Case Presentation
A 58-year-old woman with a history of cigarette smoking, chronic obstructive pulmonary disease, and recent Intensive Care Unit admission for pneumonia presented with sudden onset right-sided chest discomfort and dyspnea. On physical examination, she was tachycardic to 110 beats per minute, normotensive with a blood pressure of 128/72 mmHg, tachypneic to 24 breaths per minute, and hypoxemic to 88% on room air. She had jugular venous distension to the angle of her mandible, a II/VI holosystolic murmur that increased to III/VI with inspiration at the left lower sternal border, lung fields clear to auscultation bilaterally, and mild symmetric lower extremity edema. The electrocardiogram was notable for sinus tachycardia and T-wave inversions across the anterior precordium. Laboratory evaluation was remarkable for a D-dimer of 1,104 ng/ml (normal <500 ng/ml) and a cardiac troponin I of 1.4 ng/ml (normal <0.1 ng/ml). Contrast-enhanced chest computed tomography (CT) demonstrated thrombus filling the right main pulmonary artery and moderate right ventricular (RV) enlargement (RV-to-left ventricular [LV] dimension ratio = 1.2). Bedside transthoracic echocardiography documented moderately severe RV hypokinesis, moderate tricuspid regurgitation, and an estimated pulmonary artery systolic pressure of 55 mmHg. These clinical, laboratory, and imaging findings established the diagnosis of submassive pulmonary embolism (PE). The principal management question was whether to treat with anticoagulation alone (a “watch and wait” strategy) or whether to administer fibrinolysis immediately.
Overview
Venous thromboembolism is the third most common cardiovascular disorder after myocardial infarction and stroke.1 The mortality rate for acute PE exceeds 15% in the first three months after diagnosis and surpasses that of myocardial infarction.2 Death most commonly results from progressive RV failure culminating in cardiovascular collapse.3 Survivors of acute PE remain at risk for chronic thromboembolic pulmonary hypertension.4
Acute PE represents a spectrum of clinical syndromes with a variety of prognostic implications. Patients with acute PE who have normal systemic arterial pressure and preserved RV function have an excellent prognosis with therapeutic anticoagulation alone. In contrast, patients with massive PE present with syncope, systemic arterial hypotension, cardiogenic shock, or cardiac arrest and have an increased risk of adverse outcomes, including death.5 Normotensive patients with acute PE and evidence of RV dysfunction are classified as having submassive PE, comprise a large population at increased risk for adverse events, and warrant consultation from cardiovascular medicine specialists.6 While advanced therapy with fibrinolysis is considered a life-saving intervention in massive PE, it remains controversial in patients with submassive PE.7
Patients with submassive PE can be identified by the presence of RV dysfunction detected on physical examination, electrocardiography, cardiac biomarkers, echocardiography, and chest CT. Physical examination findings of tachycardia, elevated jugular venous pressure, right parasternal heave, accentuated sound of pulmonic valve closure (P2), and hepatomegaly suggest RV dysfunction. The electrocardiogram can provide a rapid and inexpensive indicator of RV strain and adds incremental prognostic value to echocardiographic findings of RV dysfunction in patients with submassive PE.8 Incomplete or complete right bundle branch block (RBBB), T wave inversions in leads V1-V4, and the combination of an S wave in lead I, Q wave in lead III, and T wave inversion in lead III (S1Q3T3) signify RV strain.9
Elevations in cardiac biomarkers, including troponin, brain-type natriuretic peptide (BNP) and heart-type fatty acid-binding protein (H-FABP), are associated with RV dysfunction and can noninvasively identify patients with submassive PE. Normotensive patients with acute PE and elevations in levels of cardiac troponins and BNP demonstrate an increased short-term mortality and risk of adverse outcomes.10, 11 Patients with acute PE and normal H-FABP levels have an excellent prognosis, while those with increased levels (≥ 6 ng/mL) have a higher rate of adverse events, including hemodynamic collapse, respiratory failure, cardiac arrest, and death.12, 13
Echocardiography is the best imaging study to detect RV dysfunction in the setting of acute PE. Characteristic echocardiographic findings in patients with submassive PE include RV hypokinesis and dilatation, interventricular septal flattening and paradoxical motion toward the left ventricle, abnormal transmitral Doppler flow profile, tricuspid regurgitation, pulmonary hypertension as identified by a peak tricuspid regurgitant jet velocity greater than 2.6 m/s, and loss of inspiratory collapse of the inferior vena cava (IVC).14 Regional RV dysfunction with severe free wall hypokinesis and apical sparing (McConnell sign) is a specific finding in acute PE.15 An RV-to-LV end-diastolic diameter ratio of 0.9 or greater, assessed in the left parasternal long-axis view or the subcostal view, is an independent predictor of hospital mortality.16 Echocardiography is warranted to identify RV dysfunction in patients with acute PE and clinical evidence of RV failure or elevated levels of cardiac biomarkers.3 A simple score based on clinical parameters, echocardiographic findings, and cardiac biomarkers can be used to stratify patients with acute PE according to risk of adverse outcomes (Table 1).17
Table 1.
