Abstract
Current literature suggests that serotonin (5-HT) release within the ventral horn of the spinal cord plays a role in motor function. We hypothesized that endogenous 5-HT release is involved in the recovery of motor function after spinal cord injury (SCI). In order to appreciate the functional parameters of regenerating serotonergic fibers, a microdialysis probe was stereotactically implanted in the ventral horn of sub-hemilesioned rats. Microdialysis in combination with HPLC was used to measure concentrations of 5-HT in the lumbar ventral horn during periods of rest (90 min), treadmill run (60 min) and post-exercise rest (90 min) for a one-month time period of recovery following the surgical lesion. Within the same period of time, 5-HT levels varied significantly. A significant (202%) increase was observed at day 18 post-lesion relative to day 8 and, a 16.4% decrease was observed at day 34 relative to day 18. Treadmill exercise challenge induced a 10% decrease of 5-HT release relative to rest at days 18 and 34. In conclusion, over-time treadmill locomotor recovery is parallel to amounts (rest basal levels) and patterns (exercise and post-exercise levels) of 5-HT release suggesting that changes in serotonergic system occurred within the same time frame than locomotor recovery using treadmill challenge.
Keywords: 5-HT, motor function, spinal cord injury, ventral horn, treadmill exercise
INTRODUCTION
This study addresses the question of whether or not 5-HT release improvement within the ventral horn is relevant to hindlimb locomotor movement recovery after SCI. Serotonergic pathways arise from medullary raphe nuclei. They project through the ventrolateral funiculus (VLF) and ventral funiculus (VF) ending within the ventral horn and, some making contact onto motoneurons (MN) (Basbaum et al., 1988; Holstege and Kuypers, 1987; Sharma et al., 1997; Skagerberg and Bjorklund, 1985). Several other publications stress the direct role of 5-HT modulation in motor activity such as, (1) its activation in the commissural region, (2) its variations in release within the ventral horn related to locomotor activity (Gerin et al., 1994; Schwartz et al., 2005), (3) its increased release within descending serotonergic pathways in the lumbar spinal cord correlated to treadmill exercise (Gerin et al., 1995), (4) the systemic administration of 5-HT2A,2B,2C agonists that improves locomotion in spinalized cats (Barbeau and Rossignol, 1991; Miller et al., 1996) and, (5) the 5-HT reuptake inhibitor treatment in newborn rats leading to improvement in locomotion (Kim et al., 1999). Recently, the beneficial effect of post-SCI exercise, on axonal re-growth from intact fibers above the lesion, has been demonstrated in hemisectioned mice (Goldshmit et al., 2008). As well, treadmill training improves patients with SCI (Harkema, 2001). However, specific neural pathways leading to motor function improvement need to be identified in order to administer specific corresponding neurochemical treatments to SCI patients. Furthermore, the relationship between serotonin release in the ventral horn and motor function recovery after SCI in exercising animals remains undefined. In this study we address the role of amounts (basal levels at rest) and patterns (levels during exercise and post-exercise) of 5-HT release during motor function recovery, which was assessed by treadmill exercise, after SCI. Sub-hemi-sections were performed at the low spinal thoracic level and microdialysis probes were stereotactically placed and maintained in the ipsilateral and caudal side to the lesion into layers 7–9 of Rexed and used to sample 5-HT release over a month time period.
MATERIALS AND METHODS
Experimental Animals
Seven male Sprague Dawley rats were housed in cages at a temperature of 22°C and standard food and drinking water were available ad libitum. Conditioning exercise program took place before any surgery and, exercise consisted of a progressive 5-day-a-week treadmill program, until obtaining a spontaneous and continuous run for 120 min at a speed of 26.6 m/min with a 5% slope (Bedford et al., 1979). The 120 min run included four-10 min sessions at a speed of 30 m/min with a 5 % slope (80% of VO2max). This training was maintained until surgery.
Surgical procedure (for details see Gerin et al. (1994))
Animals (235 ± 10 g) anaesthesia was induced by 50 mg/kg of sodium pentobarbital i.p.; additional doses were given i.p. (3 mg/kg), as needed. Body temperature was monitored and kept stable during surgery. A laminectomy in T9 vertebra was performed. Thereafter, a sub-hemi-transection of the spinal cord was surgically performed at T9 vertebral level (the extent of the lesion is shown in Fig. 1A).
Figure 1. Schematic representation of the lesion at T9–T10 (A) and of the microdialysis probe placement at L4–L5 (B).
