Abstract
Purpose
To demonstrate that voxels with inhomogeneously broadened water resonances, as revealed by high spectral and spatial resolution (HiSS) MRI, correlate with underlying tumor pathology findings, and thus carry diagnostically useful information.
Materials and Methods
Thirty four women with mammographically suspicious breast lesions were imaged at 1.5 T, using high resolution echo-planar spectroscopic imaging. Fourier component images (FCIs) of off-peak spectral signal were generated, and clusters of voxels with significant inhomogeneous broadening (broadened clusters) were identified and correlated to biopsy results.
Results
Inhomogeneously broadened clusters were found significantly more frequently in malignant than in benign lesions. A larger percentage of broadened cluster voxels were found inside the malignant vs. benign lesions. The high statistical significance for separation of benign and malignant lesions was robust over a large range of postprocessing parameters, with a maximum ROC area under curve of 0.83.
Conclusion
In human breast, an inhomogeneously broadened water resonance can serve as a correlate marker for malignancy, and is likely to reflect underlying anatomy or physiology.
Keywords: High spectral and spatial resolution MRI, echo-planar spectroscopic imaging, MRI of breast cancer, magnetic susceptibility imaging, sub-voxel anatomy and physiology, tumor vasculature
INTRODUCTION
Despite improvements in hardware and data analysis, diagnosis of breast cancer based on MRI results still lacks specificity, and improvements in sensitivity are also desirable. (1) Obtaining definitive clinical information within a single examination may require development of new types of contrast for breast MR imaging. This is a very active area of research, with diffusion and perfusion imaging, (2,3) elastography, (4) and equivalent cross-relaxation rate imaging (5) among techniques being explored. No technique has yet demonstrated adequate sensitivity and specificity for detection of early cancers, and a multi-parameter combination of many contrast mechanisms may be necessary.
A so-far untapped source of information is the spectral detail in complex, inhomogeneously broadened water resonances. The water resonance in small tissue voxels (on the order of 1 mm3) is often non-Lorentzian, and sometimes multiple components can be resolved. (6–11) It has been shown that different spectral components can be imaged separately, (12) that they may respond differently to changes in oxygenation, (6,13) injected contrast agents, (10) or stimulation, (14) and that they can be used to generate anatomical images. (15) A priori arguments supported by experimental evidence suggest that these components may arise from sub-voxelar, perhaps microscopic (8,10,12,16) environments that cannot be resolved by conventional imaging (e.g. intra-vs. extra-vascular water compartments). Hence, images of these separate spectral components may provide unique and potentially useful information. Conversely, important anatomic and functional information may be lost in conventional MRI, where these components are not resolved.
High spectral and spatial resolution (HiSS) MRI of the water resonance has the ability to resolve the fine structure of the water line (17,18). Data can be acquired using echo-planar spectroscopic imaging (EPSI), (19) with sub-millimeter spatial resolution. The spectral resolution that can be achieved (2.6 Hz at 1.5 T) is sufficient to identify distinct components of inhomogeneously broadened water peaks (10,20), and even to detect spectrally inhomogeneous effects of changes in blood deoxyhemoglobin (6,21,22) or injected contrast agents. (10)
In post-processing, spatial maps of individual spectral components, or “Fourier component images” (FCIs), can be produced, allowing anatomical correlation and providing a new type of MR contrast. FCIs constructed at different offsets from the water peak can differ significantly, reflecting the spectrally varying structure of the water resonance, and potentially underlying anatomical or physiological features. (18) Specifically, inhomogenous broadening of the water resonance will increase signal in off-peak components, and can be used as a source of MR contrast. (Where we, further in the text, define “broadened” voxels and clusters, inhomogeneous broadening is understood.) The high reproducibility of detailed water spectra detected by HiSS (18) supports the validity of generating individual FCIs and further analysis.
A previous report (18) was based on studies of a small number of patients (n = 13), and did not explore clinical correlates of FCIs. In the present work, FCIs of 32 patients are evaluated, and the presence of spatially correlated variations in water resonance structure is correlated to biopsy results and BIRADS rating. The ability of spatial FCI features to distinguish between benign and malignant breast lesions at a high level of statistical significance is demonstrated.
