Introduction
Nerve conduction velocity (NCV) is a reproducible measure of peripheral nerve function used to assess and diagnose human neurological disease ranging from heavy metal toxicity (15), metabolic diseases (16), inflammation (24), chemotherapy (13) and genetic disorders (22). This technique is also used to examine experimental neuropathy based on methods established in human patients and miniaturized for mice (31).
Our laboratory examines the mechanisms driving diabetic neuropathy (DN), a debilitating complication of type 1 and 2 diabetes (7). Using multiple techniques including behavior, anatomical changes and NCV, we compared the development of DN in multiple models of diabetes in mice (30). We surveyed over 20 years of literature regarding the methods and models used to examine DN (31). Of the 65 papers surveyed in 2007, over one-third used NCV as a primary measure of DN.
Multiple factors affect NCV beyond the disease condition being studied, including ambient temperature, needle placement, the intensity of the electrical stimulus and the degree and type of anesthesia. NCV in humans is performed while the patient is awake and responsive. This is usually not the case for animal experiments. By definition, anesthesia slows or blocks nerve impulses and affects synaptic transmission and neuronal function (1, 2, 27). We examined four commonly used anesthesia methods including isoflurane (ISO), pentobarbital (PB), ketamine/xylazine (KX) and 2,2,2-tribromoethanol (TBE) and documented their effects on NCV in the C57Bl6/J mouse. We report significant reductions in NCV following PB, KX and TBE induced anesthesia and significant animal mortality with TBE. Comparing across reagents, ISO had the least effect on NCV and surface temperature and was well tolerated by the mice.
Materials and Methods
Mice
Male C57Bl/6 mice (n=40) were purchased from The Jackson Laboratory (Bar harbor, ME) at 12 weeks of age. The animals were provided standard mouse chow (Lab diet 5001, Purina Mills inc.; Gray Summit, MO), had free access to food and water and were maintained on a 12:12 hour light-dark cycle. Mice were randomly assigned to one of four groups receiving the following anesthesia, isoflurane (ISO), ketamine/xylazine (KX), pentobarbital sodium (PB) and 2-2-2 tribromoethanol (TBE), n=10 per group. All animal experiments were performed in compliance with the University Committee on Use and Care of Animals at the University of Michigan.
Anesthesia and Physiological Parameters
The experimental protocol and dosages are presented in Table 1. Onset of anesthesia was judged by diminish righting reflex and decreased pedal withdrawal (3-5, 9, 28, 35, 36). Physiological parameters were recorded during the first 5 min of anesthesia. The ambient room temperature was maintained at 22° C. Monitoring included skin temperature (ear, proximolateral hind limb, tail and dorsal foot) by infrared thermometer (Fluke 63, Everett, WA), core body temperature (Cole-Parmer Instrument Company, Vernon Hills, IL), and cardiopulmonary variables (oxygen saturation, heart rate, and respiratory rate) using a pulse oximeter (Mouse Ox; Starr Life Sciences Corp, Oakmont, PA).
Table 1.
| Group (n=10) | Drug Dosage (12wk, 24wk) | Source |
|---|---|---|
| Isoflurane (ISO) | 4–5% for induction 1–2% for maintenance |
Hospira, Inc., Lake Forest, IL |
| Ketamine/Xylazine (KX) | Ketamine 100mg/kg Xylazine 5mg/kg |
Fort Dodge Animal Health, Fort Dodge, IA/Ben Venue Laboratories; Bedford, OH |
| Pentobarbital (PB) | 50mg/kg | Ovation Pharmaceutical Inc., Deerfield, IL |
| 2-2-2 tribromoethanol (TBE) |
200mg/kg | Sigma-Aldrich, St. Louis, MO |
All drugs were administrated by intraperitoneal injection with the exception of isoflurane.