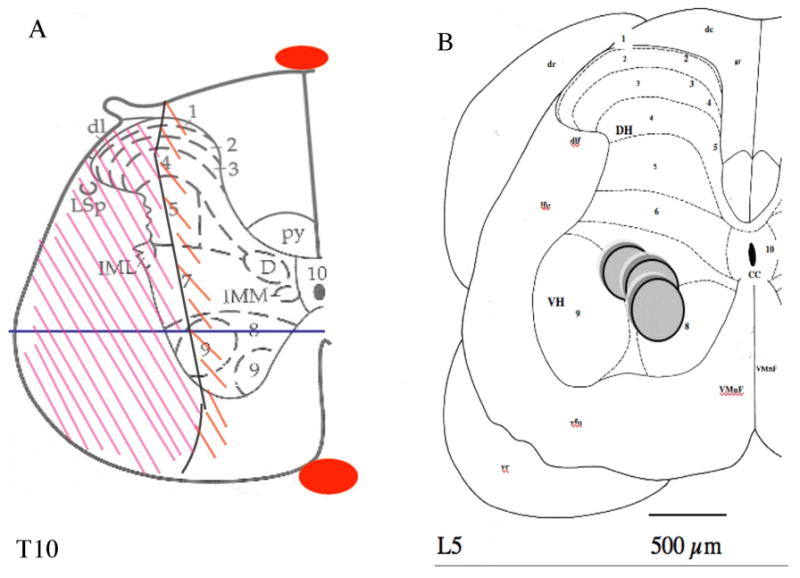
In (A) Schematic includes tissue wound margins at the T9–10 level modified from Paxinos and Watson (1997). Descending and ascending tracts interrupted include the lateral part of the Burdach bundle, the dorsolateral funiculus, the lateral funiculus, the ventrolateral funiculus, and the lateral part of the ventral funiculus. The lesion also includes grey matter ipsilateral to the lesion surrounded by the white matter already cited, such as the lateral half of the dorsal and ventral horns including the lateral half of layers 1–6 of the dorsal horn and the lateral half of lamina 7 and lamina 9 for the ventral horn, and the lateral half of the intermediate zone. The mediolateral solid line in the ventral horn represents the location where the longitudinal sections were performed in Fig. 2.
In (B) the diagram of a hemi-spinal cord section adapted from Paxinos and Watson (1997) (Gerin et al., 1994) shows the location of the dialysing part (dialysis membrane) of the microdialysis probe in the lumbar enlargement within laminae 7, 8 and, 9 of Rexed. Solid gray circles represent the microdialysis membrane of the probe inserted and maintained in place at a fixed angle (18°) towards the horizontal plane of the spinal cord (Gerin et al., 1994). Dialysis from areas farther than 150 μm fails to be measurable in this experimental set-up: therefore laminae that are dialysed are 9,7,8 (Gerin et al., 1995). L5: lumbar segment number 5; CC: central canal; dc: dorsal columns; dr: dorsal root of spinal nerve; dlf: dorsolateral funiculus of the spinal cord; lfu: lateral funiculus; vr: ventral root of spinal nerve; vfu: ventral funiculus; VMnF: ventral median fissure of the spinal cord; DH: dorsal horn of the spinal cord; VH: ventral horn. The ten layers of Rexed in the spinal cord are indicated by numerals 1–10. The probe is located in the axon terminal field of raphe obscurus and pallidus nuclei serotonergic neurons projecting to the ventral horn of the spinal lumbar enlargement. Scale bar: 500μm.
Animals were then placed in a spinal stereotactic aparatus equipped with a micromanipulator (David Kopf Instruments). A microdialysis probe (with a membrane: cut off 6000 Dalton, 240μm diameter, 1mm long, CMA/Microdialysis) was stereotactically inserted into the ipsilateral ventral horn to the lesion, in the L4 segment (vertebral body T13). The specific placement of the dialyzing part of the probe was located in layers 7-8-9 of Rexed (Gerin et al., 1994; Molander et al., 1984; Paxinos and Watson, 1997). Stereotactic coordinates (Paxinos and Watson, 1997), for the ventral horn placement of the probe, were set with reference to the apex of the spinous process of vertebra T13 for the lateral coordinate and in reference with the dura for depth (H) coordinate: AP = − 4.0 mm, L = 0.52 ± 0.14 mm, H = + 1.82 ± 0.15 mm, with an angle of 18° to the horizontal plane (Gerin et al., 1994). Dental cement and anchoring screws were used for fixation of the probes to the vertebrae. Muscles and skin were sutured plan by plan.
Post-operative care
Post-operative care and treadmill walking was initiated (usually 2h) after recovery from anaesthesia (Gerin et al., 1994). However, prior to surgery, specific and intense training was performed in order to obtain the optimal locomotor and sensory recovery after lesion. Pain management has been assessed (no prostrate behavior, weight gain, proper grooming were monitored). Treadmill exercise was continued after surgery – as a retraining program- at a speed adapted to the ability of the rats to walk or run.