Illustrations of risk stratification for acute pulmonary embolism (PE).
| Risk | Clinical Appearance | Vital Signs | Cardiac Biomarkers | Right Ventricular Function |
|---|---|---|---|---|
| LOW | Appears well | Normal | Normal | Normal right ventricular size and function |
| INTERMEDIATE | Appears well | Normal | Elevated | Moderate right ventricular dysfunction |
| HIGH | Appears ill | Transient or sustained hypotension | Elevated | Severe right ventricular dysfunction |
Submassive PE can also be diagnosed when RV enlargement on chest CT, defined by an RV-to-LV diameter ratio of greater than 0.9, is observed.18 RV enlargement on chest CT predicts increased 30-day mortality in patients with acute PE.18, 19 Detection of RV enlargement by chest CT is especially convenient for diagnosis of submassive PE because it utilizes data acquired during the initial diagnostic scan.
Pathophysiology
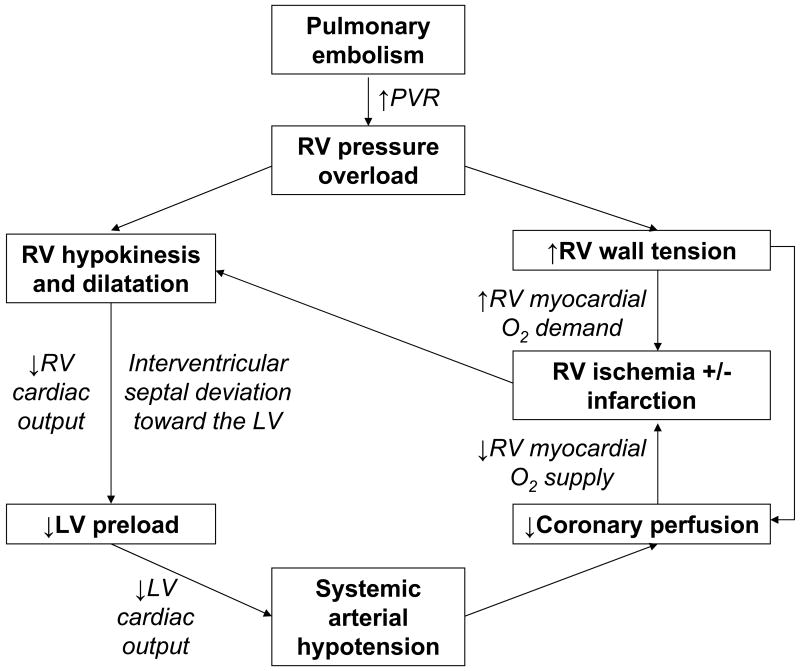
Direct physical obstruction of the pulmonary arteries, hypoxemic vasoconstriction, and release of potent pulmonary arterial vasoconstrictors increase pulmonary vascular resistance and RV afterload. Acute RV pressure overload may result in RV hypokinesis and dilation, tricuspid regurgitation, and ultimately, RV failure. Patients with submassive PE may deteriorate over the course of several hours to days and develop systemic arterial hypotension, cardiogenic shock, and cardiac arrest. Elevated diastolic pressure causes deviation of the interventricular septum toward the LV and impairs LV filling. An abnormal transmitral flow pattern on Doppler echocardiography may be observed because left atrial contraction, represented by the A wave on transmitral Doppler, makes a greater contribution to LV diastole than passive filling, signified by the E wave. RV pressure overload may also result in increased wall stress and ischemia by increasing myocardial oxygen demand while simultaneously limiting its supply (Figure 1). Severe mismatch between myocardial oxygen demand and supply may lead to RV infarction.