Nerve Conduction Studies
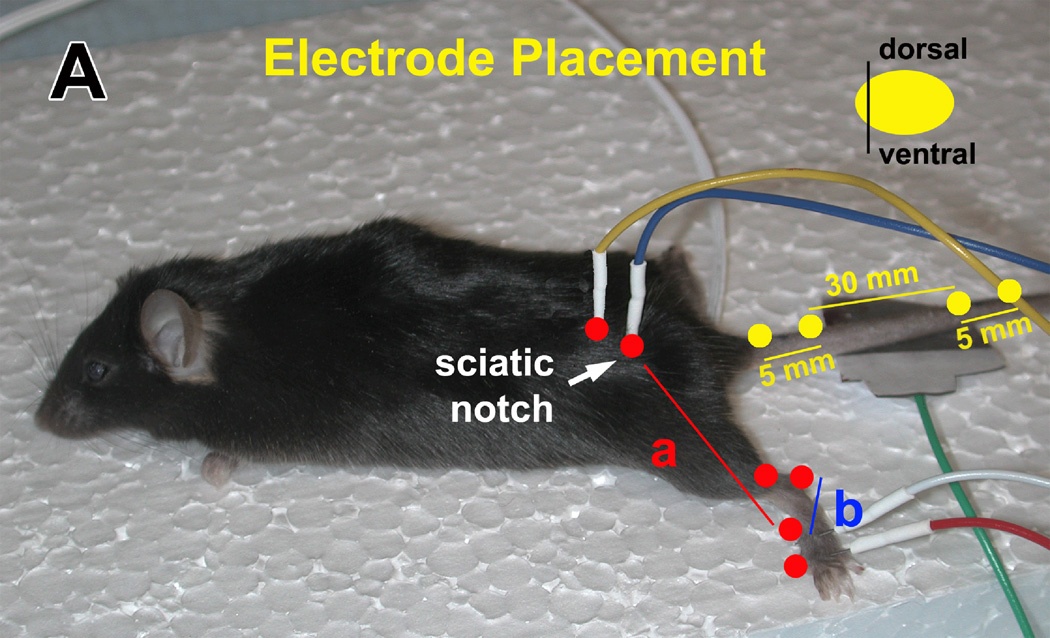
Measures of NCV were performed per our published protocols (30). Mice were anesthetized and core temperature maintained at 34° C with a heating lamp. The platinum needle electrodes (ViaSys, Madison, WI) were cleaned with 70% alcohol between animals. Tail sensory NCV (TSNCV) was determined by orthodromically stimulating the proximal 30 mm segment of tail. NCV was calculated by dividing the distance in mm by the take-off latency (measured in msec) of the sensory nerve action potential (Fig. 1). Tail motor distal latency (TDML) was determined by orthodromically stimulating the proximal 30 mm segment of the tail. Latency was measured from initial onset of the compound muscle action potential. Sural sensory NCV (SNCV) was determined by recording at the dorsum of the foot and antidromically stimulating with supramaximal stimulation at the ankle (Fig. 1). NCV was calculated by dividing the distance by the take-off latency of the sensory nerve action potential. Sciatic-tibial motor NCV (SMNCV) was determined by recording at the dorsum of the foot and orthodromically stimulating with supramaximal stimulation first at the ankle, then at the sciatic notch (Fig. 1). Latencies were measured in each case from the initial onset of the compound muscle action potential. The sciatic-tibial motor NCV was calculated by subtracting the measured ankle distance from the measured notch distance. The resultant distance was then divided by the difference in the ankle and notch latencies for a final nerve conduction velocity.
Figure 1.
The recording/stimulating electrodes in the tail (yellow dots) are placed 30 mm apart. In the sciatic nerve, the recording electrode is placed in the dorsum of the foot (b, red dots) and the stimulating electrode in the ankle and sciatic notch (a, red dots). A reference electrode is placed 5 mm distal from the recording/stimulating electrodes.
Liver Function
Following NCV measurements at 24 weeks, mice were euthanized by intraperitoneal injection of a sodium pentobarbital overdose (Fatal-plus; Dearborn, MI). Blood samples were collected by vena puncture, placed in 1.5 ml eppendorf tubes in and maintained at 22° C for 20 minutes followed by centrifugation at 10,000rpm (9.3 rcf) for 15min. The serum was collected and frozen in liquid nitrogen and stored at −80° C until analyzed for aspartate transaminase (AST) and alanine transaminase (ALT) activity.