Microdialysate sampling and locomotor assessment
Animals were subjected to three microdialysis and treadmill exercise test sessions after surgery. Animals were placed on the treadmill and, microdialyis probes were connected to a micro-perfusion pump (CMA100, CMA-Microdialysis) and, to a polyethylene (P.E.) microtube placed on ice. Microdialysis probes were perfused with sterile Ringer solution (NaCl 144 mM, CaCl2 2.0 mM, KCl 4 mM, pH = 6.25 (Westerink and Tuinte, 1986)) at a flow rate of 2.0 μl/min for 2.5 h washout period before collecting samples. The dialysate was sampled every 15 min for 4 hours divided into three periods including 90 min of rest, 60 min of exercise, and 90 min of post-exercise rest. The exercise test period consisted of a treadmill running-walking session of 60 min with a 5% slope and at a speed of 16 m/min (60 % of VO2max) when possible (Bedford et al., 1979). Experimental sessions took place 8, 18±2, 34±2 days after the chronic implantation of the microdialysis probe and after the spinal cord lesion for the measure of serotonin. The day-8 rest time point is considered as control (Gerin et al., 1994). The day-8 time point did not include the running exercise because the lesion prevented a-such one-hour exercise. Running protocol (rest, exercise, post-exercise) was maintained for days 18, 34.
Monoamine assay
Microdialysate samples and standards were injected (10 μl) onto and separated by an analytical C-18 Bondapak (300×3.9mm, 10μm silica particle diameter) chromatographic column (Waters-Millipore, USA) maintained at 20 °C. Mobile phase consisted of 0.1M sodium acetate and 1mM ethylenediaminetetraacetic acid (EDTA) adjusted to pH 4.0 and, delivered at a flow rate of 1.0ml/min using a Waters’ 501 pump. Analytes were detected electrochemically (M464 detector, Waters) with Ag/AgCl reference electrode set at 574mV. Retention times, peak areas, and concentrations of 5-HT in microdialysate were computed by comparison with known standards injected before each set of four samples. The detection limit for 5-HT was 13pg per injected sample.
Histological study
For histological analysis, animals were sacrificed at the end of the entire experimental procedure by intracardiac perfusion of 5% glutaraldehyde. Spinal cords were excised and post-fixed. Spinal cords of all animals were serially sectioned (30μm) using a vibratome. Transversal sections were performed at the site of the probe implantation to evaluate the placement of the probe. Immunocytochemistry (ICC) was performed to evaluate the density of neuronal population (5-HT) at the site where the neurotransmitter was measured by microdialysis. ICC of glial fibrillary acidic protein (GFAP) was used to evaluate the astrocytic reaction surrounding the probe (data not shown). Longitudinal (cephalo-caudal) serial dorso-ventral sections performed at the site of the lesion were immunostained for GFAP in order to evaluate the importance of the tissue astrocytic reaction (medio-laterally, dorso-ventrally, and cephalo-caudally). Prior to primary antibodies (5-HT, GFAP) incubation, sections were successively pre-incubated in 0.25% trypsine then in 5% BSA-1% hydrogen peroxide solution and in 0.04% sodium borohydride (no borohydride incubation was necessary for GFAP). Sections were incubated with primary polyclonal anti-body made in rabbit serum (5-HT 1/8000; GFAP 1/500) diluted in 0.1%TritonX-100 1% BSA overnight. The immunoreactivity (IR) of primary antibodies was detected by a 1/1000dilution anti-goat IgG peroxidase conjugated secondary antibody. For the development DAB was used as a substrate. Negative control sections were incubated with solutions made without primary anti-bodies and, were used to control for background and terminate the reaction. In addition, a few sections were stained with cresyl violet for evaluation of the inflammatory reaction of the host tissue. Primary anti-bodies, secondary peroxidase conjugated anti-body, 5-HT HCl, EDTA, DAB, glutaraldehyde were purchased from Sigma.
Data analysis
Results were expressed as mean ± S.E.M. of 5-HT concentration variations at rest, during exercise, during post-exercise with 3 time points over a post-SCI one-month period (day 8; day 18±2; day 34±2). Seven different groups were formed: rest day 8 (LR8), rest day 18 (LR18), exercise day 18 (LE18), post-exercise-rest-period day 18 (LP18), rest day 34 (LR34), exercise day 34 (LE34), post-exercise-rest-period day 34 (LP34). Basal values consisted of 6 steady state measurements at rest for LR8, LR18, LR34. Repeated-factor two-way ANOVA was used to determine statistical significance between groups for microdialysate concentrations. Student’s two-tailed distribution t-test was used for significance between groups (paired); p ≤0.05, p ≤0.01, p ≤0.001 were considered to be significant (*), very significant (**), highly significant (***) respectively. *comparison to rest mean value at day 8; $ comparison to rest at day 18; # comparison to rest of the same time period post-lesion.
We certify that all applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research (IACUC approval #04-180).
