Abstract
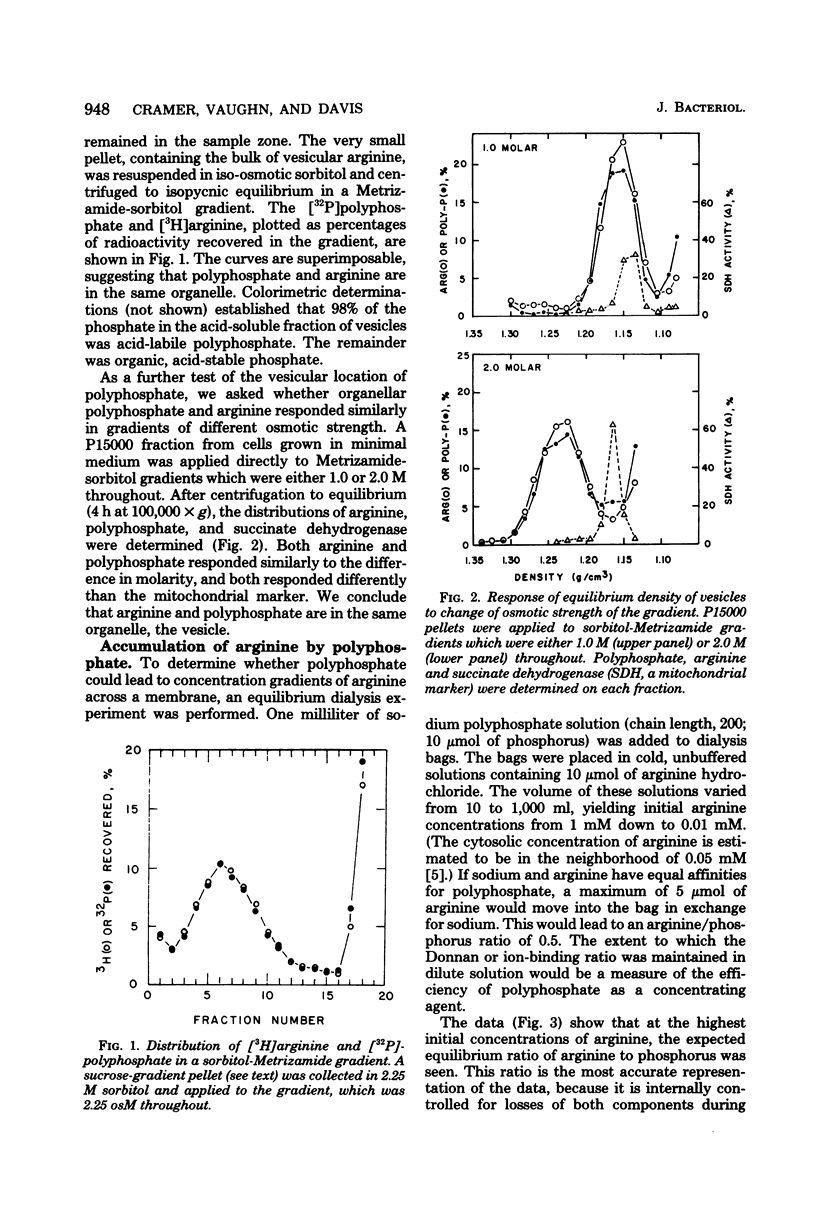
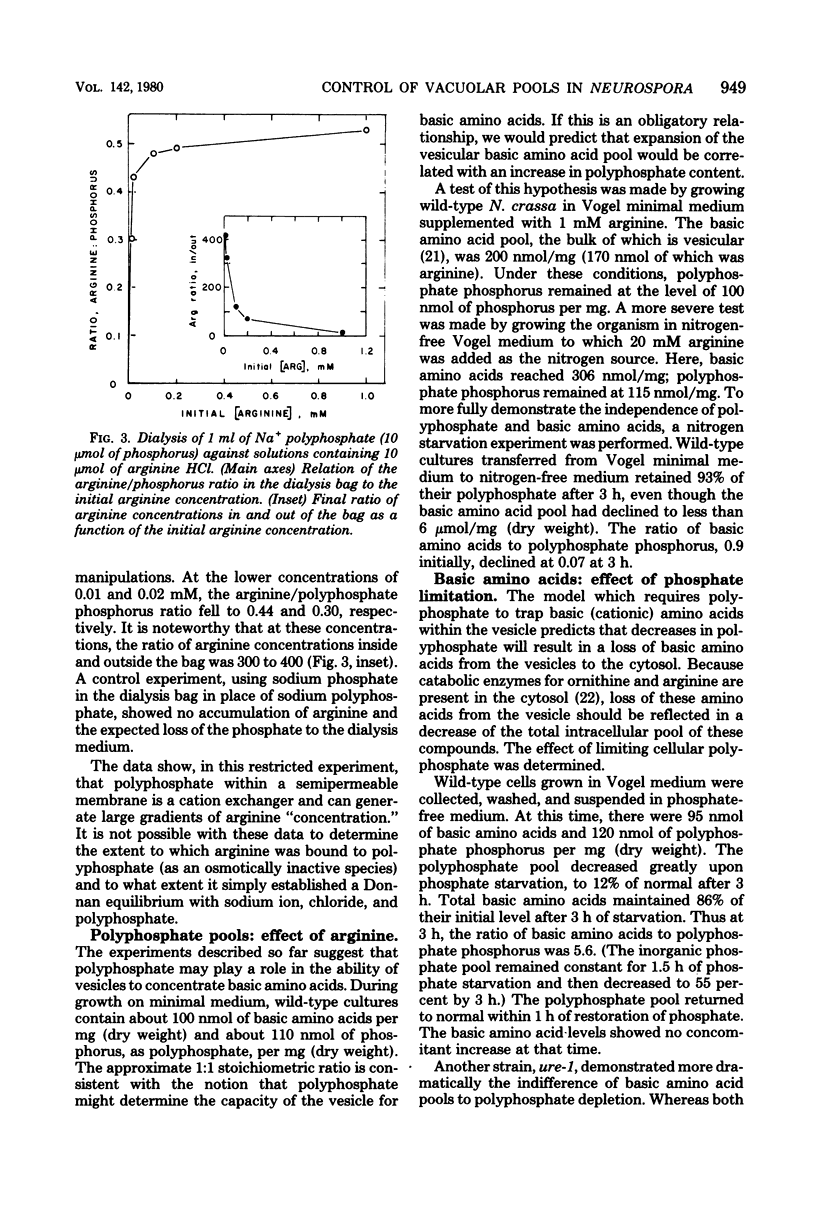
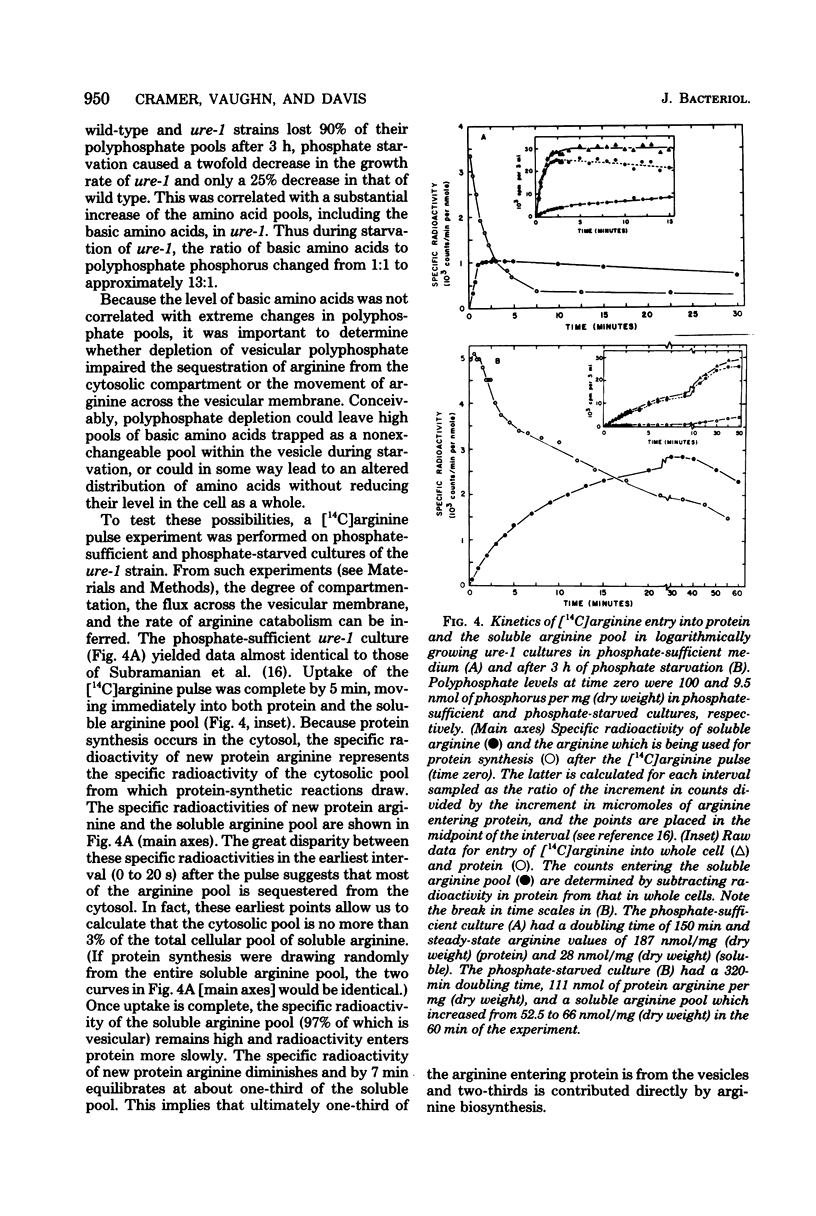
At least 78%, and perhaps all, of inorganic polyphosphate is shown to be contained within the vesicles (vacuoles) of Neurospora crassa, where over 97% of the soluble arginine, lysine, and ornithine pools are known to accumulate. Furthermore, synthetic polyphosphate can concentrate arginine up to 400-fold from dilute (0.01 mM) solutions in equilibrium dialysis. For these reasons and because the molar ratio of basic amino acids and polyphosphate phosphorus is approximately 1, we tested the hypothesis that there was an obligate physiological relationship between them. Experiments in which nitrogen starvation and arginine excess were imposed upon cells showed that polyphosphate content was insensitive to changes in the basic amino acid content. Experiments involving phosphate starvation and restoration showed that basic amino acid content was almost wholly independent of polyphosphate pools. Moreover, the normal high degree of compartmentation of arginine in vesicles was maintained despite polyphosphate depletion, and arginine was still exchanged across the vesicular membrane. We conclude that N. crassa, like yeasts, can regulate polyphosphates and basic amino acids independently, and that the accumulation of basic amino acids in vesicles may depend upon an energy-requiring mechanism in addition to the demonstrated charge interaction with polyphosphate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis R. H., Bowman B. J., Weiss R. L. Intracellular compartmentation and transport of metabolites. J Supramol Struct. 