Abstract
Athletic women are at risk for developing ovulatory dysfunction, which presents variably as menstrual irregularity or absence. Initially characterized as an isolated disruption of hypothalamic gonadotropin releasing hormone (GnRH) release, athletic amenorrhea, a form of hypogonadotropic hypogonadism, is invariably accompanied by additional neuroendocrine aberrations, including activation of adrenal and suppression of thyroidal axes. Exercise may elicit intermittent or chronic metabolic stress due to increased energy expenditure and/or insufficient or imbalanced nutrient intake. In addition, athletic activities are motivated by or serve as psychogenic stressors. Prior studies dichotomized stressors as metabolic or psychogenic. Not only is this a false dichotomy because all stressors have both a metabolic and a psychogenic component, but also stressors act synergistically rather than in isolation to compromise GnRH drive and endocrine homeostasis. To ameliorate reproductive and endocrine consequences of stress, then, requires identification and amelioration of all relevant stressors. Formal psychosocial support helps individuals to develop better coping strategies and make appropriate lifestyle changes. Our research has shown that cognitive behavior therapy restores reproductive and endocrine balance.
Keywords: athletic amenorrhea, hypogonadotropic hypogonadism, psychogenic stress, metabolic stress, energy deficit, cognitive behavioral therapy
Over the past few decades, society has changed its perception of women playing sports. With the passage of Title IX of the Educational Amendments Act of 1972 the female athlete emerged, as well as increased involvement of women in routine exercise. Women of all ages now have the opportunity to enjoy participation in both recreational and competitive sports. A 2008 survey of American time use compiled by the United States Bureau of Labor Statistics estimates that 15 percent of women participate in sports, exercise, or recreation on any given day.1 While the physiological and psychosocial health benefits of exercise have been widely reported in the literature and promoted by society, less attention has been focused on subtle and overt health concerns related to exercise. First described in 1997, the female athletic triad is a syndrome that includes disordered eating, amenorrhea and osteoporosis.2 Recent studies have highlighted that even recreational exercising women have a high prevalence of menstrual disturbances, with up to half of exercising women displaying subtle or severe menstrual disturbances.3, 4 Women with athletic amenorrhea often face persistent metabolic challenges in the form of intermittent or chronic energy imbalance due to increased energy expenditure or insufficient caloric intake. In addition, these women typically engage in a combination of behaviors that serve as psychogenic stressors, such as the rigors of competition and balancing an intense training schedule with the demands of school, work and family. It is important, therefore, to determine the roles of energy imbalance and psychogenic factors in the pathogenesis of athletic amenorrhea.
Ovulation and menstrual cyclicity are initiated and maintained by appropriate input from the hypothalamic GnRH pulse generator. The GnRH pulse generator is sensitive to central nervous system influences that impinge upon the hypothalamus, as well as target hormone feedback mechanisms. The body has developed hormonal mechanisms to allow for homeostasis in the setting of acute stress and increased energy demands. Homeostasis comes from the Greek words homeo and stasis meaning “same and stable.” Under normal conditions, after an acute stress or energy demand passes, the same stability is achieved and hormonal equilibrium is restored. In contrast, chronic stress can result in an alteration in the homeostatic set point. Allostasis comes from the Greek words allo and stasis meaning “variable and stable.” In response to chronic stress or altered energy demands, allostasis is achieved when hypothalamic function is altered to allow for preservation of the individual at the expense of physiological function. There is considerable individual variation in the ability to respond to energy imbalance and psychogenic stress. Women athletes with “hypothalamus robustus” are able to maintain homeostasis and menstrual function despite the rigors of strict training, intense competition and stringent dietary regimens. Female athletes that are less resilient to the allostatic load of chronic stress and metabolic challenges have alterations in hypothalamic functioning and concomitant amenorrhea. Potential determinants of “hypothalamus fragilis” and the differential sensitivity of stress-induced reproductive dysfunction can be explained by neuroendocrine alterations, metabolic defects and psychogenic variables.