RESULTS
Histological analysis showed the importance of the lesion and the microdialysis probe placement (Fig. 1, 2, and 3). The importance of the lesion-induced astrocytic reaction (ICC anti-GFAP) of the tissue, known to constitute a barrier to outgrowth processes, was of 500μm (Fig. 1, 2). The contralateral side to the lesion showed an absence of dense GFAP IR. On lumbar transversal sections (Fig. 3), caudal to the lesion, 5-HT IR fibers were seen within the ventral horn of the contra-lateral side to the lesion, and the IR was less intense in the ipslateral side. However, there were dense IR fibers in layer 8 as well as, within the commissural area of layer 10, and in the medio-ventral part of layer 6 in the ipsilateral side to the lesion. Axons with varicosities could be seen in these areas.
Figure 2.
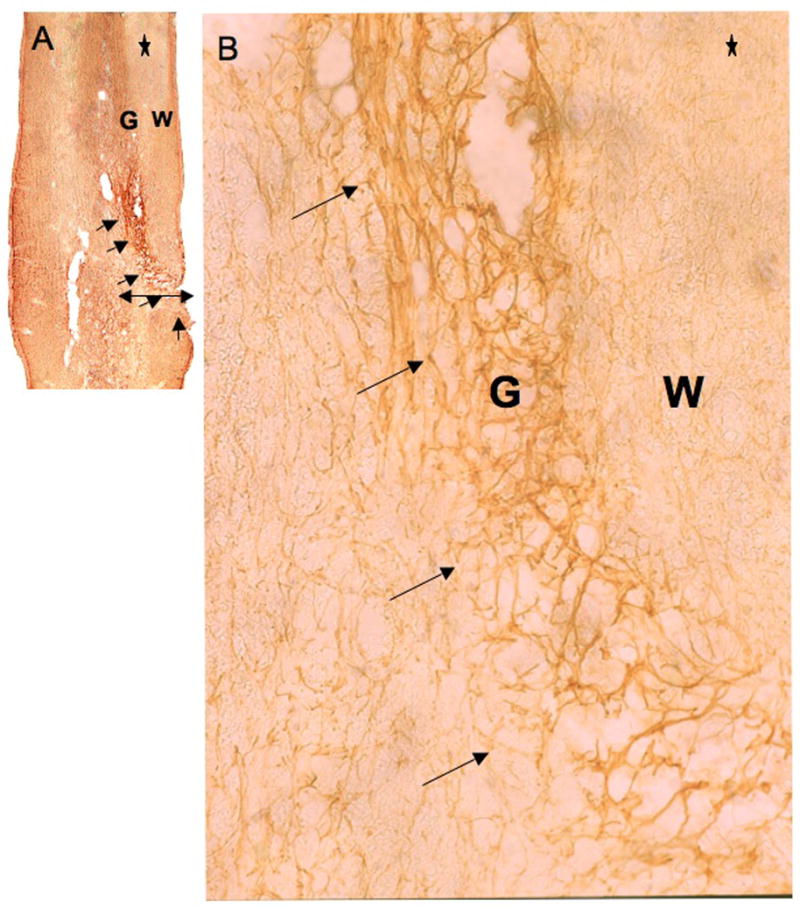
GFAP IR at the lesion site. The tissue reaction due to lesion is revealed by GFAP-ICC on 30μm cephalo-caudal sections (30μm thickness, using a vibratome) at the site of the lesion (T9). Reactive tissue goes from 1/3 medial to lateral side of the cord, including ventral gray (G) and lateral white matter (W) (as shown by the mediolateral line drawn in the schema of Fig. 1A). Arrows indicate astrocyte-dense tissue area. The extent of the scar is less than 500μm thick and appears well delineated. Astrocytes can be better observed at a higher magnification (B). * indicates cephalic side of the histological section. (A) X5, and (B) X62.5.
Figure 3.