Figure 1.
The pathophysiology of submassive pulmonary embolism (PE). PVR, pulmonary vascular resistance; RV, right ventricular; O2, oxygen; LV, left ventricular.
A combination of ventilation-to-perfusion mismatch, increases in total dead space, and right-to-left shunting explains the majority of gas exchange abnormalities observed in patients with acute PE. Arterial hypoxemia and an increased alveolar-arterial oxygen gradient are the most commonly noted abnormalities of gas exchange. Hyperventilation, especially in patients with normal baseline pulmonary function, may result in hypocapnea and respiratory alkalosis.
Management
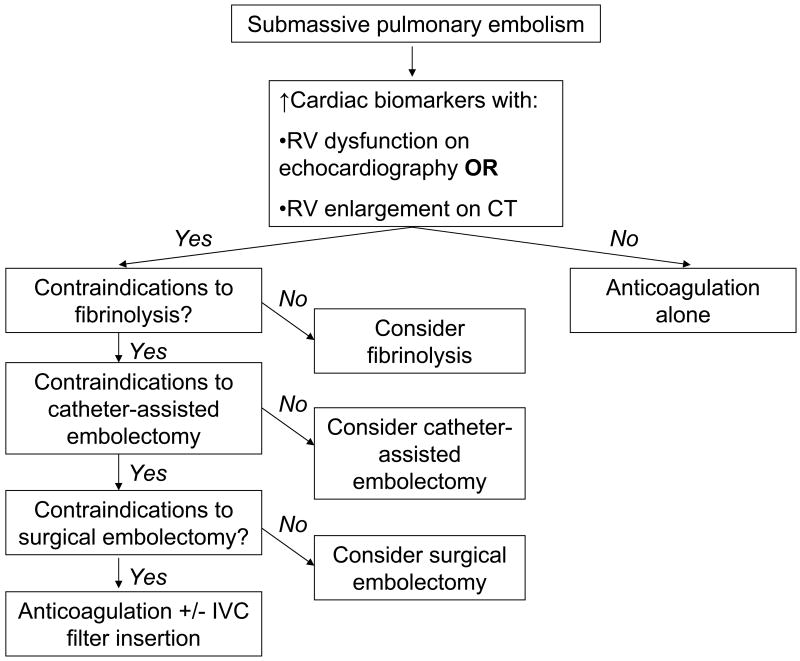
Anticoagulation remains the cornerstone of therapy. Current options for advanced therapy include fibrinolysis, catheter-assisted embolectomy, surgical embolectomy, and IVC filter insertion (Figure 2). The decision to select advanced therapy for submassive PE or to maintain anticoagulation alone must be individualized because of a paucity of trials to help guide management.
Figure 2.
An algorithm for management of patients with submassive pulmonary embolism (PE). RV, right ventricular; CT, computed tomography; IVC, inferior vena cava.
Fibrinolysis
Fibrinolysis functions as a “medical embolectomy” and when successful will rapidly reverse hemodynamic compromise and gas exchange derangements. In patients with submassive PE, fibrinolysis relieves RV pressure overload and may avert impending hemodynamic collapse and death due to progressive RV failure. Fibrinolytic therapy may reduce the likelihood of developing chronic thromboembolic pulmonary hypertension.20 The 2008 ACCP Guidelines include fibrinolysis as an option for submassive PE patients who are judged to have a low risk of bleeding (Grade 2B).21 Patients with a low risk of bleeding have normal renal function, are not frail, and are not receiving dual antiplatelet therapy.