MATERIALS AND METHODS
Patient Population
Thirty four women with suspicious breast lesions found on mammography were scheduled to receive standard clinical MRI scans, and informed consent to additional HiSS scans was obtained. Nine patients had benign findings. The biopsy confirmed fibroadenosis (n = 1), sclerosing adenosis (n = 2), or atypical ductal hyperplasia (n = 1) for 4 patients, and for 5 the biopsy was deemed unnecessary based on MRI results, and was not performed. Of these, benign-appearing lesions were found in two cases, and no enhancing lesion was found in 3 cases; all were included in data analysis. In two patients with benign findings, the target lesion was not included in the HiSS slice, and these were excluded from the data analysis. Average lesion size for patients with benign findings was 10 mm. Three patients were found to have ductal carcinoma in situ (DCIS) on biopsy (average lesion size 15 mm). Twenty patients were found to have invasive disease (average lesion size 29 mm). The biopsy results confirmed that 18 patients had invasive ductal carcinoma (IDC) with (n = 9) or without (n = 9) accompanying DCIS, one had IDC with sclerosing adenosis, and one had invasive lobular carcinoma with lobular carcinoma in situ. None of the patients presented with multiple lesions in the targeted area, and therefore there was no ambiguity as to which lesion on the MRI scans was biopsied. The patients with negative MRI findings were not recalled for follow-up MR exams as part of this study.
HiSS slice positioning
The HiSS slice was positioned by a Radiologist, based on the X-ray mamography films, and the general anatomy, as visualized on pre-contrast T2-weighted sequence. The location of the HiSS slice, with reference to other acquisition sequences, was later confirmed on the reading workstation. Two patients with benign findings were found to have enhancing lesions that were missed in the HiSS sequence, and they were not included in the data analysis.
Data acquisition
MR images were obtained on a 1.5 Tesla clinical MRI scanner (General Electric) equipped with ECHO SPEED PLUS™ gradients, using a dedicated, 2-element phased array breast coil. The HiSS sequence was implemented using echo planar spectroscopic imaging (EPSI). (19) HiSS images required an additional 2 minutes of scan time, and were acquired immediately prior to contrast agent injection, from a single sagittal slice through the lesion. The readout gradient was applied in the anterior/posterior direction to minimize respiratory and cardiac motion artifacts. Shimming was performed using the standard GE protocol, which was tested by imaging water phantoms containing 2 mM copper sulfate, using the same parameters as in breast imaging (described below). The water resonance in all image voxels sampled during testing was found to be a symmetrical Lorentzian with line width of less than 2 Hz, indicating that no artifacts due to poor shimming or eddy currents were present. Acquisition parameters were: TR = 500 ms, nominal TE ≈ 195 ms, flip angle = 60 degrees; acquisition matrix = 384 × 256 matrix, field-of-view = 24×16 cm, in-plane spatial resolution = 0.63 mm, slice thickness = 4 mm, voxel volume ≈ 1.5 mm3. For each phase-encoded line of k-space, a gradient echo train of 128 echoes was acquired, following which a crusher gradient was applied. The total sampling time was 384 ms, and echo spacing was 3 ms, resulting in spectral resolution of 2.6 Hz and spectral bandwidth of 333 Hz. This was sufficient to resolve the water and fat resonances (separated by approximately 220 Hz at 1.5 T) as well as to resolve the fine structure of the water resonance itself. Earlier studies found high reproducibility of HiSS-imaged water resonance spectra. (18)
Data analysis and synthesis of Fourier Component Images
After a 3D Fourier transform of the acquired data with respect to two k-space axes and the evolution of the FID (10,17,20,23,24), a high resolution spectrum of the water and fat resonances was obtained in each image voxel. Fat and water spectral peaks were identified, and fat and baseline signal were removed in post-processing by fitting the resonances to Lorentzian functional forms, with a linear function describing the baseline, and subtracting the fitted values from the spectrum. These robust methods were described in greater detail previously. (17,18) Images proportional to the peak signal of the water resonance (water peak height images) were synthesized. Magnitude spectra were used for all image synthesis. Data acquired during the first echo in the HiSS echo train was used to generate T1-weighted, pre-contrast images of the lesion.