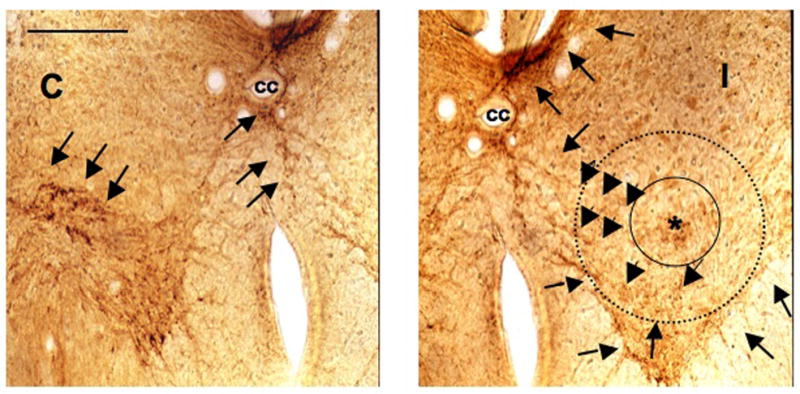
Serotonin IR caudal to lesion in the lumbar enlargement. ICC detection of 5-HT in the caudal lumbar enlargement ventral horns, in the contra- (C) and ipsilateral (I) sides to the lesion. The section was performed at the caudal end of the membrane of the microdialysis probe, marked with a star (*). The perimeter of the membrane of the microdialysis probe is artificially represented by a solid black circle (240μm diameter) surrounding the asterisk. A second dotted line circle represents the area from which neurotransmitter could diffuse up to the membrane to the probe and therefore representing the dialysed surface area. Arrowheads indicate 5-HT IR fibers and their trajectories within the contralateral side (C), the ipsilateral side (I) including the dialysed area and, the commissural area. Less dense 5-HT IR is shown in the lesioned side in layers 7 and 9 of Rexed, caudal to the lesion. Some 5-HT IR fibers can be seen in layer 8 in the ipsilateral side to the lesion. More dense ICC detection can be seen in the dorsal part of layer 10 and in the most ventromedial part of lamina 6 in the ipsilateral side to the lesion. The ipsilateral 5-HT ICC detection is compatible with both the lesion performed and some sprouting of 5-HT fibers caudal to the lesion (L4–5, S1). Scale bar 250μm. cc: central canal.
Clinical post-operative examination showed that wound margins were healed by day eight. Animals were mobile after the surgery. In previous experiments employing this preparation, no side effect has been observed as the result of microdialysis probe placement in the central nervous system (CNS) (Gerin et al., 1994, 1995, 2008). No evidence of back pain or prostrate behavior was observed after surgery (at 48 hours). Beside sensory and motor loss due to the lesion, animals did not develop additional postoperative sensory loss or motor deficit as a side effect of the surgery. Animals started gaining weight 4 days after surgery.
Post-surgical evaluation of the spinal lesion was assessed by monitoring motor and sensory hindlimb functions (Fig. 4). The sub-hemi-transection at vertebral level T9 induced sensory-motor deficit of the ipsilateral hindlimb with plantar side of the foot up at rest and during treadmill locomotion. Animal contra-lateral hindlimbs did not show evidence of motor function deficit when animals were tested on a treadmill to evaluate sensory and motor parameters during locomotion at low speed locomotion. Animals showed an absence of sensory reflexes in the ipsilateral hindlimb to the lesion, as assessed by responsiveness to needle puncture, stretch reflex, and grabbing tests (Gerin, 2003).
Figure 4.
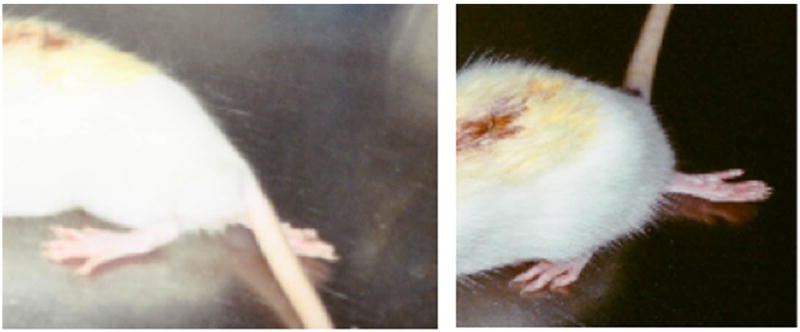
Two representative sub-hemisectioned animals show impairment of their right hindlimb. One day post spinal cord microsurgical sub-hemisection show animals with sensory-motor impairment of their right hindlimbs, inducing the plantar side of the foot to face up and the contralateral hindlimb to the lesion to appear almost as in non-lesioned animals. The contralateral hindlimb shows a little sensory deficit, but the plantar side of the foot is facing down.