The Management Strategies and Prognosis of Pulmonary Embolism Trial-3 (MAPPET-3) randomized 256 patients with submassive PE to receive recombinant tissue plasminogen activator (t-PA) 100 mg over two hours followed by unfractionated heparin infusion or placebo plus heparin anticoagulation.22 Compared with heparin anticoagulation alone, fibrinolysis resulted in a significant reduction in the primary study end point of in-hospital death or clinical deterioration requiring escalation of therapy (defined as catecholamine infusion, rescue fibrinolysis, mechanical ventilation, cardiopulmonary resuscitation, or emergency surgical embolectomy).22 The difference was largely attributable to a higher frequency of open-label fibrinolysis due to “clinical deterioration” as determined by the treating clinician.22
In a prospective study of 200 patients with submassive PE, echocardiography was performed at the time of diagnosis and after six months to determine the frequency of pulmonary hypertension.20 Estimated pulmonary artery systolic pressure at six months increased in 27% of patients receiving heparin alone, and nearly half of these patients were moderately symptomatic.20 The median decrease in pulmonary artery systolic pressure was only 2 mmHg in patients treated with heparin alone compared with 22 mmHg in those treated with t-PA plus heparin.20 Estimated pulmonary artery systolic pressure at follow-up did not increase in any of the patients treated with t-PA.20
The Pulmonary Embolism International Thrombolysis Trial (PEITHO) is a large randomized controlled trial that began in 2007. The investigators plan to enroll 1,000 patients in 12 countries to evaluate a primary clinical end point of all-cause mortality or hemodynamic collapse within seven days in patients treated with the fibrinolytic agent, tenecteplase, plus heparin versus heparin alone. As of March 31, 2010, 453 patients have been randomized. Results are expected in 2013.
The U.S. Food and Drug Administration (FDA) has approved t-PA 100 mg administered as a continuous intravenous infusion over two hours for treatment of acute massive PE. Nevertheless, t-PA is often used off-label to treat submassive PE. All patients being considered for fibrinolysis should be carefully screened for contraindications that make the bleeding risk prohibitive. The most dreaded complication of fibrinolysis is intracranial hemorrhage. An analysis from the International Cooperative Pulmonary Embolism Registry (ICOPER) observed that the risk of intracranial hemorrhage may be as high as 3%.2 A study from a center with experience in fibrinolysis for acute PE reported that the overall major bleeding rate may approach 20%.23 Major contraindications to fibrinolysis include intracranial mass, cerebrovascular event or neurosurgery within the prior two months, history of intracranial hemorrhage, recent major trauma, active or recent respiratory tract, gastrointestinal or genitourinary bleeding, severe uncontrolled hypertension, recent prolonged cardiopulmonary resuscitation, thrombocytopenia (<50,000 platelets/μL), acute pericarditis or pericardial effusion, ongoing suspicion for aortic dissection, and recent surgery, invasive procedure, or internal organ biopsy.
Fibrinolysis is most successful when administered within several days of acute PE. Although the efficacy of fibrinolysis is inversely proportional to duration of symptoms, effective fibrinolysis can be observed up to two weeks after an acute event.24, 25 Patients with more anatomically extensive PE achieve a greater response to fibrinolysis than those with smaller and peripherally-located thrombi.25 Based upon available data, local, catheter-directed delivery of the fibrinolytic agent directly into the pulmonary artery does not appear to improve efficacy or safety. Therefore, peripherally administered fibrinolytic therapy is ordinarily preferred to catheter-directed fibrinolysis.26-28
Intravenous unfractionated heparin is the preferred agent for immediate systemic anticoagulation in patients undergoing advanced therapy such as fibrinolysis (Table 2). Intravenous unfractionated heparin offers the advantage of immediate discontinuation and rapid reversal in the event of bleeding complications. In contrast to fibrinolysis in myocardial infarction, intravenous unfractionated heparin infusion is withheld during fibrinolysis in patients with acute PE.29 At the conclusion of the fibrinolytic infusion, the aPTT should be checked. Unfractionated heparin infusion should be restarted without a bolus when the aPTT is less than 80 seconds. If greater than 80 seconds, the aPTT should be rechecked every 4 hours until it falls into the range at which heparin can be safely restarted.
Table 2.
How to administer fibrinolytic therapy for submassive pulmonary embolism (PE).
|
Alternative Advanced Therapies
Alternatives to fibrinolysis may be considered when contraindications to fibrinolysis exist or when patients have failed to respond to an initial trial of fibrinolytic therapy. About half of patients with acute PE have contraindications to fibrinolysis. Alternative advanced therapies include catheter-assisted embolectomy, surgical embolectomy, and IVC filter insertion.