Statistics
ANOVA test was performed on the data using a null hypothesis set at .05. A bonferoni post hoc test was used to compare all columns. We assumed a Gaussian distribution of the data.
Results
Nerve Conduction Studies
Motor and sensory NCV were measured in the tail and hind limb. The expected increase in both motor and sensory NCV with age was noted in the hind limb and tail and was not affected by anesthesia. Sensory NCV measured in the hind limb and tail was not differentially affected by any of the anesthetics (Table 2). A significant decrease in SMNCV was detected in the KX and TBE groups at 12 weeks and the PB, KX and TBE groups at 24 weeks of age compared to ISO (Table 2). Additionally, there was a significant increase in latency in the TDML at 12 weeks in the TBE group compare to the ISO group (Table 2).
Table 2.
NCV following anesthesia
| Age | 12 weeks ISO |
PB | KX | TBE | 24 weeks ISO |
PB | KX | TBE |
|---|---|---|---|---|---|---|---|---|
| SNCV (m/sec) |
24.8±1.8 n=10 |
23.7±3.9 n=10 |
23.6±2.4 n=9 |
22.5±2.0 n=10 |
27.6±1.4 n=8 |
26.7±2.1 n=9 |
26.8±1.5 n=8 |
26.8±2.0 n=6 |
| SMNCV (m/sec) |
56.3±7.5 n=9 |
51.7±9.2 n=10 |
44.7±4.2* n=10 |
43.1±3.5* n=10 |
62.9±5.7 n=8 |
49.6±4.6* n=9 |
52.4±4.6* n=8 |
49.7±5.2* n=6 |
| TSNCV (m/sec) |
32.1±1.4 n=9 |
30.6±1.3 n=10 |
32.6±1.2 n=9 |
31.2±2.1 n=10 |
33.0±1.9 n=6 |
30.6±4.1 n=7 |
31.9±2.5 n=7 |
30.0±.7 n=5 |
| TDML msec |
1.7±.1 n=10 |
1.9±.1 n=9 |
1.9±.1 n=9 |
1.9±.1* n=10 |
1.8±.2 n=7 |
1.9±.1 n=9 |
1.8±.2 n=8 |
2.0±.1 n=5 |
Motor and sensory NCV were measured in the sural, sciatic-tibial and caudal tail nerves. The type of anesthesia did not result in significant differences in SMNCV or TSNCV. ± SD, ISO = Isoflurane, PB = Pentobarbital, KX = Ketamine/Xylazine, TBE = 2-2-2 tribromoethanol.
p < 0.05 compared to ISO.
Surface and Core Temperature
Peripheral nerves lie close to the surface; therefore, we assessed the effects of anesthesia on surface temperature. When compared to ISO, mice anesthetized with KX exhibited a significantly lower surface temperature measured at the ear, dorsal foot and hind limb at 12 weeks of age (Table 3). This effect was also observed in the hind limb at 24 weeks of age (Table 3). At 24 weeks of age, PB, KX and TBE treated animals exhibited significantly decreased surface temperature measured at the hind limb (Table 3). Core temperature measured in mice at 24 weeks of age demonstrated similar effects of anesthesia as surface measures with significant decreases in animals treated with PB, KX and TBE (Table 3).
Table 3.