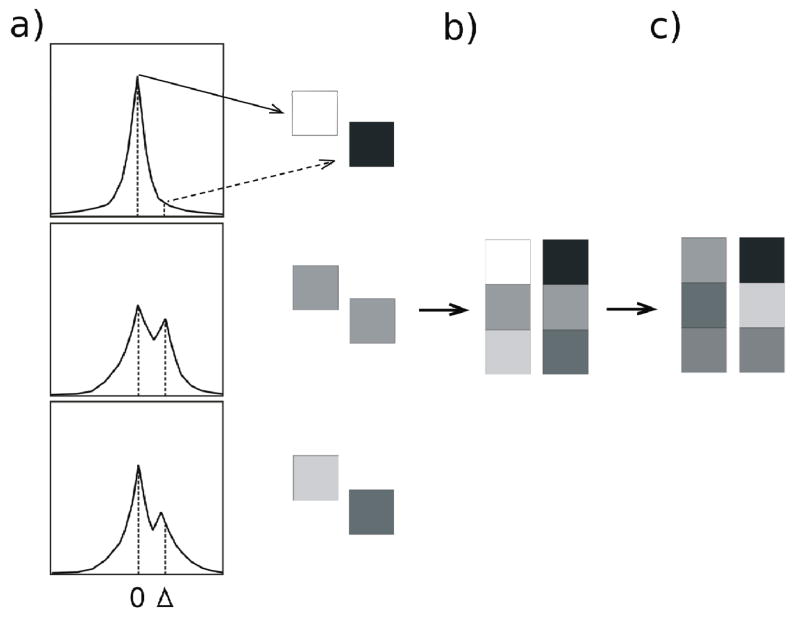
The process of FCI synthesis is explained in Fig. 1, where Fig. 1a shows schematically the water spectra in three voxels with different water resonance structure, typical of lineshapes observed experimentally. The spectra are centered at the peak frequency, and the vertical lines correspond to a zero-offset, and an arbitrary offset Δ from the peak. In b), 3-voxel ‘images’ are generated, with intensities proportional to spectral intensities at the spectral peak (FCI0, left) and at offset Δ (FCIΔ right). In order to be meaningfully compared, Fourier component images are normalized to same average intensity (c), revealing strikingly different inherent contrast which arises from differences in the water resonance structure. Inhomogeneously broadened voxels will have higher intensity FCIΔ than voxels with Lorentzian water lineshapes.
Figure 1.
a) A schematic view of the isolated water resonance in three different voxels is shown (top to bottom). The intensity and frequency units are arbitrary, and spectra are centered on the intensity peaks. The vertical lines correspond to the center frequency (zero offset from peak), and an arbitrary frequency offset Δ from the peak. b) Peak intensity (solid arrow) and off-peak intensity (at offset Δ from spectral peak, dashed arrow) values can be used to generate Fourier component images (FCI0 and FCIΔ). c) When FCIs are normalized to same average intensity, they reveal strikingly different inherent contrast, reflecting differences in spatial variation of different water components.
FCIs were generated at various offsets from the peak, in increments of 2.6 Hz (1 frequency bin), and are referred to as ‘FCIΔ’, where Δ is the offset from spectral peak, in Hz. For example, the water peak height images (0 Hz offset) are denoted as FCI0, and FCIs generated at 10.4 Hz offset are denoted as FCI10. Each FCIΔ was locally normalized, so that its average value was 1:
| (1) |
where S is the spectral intensity at spectral offset Δ from peak frequency f0, at location (x,y). The sum in the denominator is over a 5 × 5 cm area surrounding the lesion (80 × 80 voxels) in which FCIs were constructed. “FCI difference images” (FCIΔ − FCI0) were produced and found previously to be highly reproducible. (18)
Feature Segmentation
Automatic segmentation software was applied to FCI difference images over a 5 × 5 cm area surrounding the lesion (80 × 80 voxels) in the following manner: Standard deviation σ of noise in FCI difference images was calculated from a manually selected region of interest (ROI) positioned close to the lesion and containing negligible water signal. Voxels were considered “broadened” if the intensity was higher than a certain multiple of σ, i.e. Nσ * σ. In analogy to functional MRI, connected clusters of at least Nc broadened voxels were selected as “broadened clusters”. Broadened clusters reflect anatomic regions with higher normalized intensity in the off-peak FCI, compared to FCI0, and also compared to other voxels. This could be due to spectral shoulders or partially resolved off-peak components, or any kind of inhomogeneous broadening of the water resonance.
Statistical Considerations
The noise distribution in FCI difference images is assumed to be Gaussian. Then the significance level of each broadened voxel is 5e-2 for Nσ = 2, 3e-3 for Nσ = 3, 6e-5 for Nσ = 4, etc. Thus starting with Nσ = 3, the existence of each broadened voxel is very highly statistically significant. The statistical significance of broadened clusters is harder to quantify, due to geometrical considerations (contiguity, lesion size and shape, spatial correlation due to underlying anatomy), but is not less than that of a single broadened voxel. Using the presence of the broadened clusters as a classifier, performance for the task of distinguishing malignant and benign lesions was assessed using the ROC method. Curves were generated by varying 3 <= Nc <= 12, and for 3 <= Nσ <= 9. ROC area under curve (AUC) values were calculated using the trapezoidal method, and used as a metric for classifier performance. The statistical significance for the task of separating benign and malignant lesions separation was calculated using a one-sided Student’s test for proportions for both the percentages exhibiting broadened clusters, and percentage of broadened cluster voxels located within lesions,.
