Abstract
The sensory circuit of the stretch reflex arc, composed of specialized intrafusal muscle fibers and type Ia proprioceptive sensory neurons, converts mechanical information regarding muscle length and stretch to electrical action potentials and relays them to the central nervous system. Utilizing a non-biological substrate, surface patterning photolithography and a serum-free medium formulation a co-culture system was developed that facilitated functional interactions between intrafusal muscle fibers and sensory neurons. The presence of annulospiral wrappings (ASWs) and flower spray endings (FSEs), both physiologically relevant morphologies in sensory neuron-intrafusal fiber interactions, were demonstrated and quantified using immunocytochemistry. Furthermore, two proposed components of the mammalian mechanosensory transduction system, BNaC1 and PICK1, were both identified at the ASWs and FSEs. To verify functionality of the mechanoreceptor elements the system was integrated with a MEMS cantilever device, and Ca2+ currents were imaged along the length of an axon innervating an intrafusal fiber when stretched by cantilever deflection. This system provides a platform for examining the role of this mechanosensory complex in the pathology of myotonic and muscular dystrophies, peripheral neuropathy, and spasticity inducing diseases like Parkinson's. These studies will also assist in engineering fine motor control for prosthetic devices by improving our understanding of mechanosensitive feedback.
Keywords: BNaC1, mechanosensation, DETA, DRG neurons, synapse
Introduction
The ability of multi-cellular organisms to function in cohesive synergy depends on the integration and communication of sensory information throughout the body. One principle type of sensory information is mechanical in nature. Organisms from bacteria and worms to humans have developed mechanisms to detect mechanical force dynamics in the environment and their function plays a role in activities as diverse as development, immunity and fracture repair to balance and hearing [1-6]. Consequently, the vertebrate body has developed several conserved mechanisms for the transduction of mechanical forces to sensory neural impulses, this process is also known as mechanotransduction [7]. These include groups of mechanoreceptors, mainly cutaneous in nature, that respond to touch, pressure and vibration as well as proprioceptors sensitive to changes in muscle length, stretch and tension [8-10]. The activity of this reflex mechanism assists in coordinating a diverse range of muscular activities including eye movement, respiration and fine motor control. Both mechanoreceptors and proprioceptors are composed of specialized receptors innervated by a specific type of sensory neuron [8, 9, 11]. Physical deformation of the receptor results in depolarization of the sensory neuron and action potential generation, by which information is transferred to this important system.
In the case of muscle spindles, a major type proprioceptor, the sensory receptor, is composed of an encapsulated group of specialized muscle fibers referred to as intrafusal fibers. Within the spindle, the intrafusal fibers are of two main types: 1) nuclear bag fibers, so named because their nuclei cluster in a bulging equatorial region, and 2) nuclear chain fibers, so named because their nuclei align linearly next to each other. Several different neuronal types innervate these fibers. The sensory innervation is accomplished by type Ia and type II neurons located in the dorsal root ganglia. These sensory neurons form specialized synapses on intrafusal muscle fibers called annulospiral wrappings (ASWs) (type Ia) and flower-spray endings (FSEs) (type II) [12]. Muscle spindles also receive motor innervation from γ-motoneurons and βmotoneurons. This motor innervation occurs at the polar regions of the intrafusal fibers and functions to maintain the sensitivity of the spindle during extrafusal fiber contraction [13, 14].
Our basic knowledge of these mechanosensory systems comes from years of physiological analysis using vertebrates from frogs and cats to humans [11, 15-17]. However, the molecular components and processes of the vertebrate mechanosensory transduction system are poorly understood due to difficulty in directly observing the sensory structures in vivo. This is due to their sparse and random distribution in the body. Additionally, their encapsulated structure spatially limits electrical and biochemical examination. Therefore, some of the most interesting information gathered regarding the molecular components of the system have come from experiments done in the worm Caenorhabditis elegans [18]. Ultimately, the work resulted in the discovery of one such mechanoreceptive complex, including the identification of a mechanically sensitive ion channel labeled as MEC-4/10 [19-21]. Using a genetic screen, homologous vertebrate sequences were identified and determined to be members of the degenerin/epithelial sodium channel (DEG/ENaC) superfamily [22-24]. These proteins, brain sodium channels 1 and 2 (BNaC1 & BNaC2), form an additional branch of the superfamily and have been shown to localize at mechanosensory terminals of dorsal root ganglion neurons [25-27]. Specifically, BNaC1 is expressed in large diameter sensory neurons and BNaC2 in medium and small diameter sensory neurons [27]. Furthermore, the channels were found localized at specialized mechanosensory terminals like Meissner corpusles and Merkel discs [25]. Also, BNaC1 has been shown to interact with PRKCA 1 (PICK1) a structural protein that anchors ion channels in the membrane and both have been shown to localize with mechanosensory terminals of type Ia sensory neurons [25, 28]. However, further progress in identifying additional functional or structural proteins present in the vertebrate stretch receptor complex has not been successful.
The development of a functional in vitro system capable of supporting the growth and differentiation of both intrafusal fibers and sensory neurons would provide a critical bridge/transition/segue in the study of vertebrate mechanosensory complexes. Furthermore, systems using serum-free, defined medium formulations and nonbiological, uniform growth substrates provide an environment ideal for manipulation of specific factors in order to generate specific cell phenotypes. These systems also provide the added benefit of being readily modified using photolithography to generate patterned adhesive sites for cells. These systems provide the added ease of evaluating the manipulated factor's effect with less concern for the confounding variability associated with systems based on biological substrates and serum containing medium, especially when constructing hybrid biological/non-biological systems.
MEMS devices are mechanical technologies made up of components ranging in size from 1 and 1000 micrometers that can be combined with biological systems to create hybrid devices. These devices are an integration of mechanical and electronic elements on a substrate built using microfabrication technologies. Cantilevers, a type of MEMS device fabricated from silicon wafers, are diving board-like structures organized in an array, which can be used for biosensing or bending detection [29]. For bending detection, the deflection of an optical beam aimed at the free end of the cantilever is monitored by a detector [30]. Previously, it had been shown that myotubes could be integrated with myotubes and the bending caused by myotube contraction, monitored by beam deflection, could be used to calculate myotube contractile force [31].
We previously had shown the ability to grow embryonic skeletal muscle on trimethoxysilylpropyl-diethylenetriamine (DETA) surfaces and direct myotube differentiation towards intrafusal fiber formation [32]. Also, we have shown that the same culture conditions will support the growth and phenotypic development of DRG neurons [33]. The next critical development for the design and engineering of neural and myoskeletal tissue based devices was the formulation of a medium that provided an environment conducive to the growth and differentiation of both cell types simultaneously. This work documents the development of an in vitro system that supports the growth and differentiation of both intrafusal muscle fibers and sensory neurons and has enabled the development of the specialized mechanosensory endings seen in vivo. The system was also integrated with a cantilever MEMS device in order to investigate and confirm the functionality of the immunocytochemically observed connections. The development of the sensory circuit of the stretch reflex arc, as demonstrated by these results, facilitates further analysis of the as yet undefined interacting mechanosensory structures as well as how the mechanical inputs get converted to electrical outputs.
Engineering an in vitro system to study the mechanical interactions between intrafusal muscle fibers and Ia sensory neurons and the subsequent generation of electrical activity is an integral step in building a functional stretch reflex arc in vitro. Such a system would capacitate studies of nerve-muscle and nerve-nerve communication, aiding in the enhanced functioning of prosthetic devices by improving our understanding of mechanosensitive feedback and by unraveling the conversion mechanism between electrical and mechanical signaling in mammalian systems. It would also provide insight into the pathologies of neuromuscular diseases such as infantile spinal muscular atrophy type I (Werdnig-Hoffman disease), peripheral neuropathy, and spasticity inducing diseases like Parkinson's, muscular dystrophy and myasthenia gravis [34-39].