Insight into the affect of stress on neuroendocrine function can be gleaned from studies done in patients with functional hypothalamic amenorrhea (FHA). FHA is estimated to affect up to 5% of women of reproductive age and is the underlying cause of 35% of women seeking evaluation for secondary amenorrhea.5, 6 It is also more commonly observed in women athletes and those with clinical and subclinical eating disorders.7 A constellation of neuroendocrine secretory aberrations has been observed in women with functional hypothalamic amenorrhea.8 Women with FHA have reduced central GnRH drive resulting in low FSH and LH levels which causes them to be anovulatory. While prior studies have focused on targets that disrupt GnRH drive, there is also activation of the hypothalamic-pituitary-adrenal (HPA) axis and suppression of the hypothalamic-pituitary-thyroidal (HPT) axis which contributes to the disruption of the hypothalamic-pituitary-gonadal (HPG) axis in these women. In addition, other hypothalamic perturbations are observed including elevated night time serum growth hormone levels and lower 24 hour prolactin levels.8
Allostatic alterations in the metabolic axis are observed in patients with FHA. Critical to the mobilization of energy in response to stress is activation of the hypothalamic-pituitary-adrenal (HPA) axis and suppression of the hypothalamic-pituitary-thyroidal axis. Orexigenic peptides such as ghrelin also play a role in communicating energy status to the brain areas that modulate metaboslism. The HPA axis is universally activated in patients with FHA and associated with increased circulating cortisol levels.8 The increased cortisol likely indicates that FHA and its variants are stress-induced and can be referred to as stress-induced anovulation (SIA). Women with functional hypothalamic amenorrhea have elevated levels of cortisol in both cerebral spinal fluid (CSF) and peripheral circulation as compared to eumenorrheic women.8, 9 Interestingly, corticotropin-releasing hormone (CRH) concentrations in the CSF of FHA and eumenorrheic women are similar.10 This suggests that the elevated cortisol levels are not maintained by excess CRH production, but rather a consequence of allostatic resistance to negative feedback suppression by cortisol.9, 10 In addition, women with FHA who are relatively hypercortisolemic before exercise, generate a larger rise in cortisol during exercise as compared to eucortisolemic, eumenorrheic women.11 The exaggerated cortisol response to exercise in FHA may provoked by the decline in glucose levels seen in women with FHA while exercising as compared to a rise in glucose levels in eumenorrheic women. Recent studies in macaques suggest that stress-induced reproductive dysfunction is linked to decreased activity of serotonin, as well as modulated by gamma-aminobutyric acid (GABA).12–14 While not as extensively studied, women with athletic amenorrhea show many of the same homeostatic alterations, including suppression of the hypothalamic-pituitary-ovarian axis, suppression of the thyroidal axis, and activation of the hypothalamic-pituitary-adrenal axis. Similar to women with FHA, women with athletic amenorrhea have fewer LH pulses indicative of reduced GnRH drive, elevated serum cortisol levels, and blunted responses to GnRH and CRH.15 The hypothalamic-pituitary-thyroidal axis responds to metabolic stressors by conserving energy. While TSH levels are preserved in women with FHA, triiodothyronine (T3) and thyroxine (T4) are reduced as compared to eumenorrheic ovulatory women reflecting a change in the set point for TRH drive to the pituitary and thyroidal responsivity to TSH.8 Athletic woman with amenorrhea similarly demonstrate activation of the HPA axis, which mobilizes glucose, and a relative hypothalamic hypothyroidism, which minimizes energy expenditure. The energy drain and metabolic mobilization that accompanies both acute and chronic forms of stress are communicated to the brain, particularly the hypothalamus. The result is reproductive compromise via disruption of GnRH drive that further conserves energy and allows for energy to be diverted from reproduction to meeting the immediate environmental demands.