Surface and Core Temperature
| Age | 12 weeks ISO |
PB | KX | TBE | 24 weeks ISO |
PB | KX | TBE |
|---|---|---|---|---|---|---|---|---|
| Ear | 29.1±1.0 n = 8 |
28.0±2.2 n = 10 |
26.9±1.3* n = 10 |
27.8±.7 n=10 |
29.8±1.7 n=7 |
27.7±.4 n=9 |
27.6±1.5 n=9 |
28.5±.6 n=6 |
| Tail | 23.7±.6 n = 8 |
22.8±1.1 n = 10 |
23.6±1.4 n = 10 |
23.4±.4 n=10 |
25.7±4.2 n=7 |
24.4±3.5 n=9 |
23.9±.6 n=9 |
23.6±.7 n=6 |
| Foot | 24.2±.6 n = 8 |
22.3±.9* n = 10 |
22.9±1.1* n = 10 |
23.9±.5 n=10 |
23.9±.4 n=7 |
23.0±.7 n=9 |
23.8±1.0 n=9 |
23.4±1.0 n=6 |
| Hind Limb |
25.0±.4 n = 8 |
23.2±.5* n = 10 |
23.2±1.0* n = 10 |
24.1±.5 n=10 |
27.3±1.0 n=7 |
24.5±1.2* n=8 |
24.3±.9* n=8 |
24.1±.8* n=5 |
| Core | NA | NA | NA | NA | 35.0±.5 n=8 |
31.2±1.0 n=9 |
29.6±2.0 n=9 |
31.5±.7 n=6 |
The effect of anesthetic agents on surface temperature measured on the ear, dorsal hind paw, tail and lateral hind limb and rectal core temperature. For each drug temperatures were monitored at 12 week and 24 weeks of age. ± SD, ISO = Isoflurane, PB = Pentobarbital, KX = Ketamine/Xylazine, TBE = 2-2-2 tribromoethanol.
p < 0.05 compared to ISO.
Cardiopulmonary Function
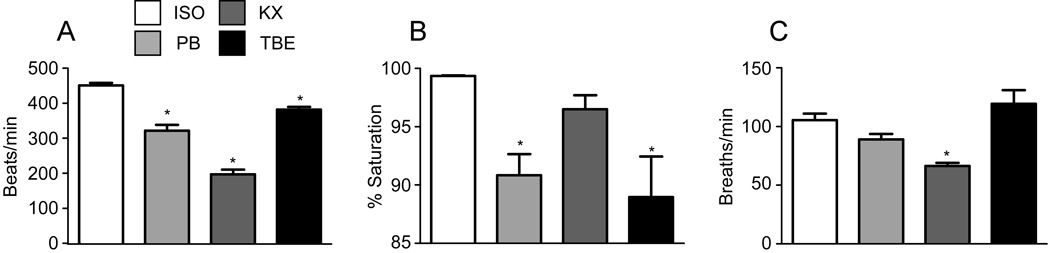
Anesthesia depresses heart function and blood flow (26) which may affect both surface temperature and potentially blood flow to the peripheral nervous system. We assessed the effects of ISO, PB, KX and TBE on heart rate, arterial oxygen saturation and respiratory rate. Compared to ISO anesthesia (451.1±7.109), heart rate (HR) was significantly reduced by PB (321.7±16.28), KX (197.2±13.46) and TBE (381.7±8.125) (Fig 2a). Oxygen saturation was also significantly decreased by PB (90.85±1.8) and TBE (88.97±3.5) compared to ISO (99.36±.04) (Fig. 2b). The method of anesthesia also had an impact on respiratory rate. Compared to ISO, KX anesthesia significantly decreased respiratory rate (Fig. 2c).
Figure 2.
The effects of anesthesia on cardiopulmonary function (heart rate, arterial oxygen saturation and respiratory rate). All parameters were measured under anesthesia in mice 24 weeks of age and within 5 min of anesthesia. * = p < 0.05
Liver Function and Mortality
When performed carefully, NCV may be measured at multiple time points throughout the developmental time course of a disease. Therefore, any long term toxicity may affect both the disease under study and independently affect NCV. We assessed liver toxicity following the two doses of anesthesia. Serum activities of aspartate transaminase (AST) and alanine transaminase (ALT) were measured (data not shown) to determine the effect of anesthetic agents on liver function. All four agents, at the recommended dosage, did not cause significant changes and were within the reference levels (32). Increased animal mortality was observed following treatment with TBE (6, 38). Five of these animals developed peritonitis and died (6, 38). Five other mice, two from the ISO group, one from the PB group and two from the KX group died while anesthetized.
Discussion
We compared the effects of ISO, KX, PB and TBE on motor and sensory NCV. We also documented their effects on surface and core temperature, heart and respiratory rates and oxygen saturation and performed simple tests of liver function. We determined that motor NCV was decreased following anesthesia induced by KX and TBE compared to ISO, while sensory NCV was consistent. Surface temperature in the hind limb, heart rate and oxygen saturation were also affected by the different modes of anesthesia.