Catheter-assisted embolectomy is an emerging technique for advanced therapy when full dose fibrinolysis has failed or is contraindicated.30, 31 Catheter-assisted techniques, such as low-dose “local” fibrinolysis and thrombus fragmentation or aspiration, have the greatest success when applied to large, centrally located thrombi within the first five days of symptoms. The combination of local fibrinolysis with mechanical thrombectomy is called “pharmacomechanical therapy.”32
Surgical embolectomy requires a median sternotomy and cardiopulmonary bypass. Surgical embolectomy is most effective in patients with large centrally located thrombi. Perioperative mortality for patients undergoing surgical embolectomy has declined over the last two decades.33 Surgical embolectomy has been shown to be a safe and effective technique in the treatment of acute PE when performed by experienced surgeons.34, 35
Consider IVC filter insertion in patients with submassive PE in whom fibrinolysis and embolectomy are contraindicated or unavailable. IVC filter insertion reduces the incidence of recurrent PE but has not been shown to lower long-term mortality.36 IVC filters do not halt ongoing thrombogenesis and appear to increase the risk of DVT.36 Retrievable IVC filters offer a safe and effective alternative to permanent filters and may be removed up to several months after insertion.37
Case Presentation
The patient immediately received unfractionated heparin by intravenous bolus and then continuous infusion. After screening for contraindications to fibrinolysis and following discussion with the patient and her family about their preferences, the decision was made to proceed with fibrinolysis for submassive PE. Our recommendation to proceed with fibrinolysis was predicated upon a low risk of bleeding and an increased risk of death from recurrent PE and RV failure with anticoagulation alone. Unfractionated heparin infusion was discontinued and 100 mg of t-PA was administered over two hours. She began having gingival oozing during t-PA administration. This was managed with gauze packing. At about one hour into fibrinolysis, her chest pain and dyspnea abated. After completion of the fibrinolytic infusion and when the aPTT was less than twice the upper limit of normal, unfractionated heparin infusion was restarted without a bolus as a “bridge” to anticoagulation with warfarin. At her two-week office visit, she felt well and had no chest pain or shortness of breath. A follow-up transthoracic echocardiogram six weeks later demonstrated normal pulmonary artery systolic pressures and normal RV size and systolic function.
Acknowledgments
Funding Sources: Dr. Piazza is supported by a Research Career Development Award (K12 HL083786) from the National Heart, Lung, and Blood Institute (NHLBI).
Abbreviations
- BNP
brain-type natriuretic peptide
- CT
computed tomography
- DVT
deep vein thrombosis
- H-FABP
heart-type fatty acid-binding protein
- IVC
inferior vena cava
- LV
left ventricular
- PE
pulmonary embolism
- RV
right ventricular
Footnotes
Conflict of Interest Disclosures: Drs. Piazza and Goldhaber have no conflicts to disclose.
References
- 1.Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Ridker PM. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–1861. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 3.Piazza G, Goldhaber SZ. The acutely decompensated right ventricle: pathways for diagnosis and management. Chest. 2005;128:1836–1852. doi: 10.1378/chest.128.3.1836. [DOI] [PubMed] [Google Scholar]
- 4.Fanikos J, Piazza G, Zayaruzny M, Goldhaber SZ. Long-term complications of medical patients with hospital-acquired venous thromboembolism. Thromb Haemost. 2009;102:688–693. doi: 10.1160/TH09-04-0266. [DOI] [PubMed] [Google Scholar]