RESULTS
Strong differences from the water peak height image (FCI0) were observed in FCIs constructed at offsets ranging from −15 Hz to +15 Hz. However, they were most frequently seen in FCI10 images, as observed in a previous study, (17,18) and hence FCI10 images were selected for further calculations. Notably, the differences were less prominent in images generated at −10Hz from the peak (in FCI-10), pointing to asymmetry in the broadened water line shape.
We examined the potential of broadened clusters to serve as marker of malignancy. ROC curves were generated by varying Nc, for a range of Nσ. Given the high statistical significance of broadened voxels, little variation in ROC AUCs with respect to Nσ was expected. Indeed, the ROC AUC varied between 0.75 and 0.83 for 3 ≤ Nσ ≤ 9, and was maximized for Nσ = 6. The corresponding ROC curve is shown in Figure 2. At Nσ = 6, the highest statistical significance (p = 0.0002) for separation of benign and malignant lesions was achieved at minimum cluster size Nc = 3 (solid black point). This value of Nc was used to generate FCIs depicted in Figs 3 and 4. However, it is important to note that the separation at almost all Nc tested (3 ≤ Nc ≤ 12) was significant at the p = 0.02 level.
Figure 2.
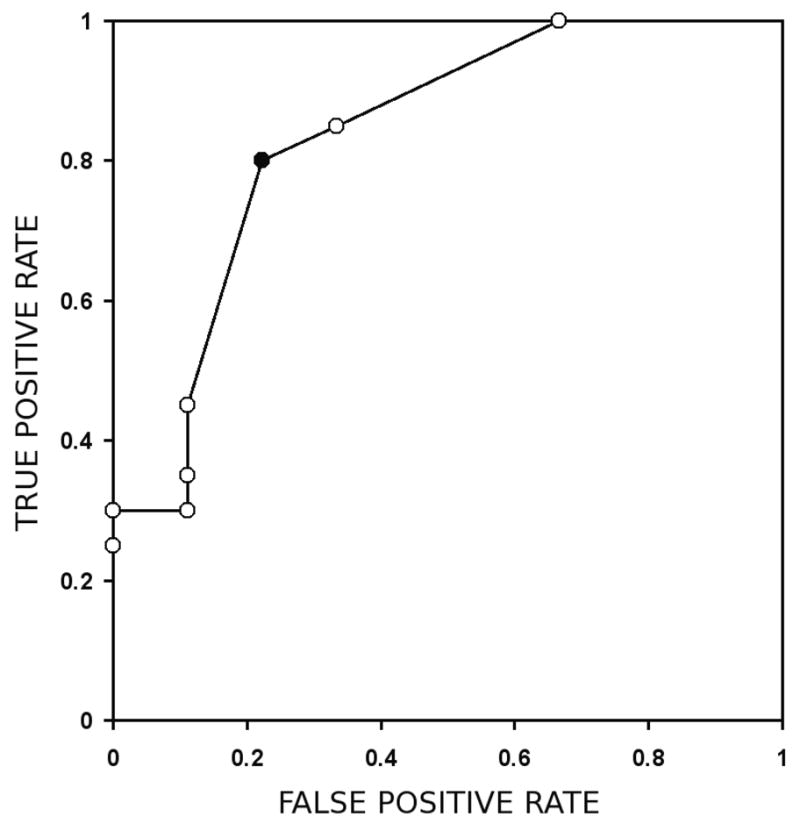
The ROC curve with a maximum AUC is obtained for Nσ = 6 and, with a ROC AUC of 0.83. The black data point corresponds to the data point with the separation of benign and malignant lesions to the highest level of statistical significance, and is obtained for Nc = 3.
Figure 3.