Age related normative data for motor and sensory nerve conduction velocities are available for human patients; unfortunately, this type of information is not widely available for mice. Osuchowski et al 2009 and Xia et al 2010 recently published normative data for female ICR and male and female Swiss Jim Lambert/Jackson (SJL/L) mice respectively (21, 37). These values were established with the induction of experimental neuropathy. This type of information is available as control data in reports of experimental neuropathy. The establishment of strain, age and gender specific normative values could be obtained from published values; however, these comparisons are limited by a lack of technical detail in many reports including the type and dosage of anesthesia (31).
A direct comparison of awake and ISO anesthesia was conducted by Osuchowski et al. For awake measures, animals were restrained and the hind limb treated with lidocaine 10 min prior to needle insertion. Anesthetized time zero was defined as immediately following induction of anesthesia (21). The authors report that anesthetized time zero NCV is identical to waking values and report a greater than 20% reduction in NCV 5 min following ISO treatment (21). Even with the application of lidocaine, animal restraint is stressful and in most animal models of disease could contribute more to mortality than short term anesthesia. We consistently collect NCV data within the first 5 min following the onset of anesthesia and though these values are potentially decreased compared to waking animals, they are consistent within experimental groups and across multiple experiments (23, 30, 33, 34).
The C57Bl/6J mouse is the most commonly used models of human disease and is frequently used as a model of type 1 diabetes. In conjunction with our own studies of DN, we reported average values of 38 m/sec for sciatic motor NCV in control C57Bl/6J mice under KX anesthesia (30, 33). These data are similar to control data published by a number of investigators in the field of DN using C57Bl/6J mice and KX anesthesia. Values range from 47 m/sec (10, 18) to 34.8 m/sec (19, 20). KX anesthesia of varying doses was used in all of these studies; however, we contend that differences in NCV are likely due to the age of the animals. While the duration of diabetes and treatment are included, many reports provide animal weight (usually 25–30 g) or state that they are mature. Data presented in this study document an increase in sural sensory NCV and sciatic motor NCV from 12 to 24 weeks of age in agreement with previous reports of increased velocity in rats, dogs and humans (12, 17, 29).
Surface temperature may be another source of variability. In our own work and the studies mentioned above, surface temperatures are maintained between 32 and 37° C usually via warming light or heating pad. We examined the effect of anesthesia on surface temperature measured at the ear and dorsum of the foot. These measures were relatively stable but decreased by anesthesia at the proximal hind limb with the exception of ISO. Temperature is an important factor with regard to NCV (11). The peripheral nerves most often analyzed including the sural, tibial and caudal tail, lie close to the surface and are therefore susceptible to changes in ambient room temperature. Clinical guides report that a decrease of 1° C may result in a 1 m/sec reduction in velocity (11). The reverse is also true and temperatures exceeding 45° C lead to conduction block (25). This sensitivity is also observed in rodents. Surface temperature is relatively easy to control by monitoring the temperature in the room and warming the animal with a light source. Anesthesia often lowers core body temperature which may in turn affect surface temperature; however, we were able to maintain a consistent surface temperature to record accurate NCV.
Anesthesia also has profound effects on cardiopulmonary function (1, 2, 6, 8, 26, 27). Changes in surface blood flow may affect surface temperature and changes in cardiac output may also affect nerve blood flow. Our data revealed a significant reduction in heart rate following anesthesia induced with PB, KX and TBE and significantly reduced arterial oxygen saturation with PB and TBE. Surprisingly, these reductions were not reflected by reductions in sensory NCV but are paralleled by reduced motor NCV in mice at 24 weeks of age. We conclude that cardiopulmonary function does not have a significant impact on NCV within the short duration of these experiments.