- 5.Kucher N, Goldhaber SZ. Management of massive pulmonary embolism. Circulation. 2005;112:e28–32. doi: 10.1161/CIRCULATIONAHA.105.551374. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez O, Trinquart L, Colombet I, Durieux P, Huisman MV, Chatellier G, Meyer G. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29:1569–1577. doi: 10.1093/eurheartj/ehn208. [DOI] [PubMed] [Google Scholar]
- 7.Piazza G, Goldhaber SZ. Acute Pulmonary Embolism, Part II: Treatment and Prophylaxis. Circulation. 2006;114:e42–e47. doi: 10.1161/CIRCULATIONAHA.106.620880. [DOI] [PubMed] [Google Scholar]
- 8.Vanni S, Polidori G, Vergara R, Pepe G, Nazerian P, Moroni F, Garbelli E, Daviddi F, Grifoni S. Prognostic value of ECG among patients with acute pulmonary embolism and normal blood pressure. Am J Med. 2009;122:257–264. doi: 10.1016/j.amjmed.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Piazza G, Goldhaber SZ. Acute Pulmonary Embolism, Part I: Epidemiology and Diagnosis. Circulation. 2006;114:e28–e32. doi: 10.1161/CIRCULATIONAHA.106.620872. [DOI] [PubMed] [Google Scholar]
- 10.Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med. 2008;178:425–430. doi: 10.1164/rccm.200803-459OC. [DOI] [PubMed] [Google Scholar]
- 11.Jimenez D, Uresandi F, Otero R, Lobo JL, Monreal M, Marti D, Zamora J, Muriel A, Aujesky D, Yusen RD. Troponin-based risk stratification of patients with acute nonmassive pulmonary embolism: systematic review and metaanalysis. Chest. 2009;136:974–982. doi: 10.1378/chest.09-0608. [DOI] [PubMed] [Google Scholar]
- 12.Puls M, Dellas C, Lankeit M, Olschewski M, Binder L, Geibel A, Reiner C, Schafer K, Hasenfuss G, Konstantinides S. Heart-type fatty acid-binding protein permits early risk stratification of pulmonary embolism. Eur Heart J. 2007;28:224–229. doi: 10.1093/eurheartj/ehl405. [DOI] [PubMed] [Google Scholar]
- 13.Dellas C, Puls M, Lankeit M, Schafer K, Cuny M, Berner M, Hasenfuss G, Konstantinides S. Elevated heart-type fatty acid-bindng protein levels on admission predict an adverse outcome in normotensive patients with acute pulmonary embolism. J Am Coll Cardiol. 2010 doi: 10.1016/j.jacc.2009.10.078. in press. [DOI] [PubMed] [Google Scholar]
- 14.Goldhaber SZ. Echocardiography in the management of pulmonary embolism. Ann Intern Med. 2002;136:691–700. doi: 10.7326/0003-4819-136-9-200205070-00012. [DOI] [PubMed] [Google Scholar]
- 15.McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78:469–473. doi: 10.1016/s0002-9149(96)00339-6. [DOI] [PubMed] [Google Scholar]
- 16.Fremont B, Pacouret G, Jacobi D, Puglisi R, Charbonnier B, de Labriolle A. Prognostic value of echocardiographic right/left ventricular end-diastolic diameter ratio in patients with acute pulmonary embolism: results from a monocenter registry of 1,416 patients. Chest. 2008;133:358–362. doi: 10.1378/chest.07-1231. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez O, Trinquart L, Caille V, Couturaud F, Pacouret G, Meneveau N, Verschuren F, Roy PM, Parent F, Righini M, Perrier A, Lorut C, Tardy B, Benoit MO, Chatellier G, Meyer G. Prognostic factors for pulmonary embolism: the prep study, a prospective multicenter cohort study. Am J Respir Crit Care Med. 2010;181:168–173. doi: 10.1164/rccm.200906-0970OC. [DOI] [PubMed] [Google Scholar]
- 18.Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110:3276–3280. doi: 10.1161/01.CIR.0000147612.59751.4C. [DOI] [PubMed] [Google Scholar]
- 19.van der Meer RW, Pattynama PM, van Strijen MJ, van den Berg-Huijsmans AA, Hartmann IJ, Putter H, de Roos A, Huisman MV. Right ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3-month follow-up in patients with acute pulmonary embolism. Radiology. 2005;235:798–803. doi: 10.1148/radiol.2353040593. [DOI] [PubMed] [Google Scholar]
- 20.Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest. 2009;136:1202–1210. doi: 10.1378/chest.08-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kearon C, Kahn SR, Agnelli G, Goldhaber SZ, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133:454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 22.Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347:1143–1150. doi: 10.1056/NEJMoa021274. [DOI] [PubMed] [Google Scholar]