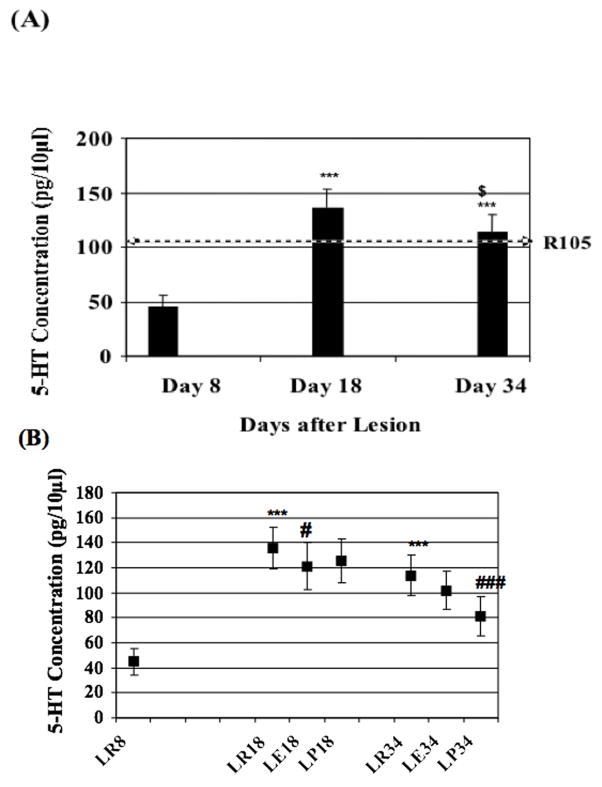
Release of 5-HT during rest, within the ventral horn, evidenced a role for the neurotransmitter in regenerative processes. Release of 5-HT was measured during rest periods 8, 18 and, 34 days after SCI (Table 1; Fig. 5A). Eight days after the lesion (LR8) 5-HT concentration averaged 45 ± 10.52 pg/10μl in the microdialysate (n=7). At LR18 5-HT concentration was of 135.8 ± 16.82 pg/10μl representing a highly significant (p ≤0.001) increase of 202% when compared to LR8. At LR34 5-HT concentration significantly (p ≤0.001) increased of 153% when compared to LR8 and, significantly (p ≤0.05) decreased of 16.4% when compared to LR18. Therefore, 5-HT release showed significant variations during motor function recovery after the lesion.
Table 1.
Serotonin concentrations in the ventral horn ipsilateral to the lesion, over recovery time period. Extracellular concentration (pg/10μl) of 5-HT from the lumbar enlargement ventral horn of the rat spinal cord is expressed as mean±S.E.M. of time point concentrations during rest (6 time points), exercise (4 time points) and post-exercise rest periods (6 time points) at different post-lesion times (8 days, 18 days, 34 days). *Comparison to rest mean value at day 8; $ comparison to rest at day 18; # comparison to rest of the same time period post-lesion.
| CONCENTRATIONS OF 5-HT WITHIN VENTRAL HORN CAUDAL AND IPSILATERAL TO LESION (pg/10μl) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| DURING REST CONDITIONS | DURING EXERCISE CONDITIONS | DURING POST-EXERCISE CONDITIONS | |||||||
| DAY 8 POST- LESION | DAY 18 POST- LESION | DAY 34 POST- LESION | DAY 8 POST- LESION | DAY 18 POST- LESION | DAY 34 POST- LESION | DAY 8 POST- LESION | DAY 18 POST- LESION | DAY 34 POST- LESION | |
| Number of Rats | n = 7 | n = 7 | n = 7 | n = 7 | n = 7 | n = 7 | n = 7 | n = 7 | n = 7 |
| Concentration | 45±10.52 | 135.8±16.82*** | 113.9±16.5*** | N/A (No Run) | 121.3±19.16# | 102 ± 15.2 | N/A (No Run) | 125.4 ± 17.42 | 81 ± 15.6### |
Figure 5.
(A) Serotonin concentrations variations at rest after spinal cord lesion. Microdialysate concentrations of 5-HT (mean ± S.E.M.) during rest, 8, 18±2, 34±2 days after spinal cord lesion reflecting the variation of 5-HT release in correlation with the motor function recovery time from the lesion. The horizontal arrow (R105) represents the concentration of 5-HT from the literature (Gerin, 2003; Gerin et al., 1994) in non-lesion animals (105μg/10μl injected). (B) Microdialysate concentration variations induced by exercise at different time points after spinal cord lesion. At day 8 after lesion no running exercise was performed. At day 18 exercise (LE18) induced a decrease of 5-HT release followed by an increase during the post-exercise rest period (LP18). At days 34, when animals are able to sustain a “normal” running exercise, the pattern of 5-HT release (R-E-PE) is different than the one at day 18 when animals could not sustain a running exercise similar to non-lesioned animals. P ≤ 0.05, P ≤ 0.01, P ≤ 0.001 were considered to be significant (*), very significant (**), highly significant (***) respectively. (*) comparison to rest mean value of day 8, ($) comparison to rest day 18, (#) comparison to rest of the same time period post-lesion.
Variations of 5-HT release in the ventral horn were induced by exercise during the recovery period after SCI (Table 1, Figure 5B). At day 18, the release of 5-HT was significantly (p ≤0.05) decreased by 10.6% during exercise (LE18) and, by 7.7% during LP18 when compared to LR18. At LE34 release of 5-HT was decreased by 10.5% and, by a high significant (p ≤0.001) 29% decrease during LP34 when compared to LR34.