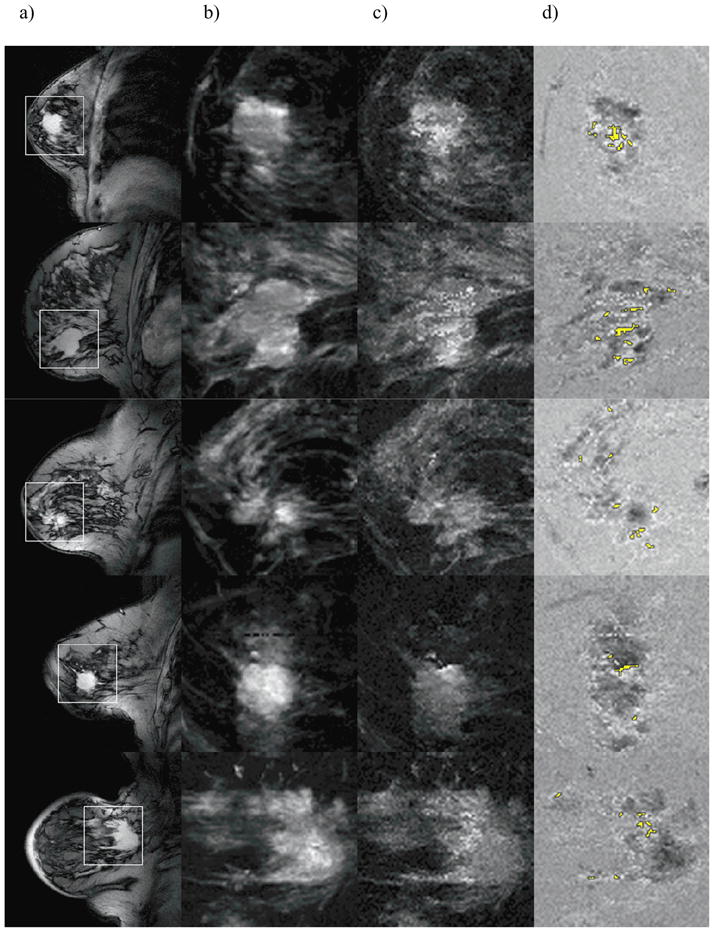
A T1-weighted (a) image is shown for 5 patients with IDC lesions confirmed on biopsy, and a 5 × 5 cm region surrounding the lesion is outlined by the white square. FCI0 and FCI10 in the outlined region are shown (b and c), and their difference is shown in (d). Broadened clusters are highlighted in yellow. The difference image in (d) was calculated after FCIs were normalized to same average intensity.
Figure 4.
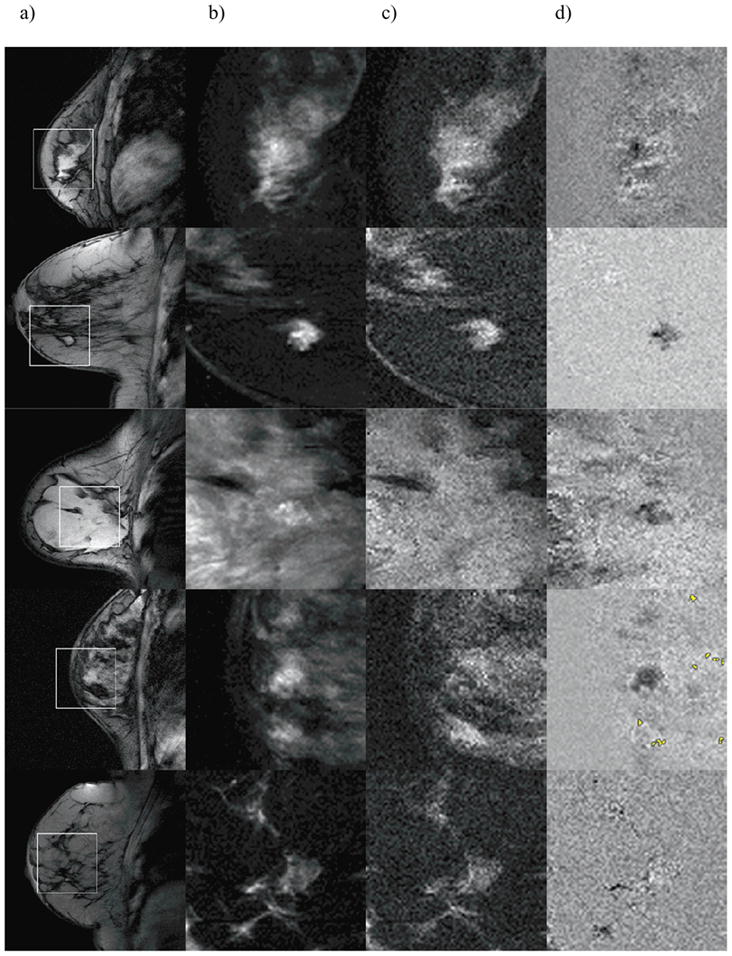
T1-weighted image (a), FCI0 (b) and FCI10 (c), as well as their difference (d) are shown for five patients with benign findings, analogously to Figure 2. Broadened clusters are highlighted in yellow in row 4. No broadened clusters were found in rows 1–3 and 5.