What constitutes psychogenic stress is variable and reflects individual factors, including valences. Women with functional hypothalamic amenorrhea have increased cognitive dysfunction and psychiatric morbidity including more depressive symptoms and difficulty dealing with stressors.16 More dysfunctional attitudes and unrealistic expectations were noted in a selected population of women with FHA that excluded women with eating disorders, significant weight changes and or extreme exercisers when compared to eumenorrheic women and those with other causes of amenorrhea.17 The FHA group only included women within 90–110% of their ideal body weight, with less than a 10 pound weight fluctuation in the previous year and who exercised less than 10 hours a week. This selected group of women with FHA without overt eating disorders was noted to have attitudes commonly observed in women with disordered eating. In addition, these women had a high degree of perfectionism and need for social approval, both of which seem to increase sensitivity to life’s many stressors.17 Response to psychogenic stressors also depends on the cognitive abilities and personality style of the individual. The current psychological milieu, as well as sensitization from past experiences, may modulate mood, attitudes and coping skills. Athletes are not immune to commonplace stressors, and in addition, face sports-related psychodynamic factors including performance pressure and anxiety, coping with injuries, training obstacles, and interpersonal problems with coaches and teammates.18 Athletes lacking insight into the mind-body connection may be particularly sensitive to stressors that alter both neural and endocrine mechanisms.
Most prior studies have attempted to dichotomize stressors that result in reproductive compromise as either metabolic or psychogenic stresses.19 Early studies focused on inducing menstrual dysfunction by strenuous exercise and altering dietary intake in humans and monkeys.20, 21 While metabolic variables are more readily quantifiable and arguably easier to manipulate, focusing on the metabolic mechanisms precluded understanding the impact of psychosocial stressors. Synergistic impairment of reproductive function was recently demonstrated in response to mild metabolic and psychosocial stressors in cynomolgus monkeys.22 Eumenorrheic monkeys were given either a metabolic challenge in the form of mild undernutrition and increased energy expenditure or a social challenge in the form of social disruption, or a combination. Neither the metabolic nor the social challenge alone had much impact, but when the stressors were combined nearly 75% of the monkeys developed reproductive compromise that manifested as prolongation of the menstrual interval. It was thus concluded that the stressor interacted synergistically to compromise the hypothalamic-pituitary-ovarian axis. Furthermore, the complex interaction between reproductive compromise, energy imbalance, and HPA activation has been demonstrated in ovariectomized rhesus monkeys.23 In this study monkeys were given ghrelin, a key modulator of appetite and energy homeostasis that acts centrally and suppresses LH pulse frequency and elevates cortisol levels by activating the HPA axis. The addition of a nonselective corticotropin-releasing hormone receptor antagonist not only prevented an increase in cortisol, but also prevented the inhibitory effect of ghrelin on GnRH/luteinizing hormone pulse frequency. This study suggests that the deleterious impact of energy imbalance on the GnRH pulse generator is mediated by the HPA axis. Novel treatment strategies with CRH antagonists may be useful in the treatment of stress-induced reproductive dysfunction seen in patients with FHA. In this context, exercise-induced amenorrhea may be viewed as a variant form of FHA in which the recognizable stressor is exercise. While exercise is a commonplace stressor, it is also a commonplace coping mechanism. Unfortunately, when exercise is initiated as a means to cope with psychosocial stressors the exercise may actually exacerbate, rather than ameliorate, the neuroendocrine alterations that sustain FHA. Thus, it is better to cope with psychosocial stress by changing the stressors rather than by initiating a behavior that induces energy deficiency.
Metabolic and psychosocial factors that promote and sustain neuroendocrine allostasis may have profound health implications for the female athlete. Disturbances in gonadal, adrenal and thyroid function have both immediate and long term health consequences. A disruption of GnRH drive results in reduced LH and FSH which are necessary for folliculogenesis and ovulation and thus results in oligomenorrhea or amenorrhea. Ovulatory dysfunction can result in difficulty conceiving. In addition, estrogen deficiency may increase a woman’s long-term risk for osteoporosis, cardiovascular disease, dementia, depression, and other neurodegenerative and psychiatric disorders.24
Profound detrimental health consequences of athletic amenorrhea are not only limited to amenorrheic, hypoestrogenic women, but also to their future pregnancies, whether spontaneous or assisted. The consequences of alterations to the adrenal and thyroid axes may be less clinically appreciated in women who are not pregnant. However, adrenal and thyroid dysfunction can have a profound impact, particularly in the setting of pregnancy. Several studies have demonstrated that maternal stress is associated with low birth weights and preterm delivery.25, 26 In addition, a recent study showed prenatal exposure to maternal psychosocial stress altered subsequent regulation of the HPA axis in young adults.27 Healthy young adults whose mothers experienced severe stress during pregnancy were noted to have higher ACTH levels and increased cortisol production in response to a social stress test. Prior to the social stress test, cortisol levels in the prenatally stressed group were noted to be lower when compared to the comparison group. Interesting, the prenatally stressed group had lower cortisol levels in response to a 1μg ACTH stimulation test. The elevated ACTH levels in response to stress suggests that there is increased reactivity at the level of the pituitary, while the lower baseline cortisol levels and blunted response to the ACTH stimulation test suggest counter-regulatory adaptations with dampening of signaling at the level of the adrenal gland.