Materials & Methods
DETA surface modification
Glass coverslips (VWR 48366067, 22×22 mm2 No. 1) were first cleaned using 1:1 HC lmethanol followed by a concentrated H2SO4 soak for 2 hours. The DETA (United Chemical Technologies Inc. T2910-KG) modified surface was formed by the reaction of the cleaned surfaces with 0.1% (v/v) mixture of the organosilane in freshly distilled toluene (VWR BDH1151). The cleaned surfaces were heated to about 100°C in the organosilane mixture, rinsed with toluene, reheated to about 100°C in toluene, and then dried in the oven overnight (100°C).
PEG surface modification
Glass coverslips (VWR 48366067, 22×22 mm2 No. 1) were first cleaned using a 1:1 HCl-methanol followed by a concentrated H2SO4 soak for 2 hours. The cantilevers were then coated with a PEG-terminated silane by a modified protocol from Papra et al. [40]. Dry toluene was prepared by distillation over metallic sodium to remove water or any other contaminants. The alkylsilane 2-[Methoxypoly (ethyleneoxy)propyl] trimethoxysilane (Gelest, Tullytown, PA), was added to the toluene at a final concentration of 0.1% by volume in an MBraun glove box (MBraun, Stratham, NH). Concentrated HCl was added to the PEG-toluene solution to a final volume of 0.08 % (0.8 ml HCl/L) and the solution briefly stirred. The cantilevers were then incubated in the PEG-toluene solution for 1 hour at room temperature. After 1 hour the samples were removed and rinsed in serial washes of toluene (1×), ethanol (2×), and diH2O (1×). The washed samples were blown dry under a stream of ultrapure nitrogen and were either used immediately or stored in a desiccator until needed.
Deep-UV photolithography of silane monolayers
Boxed line pattern surface fabrication
Boxed line patterned quartz photomasks were designed using the CleWin layout editor (WieWeb, Hengelo, The Netherlands) and fabricated through a commercial vendor (Bandwidth Foundry Pty Ltd., Australia). The surface of the DETA coated coverslips not covered by the photomask were ablated using a 193 nm Ar/F LPX200i laser (Lambda Physik, Ft. Lauderdale, FL) combined with a beam homogenizer (Microlas, Ft. Lauderdale, FL, Energy density: 50 mJ/cm2) to create the patterns. Samples were placed on the stage of the mask aligner under a 5x5 inch chrome plated photomask, which contained the pattern, to be ablated. The substrate was then brought into contact with the mask to ensure a hard contact. A hard contact was used to minimize the gap between the substrate and mask to enable a high contrast pattern with minimal edge effects due to refraction of the laser light. The ablated regions of the DETA coverslips were then rederivatized with the fluorinated silane tridecafluoro-1,1,2,2-tetrahydroctyl-1-dimethylchlorosilane (13F) as previously described [41, 42].
Deep-UV photolithography of PEG-silane monolayers
PEG-silane modified cantilevers were patterned using deep ultraviolet (DUV) photolithography. The masks were written in dark-field polarity such that the areas corresponding to the ablated pattern were transparent and the remaining areas were opaque. The pattern utilized contained somal adhesion sites for DRG neurons on the off-cantilever region and myoblast adhesion sites in the cantilever region with pathways connecting the two areas. The substrate was precision aligned using the aligner stage to ensure micrometer precision placement of the pattern in reference to the cantilevers. The substrates were then exposed to 193 nm ultraviolet laser light for 45 seconds with a pulse intensity of 200 mJ/pulse and a frequency of 10 Hz. After ablation the patterned PEG-silane substrates were backfilled with the alkylsilane (3-Trimethoxysilyl propyl) diethylenetriamine (DETA). Fresh distilled dry toluene was prepared as discussed previously. DETA was added to the toluene to a final concentration of 0.1% (v:v) inside the glove box. The DETA-toluene solution was removed from the glove box and transferred to a beaker and the samples were immersed in the solution. To drive the reaction forward the solution was gently heated to no more than 65°C for 30 minutes. After reaction with DETA the samples were allowed to cool to room temperature, washed 3 times with dry tolune and heated to 65°C for 30 more minutes.
Palladium-catalyzed metallization of patterned silane monolayers
Patterned samples were visualized using a palladium-catalyzed copper reduction reaction, modified from Kind et al. [43], that specifically deposits metallic copper on regions containing the amine terminated silane DETA. The patterned substrates were immersed in a solution containing 0.8 mM Palladium chloride and 0.6 mM NaCl for 10 minutes. The substrates were then rinsed 3 times in diH20. A solution of 1 part 0.6 M dimethylamiobutyrate (DMAB) and 4 parts diH2O was prepared, and the samples were immersed for 10 minutes. The samples were again washed in diH20, then immersed in 10 ml of a 0.2 M solution of Copper (II) sulfate with 10 ml of formaldehyde (37.2%) until copper deposition was observed. Metallized patterns were imaged and analyzed using light microscopy.
Surface Characterization
Surfaces were characterized by static water contact angle measurements using a Rame-Hart Model 250 goniometer, and by X-ray photoelectron spectroscopy (XPS) using an Escalab 200i spectrometer (VG Scientific) by monitoring the N 1s peak [42, 44, 45]. The values are reported as the mean ± SEM.
Cantilever fabrication
Bio-MEMS cantilevers were fabricated as previously described [30, 31]. Briefly, a four-inch double-sided polished SOI wafer containing a 5 μm thick top layer of crystalline silicon bonded onto a 500 μm thick silicon dioxide layer was photoresist spun and developed in a Suss ACS200 wafer coater/developer. First, the 5 μm layer was primed with 100Å layer of hexamethyldisilazaine (HMDS). Then was placed silicon side up on the resist spinner and the AZ5214e photoresist was applied and spun at 1,900 rpm to a final thickness of 2.1μm and the resist baked at 110°C. Next, the wafer was exposed on the Suss MA6 aligner to the photomask containing the cantilever pattern and the resist developed in a 1:1 mixture of AZ320 and diH2O. Deep reactive ion etching (DRIE) was performed on the wafer in a PlasmaTherm 770 DRIE at a rate of 4 μm/min. The 5 μm device layer was etched through to the silicon dioxide layer and the photoresist was removed using a Metroline M4L plasma resist etcher. Next, the wafer was inverted and the silicon dioxide layer was primed with a 100Å layer of HMDS and then coated with AZ9245 photoresist to a final thickness of 10 μm (1,000 rpm) and soft baked at 100°C for 2 min. The coated wafer was placed in a Suss MA6 aligner and exposed with the mask containing the backside pattern. After exposure, the wafer was immersed in AX400K developer to develop the patterned resist followed by a hard bake at 120°C for 30 min. It was then etched until approximately 50 μm etch depth was left and then mounted on a dummy wafer using Nitto Model 3195V thermal release tape and then the DRIE was completed. The wafer was demounted by heating to 170°C on a hotplate and the photoresist was removed by plasma etching in a Metroline M4L plasma resist etcher. A final etch in 49% hydrofluoric acid for 10 min was performed to remove the buried oxide layer.
Animals
Dated pregnant Sprague-Dawley rats were housed in the animal facility at the University of Central Florida. All research was approved by the Institutional Animal Care and Use Committee at the University of Central Florida and conformed to NIH guidelines. Pregnant rats were anesthetized and sacrificed at embryonic day 14 (for DRG neurons) or 18 (for myocytes) (E14 / E18). The embryos were removed by caesarean section and fetuses dissected under a stereomicroscope (Carl Zeiss, Stemi 2000).
Primary culture of Rattus norvegicus myocytes
Skeletal muscle was removed from the hind limb of E18 rat fetuses, collected in a 15 ml tube in cold Hibernate E + GlutaMAX™ + antibiotic-antimycotic + B27 (dissection medium) and incubated in 0.05% trypsin-EDTA for 45 minutes in a 37°C water bath. Following incubation, the trypsin-EDTA was removed and the cells suspended in dissection media + 10% FBS and the tissue gently triturated. The dissociated cell suspension was then centrifuged at 500g for 10 minutes at 4°C to pellet the cells. Following centrifugation, the supernatant was aspirated and the cells resuspended in dissection media + 10% FBS. Fibroblasts were removed by panning the cell suspension in a 100 mm cell culture dish containing dissection media + 10% FBS for 20 min [46]. After panning, the myocytes were aspirated off the panning dish and then pelleted by centrifugation at 500g for 10 minutes at 4°C [47]. Finally, the supernatant was removed and the myocytes suspended in the serum-free culture medium and a cell count was conducted using the trypan blue method. Myocytes were then plated on DETA coverslips at a density of 600-700 cells/mm2.