The use of anesthetics for rodent survival surgery is well documented (3–5, 32, 38). For these procedures, the animals are often anesthetized for periods longer than half an hour and KX is the preferred anesthesia (3–5, 32, 38). It is not, however, without side-effects including lymphocyte injury (32), hypotension (5) and elevated blood glucose (4). This last point is especially relevant to studies of DN. Both KX and TBE cause a significant and sustained (60 min) increase in blood glucose (4). To examine potential toxicity or procedural problems, the mice were anesthetized and NCV measured at 2 time points, 12 and 24 weeks of age. This is typical for studies documenting the longitudinal progression of DN. We did not detect any negative effects of multiple measures of NCV. Indeed, we detected an often reported increase in NCV associated with maturation. Liver enzymes, aspartate transaminase (AST) and alanine transaminase (ALT), were within the normal range for mice indicating that the two brief drug exposures did not have any lasting effects on liver function. Animals were more likely to die following treatment with TBE than the other three agents tested. Previous reports regarding the use of TBE describe increase apoptosis within the spleen and liver and necrotic lesions of the abdominal wall (14, 32, 38) Similar to these studies, peritonitis developed in four of the ten mice in the original group and they died within a week of anesthesia. The toxic effects of TBE toxicity may be related to a breakdown product, dibromoacetaldhyde.
Due to the increase in circulating blood glucose, decreased oxygen saturation and increased mortality following TBE administration, we would discourage its use for any rodent study. KX anesthesia is safe, effective and relatively easy to administer for procedures of longer duration, e.g. survival surgery; however, its effects on blood glucose should be considered in models of diabetic complications. PB is similar to KX with no effect on blood glucose but significantly decreases oxygen saturation. All of the injectable anesthetics require time to take effect and often require supplemental dosing leading to accidental overdose. ISO anesthesia is fast, easily monitored, has the least impact on NCV, surface and core temperatures and cardiopulmonary function and the least toxic effects. We highly recommend its use for short term procedures such as measures of NCV in all models of peripheral nerve disease.
Acknowledgements
The authors wish to acknowledge Ms. Judith Boldt for expert manuscript preparation and Janet Hoff for expert assistance regarding measures of cardiopulmonary function. This work was supported by the National Institutes of Health (NIH 2 PO1 AG020591-06A1, NIH UO1 DK076160, NIH T32 NS07222 and T32-AG000114) and the Program for Neurology Research and Discovery (www.pfund.umich.edu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antkowiak B. Different actions of general anesthetics on the firing patterns of neocortical neurons mediated by the GABA(A) receptor. Anesthesiology. 1999;91:500–511. doi: 10.1097/00000542-199908000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Antkowiak B. How do general anaesthetics work? Naturwissenschaften. 2001;88:201–213. doi: 10.1007/s001140100230. [DOI] [PubMed] [Google Scholar]
- 3.Arras M, Autenried P, Rettich A, Spaeni D, Rulicke T. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adnerves effets and anesthesia depth. Comp Med. 2001;51:443–456. [PubMed] [Google Scholar]
- 4.Brown ET, Umino Y, Loi T, Solessio E, Barlow R. Anesthesia can cause sustained hyperglycemia in C57/BL6J mice. Vis Neurosci. 2005;22:615–618. doi: 10.1017/S0952523805225105. [DOI] [PubMed] [Google Scholar]