DISCUSSION
5-HT immunoreactivity is compatible with local sampling
Histological analysis shows an increased 5-HT IR density within the commissural area, and the ventromedial region of layer 6. Medial and ventral 5-HT IR fibers could be seen within layer 8 as well (Fig. 3). This suggests a spatial re-arrangement and sprouting, as previously shown (Goldshmit et al., 2008) that correlates with the improved 5-HT release over the recovery time period of a month. 5-HT IR fibers within the dialyzing perimeter of the probe suggests that at the end of the motor recovery from the lesion, 5-HT detected through the microdialysis probe is provided by IR axons from layers 8, commissural areas including layers 10 and 6. However, the general density of 5-HT fibers is lower in the ipsilateral side, which suggests the involvement of regenerative compensatory mechanisms of release such as (but not limited to) increase of non-synaptic junctions, increase of 5-HT receptors onto MN, onto interneuronal populations and, onto serotonergic terminals (Kim et al., 1999; Miller et al., 1996).
5-HT descending innervation impacts motor function
The lesion performed interrupted the medullary spinal neurons from the raphe magnus projecting ipsilaterally (Basbaum et al., 1988; Skagerberg and Bjorklund, 1985). Theses neurons do not directly influence locomotor activity. In contrast, the medullary reticulospinal neuronal pathway running bilaterally within the ventral and lateral funiculi and terminating in laminae 3 to 10 has been interrupted by the lesion of the lateral funiculus and the lateral part of the ventral funiculus, leaving only intact fibers coming from the contralateral side and the medial part of the ventral funiculus on the ipsilateral side. Therefore, the lesion impairs the ipsilateral excitatory modulation on MN. Another pathway also involved in motor activity and, terminating in the ventral horn that makes contact onto MN is the serotonergic raphe spinal pathway from raphe obscurus and pallidus nuclei. The latter pathway, when interrupted, impairs modulation of motor function in the ipsilateral hindlimb to the lesion (Holstege and Kuypers, 1987). Therefore, motor function and 5-HT release improvement after lesion might be dependent upon neural reorganization within layers that have not been deprived of their original inputs, such as layers 10, 8, the ventromedial part of layer 6 and the contralateral ventral horn.
5-HT pattern of release changes during recovery
5-HT pattern of release showed significant variations during recovery after the lesion (Table 1, Figure 5B). Variations of 5-HT release, induced by exercise, within the ventral horn during recovery period after spinal cord lesion (Table 1, Figure 5B) indicates differential 5-HT implication in motor function. 5-HT decreased release observed at LR8 after the lesion is probably due to the interruption of descending pathways terminating within layers 7–9 of the ipsilateral ventral horn lumbar enlargement. Later improvement after lesion might involve two steps: first, the amount of serotonin able to be released (such as the over-release we observed at LR18), and second, the regulation during exercise for which serotonin release is decreased and continues to decrease after the exercise stops. The comparison of LE-, LP-, 18 and 34 indicates that regulation of 5-HT release mechanisms might be different during exercise and post-exercise. Exercise time point releases LE18 and LE34 might reflect that a negative feedback mechanism acting through interneuronal network regulation is effective as early as 18 days after the lesion. At L34, animals were able to run as well as intact animals, suggesting that motor function recovery is linked to serotonergic recovery, as suggested earlier in graft studies (Kim et al., 1999; Barbeau and Rossignol, 1991). Comparison between LP 18 and 34 and previous data (Gerin et al., 1994) indicate that the pattern of release at day 34 is similar to the one in intact animals. LP34 release regulation suggest that recovery, perhaps involving 5-HT receptors (Kim et al., 1999; Schwartz et al., 2005) and uptake and degradation mechanisms, might only occur later in time, 34 days after the inflicted lesion.
Conclusion
5-HT release during neural regeneration might play an important role in neuronal network re-arrangement, which later regulates variations of 5-HT release necessary to locomotion. Although microdialysis allows to monitor the release of neurotransmitters during long neurological recovery processes corresponding to anatomical changes after a lesion, further detailed neuroanatomical studies should be performed to fully explain the differential pattern of 5-HT release during the motor function recovery period after a spinal lesion. Furthermore, our results, along with others (Dietz and Harkema, 2004) suggest that locomotor exercise after SCI triggers neural plasticity and synaptic efficiency occurring within 2–3 weeks after SCI in rats and, in turns improves the motor function occurring after 3 to 4 weeks (this study) (Gerin, 2003; Kim et al., 1999) or later (depending on the importance of the SCI). These results explain some of the mechanisms of late recovery, years after the trauma, in some SCI patients and, might have implications on future long-term chronic treatments of paraplegic patients such as administration of serotonin neurotransmitter release enhancing drugs, combined with treadmill exercise.
Acknowledgments
This work was supported by INSERM and NIH grants R01NS023868, R25GM059218, G11HD039879.
Authors thank B. Haley and Drs. E. Morris, T. Paunesku, L. Walter for helpful discussions.