Primary culture of Rattus norvegicus sensory neurons from dorsal root ganglia
Dorsal root ganglia (DRGs) were removed from E14 rats and collected in dissection medium. Following dissection, the DRGs were incubated in 0.05% trypsin-EDTA for 15 minutes in a 37°C water bath. Following incubation, the trypsin-EDTA was removed and the cells suspended in dissection media + 10% FBS and the tissue gently triturated. The dissociated cell suspension was then centrifuged at 500g for 10 minutes at 4°C to pellet the cells. Following centrifugation, the supernatant was aspirated and the cells resuspended in the serum-free culture medium and a cell count was conducted using the trypan blue method. DRG cells were then plated on DETA coverslips at a density of 100 cells/mm2.
Preparation BA-G5 alpha cardiac myosin heavy chain
The mouse B lymphocyte hybridoma cell line HB-276 was cultured according to ATCC guidelines [48]. Briefly, cells were grown in DMEM (Gibco 10313-021) + 10% FBS (Gibco 16000-077) or Hybridoma-SFM (Gibco 12045-076) in 75 mm2 tissue culture flasks and placed in an incubator at 37°C and 5% CO2 at a concentration of between 1×105 and 1×106 cells/ml. The medium was changed twice weekly. The BA-G5 antibody is constantly secreted by the cells in culture and was harvested by removing 12 ml of conditioned culture medium, transferring it to a 15 ml tube, followed by centrifugation at 4000g for 15 minutes at 4°C. The antibody containing supernatant was then removed and the concentration quantified using the microBCA method (23235, Pierce). The antibody concentration ranged from 9-12 μg/ml (data not shown) and was used 4 to 1 in blocking solution (see below).
Co-culture assessment: immunocytochemistry and laser scanning confocal microscopy
The co-cultures were fixed in fresh 4% paraformaldehyde in PBS for 10 minutes and then rinsed twice with phosphate buffered saline (PBS). Next, cells were permeabilized with a solution of 0.05% triton-X 100 in PBS + 5% bovine serum albumin (BSA) for 5 minutes, rinsed once with PBS and then blocked with permeabilization solution + 5% donkey serum [49]. The cells were then incubated with primary antibodies diluted in blocking solution overnight at 4°C. The primary antibodies used include: mouse anti-alpha cardiac-like myosin heavy chain (HB-276, ATCC), anti-neurofilament heavy chain (AB5539, Millipore), rabbit anti-ACCN1 (ab65699, Abcam). The next day primary antibody solutions were aspirated and the cells rinsed three times with PBS. Then, donkey AlexaFluor® secondary antibodies (Molecular Probes) diluted 1:200 in blocking solution were added to the cells and incubated for 2 hours at room temperature in the dark. The secondary antibody solution was then aspirated and the coverslips rinsed three times in PBS. Finally, coverslips were mounted on glass slides using VectaShield mounting medium with DAPI (H-1200, Vector Labs) and fixed using clear nail polish.
Calcium imaging of type Ia sensory neuron currents in co-culture with myotubes integrated on cantilevers
Co-cultures were grown for 10-14 days in serum-free medium (Table 1). The calcium sensitive dye, Fluo-4 AM (F14201, Invitrogen), was prepared as described by the manufacturer. Briefly, a 5 mM dye stock was prepared in DMSO, and then mixed 1:1 with DMSO + 20% pluronic F-127 to prepare a 2.5 mM working solution. Next, the working solution was added to the co-cultures for a final concentration of 10 μM and incubated for 30 minutes. The co-cultures were washed twice with fresh medium and placed in the field stimulation chamber in fresh medium. The field stimulator was run using the following parameters to specifically stimulate myotube contraction: 1 Hz, 40 millisecond pulse width and 2 volts peak-to-peak [50]. In the DRG only control experiments, field stimulation did not result in DRG neuronal electrical activity. During field stimulation of the co-cultures, the confocal microscope was set to record video files of Fluo-4 AM conformational change due to spatio-temporal Ca2+ binding.
Table 1.
Co-culture conformity to box line pattern
| Culture Day | Number of Patterns | Conforming Patterns | %Conformity |
|---|---|---|---|
| Day 2 | 56 | 49.03 ± 1.57 | 88 |
| Day 8 | 56 | 38.33 ± 1.39 | 69 |
| Day 16 | 56 | 4.11 ± 0.25 | 8 |
Quantified by counting myotube and Ia sensory neuron conformity to box line pattern using phase contrast microscopy. Data is the average ± SEM of at least five experiments performed on six coverslips / experiment at each time point.
Amiloride blockage of type Ia sensory neuron currents in co-culture
Amiloride hydrochloride is a known blocker of mechanotransduction and has been shown to block BNaC1, the proposed mechanosensitive ion channel [26, 27]. Co-cultures were grown and Ca2+ flux was monitored as previously described [51]. After imaging the Ca2+ flux generated in type Ia sensory neurons in co-culture, amiloride hydrochloride (A7410, Sigma-Aldrich) was added to the bath solution at a final concentration of 500 μM with the field stimulation of the intrafusal muscle fibers still in progress.
Results
DETA surface modification
The aminosilane, trimethoxy-silylpropyl-diethylenetriamine (DETA), functions efficiently as a non-biological substrate due to its self-assembling monolayer properties and the multiple amines contained in the terminal group. This group confers hydrophilic properties to the surface, and that combined with the partial positive change on the amines at physiological pH make it an ideal surface for cellular attachment and survival. It also resembles the structure of spermamin, a growth factor, and this may also be involved in its unique property to support cells in vitro. The system is similar to poly-Dlysine, but has been found to be more robust and consistent in terms of cell attachment and survival [52]. XPS measurements of the DETA coated coverslips indicated a reaction site limited monolayer formed during the self-assembly process. The normalized area values of N 1s (401 and 399 eV) to the Si 2p3/2 peaks were stable throughout the study at 1500 ± 200 and were similar to previously published results [41, 42, 45, 52, 53]. Static contact angle measurements of 44 ± 2° validated the hydrophilicity of the DETA surfaces. Stable XPS readings and contact angles across coverslips throughout the study indicate uniformity and reproducibility of the self-assembly of the DETA monolayer.
Box line pattern surface preparation and cellular pattern conformity
The DETA foreground surrounded by 13F background provided a pattern supporting differential cell adhesion to the surface (Fig. 1A). Two control coverslips were used in order to test the quality of the DETA/13F patterns: (1) one DETA coverslip was ablated without the photomask and rederivatized with 13F, and (2) a second DETA coverslip was rederivatized with 13F only. Laser irradiation and 13F rederivatization were done in the same conditions as for the DETA/13F patterns.
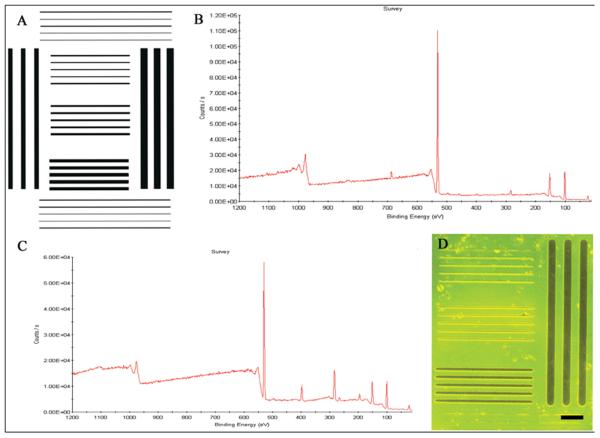
Figure 1. Photomask image and chemical analysis of box line patterns.