- 23.Fiumara K, Kucher N, Fanikos J, Goldhaber SZ. Predictors of major hemorrhage following fibrinolysis for acute pulmonary embolism. Am J Cardiol. 2006;97:127–129. doi: 10.1016/j.amjcard.2005.07.117. [DOI] [PubMed] [Google Scholar]
- 24.Parker JA, Drum DE, Feldstein ML, Goldhaber SZ. Lung scan evaluation of thrombolytic therapy for pulmonary embolism. J Nucl Med. 1995;36:364–368. [PubMed] [Google Scholar]
- 25.Daniels LB, Parker JA, Patel SR, Grodstein F, Goldhaber SZ. Relation of duration of symptoms with response to thrombolytic therapy in pulmonary embolism. Am J Cardiol. 1997;80:184–188. doi: 10.1016/s0002-9149(97)00315-9. [DOI] [PubMed] [Google Scholar]
- 26.Verstraete M, Miller GA, Bounameaux H, Charbonnier B, Colle JP, Lecorf G, Marbet GA, Mombaerts P, Olsson CG. Intravenous and intrapulmonary recombinant tissue-type plasminogen activator in the treatment of acute massive pulmonary embolism. Circulation. 1988;77:353–360. doi: 10.1161/01.cir.77.2.353. [DOI] [PubMed] [Google Scholar]
- 27.Leeper KV, Jr, Popovich J, Jr, Lesser BA, Adams D, Froelich JW, Burke MW, Shetty PC, Thrall JH, Stein PD. Treatment of massive acute pulmonary embolism. The use of low doses of intrapulmonary arterial streptokinase combined with full doses of systemic heparin. Chest. 1988;93:234–240. doi: 10.1378/chest.93.2.234. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Juanatey JR, Valdes L, Amaro A, Iglesias C, Alvarez D, Garcia Acuna JM, de la Pena MG. Treatment of massive pulmonary thromboembolism with low intrapulmonary dosages of urokinase. Short-term angiographic and hemodynamic evolution. Chest. 1992;102:341–346. doi: 10.1378/chest.102.2.341. [DOI] [PubMed] [Google Scholar]
- 29.Goldhaber SZ, Come PC, Lee RT, Braunwald E, Parker JA, Haire WD, Feldstein ML, Miller M, Toltzis R, Smith JL, Taveira da Silva AM, Mogtader A, McDonough TJ. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet. 1993;341:507–511. doi: 10.1016/0140-6736(93)90274-k. [DOI] [PubMed] [Google Scholar]
- 30.Kucher N. Catheter embolectomy for acute pulmonary embolism. Chest. 2007;132:657–663. doi: 10.1378/chest.07-0665. [DOI] [PubMed] [Google Scholar]
- 31.Goldhaber SZ. Percutaneous mechanical thrombectomy for acute pulmonary embolism: a double-edged sword. Chest. 2007;132:363–365. doi: 10.1378/chest.07-0591. [DOI] [PubMed] [Google Scholar]
- 32.Kuo WT, Gould MK, Louie JD, Rosenberg JK, Sze DY, Hofmann LV. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol. 2009;20:1431–1440. doi: 10.1016/j.jvir.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Stein PD, Alnas M, Beemath A, Patel NR. Outcome of pulmonary embolectomy. Am J Cardiol. 2007;99:421–423. doi: 10.1016/j.amjcard.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 34.Leacche M, Unic D, Goldhaber SZ, Rawn JD, Aranki SF, Couper GS, Mihaljevic T, Rizzo RJ, Cohn LH, Aklog L, Byrne JG. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg. 2005;129:1018–1023. doi: 10.1016/j.jtcvs.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Aklog L, Williams CS, Byrne JG, Goldhaber SZ. Acute pulmonary embolectomy: a contemporary approach. Circulation. 2002;105:1416–1419. doi: 10.1161/01.cir.0000012526.21603.25. [DOI] [PubMed] [Google Scholar]
- 36.Decousus H, Leizorovicz A, Parent F, Page Y, Tardy B, Girard P, Laporte S, Faivre R, Charbonnier B, Barral FG, Huet Y, Simonneau G. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338:409–415. doi: 10.1056/NEJM199802123380701. [DOI] [PubMed] [Google Scholar]
- 37.Mismetti P, Rivron-Guillot K, Quenet S, Decousus H, Laporte S, Epinat M, Barral FG. A prospective long-term study of 220 patients with a retrievable vena cava filter for secondary prevention of venous thromboembolism. Chest. 2007;131:223–229. doi: 10.1378/chest.06-0631. [DOI] [PubMed] [Google Scholar]