References
- Barbeau H, Rossignol S. Initiation and modulation of locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Res. 1991;546:250–260. doi: 10.1016/0006-8993(91)91489-n. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Zahs K, Lord B. The fiber caliber of 5-HT immunoreactive axons in the dorsolateral funiculus of the spinal cord of the rat and cat. Somatosens Res. 1988;5:177–185. doi: 10.3109/07367228809144625. [DOI] [PubMed] [Google Scholar]
- Bedford TG, Tipton CM, Wison NC, Oppliger RA, Gisolfi CV. Maximum oxygen consumption of rats and its changes with various experimental procedures. J Appl Physiol. 1979;47:1278–1283. doi: 10.1152/jappl.1979.47.6.1278. [DOI] [PubMed] [Google Scholar]
- Dietz V, Harkema SJ. Locomotor activity in spinal cord-injured persons. J Appl Physiol. 2004;5(96):1954–1960. doi: 10.1152/japplphysiol.00942.2003. [DOI] [PubMed] [Google Scholar]
- Gerin C. Possible role of 5-HT release in spinal cord regenerative processes. Molecular aspects of neuro-degenerative diseases conference; Sweden. 2003. [Google Scholar]
- Gerin C, Becquet D, Privat A. Direct evidence for the link in-between monoaminergic descending pathways and motor activity. I. A study with microdialysis probes implanted in the ventral funiculus of the spinal cord. Brain Res. 1995;704:191–201. doi: 10.1016/0006-8993(95)01111-0. [DOI] [PubMed] [Google Scholar]
- Gerin C, Legrand A, Privat A. Study of 5-HT release with a chronically implanted microdialysis probe in the ventral horn of the spinal cord of unrestrained rats during exercise on a treadmill. J Neurosci Methods. 1994;52:129–141. doi: 10.1016/0165-0270(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Gerin C, Teilhac J-R, Smith K, Privat A. Motor activity induces release of serotonin in the dorsal horn of the rat lumbar spinal cord. Neurosci Lett. 2008;436:91–95. doi: 10.1016/j.neulet.2008.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, Lythgo N, Galea MP, Turnley AM. Treadmill training after spinal cord hemisection in mice promotes axonal sprouting and synapse formation and improves motor recovery. J Neurotrauma. 2008;(25):449–446. doi: 10.1089/neu.2007.0392. [DOI] [PubMed] [Google Scholar]
- Harkema SJ. Neural plasticity after human spinal cord injury: application of the locomotor training to the rehabilitation of walking. Neuroscientist. 2001;(7):455–468. doi: 10.1177/107385840100700514. [DOI] [PubMed] [Google Scholar]
- Holstege JC, Kuypers HGJM. Brainstem Projections to spinal motoneurons: An update. Neuroscience. 1987;23:809–821. doi: 10.1016/0306-4522(87)90160-6. [DOI] [PubMed] [Google Scholar]
- Kim D, Adipudi V, Shibayama M, Giszter S, Tessler A, Murray M, Simansky KJ. Direct Agonists for Serotonin Receptors Enhance Locomotor Function in Rats that Received Neural Transplants after Neonatal Spinal Transection. J Neurosci. 1999;19(14):6213–6224. doi: 10.1523/JNEUROSCI.19-14-06213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JF, Paul KD, Lee RH, Rymer WZ, Heckman CJ. Restoration of extensor excitability in the acute spinal cat by the 5-HT2 agonist DOI. J Neurophysiol. 1996;75:620–628. doi: 10.1152/jn.1996.75.2.620. [DOI] [PubMed] [Google Scholar]
- Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol. 1984;230:133–141. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, editors. Compact. 3. San Diego: Academic Press; 1997. The rat brain in stereotaxic coordinates. [Google Scholar]
- Schwartz EJ, Gerachshenko T, Alford S. 5-HT Prolongs Ventral Root Bursting Via Presynaptic Inhibition of Synaptic Activity During Fictive Locomotion in Lamprey. J Neurophysiol. 2005;93:980–988. doi: 10.1152/jn.00669.2004. [DOI] [PubMed] [Google Scholar]
- Sharma A, Punhani T, Fone K. Distribution of 5-hydroxytryptamine2C receptor protein in adult rat brain and spinal cord determined using a receptor-directed antibody: effect of 5,7-dihydroxy-tryptamine. Synapse. 1997;27:45–56. doi: 10.1002/(SICI)1098-2396(199709)27:1<45::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Skagerberg G, Bjorklund A. Topographic principles in the spinal projections of serotonergic and non-serotonergic brainstem neurons in the rat. Neuroscience. 1985;15:445–480. doi: 10.1016/0306-4522(85)90225-8. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Tuinte MH. Chronic use of intracerebral dialysis for the in vivo measurement o f 3, 4-dihydroxyphenylethylamine and its metabolite 3,4-dihydroxyphenylacetic acid. J Neurochem. 1986;46:181–185. doi: 10.1111/j.1471-4159.1986.tb12942.x. [DOI] [PubMed] [Google Scholar]