(A) CleWin drawing of the box line pattern, (B) XPS survey of an ablated DETA monolayer exposed to 13F solution, (C) XPS survey of a non-ablated DETA monolayer exposed to 13F solution, (D) image of a box line pattern visualized using palladium catalyzed copper reduction metallization (dark bars and lines indicate DETA regions). Scale bar = 90 μm
The XPS measurements of the control coverslips show that 13F was incorporated in the laser exposed DETA control coverslip (Fig. 1B), but it was not incorporated (or only incorporated in trace amounts) in the unexposed DETA control coverslip (Fig. 1C). Further, static water contact angle measurements of 92 ± 2 indicated the hydrophobicity of the laser exposed DETA control coverslip after 13F rederivatization. However, the non-ablated DETA monolayer was not affected by the reaction with 13F, as was revealed by the contact angle values of 45 ± 3 of the unexposed DETA control coverslip; values that are close to the ones for pure DETA.
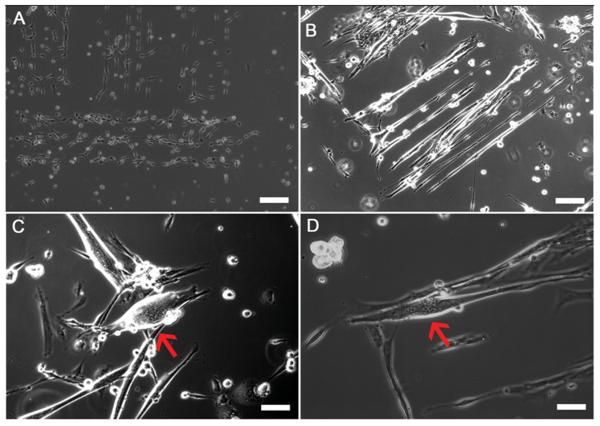
The pattern uniformity was verified by palladium catalyzed copper reduction metallization (Fig. 1D). These results were stable throughout the study indicating the reproducibility of the box line patterns. The cellular conformity to the patterns was also analyzed throughout the study. As shown in Table 1, pattern conformity decreased throughout the time in culture. During Days 0-10, pattern conformity was at its highest, with 49.03 ± 1.57 out of 56 boxes (88%) indicating cells only on the lines (Fig. 2). As the cultures aged, conformity to the pattern decreased with only 4.11 ± 0.25 boxes out of 56 (8%) showing cells conforming to the pattern (Fig. 2).
Figure 2. Phase contrast images of Intrafusal myotubes and DRG sensory neuron in co-culture on box line patterns.
(A) Day 1 image illustrating myocytes conforming to DETA patterns, (B) Day 7 image illustrating developing myotubes conforming to the box line pattern, (C-D) Day 16 images showing developed intrafusal myotubes (red arrows) on box line patterned DETA coverslips. Scale bars = 60 μm.
Cantilevers and PEG-DETA pattern analysis
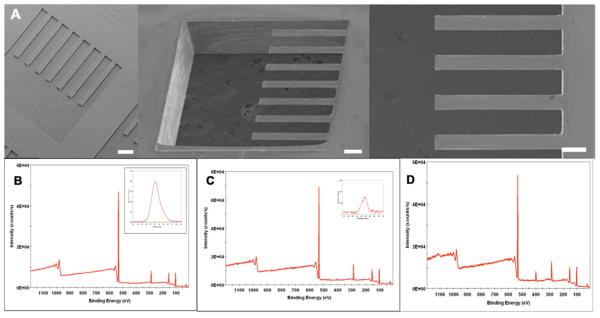
The quality of cantilever fabrication was verified using electron microscopy (Fig. 3). After verification of cantilever fabrication, the devices were exposed to a solution of PEG for SAM formation. As shown in Figure 3, PEG forms a SAM on the cantilever devices (Fig. 3B). The PEG was then laser ablated and DETA was back-filled in the ablated region forming a cell adhesive region on the cantilevers (Fig. 3D). No DETA incorporation into PEG coated regions of the cantilever could be detected (Fig. 3C).
Figure 3. Cantilever analysis and formation of PEG-DETA self-assembling monolayer patterns.
(A) Electron micrograph images of the bio-MEMS cantilevers, (B) XPS survey spectrum of PEG-coated cantilever (insert shows high resolution C1s spectrum), (C) XPS survey spectrum of DETA on PEG-coated cantilever (insert shows high resolution N1s spectrum), (D) XPS survey spectrum of DETA on ablated PEG-coated cantilever.
Serum-free medium and defined system formulation
The basic serum-free medium composition developed in our laboratory for the co-culture of embryonic motoneurons and myocytes was used as a starting point for the development of a medium that enhanced synaptic connectivity between neurons and myotubes in vitro [54-56]. The addition of 10 new growth factors known to play a role in neuromuscular junction formation and synaptic maintenance led to increased recovery and development of neurons and myocytes as well as increased development of synaptic structures in the system [57] (Table 2).
Table 2.
Serum-free medium for enhanced synaptic connectivity in NB4A (500 mL)
| Component | Concentration | Company | Catalog Number |
|---|---|---|---|
| NAP2 | 1 ug/ml | AnaSpec | 61170 |
| h BDNF1 | 20 ng/ml | Cell Sciences | CRB 600B |
| h GDNF1 | 20 ng/ml | Cell Sciences | CRG 400B |
| r CNTF1 | 50 ng/ml | Cell Sciences | CRC 401B |
| h CT-11 | 20 ng/ml | Cell Sciences | CRC 700B |
| NT-31 | 20 ng/ml | Cell Sciences | CRN 500B |
| NT-41 | 20 ng/ml | Cell Sciences | CRN 501B |
| Antibiotic/Antimycotic1 | 10 μl/ml | Invitrogen | 15240-062 |
| aFGF1 | 20 ng/ml | Invitrogen | 13241-013 |
| VEGF 1651 | 20 ng/ml | Invitrogen | P2654 |
| G5 (100×)1 | 2 ul/ml | Invitrogen | 17503-012 |
| Cholesterol2 | 10 ug/ml | Invitrogen | 12531 |
| Laminin2 | 4 ug/ml | Millipore | 08-125 |
| Beta-amyloid2 | 200 ng/ml | Millipore | AG966 |
| h Tenascin-C2 | 200 ng/ml | Millipore | CC065 |
| Recomb. ApoE22 | 100 ng/ml | Panvera | P2002 |
| h β-NGF1 | 10 ng/ml | R&D Systems | 256-GF |
| SHh2 | 100 ng/ml | R&D Systems | 1314-SH |
| Agrin2 | 100 ng/ml | R&D Systems | 550-AG |
| Heparan sulfate1 | 80 ng/ml | Sigma | D9809 |
| Vitronectin1 | 100 ng/ml | Sigma | V0132 |
| LIF1 | 20 ng/ml | Sigma | L5158 |
| TNF-α2 | 20 ng/ml | Sigma | T6674 |
| PDGF2 | 100 ng/ml | Sigma | P4056 |
| VIP2 | 500 ng/ml | Sigma | V6130 |
| IGF-12 | 50 ng/ml | Sigma | I2656 |
component of original medium 1
component of original medium 2
Immunocytochemical evaluation of Ia sensory neuron endings on intrafusal fibers
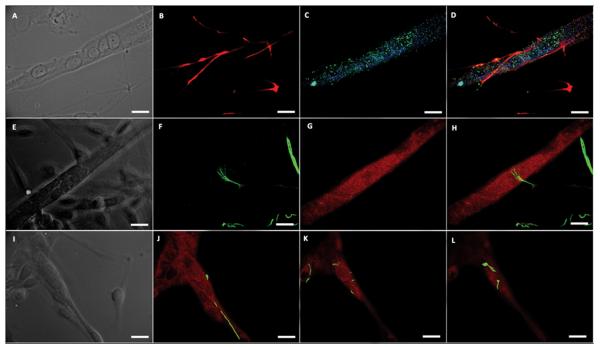
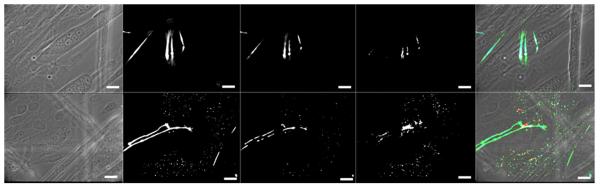
The synapses formed between Ia sensory neurons and intrafusal fibers are morphologically distinct (unique) structures [13]. Annulospiral wrappings (ASWs) and flower-spray endings (FSEs) terminate on intrafusal fibers in vivo and form a mechanosensitive complex capable of relaying muscle stretch information to spinal cord motoneurons. In order to determine if the co-culture of sensory neurons and skeletal muscle would result in the formation of ASWs and/or FSEs, cultures were evaluated using immunocytochemistry (Figure 4). Additionally, in order to evaluate the rapidity of Ia sensory neuron innervation in this system, the cultures were evaluated at three different time points (Table 3). As shown in Figure 4, ASWs are evident on nuclear chain fibers as shown by sensory neuron axon wrapping around the intrafusal fiber (Fig. 4A-D). Flower-spray endings were also observed in the co-culture as shown by axonal “flower-spray” termination morphology upon contact with the equatorial region of the nuclear chain fiber (Fig. 4E-H). Annulospiral wrappings were also observed on nuclear bag fibers as shown by the Z-stack slices of an axon wrapping around an intrafusal fiber and with termination near the equatorial region (Fig. 4I-L). As shown in Table 3, a small number of ASWs and FSEs were observed at six days in culture, 7% and 5% respectively. As the cultured aged, the number of endings increased, reaching their maximal values at day 23 with 18% ASWs and 15% FSEs.