Thyroid dysfunction, including overt and subclinical hypothyroidism, also has adverse affects on both the course of pregnancy and the developing fetus. Increased risks of miscarriage, preeclampsia, preterm delivery, and stillbirth are observed during pregnancy in women with thyroid dysfunction.28–30 Another study in children ages 7 to 9 years born to mothers with subclinical hypothyroxinemia showed impaired neuropsychological development with full-scale IQ scores averaging 7 points below matched controls.31 Thyroxine is known to be critical to neurogenesis and neuronal migration.
Given the potential negative health consequences of athletic amenorrhea, it is imperative to develop comprehensive treatment strategies to promote recovery of gonadal function and restoration of the HPA and HPT axes. Former treatment approaches focused on hormone replacement with the rationale based on the mindset that functional forms of hypothalamic hypogonadism represent only or primarily an alteration in the hypothalamic-pituitary-ovarian (HPO) axis. However, hormone replacement strategies have limited benefit because they do not promote recovery of the complex allostatic endocrine alterations. Furthermore, use of sex hormones masks deficits that accrue from altered adrenal and thyroid function. While fertility can be restored with exogenous administration of gonadotropins or pulsatile GnRH, fertility management alone will not permit recovery of the HPA and HPT axes. Failure to reverse the hormonal milieu induced by combinations of psychogenic and metabolic stress may increase the likelihood of poor obstetrical, fetal, or neonatal outcomes. In contrast, behavioral and psychological interventions that address problematic behaviors and attitudes have the potential to permit resumption of ovarian function in juxtaposition with the recovery of the adrenal and thyroid axes to restore endocrine balance and promote better individual, maternal, and child health.
Appropriate intervention depends on determining which behaviors need to be modified to promote full endocrine, including reproductive, recovery. One approach directed at reducing psychogenic stress in women with athletic amenorrhea is cognitive behavior therapy (CBT). This approach has been utilized quite affectively to address problematic attitudes and promote psychosocial harmony in order to restore ovulation and menstrual cyclicity in women with FHA.32 In a small study of 16 women with FHA randomized to CBT or observation, 6 out of 8 women randomized to CBT resumed ovulating within 20 weeks of the initiation of treatment as compared to 1 out of 8 in the observational group.33 Given the success observed in women with FHA, CBT might have a similar impact in women with athletic amenorrhea, although, to the best of our knowledge, CBT has not been tested in this population. Ultimately, a combination of CBT, stress management, and relaxation techniques coupled with adequate caloric intake may work best to restore internal homeostasis. With proper nutritional support, attention to avoiding excessive energy expenditure and the ability to cope with multiple everyday stressors women may continue to enjoy the benefits of exercise without detriment to their general and reproductive health.
Figure 1.
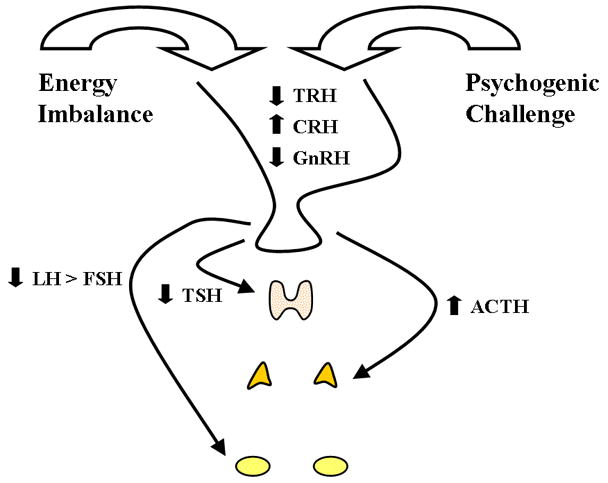
Diagram showing the major neuroendocrine alterations produced by the synergistic combination of metabolic and psychogenic stress. The proximate cause of athletic amenorrhea is reduced GnRH drive leading to reduced LH and FSH and secondary compromise of ovarian activity and hypoestrogenism. Athletic amenorrhea is a variant of functional hypothalamic anovulation (FHA) and concomitant neuroendocrine alterations include hypothalamic-pituitary-adrenal axis activation leading to increased circulatory and cerebrospinal fluid levels of cortisol and hypothalamic-pituitary-thyroidal suppression leading to variable degrees of hypothyroxinemia.