1978;9(4):473–488. doi: 10.1002/jss.400090403. [DOI] [PubMed] [Google Scholar]
- Davis R. H., Lawless M. B., Port L. A. Arginaseless Neurospora: genetics, physiology, and polyamine synthesis. J Bacteriol. 1970 May;102(2):299–305. doi: 10.1128/jb.102.2.299-305.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H. Utilization of exogenous and endogenous ornithine by Neurospora crassa. J Bacteriol. 1968 Aug;96(2):389–395. doi: 10.1128/jb.96.2.389-395.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAROLD F. M. Accumulation of inorganic polyphosphate in mutants of Neurospora crassa. Biochim Biophys Acta. 1960 Dec 4;45:172–188. doi: 10.1016/0006-3002(60)91438-4. [DOI] [PubMed] [Google Scholar]
- HAROLD F. M. Binding of inorganic polyphosphate to the cell wall of Neurospora crassa. Biochim Biophys Acta. 1962 Feb 12;57:59–66. doi: 10.1016/0006-3002(62)91077-6. [DOI] [PubMed] [Google Scholar]
- HESS J., KITO E., MARTIN R. P., VAN PILSUM J. F. Determination of creatine, creatinine, arginine, guanidinoacetic acid, guanidine, and methylguanidine in biological fluids. J Biol Chem. 1956 Sep;222(1):225–235. [PubMed] [Google Scholar]
- Karlin J. N., Bowman B. J., Davis R. H. Compartmental behavior of ornithine in Neurospora crassa. J Biol Chem. 1976 Jul 10;251(13):3948–3955. [PubMed] [Google Scholar]