Figure 4. Ia sensory neuron annulospiral and flower-spray endings on intrafusal myotubes.
(A) phase contrast image of nuclear chain fiber, (B) neurofilament-H (NF-H) illustrating classic annulospiral wrapping morphology (C) Mcadherin (green) and alpha cardiac-like MHC (blue) staining verifying nuclear chain fiber phenotype, (D) composite image, (E) phase contrast image of a nuclear bag fiber, (F) NF-H displaying classic flower-spray morphology (G) alpha cardiac-like MHC staining verifying nuclear bag fiber phenotype, (H) composite image, (I) phase contrast image of nuclear bag fiber, (J-L) rising slice confocal stack showing a Ia sensory neuron axon (NF-H – green) wrapping around a nuclear bag fiber (alpha cardiac-like MHC – red) to form an annulospiral wrapping. Scale bars = 50 μm.
Table 3.
Annulospiral and flower-spray endings on box line patterns
| Culture Day | # of Patterns | #ASEs | % ASEs | # of FSE | % of FSE |
|---|---|---|---|---|---|
| Day 6 | 56 | 4.00 ± 0.41 | 07 | 3.00 ± 0.71 | 05 |
| Day 16 | 56 | 8.50 ± 1.06 | 15 | 7.25 ± 0.63 | 13 |
| Day 23 | 56 | 10.09 ± 0.36 | 18 | 8.59 ± 0.32 | 15 |
Quantified by counting the number of respective ending morphologies on box line patterned coverslips using immunocytochemistry and laser scanning confocal microscopy. Data is the mean ± SEM of at least three experiments performed on four coverslips / experiment at each time point.
Immunocytochemical evaluation of the mechanosensory complex
In order for the action potential generation to occur in the mechanosensitive terminals, stretch sensitive ion channels located in Ia sensory neurons must open, thereby depolarizing the axolemma. Due to the localization of BNaC1 to the nerve terminals of mechanosensitive skin structures as well as the channels sensitivity to amiloride and the attenuation of stretch activated spindle firing by amiloride, the ion channel's presence at ASWs and FSEs was investigated in vitro [58]. Using immunocytochemistry, clusters of BNaC1 ion channels were shown to localize at the terminals of ASWs and FSEs (Fig. 5A-J). We also evaluated the localization of PICK1 with BNaC1 at the proposed mechanosensory ASWs of the type Ia sensory neurons.
Figure 5. BNaC1 and PICK1 localize to Ia sensory neuron annulospiral and flower-spray endings.
(A-D) localization at an annulospiral ending, (A) phase contrast image, (B) NF-H illustrating annulospiral wrapping, (C) PICK1 localized at the superior surface wrapping, (D) BNaC1 localized at the superior surface wrappings, (E) color composite image, (F-I) localization at a flower-spray ending, (F) phase contrast image, (G) NF-H illustrating flower-spray ending, (H) PICK1 localized at the flower-spray terminal, (I) BNaC1 localized at the flower-spray terminal, (J) color composite image. Scale bars = 50 μm.
As shown in Figure 5, the annulospiral wrappings associated with intrafusal fibers contain BNaC1 and PICK1 colocalized in the axonal terminals (Fig. 5A-E). Additionally, BNaC1 and PICK1 colocalized with flower-spray endings terminating on intrafusal myotubes (Fig. 5F-J).
Calcium imaging of type Ia sensory neuron currents in co-culture on cantilevers
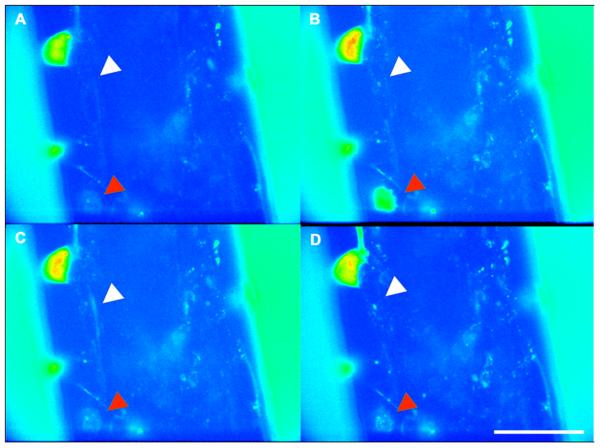
Electrical signals generated in the different processes of the neurons results in spatio-temporal calcium flux. Due to the fact that Ca2+ flux has been shown to be important for muscle spindle afferent activity [51], Ca2+ dynamics in this model system was investigated in order to evaluate the functionality of the neurons in co-culture using Fluo-4 AM dye. Fluo-4 AM reversibly binds to intracellular Ca2+ resulting in a conformational change that with an excitation emission spectra of 488nm / 516nm. We hypothesized that a change in length of the intrafusal fibers due to contraction would result in electrical activity by the innervating type Ia sensory neuron axon that could be visualized as a spatio-temporal Ca2+ flux. Using the Fluo-4 AM dye, we visualized electrical activity in real time, and in this case, the type Ia sensory neuron currents generated as a result of intrafusal fiber innervation. Using field stimulation, intrafusal muscle fibers contracted and the subsequent Ca2+ flux moving along a type Ia sensory neuron axon resulting from innervation was visualized (Fig. 6) (Vid. 1). Conversely, in control experiments conducted with only DRG neurons no Ca2+ flux could be observed, suggesting that field stimulation in the absence of myotubes does not result in any form of electrical activity with the type Ia sensory neurons.
Figure 6. Ca2+ flux in a type Ia sensory neuron generated after field stimulation of intrafusal myotubes on cantilevers.
(A-D) time course images in sequence from the beginning to the end the Ca2+ wave traveling through a type Ia sensory neuron. (red arrowheads indicate location of the soma) (white arrowheads indicate location of the axon) Scale bars = 50 μm.
Amiloride attenuation of Ca2+ flux imaging in type Ia sensory neurons
After establishing that field stimulation resulted in intrafusal myotube activity and Ca2+ currents in type Ia sensory neurons, we hypothesized that the activity was generated by mechanically gated ion channels present located at the site of sensory neurons innervation of the intrafusal myotubes. Amiloride hydrochloride is a potent inhibitor of members of the degenerin/epithelial sodium channel (DEG/ENaC) superfamily. The mechanosensitive ion channel BNaC1, a member of the DEG/ENaC family has been shown to be sensitive to amiloride. After amiloride injection into the system, Ca2+ electrical activity in the sensory neuron ceased even while field stimulation of the intrafusal fibers continued, suggesting the functional connection between the two elements had been blocked (Vid. 2).