- 5.Buitrago S, Martin TE, Tetens-Woodring J, Belicha-Villanueva A, Wilding GE. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J Am Assoc Lab Anim Sci. 2008;47:11–17. [PMC free article] [PubMed] [Google Scholar]
- 6.Chu DK, Jordan MC, Kim JK, Couto MA, Roos KP. Comparing isoflurane with tribromoethanol anesthesia for echocardiographic phenotyping of transgenic mice. J Am Assoc Lab Anim Sci. 2006;45:8–13. [PubMed] [Google Scholar]
- 7.Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: Mechanisms to management. Pharmacol Ther. 2008;120:1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart CY, Burnett JC, Jr., Redfield MM. Effects of avertin versus xylazine-ketamine anesthesia on cardiac function in normal mice. Am J Physiol Heart Circ Physiol. 2001;281:H1938–H1945. doi: 10.1152/ajpheart.2001.281.5.H1938. [DOI] [PubMed] [Google Scholar]
- 9.Henke J, Astner S, Brill T, Eissner B, Busch R, Ekrhardt W. Comparative study of three intramuscular anesthetic combinations (medetomidine/ketamine, medetomidine/fentanyl/midazolam and xylazine/ketamine) in rabbits. Vet Anaesth Analg. 2003;32:261–270. doi: 10.1111/j.1467-2995.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- 10.Kellogg AP, Pop-Busui R. Peripheral nerve dysfunction in experimental diabetes is mediated by cyclooxygenase-2 and oxidative stress. Antioxid.Redox Signal. 2005;7:1521–1529. doi: 10.1089/ars.2005.7.1521. [DOI] [PubMed] [Google Scholar]
- 11.Kimura J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice. Philadelphia: F.A. Davis Company; 1989. [Google Scholar]
- 12.Lang HA, Puusa A, Hynninen P, Kuusela V, Jantti V, Sillanpaa M. Evolution of nerve conduction velocity in later childhood and adolescence. Muscle Nerve. 1985;8:38–43. doi: 10.1002/mus.880080108. [DOI] [PubMed] [Google Scholar]
- 13.Levy MH, Chwistek M, Mehta RS. Management of chronic pain in cancer survivors. Cancer J. 2008;14:401–409. doi: 10.1097/PPO.0b013e31818f5aa7. [DOI] [PubMed] [Google Scholar]
- 14.Lieggi CC, J.D. F, R.A. K, V. S, Anderson JA, Brown CE, Artwohl JE. An evaluation of preparation methods and storage conditions of tribromoethanol. Contemporary Topics in Laboratory Animal Medicine. 2005;44:11–16. [PubMed] [Google Scholar]
- 15.London Z, Albers JW. Toxic neuropathies associated with pharmaceutic and industrial agents. Neurol Clin. 2007;25:257–276. doi: 10.1016/j.ncl.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Mahmood D, Singh BK, Akhtar M. Diabetic neuropathy: therapies on the horizon. J Pharm Pharmacol. 2009;61:1137–1145. doi: 10.1211/jpp/61.09.0002. [DOI] [PubMed] [Google Scholar]
- 17.Malone JI, Lowitt S, Korthals JK, Salem A, Miranda C. The effect of hyperglycemia on nerve conduction and structure is age dependent. Diabetes. 1996;45:209–215. doi: 10.2337/diab.45.2.209. [DOI] [PubMed] [Google Scholar]
- 18.Ng TF, Lee FK, Song ZT, Calcutt NA, Lee AY, Chung SS, Chung SK, Ng DT, Lee LW. Effects of sorbitol dehydrogenase deficiency on nerve conduction in experimental diabetic mice. Diabetes. 1998;47:961–966. doi: 10.2337/diabetes.47.6.961. [DOI] [PubMed] [Google Scholar]
- 19.Obrosova IG, Li F, Abatan OI, Forsell MA, Komjati K, Pacher P, Szabo C, Stevens MJ. Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes. 2004;53:711–720. doi: 10.2337/diabetes.53.3.711. [DOI] [PubMed] [Google Scholar]
- 20.Obrosova IG, Mabley JG, Zsengeller Z, Charniauskaya T, Abatan OI, Groves JT, Szabo C. Role for nitrosative stress in diabetic neuropathy: evidence from studies with a peroxynitrite decomposition catalyst. FASEB J. 2005;19:401–403. doi: 10.1096/fj.04-1913fje. [DOI] [PubMed] [Google Scholar]
- 21.Osuchowski MF, Teener J, Remick D. Noninvasive model of sciatic nerve conduction in healthy and septic mice: reliability and normative data. Muscle Nerve. 2009;40:610–616. doi: 10.1002/mus.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pareyson D, Marchesi C. Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neurol. 2009;8:654–667. doi: 10.1016/S1474-4422(09)70110-3. [DOI] [PubMed] [Google Scholar]