Acknowledgments
Funded by NCRR 00056 to the University of Pittsburgh School of Medicine and NIH RO1 MH50748 to SLB
References
- 1.U.S. Bureau of Labor Statistics. http://www.bls.gov/news.release/atus.nr0.htm.
- 2.Otis CL, et al. American College of Sports Medicine position stand. The Female Athlete Triad. Med Sci Sports Exerc. 1997;29:i–ix. doi: 10.1097/00005768-199705000-00037. [DOI] [PubMed] [Google Scholar]
- 3.De Souza MJ, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220–4232. doi: 10.1210/jcem.83.12.5334. [DOI] [PubMed] [Google Scholar]
- 4.De Souza MJ, et al. High prevalence of subtle and severe menstrual disturbances in exercising women: confirmation using daily hormone measures. Hum Reprod. 2009;25:491–503. doi: 10.1093/humrep/dep411. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan JR, Manuck SB. Ovarian dysfunction, stress, and disease: a primate continuum. ILAR J. 2004;45:89–115. doi: 10.1093/ilar.45.2.89. [DOI] [PubMed] [Google Scholar]
- 6.Reindollar RH, et al. Adult-onset amenorrhea: a study of 262 patients. Am J Obstet Gynecol. 1986;155:531–543. doi: 10.1016/0002-9378(86)90274-7. [DOI] [PubMed] [Google Scholar]
- 7.Laughlin GA, Dominguez CE, Yen SS. Nutritional and endocrine-metabolic aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1998;83:25–32. doi: 10.1210/jcem.83.1.4502. [DOI] [PubMed] [Google Scholar]
- 8.Berga SL, et al. Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1989;68:301–308. doi: 10.1210/jcem-68-2-301. [DOI] [PubMed] [Google Scholar]
- 9.Brundu B, et al. Increased cortisol in the cerebrospinal fluid of women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 2006;91:1561–1565. doi: 10.1210/jc.2005-2422. [DOI] [PubMed] [Google Scholar]
- 10.Berga SL, et al. Cerebrospinal fluid levels of corticotropin-releasing hormone in women with functional hypothalamic amenorrhea. Am J Obstet Gynecol. 2000;182:776–781. doi: 10.1016/s0002-9378(00)70326-7. discussion 781–774. [DOI] [PubMed] [Google Scholar]
- 11.Berga SL, Daniels TL, Giles DE. Women with functional hypothalamic amenorrhea but not other forms of anovulation display amplified cortisol concentrations. Fertil Steril. 1997;67:1024–1030. doi: 10.1016/s0015-0282(97)81434-3. [DOI] [PubMed] [Google Scholar]
- 12.Mirkes SJ, Bethea CL. Oestrogen, progesterone and serotonin converge on GABAergic neurones in the monkey hypothalamus. J Neuroendocrinol. 2001;13:182–192. doi: 10.1046/j.1365-2826.2001.00612.x. [DOI] [PubMed] [Google Scholar]
- 13.Bethea CL, et al. Sensitivity to stress-induced reproductive dysfunction linked to activity of the serotonin system. Fertil Steril. 2005;83:148–155. doi: 10.1016/j.fertnstert.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 14.Bethea CL, Centeno ML, Cameron JL. Neurobiology of stress-induced reproductive dysfunction in female macaques. Mol Neurobiol. 2008;38:199–230. doi: 10.1007/s12035-008-8042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loucks AB, et al. Alterations in the hypothalamic-pituitary-ovarian and the hypothalamic-pituitary-adrenal axes in athletic women. J Clin Endocrinol Metab. 1989;68:402–411. doi: 10.1210/jcem-68-2-402. [DOI] [PubMed] [Google Scholar]
- 16.Giles DE, Berga SL. Cognitive and psychiatric correlates of functional hypothalamic amenorrhea: a controlled comparison. Fertil Steril. 1993;60:486–492. [PubMed] [Google Scholar]