Discussion
The formulation of a set of conditions resulting in the development of annulospiral wrappings and flower-spray endings on intrafusal muscle fibers in vitro is a critical step in tissue engineering the stretch reflex arc as well as muscle repair and regeneration. In this work, we showed that temporal application of growth factors in NBActiv4 medium resulted in the development of annulospiral wrappings, indicative of type 1a sensory neuron innervations, and flower-spray, for type II sensory neuron innervation, endings using morphological and immunocytochemical evaluation criteria. Furthermore, we were able to identify the proteins BNaC1 and PICK1 localized to the terminals of type Ia sensory neuron ASWs and FSEs, both components of the proposed mammalian mechanosensory complex and demonstrated the functionality of these connections using Ca2+ imaging to visualize ion currents in the sensory neurons after intrafusal myotube stretch deformation. Subsequently, the stretch sensitive ion channels were blocked with amiloride and an attenuation of the Ca2+ flux was observed indicating a functional connection between the sensory neurons and the intrafusal myotubes.
The utility of each growth factor present in the medium formulation was derived from previous experiments done by our group and others and was developed for the purpose of enhancing neuromuscular junction (NMJ) development. Due to the similarities between traditional NMJs and the terminations made by type Ia sensory neurons on intrafusal muscle fibers (ASWs and FSEs), we hypothesized the same growth factor cocktail would support their development [59]. Additionally, similarities in myoblast origin, myosin heavy chain isoforms expression and encapsulation of the fibers further supported the idea that similar developmental cues would facilitate the formation of ASWs and FSEs [60-63]. Essentially, both ASWs and FSEs are alternative types of neuromuscular junctions; in this manuscript, mechanosensory junctions formed between sensory neurons and intrafusal muscle fibers.
The use of photolithography and surface chemistry modification to pattern cytophilic and cytophobic regions on glass coverslips resulted in distinct cell adhesion areas and helped direct cell-cell interactions. Pattern conformity decreased over time in culture, primarily due to myotube overgrowth from the DETA regions of the pattern area onto the 13F regions. Regardless, the initial patterning enhanced localized interaction between sensory neurons and intrafusal muscle fibers, resulting in easily quantified ASWs and FSEs. One advantage of surface chemistry modification is the potential to use a variety of silanes for cell repulsion at plating. One silane in particular, polyethylene glycol (PEG), has been shown to minimize protein adhesion while maintaining biocompatibility and work done in our lab suggests it might also enhance cellular pattern conformity [64, 65].
The observation that BNaC1 and PICK1 colocalize at the sensory neuron terminals associated with intrafusal myotubes demonstrates the utility of this system to investigate basic developmental questions regarding the formation of the mammalian mechanosensory complex. Consequently, this in vitro system will be a useful tool for identifying additional components of the mechanosensory complex. This is due to the ease of access to both individual intrafusal fibers and their associated axonal endings.
For example, while the presence of proteins linking the stretch activity of the intrafusal fibers to the mechanically sensitive ion channels in the sensory neurons is postulated, these proteins have yet to be identified. It also provides for an efficient way to analyze electrical impulses generated in the sensory neurons caused by stretch deformation of the intrafusal fiber. Also, by integrating this system with a cantilever micro-electrical mechanical systems (MEMS) device, it would be possible to determine how intrafusal fiber stretching due to measurable cantilever deflection is converted to electrical impulses in the type Ia sensory neurons.
Building on our DRG+ESM model grown on patterned coverslips, the integration of the sensory circuit of the stretch reflex arc on bio-MEMS cantilevers represents a significant step towards understanding the physiology of this most basic of neuromuscular circuits. The Ca2+ flux imaging of sensory neuron electrical activity after field stimulation of intrafusal fibers and flux attenuation after amiloride treatment strongly suggests a functional innervation between the neuron and myotube. When combined with the immunocytochemistry data, these results indicate the development of the sensory circuit of the stretch reflex arc on a MEMS cantilever device. This is significant because it provides for an efficient way to analyze electrical impulses generated in the sensory neurons caused by stretch deformation of the intrafusal fiber. Also, by integrating this system with a cantilever micro-electrical mechanical systems (MEMS) device, it is possible to quantify how intrafusal fiber stretch due to measurable cantilever deflection is converted to electrical impulses in the type Ia sensory neurons. It will also be useful in understanding muscle repair and regeneration in tissue engineering applications and for wound repair by providing a functional in vitro test-bed for understanding how different growth factors and putative drug candidates enhance this process.
Conclusion
In this report we document the development of a tissue-engineered system resulting in physiologically relevant interactions between Ia sensory neurons and intrafusal muscle fibers on patterned DETA coverslips and MEMS cantilever devices. Using photolithographic patterning, the formation of annulospiral wrapping and flower-spray endings were documented and quantified. Furthermore, two components of the proposed mechanosensory system, the stretch sensitive ion channel BNaC1 and the membrane support protein PICK1, were also shown to be localized at the Ia sensory neuron terminals interacting with the intrafusal fibers. Ca2+ flux in Ia sensory neurons following intrafusal fiber stimulation was visualized and then blocked using the stretch sensitive ion channel blocker amiloride verifying the formation of physiologically relevant endings. This engineered system provides a platform to investigate the mechanosensory activity of muscle spindles in a controlled and reproducible environment. This system is an integral component of an in vitro model of the stretch reflex arc, which has applications in functional prosthetic device design and the study of spasticity inducing diseases such as Parkinson's, muscular dystrophy and myasthenia gravis as well as muscle repair and regeneration.
Supplementary Material
Ca2+ flux in a type Ia sensory neuron generated after field stimulation of an intrafusal myotube on a cantilever.
Ca2+ flux attenuation after amiloride blockage of the mechanically sensitive ion channels in a DRG+ESM co-cultures on cantilever.