- 23.Pop-Busui R, Sullivan KA, Van Huysen C, Bayer L, Cao X, Towns R, Stevens MJ. Depletion of taurine in experimental diabetic neuropathy: implications for nerve metabolic, vascular, and functional deficits. Experimental Neurology. 2001;168:259–272. doi: 10.1006/exnr.2000.7591. [DOI] [PubMed] [Google Scholar]
- 24.Ramchandren S, Lewis RA. Chronic neuropathies - chronic inflammatory demyelinating neuropathy and its variants. Front Neurol Neurosci. 2009;26:12–25. doi: 10.1159/000212313. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg PH, Heavner JE. Temperature-dependent nerve-blocking action of lidocaine and halothane. Acta Anaesthesiol Scand. 1980;24:314–320. doi: 10.1111/j.1399-6576.1980.tb01555.x. [DOI] [PubMed] [Google Scholar]
- 26.Roth DM, Swaney JS, Dalton ND, Gilpin EA, Ross J., Jr. Impact of anesthesia on cardiac function during echocardiography in mice. Am J Physiol Heart Circ Physiol. 2002;282:H2134–H2140. doi: 10.1152/ajpheart.00845.2001. [DOI] [PubMed] [Google Scholar]
- 27.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 28.Saha DC, Saha AC, Malik G, Astiz ME, Rackow EC. Comparison of cardiovascular effects of tiletamine-zolazepam, pentobarbital and ketamine-xylazine in male rats. J Am Assoc Lab Anim Sci. 2007;46:74–80. [PubMed] [Google Scholar]
- 29.Sims MH, Redding RW. Maturation of nerve conduction velocity and the evoked muscle potential in the dog. Am J Vet Res. 1980;41:1247–1252. [PubMed] [Google Scholar]
- 30.Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI, Brosius F, 3rd, Feldman EL. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28:276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan KA, Lentz SI, Roberts JL, Jr., Feldman EL. Criteria for creating and assessing mouse models of diabetic neuropathy. Curr Drug Targets. 2008;9:3–13. doi: 10.2174/138945008783431763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson JS, Brown SA, Khurdayan V, Zeynalzadedan V, Sullivan PG, Scheff SW. Early effects of tribromoethanol, ketamine/xylazine, pentobarbital and isoflurane anesthesia on hepatic and lymphoid tissue in ICR mice. Comp Med. 2002;52:63–67. [PubMed] [Google Scholar]
- 33.Vincent AM, Russell JW, Sullivan KA, Backus C, Hayes JM, McLean LL, Feldman EL. SOD2 protects neurons from injury in cell culture and animal models of diabetic neuropathy. Exp Neurol. 2007;208:216–227. doi: 10.1016/j.expneurol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiggin TD, Kretzler M, Pennathur S, Sullivan KA, Brosius FC, Feldman EL. Rosiglitazone Treatment Reduces Diabetic Neuropathy in STZ Treated DBA/2J Mice. Endocrinology. 2008 doi: 10.1210/en.2008-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wixson SK, White WJ, Hughes HC, Jr., Lang CM, Marshall WK. The effects of pentobarbital, fentanyl-droperidol, ketamine-xylazine and ketamine-diazepam on arterial blood pH, blood gases, mean arterial blood pressue and heart rate in adult male rats. Lab Anim Sci. 1987;37:736–742. [PubMed] [Google Scholar]
- 36.Wixson SK, White WJ, Hughes HC, Jr., Lang CM, Marshall WK. The effects of pentobarbital, fentanyl-droperidol, ketamine-xylazine and ketamine-diazepam on core and surface body tmperautre regulation in adult male rats. Lab Anim Sci. 1987;37:743–749. [PubMed] [Google Scholar]
- 37.Xia RH, Yosef N, Ubogu EE. Dorsal caudal tail and sciatic motor nerve conduction studies in adult mice: Technical aspects and normative data. Muscle Nerve. doi: 10.1002/mus.21588. [DOI] [PubMed] [Google Scholar]
- 38.Zeller W, Meier G, Burki K, Panoussis B. Adverse effects of tribromoethanol as used in the production of transgenic mice. Lab Anim. 1998;32:407–413. doi: 10.1258/002367798780599811. [DOI] [PubMed] [Google Scholar]