.1983.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 27.Myers SA, Wolowacz RG. Tetracycline-based MMP inhibitors can prevent fibroblast-mediated collagen gel contraction in vitro. Adv Dent Res. 1998;12(2):86–93. doi: 10.1177/08959374980120012701. [DOI] [PubMed] [Google Scholar]
- 28.Gioia M, Monaco S, Fasciglione GF, Coletti A, Modesti A, Marini S, et al. Characterization of the mechanisms by which gelatinase A, neutrophil collagenase, and membrane-type metalloproteinase MMP-14 recognize collagen I and enzymatically process the two alpha-chains. J Mol Biol. 2007;368(4):1101–13. doi: 10.1016/j.jmb.2007.02.076. [DOI] [PubMed] [Google Scholar]
- 29.Patel YG, Nehal KS, Aranda I, Li Y, Halpern AC, Rajadhyaksha M. Confocal reflectance mosaicing of basal cell carcinomas in Mohs surgical skin excisions. J Biomed Opt. 2007;12(3):034027. doi: 10.1117/1.2750294. [DOI] [PubMed] [Google Scholar]
- 30.Gareau DS, Li Y, Huang B, Eastman Z, Nehal KS, Rajadhyaksha M. Confocal mosaicing microscopy in Mohs skin excisions: feasibility of rapid surgical pathology. J Biomed Opt. 2008;13(5):054001. doi: 10.1117/1.2981828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gareau DS. Feasibility of digitally stained multimodal confocal mosaics to simulate histopathology. J Biomed Opt. 2009;14(3):034050. doi: 10.1117/1.3149853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bohren CF, Huffman DR. Absorption and ccattering of light by small particles. 1983 [Google Scholar]
- 33.Levitz D, Choudhury N, Vartanian K, Hinds MT, Hanson SR, Jacques SL. Optically characterizing collagen gels made with different cell types. In: Kirkpatrick S, Wang R, editors. Proc SPIE; 2009 Mar-Apr; San Jose, CA. 2009. p. 717905. [Google Scholar]
- 34.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97(11):1093–107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 35.Brown RA, Wiseman M, Chuo CB, Cheema U, Nazhat SN. Ultrarapid engineering of biomimetic materials and tissues: fabrication of nano- and microstructures by plastic compression. Adv Func Mater. 2005;15:1762–1770. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
acroscopic compaction in collagen gels calculated as a percent reduction in volume (B) and diameter (C). At day 0, the gel volume was 1 ml and the gel area was 4 cm2. Error bars represent the standard deviation. *: P<0.05; ‡: P<0.001.
Confocal mosaics of day 5, dox+ gels, with the reflectance signal (matrix) false-colored green and the cells false-colored red. (A, C) Representative 1 × 1 cm mosaics of 200 μM and 450 μM dox+ gels, respectively, with representative 500 × 500 μm tiles shown at higher magnification in (B, D), respectively. Similar to Fig. 1, the reflectance signal was digitally boosted 3-fold. Note that for both doxycycline doses the reflectance signal from the matrix is weak, similar to day 1 gels (Fig. 1A and B).