- 17.Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalamic amenorrhea. Fertil Steril. 2001;76:310–316. doi: 10.1016/s0015-0282(01)01921-5. [DOI] [PubMed] [Google Scholar]
- 18.Begel D. An overview of sport psychiatry. Am J Psychiatry. 1992;149:606–614. doi: 10.1176/ajp.149.5.606. [DOI] [PubMed] [Google Scholar]
- 19.Berga SL. Stress and reprodution: a tale of false dichotomy? Endocrinology. 2008;149:867–868. doi: 10.1210/en.2008-0004. [DOI] [PubMed] [Google Scholar]
- 20.Bullen BA, et al. Induction of menstrual disorders by strenuous exercise in untrained women. N Engl J Med. 1985;312:1349–1353. doi: 10.1056/NEJM198505233122103. [DOI] [PubMed] [Google Scholar]
- 21.Williams NI, et al. Longitudinal changes in reproductive hormones and menstrual cyclicity in cynomolgus monkeys during strenuous exercise training: abrupt transition to exercise-induced amenorrhea. Endocrinology. 2001;142:2381–2389. doi: 10.1210/endo.142.6.8113. [DOI] [PubMed] [Google Scholar]
- 22.Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab. 2007;293:E270–276. doi: 10.1152/ajpendo.00108.2007. [DOI] [PubMed] [Google Scholar]
- 23.Vulliemoz NR, et al. Astressin B, a nonselective corticotropin-releasing hormone receptor antagonist, prevents the inhibitory effect of ghrelin on luteinizing hormone pulse frequency in the ovariectomized rhesus monkey. Endocrinology. 2008;149:869–874. doi: 10.1210/en.2007-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Souza MJ, Williams NI. Physiological aspects and clinical sequelae of energy deficiency and hypoestrogenism in exercising women. Hum Reprod Update. 2004;10:433–448. doi: 10.1093/humupd/dmh033. [DOI] [PubMed] [Google Scholar]
- 25.Paarlberg KM, et al. Psychosocial predictors of low birthweight: a prospective study. Br J Obstet Gynaecol. 1999;106:834–841. doi: 10.1111/j.1471-0528.1999.tb08406.x. [DOI] [PubMed] [Google Scholar]
- 26.Wadhwa PD, Sandman CA, Garite TJ. The neurobiology of stress in human pregnancy: implications for prematurity and development of the fetal central nervous system. Prog Brain Res. 2001;133:131–142. doi: 10.1016/s0079-6123(01)33010-8. [DOI] [PubMed] [Google Scholar]
- 27.Entringer S, et al. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Horm Behav. 2009;55:292–298. doi: 10.1016/j.yhbeh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Leung AS, et al. Perinatal outcome in hypothyroid pregnancies. Obstet Gynecol. 1993;81:349–353. [PubMed] [Google Scholar]
- 29.Allan WC, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen. 2000;7:127–130. doi: 10.1136/jms.7.3.127. [DOI] [PubMed] [Google Scholar]
- 30.Abalovich M, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2002;12:63–68. doi: 10.1089/105072502753451986. [DOI] [PubMed] [Google Scholar]
- 31.Haddow JE, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 32.Berga SL, Loucks TL. Use of cognitive behavior therapy for functional hypothalamic amenorrhea. Ann N Y Acad Sci. 2006;1092:114–129. doi: 10.1196/annals.1365.010. [DOI] [PubMed] [Google Scholar]
- 33.Berga SL, et al. Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertil Steril. 2003;80:976–981. doi: 10.1016/s0015-0282(03)01124-5. [DOI] [PubMed] [Google Scholar]