- Matile P., Jost M., Moor H. Intrazelluläre Lokalisation proteolytischer Enzyme von Neurospora crassa. Z Zellforsch Mikrosk Anat. 1965 Oct 12;68(2):205–216. [PubMed] [Google Scholar]
- Ohnishi T., Gall R. S., Mayer M. L. An improved assay of inorganic phosphate in the presence of extralabile phosphate compounds: application to the ATPase assay in the presence of phosphocreatine. Anal Biochem. 1975 Nov;69(1):261–267. doi: 10.1016/0003-2697(75)90585-0. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K. N., Weiss R. L., Davis R. H. Use of external, biosynthetic, and organellar arginine by Neurospora. J Bacteriol. 1973 Jul;115(1):284–290. doi: 10.1128/jb.115.1.284-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urech K., Dürr M., Boller T., Wiemken A., Schwencke J. Localization of polyphosphate in vacuoles of Saccharomyces cerevisiae. Arch Microbiol. 1978 Mar;116(3):275–278. doi: 10.1007/BF00417851. [DOI] [PubMed] [Google Scholar]
- Weiss R. L. Compartmentation and control of arginine metabolism in Neurospora. J Bacteriol. 1976 Jun;126(3):1173–1179. doi: 10.1128/jb.126.3.1173-1179.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Intracellular localization of enzymes of arginine metabolism in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5403–5408. [PubMed] [Google Scholar]
- Weiss R. L. Intracellular localization of ornithine and arginine pools in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5409–5413. [PubMed] [Google Scholar]