Acknowledgements
We would like to acknowledge that support for this research was provided by the University of Central Florida and NIH Grant no. 5 R01 NS 050452. We confirm that any aspect of the work covered in this manuscript that has involved experimental animals has been conducted with the ethical approval of all relevant bodies. We would also like to thank Dr. Stephen Lambert for his feedback and guidance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kendal-Wright CE. Stretching, mechanotransduction, and proinflammatory cytokines in the fetal membranes. Reprod Sci. 2007;14:35–41. doi: 10.1177/1933719107310763. [DOI] [PubMed] [Google Scholar]
- 2.Kloda A, Martinac B. Mechanosensitive channels in archaea. Cell Biochem Biophys. 2001;34(3):349–381. doi: 10.1385/CBB:34:3:349. [DOI] [PubMed] [Google Scholar]
- 3.Makino A, Shin HY, Komai Y, Fukuda S, Coughlin M, Sugihara-Seki M, et al. Mechanotransduction in leukocyte activation: A review. Biorheology. 2007;44(4):221–249. [PubMed] [Google Scholar]
- 4.Martinac B, Kloda A. Evolutionary origins of mechanosensitive ion channels. Prog Biophys Mol Biol. 2003;82(1-3):11–24. doi: 10.1016/s0079-6107(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 5.Morgan EF, Gleason RE, Hayward LNM, Leong PL, Palomares KTS. Mechanotransduction and fracture repair. J Bone Joint Surg Am. 2008;90(Supplement_1):25–30. doi: 10.2106/JBJS.G.01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vollrath MA, Kwan KY, Corey DP. The micromachinery of mechanotransduction in hair cells. Annu Rev Neurosci. 2007;30(1):339–365. doi: 10.1146/annurev.neuro.29.051605.112917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kloda A, Martinac B. Common evolutionary origins of mechanosensitive ion channels in archaea bacteria and cell-walled eukarya. Archaea. 2002;1(1):35–44. doi: 10.1155/2002/419261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt CC. Mammalian muscle spindle: Peripheral mechanisms. Physiol Rev. 1990;70(3):643–663. doi: 10.1152/physrev.1990.70.3.643. [DOI] [PubMed] [Google Scholar]
- 9.Hunt CC, Ottoson D. Initial burst of primary endings of isolated mammalian muscle spindles. J Neurophysiol. 1976;39(2):324–330. doi: 10.1152/jn.1976.39.2.324. [DOI] [PubMed] [Google Scholar]
- 10.Hunt CC, Wilkinson RS, Fukami Y. Ionic basis of the receptor potential in primary endings of mammalian muscle spindles. J Gen Physiol. 1978;71(6):683–698. doi: 10.1085/jgp.71.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt CC, Ottoson D. Responses of primary and secondary endings of isolated mammalian muscle spindles to sinusoidal length changes. J Neurophysiol. 1977;40(5):1113–1120. doi: 10.1152/jn.1977.40.5.1113. [DOI] [PubMed] [Google Scholar]
- 12.Schroder JM, Bodden H, Hamacher A, Verres C. Scanning electron microscopy of teased intrafusal muscle fibers from rat muscle spindles. Muscle Nerve. 1989;12(3):221–232. doi: 10.1002/mus.880120311. [DOI] [PubMed] [Google Scholar]
- 13.Kucera J, Walro JM, Reichler J. Innervation of developing intrafusal muscle fibers in the rat. Am J Anat. 1988;183(4):344–358. doi: 10.1002/aja.1001830408. [DOI] [PubMed] [Google Scholar]
- 14.Kucera J, Walro JM, Reichler J. Motor and sensory innervation of muscle spindles in the neonatal rat. Anat Embryol (Berl) 1988;177(5):427–436. doi: 10.1007/BF00304740. [DOI] [PubMed] [Google Scholar]
- 15.Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol. 2007;581(3):1271–1282. doi: 10.1113/jphysiol.2007.129197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunt CC, Wilkinson RS. An analysis of receptor potential and tension of isolated cat muscle spindles in response to sinusoidal stretch. J Physiol. 1980;302(1):241–262. doi: 10.1113/jphysiol.1980.sp013240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Querfurth H. Action-potential initiation and maintained activity of the isolated frog muscle spindle. Eur J Neurosci. 2006;24(4):1147–1156. doi: 10.1111/j.1460-9568.2006.04983.x. [DOI] [PubMed] [Google Scholar]
- 18.Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature. 2001;413(6852):194–202. doi: 10.1038/35093011. [DOI] [PubMed] [Google Scholar]
- 19.Bianchi L. Mechanotransduction: Touch and feel at the molecular level as modeled in caenorhabditis elegans. Mol Neurobiol. 2007;36(3):254–271. doi: 10.1007/s12035-007-8009-5. [DOI] [PubMed] [Google Scholar]
- 20.Tavernarakis N, Driscoll M. Molecular modeling of mechanotransduction in the nematode caenorhabditis elegans. Annu Rev Physiol. 1997;59(1):659–689. doi: 10.1146/annurev.physiol.59.1.659. [DOI] [PubMed] [Google Scholar]
- 21.Tavernarakis N, Driscoll M. Mechanotransduction in caenorhabditis elegans. Cell Biochem Biophys. 2001;35(1):1–18. doi: 10.1385/CBB:35:1:01. [DOI] [PubMed] [Google Scholar]
- 22.Driscoll M, Chalfie M. The mec-4 gene is a member of a family of caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature. 1991;349(6310):588–593. doi: 10.1038/349588a0. [DOI] [PubMed] [Google Scholar]
- 23.Emtage L, Gu G, Hartwieg E, Chalfie M. Extracellular proteins organize the mechanosensory channel complex in c. Elegans touch receptor neurons. Neuron. 2004;44(5):795–807. doi: 10.1016/j.neuron.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Lai CC, Hong K, Kinnell M, Chalfie M, Driscoll M. Sequence and transmembrane topology of mec-4, an ion channel subunit required for mechanotransduction in caenorhabditis elegans. J Cell Biol. 1996;133(5):1071–1081. doi: 10.1083/jcb.133.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duggan A, Garcia-Anoveros J, Corey D. The pdz domain protein pick1 and the sodium channel bnac1 interact and localize at mechanosensory terminals of dorsal root ganglion neurons and dendrites of central neurons. J Biol Chem. 2002;277(7):5203–5208. doi: 10.1074/jbc.M104748200. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Anoveros J, Derfler B, Neville-Golden J, Hyman BT, Corey DP. Bnac1 and bnac2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci USA. 1997;94(4):1459–1464. doi: 10.1073/pnas.94.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Anoveros J, Samad TA, Zuvela-Jelaska L, Woolf CJ, Corey DP. Transport and localization of the deg/enac ion channel bnac1{alpha} to peripheral mechanosensory terminals of dorsal root ganglia neurons. J Neurosci. 2001;21(8):2678–2686. doi: 10.1523/JNEUROSCI.21-08-02678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hruska-Hageman AM, Wemmie JA, Price MP, Welsh MJ. Interaction of the synaptic protein pick1 (protein interacting with c kinase 1) with the non-voltage gated sodium channels bnc1 (brain na+ channel 1) and asic (acid-sensing ion channel) Biochem J. 2002;361(3):443–450. doi: 10.1042/0264-6021:3610443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fritz J, Baller MK, Lang HP, Rothuizen H, Vettiger P, Meyer E, et al. Translating biomolecular recognition into nanomechanics. Science. 2000;288:316–318. doi: 10.1126/science.288.5464.316. [DOI] [PubMed] [Google Scholar]
- 30.Das M, Wilson K, Molnar P, Hickman JJ. Differentiation of skeletal muscle and integration of myotubes with silicon microstructures using serum-free medium and a synthetic silane substrate. Nat Protoc. 2007;2(7):1795–1801. doi: 10.1038/nprot.2007.229. [DOI] [PubMed] [Google Scholar]
- 31.Wilson K, Das M, Wahl KJ, Colton RJ, Hickman JJ. Measurement of contractile stress generated by cultured muscle on silicon cantilevers. PLoS One. 2010;5(6):e11042. doi: 10.1371/journal.pone.0011042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rumsey JW, Das M, Kang JF, Wagner R, Molnar P, Hickman JJ. Tissue engineering intrafusal fibers: Dose and time dependent differentiation of nuclear bag fibers in a defined in vitro system using neuregulin 1-β-1. Biomaterials. 2008;29:994–1104. doi: 10.1016/j.biomaterials.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Rumsey JW, Molnar P, Das M, Gregory C, Riedel L, et al. Electrophysiological and immunocytochemical characterization of drg neurons on an organosilane surface in serum free medium. In Vitro Cell Dev Biol. 2008;44:162–168. doi: 10.1007/s11626-008-9097-x. [DOI] [PubMed] [Google Scholar]
- 34.Dietz V. Gait disorder in spaciticity and parkinson's disease. In: Ruzieka E, Hallett M, Jankovic J, editors. Gait disorders: Advance in neurology. Lippincott, Williams and Wilkins; Philadelphia: 2001. pp. 143–154. [Google Scholar]
- 35.Kararizou E, Manta P, Kalfakis N, Gkiatas K, Vassilopoulos D. Morphological and morphometrical study of human muscle spindles in werdnig-hoffmann disease (infantile spinal muscular atrophy type i) Acta Histochemica. 2006;108(4):265–269. doi: 10.1016/j.acthis.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Kararizou EG, Manta P, Kalfakis N, Gkiatas KA, Vassilopoulos D. Morphologic and morphometrical study of the muscle spindle in muscular dystrophy. Anal Quant Cytol Histol. 2007;29(3):148–152. [PubMed] [Google Scholar]
- 37.Lunn MR, Wang CH. Spinal muscular atrophy. Lancet. 2008;371(9630):2120–2133. doi: 10.1016/S0140-6736(08)60921-6. [DOI] [PubMed] [Google Scholar]
- 38.Saito M, Tomonaga M, Narabayashi H. Histochemical study of the muscle spindles in parkinsonism, motor neuron disease and myasthenia. J Neurol. 1978;219(4):261–271. doi: 10.1007/BF00312979. [DOI] [PubMed] [Google Scholar]
- 39.Swash M, Fox KP. The pathology of the muscle spindle in duchenne muscular dystrophy. J Neurol Sci. 1976;29(1):17–32. doi: 10.1016/0022-510x(76)90077-0. [DOI] [PubMed] [Google Scholar]
- 40.Papra A, Gadegaard N, Larsen NB. Characterization of ultrathin poly(ethylene glycol) monolayers on silicon substrates. Langmuir. 2001;17(5):1457–1460. [Google Scholar]
- 41.Stenger DA, Georger JH, Dulcey CS, Hickman JJ, Rudolph AS, Nielsen TB, et al. Coplanar molecular assemblies of aminoalkylsilane and perfluorinated alkylsilane - characterization and geometric definition of mammalian-cell adhesion and growth. J Am Chem Soc. 1992;114(22):8435–8442. [Google Scholar]
- 42.Stenger DA, Hickman JJ, Bateman KE, Ravenscroft MS, Ma W, Pancrazio JJ, et al. Microlithographic determination of axonal/dendritic polarity in cultured hippocampal neurons. J Neurosci Methods. 1998;82(2):167–73. doi: 10.1016/s0165-0270(98)00047-8. [DOI] [PubMed] [Google Scholar]
- 43.Kind H, Bittner AM, Cavalleri O, Kern K, Greber T. Electroless deposition of metal nanoislands on aminothiolate-functionalized au(111) electrodes. J Phys Chem B. 1998;102(39):7582–7589. [Google Scholar]
- 44.Spargo BJ, Testoff MA, Nielsen TB, Stenger DA, Hickman JJ, Rudolph AS. Spatially controlled adhesion, spreading, and differentiation of endothelial cells on self-assembled molecular monolayers. Proc Natl Acad Sci U S A. 1994;91(23):11070–11074. doi: 10.1073/pnas.91.23.11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stenger DA, Pike CJ, Hickman JJ, Cotman CW. Surface determinants of neuronal survival and growth on self-assembled monolayers in culture. Brain Res. 1993;630(1-2):136–47. doi: 10.1016/0006-8993(93)90651-3. [DOI] [PubMed] [Google Scholar]
- 46.Kuhl U, Timpl R, von der Mark K. Synthesis of type iv collagen and laminin in cultures of skeletal muscle cells and their assembly on the surface of myotubes. Dev Biol. 1982;93(2):344–54. doi: 10.1016/0012-1606(82)90122-1. [DOI] [PubMed] [Google Scholar]
- 47.Daniels MP, Lowe BT, Shah S, Ma JX, Samuelson SJ, Lugo B, et al. Rodent nerve-muscle cell culture system for studies of neuromuscular junction development: Refinements and applications. Microsc Res Tech. 2000;49(1):26–37. doi: 10.1002/(SICI)1097-0029(20000401)49:1<26::AID-JEMT4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 48.Rudnicki MA, Jackowski G, Saggin L, McBurney MW. Actin and myosin expression during development of cardiac muscle from cultured embryonal carcinoma cells. Dev Biol. 1990;138(2):348–358. doi: 10.1016/0012-1606(90)90202-t. [DOI] [PubMed] [Google Scholar]
- 49.Daniels MP. Localization of actin, beta-spectrin, 43 × 10(3) mr and 58 × 10(3) mr proteins to receptor-enriched domains of newly formed acetylcholine receptor aggregates in isolated myotube membranes. J Cell Sci. 1990;97(Pt 4):615–26. doi: 10.1242/jcs.97.4.615. [DOI] [PubMed] [Google Scholar]
- 50.Wilson K, Molnar P, Hickman JJ. Integration of functional myotubes with a bio-mems device for non-invasive interrogation. Lab Chip. 2007;7:920–922. doi: 10.1039/b617939h. [DOI] [PubMed] [Google Scholar]
- 51.Fischer M, Schafer SS. Effects of the calcium antagonist nifedipine on the afferent impulse activity of isolated cat muscle spindles. Brain Res. 2002;954(2):256–276. doi: 10.1016/s0006-8993(02)03353-x. [DOI] [PubMed] [Google Scholar]
- 52.Schaffner AE, Barker JL, Stenger DA, Hickman JJ. Investigation of the factors necessary for growth of hippocampal neurons in a defined system. J Neurosci Methods. 1995;62(1-2):111–9. doi: 10.1016/0165-0270(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 53.Ravenscroft MS, Bateman KE, Shaffer KM, Schessler HM, Jung DR, Schneider TW, et al. Developmental neurobiology implications from fabrication and analysis of hippocampal neuronal networks on patterned silane- modified surfaces. J Am Chem Soc. 1998;120(47):12169–12177. [Google Scholar]
- 54.Copray S, Liem R, Mantingh-Otter IJ, Brouwer N. Coculture of rat embryonic proprioceptive sensory neurons and myotubes. Muscle Nerve. 1996;19(11):1401–1412. doi: 10.1002/(SICI)1097-4598(199611)19:11<1401::AID-MUS4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 55.Das M, Rumsey JW, Gregory CA, Bhargava N, Kang JF, Molnar P, et al. Embryonic motor neuron-skeletal muscle co-culture in a defined system. Neuroscience. 2007;146:481–488. doi: 10.1016/j.neuroscience.2007.01.068. [DOI] [PubMed] [Google Scholar]
- 56.Murphy RA, Singer RH, Saide JD, Pantazis NJ, Blanchard MH, Byron KS, et al. Synthesis and secretion of a high molecular weight form of nerve growth factor by skeletal muscle cells in culture. Proc Natl Acad Sci USA. 1977;74(10):4496–5000. doi: 10.1073/pnas.74.10.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Das M, Rumsey JW, Bhargava N, Stancescu M, Hickman JJ. A defined long-term in vitro tissue engineered model of neuromuscular junctions. Biomaterials. 2010;31:4880–4888. doi: 10.1016/j.biomaterials.2010.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simon A, Banks RW, Bewick GS. Proc Physiol Soc 10. University of Leeds; 2008. Attenuation of stretch-activated discharge of rat muscle spindle afferents by enac channel inhibitors. [Google Scholar]
- 59.Iino S, Kobayashi S, idaka H. Neurocalcin-immunopositive nerve terminals in the muscle spindle, golgi tendon organ and motor endplate. Brain Res. 1998;808(2):294–299. doi: 10.1016/s0006-8993(98)00750-1. [DOI] [PubMed] [Google Scholar]
- 60.Kucera J, Walro JM. Origin of intrafusal muscle fibers in the rat. Histochemistry. 1990;93(6):567–580. doi: 10.1007/BF00272199. [DOI] [PubMed] [Google Scholar]
- 61.Kucera J, Walro JM, Gorza L. Expression of type-specific mhc isoforms in rat intrafusal muscle fibers. J Histochem Cytochem. 1992;40(2):293–307. doi: 10.1177/40.2.1552171. [DOI] [PubMed] [Google Scholar]
- 62.Soukup T, Pedrosa-Domellof F, Thornell LE. Expression of myosin heavy chain isoforms and myogenesis of intrafusal fibres in rat muscle spindles. Microsc Res Tech. 1995;30(5):390–407. doi: 10.1002/jemt.1070300506. [DOI] [PubMed] [Google Scholar]
- 63.Walro JM, Kucera J. Why adult mammalian intrafusal and extrafusal fibers contain different myosin heavy-chain isoforms. Trends Neurosci. 1999;22(4):180–184. doi: 10.1016/s0166-2236(98)01339-3. [DOI] [PubMed] [Google Scholar]
- 64.Tessmar JK, Göpferich AM. Customized peg-derived copolymers for tissue-engineering applications. Macromol Biosci. 2007;7(1):23–39. doi: 10.1002/mabi.200600096. [DOI] [PubMed] [Google Scholar]
- 65.Zhang M, Li XH, Gong YD, Zhao NM, Zhang XF. Properties and biocompatibility of chitosan films modified by blending with peg. Biomaterials. 2002;23(13):2641–2648. doi: 10.1016/s0142-9612(01)00403-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ca2+ flux in a type Ia sensory neuron generated after field stimulation of an intrafusal myotube on a cantilever.
Ca2+ flux attenuation after amiloride blockage of the mechanically sensitive ion channels in a DRG+ESM co-cultures on cantilever.