Table 1 summarizes the number and percentage of patients in each diagnostic category for whom broadened clusters were detected, regardless of whether they appeared in- or outside the lesion. The presence of broadened clusters in the difference images is highly statistically significant because of the very conservative selection criteria (see ‘Methods and Materials’ section). Broadened clusters were significantly more frequent in patients with biopsy-confirmed invasive disease, than in patients with benign findings (p = 0.0002). When only broadened clusters within the lesion were taken into account, the difference was significant at the p = 10e-6 level. No broadened clusters were detected within benign or DCIS lesions, while for patients with an invasive finding, 55% of the patients had broadened clusters detected within the lesion, and 84% of total broadened cluster voxels were found within lesions.
Table 1.
The statistics for number of patients with broadened clusters and number of broadened cluster voxels in each diagnostic category
| Diagnosis | Np | Np with BCs | Np with BCs (in lesion only) | BC voxels in lesion |
|---|---|---|---|---|
| Benign | 9 | 1 (11%) | 0 (0%) | 0% |
| DCIS | 3 | 1 (33%) | 0 (0%) | 0% |
| IDC | 20 | 16 (80%) | 11 (55%) | 84% |
| Benign/IDC Significance level | p = 0.0002 | p ~ 10e-6 |
Np: number of patients
BC: broadened cluster
DCIS: ductal carcinoma in situ
IDC: invasive ductal carcinoma
In Fig. 3, T1-weighted pre-contrast images, derived from first-echo HiSS data, of five patients with IDC confirmed by biopsy are shown (a). The white squares outline a 5 × 5 cm region surrounding the lesion, shown under magnification in panels (b–d). Figure 2b shows FCI0 (water peak height image), and Fig. 2c shows FCI10. In Fig. 2d, FCI difference images (FCI10 − FCI0) are shown. The marked non-uniformity in difference images reflects differences in inherent contrast between FCI0 and FCI10. Specifically, broadened water resonances, where an off-resonance component is present at 10Hz offset from the peak, produce bright voxels. Broadened clusters segmented using Nσ = 6 and Nc = 3, and containing groups of voxels with strong broadening are colored yellow. They typically appear within or on the boundary of a lesion, although in many cases, broadened clusters were found in regions believed to contain parenchyma (see Fig. 4).
Figure 4 shows the same information as Fig. 3, for five patients with benign findings (rows 1 to 3: biopsy confirmed fibroadenoma, sclerosing adenosis, and atypical ductal hyperplasia; rows 4 and 5: no biopsy was performed). Only one of nine patients (row 4) with a benign finding showed broadened clusters, either in or outside the lesion.
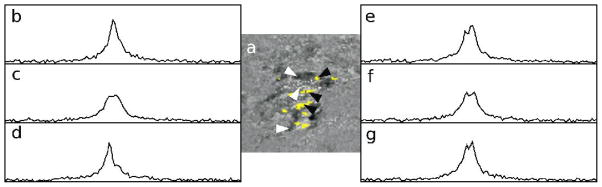
In Fig. 5, examples of water resonance spectra at voxels that are marked, and that are not marked as broadened are shown. The image in a) is an FCI difference image of an IDC lesion, with broadened voxels highlighted in yellow. It is identical to the image in Fig. 3d, second row. The spectra in non-broadened voxels typically have a narrow, Lorentzian peak, as shown in b), although minor splitting (c), or asymmetry (d) are possible. In broadened voxels, splitting of the water resonance is strong (e–g), and when compared to voxels in which there is no second peak, gives rise to the differential contrast in FCI0 and FCI10 images.
Figure 5.
a) FCI difference image of an IDC lesion, with broadened voxels highlighted in yellow is shown. This is the same image as Fig 3d, second row. b–g) Samples of water resonance spectra obtained using HiSS imaging is shown for three voxels not marked as ‘broadened’ (b–d, white arrowheads, top to bottom), and three voxels that are marked as broadened (e–g, black arrowheads, top to bottom).
The outcomes of the standard clinical imaging, with the numbers of patients in each BIRADS category, are presented in Table 2. The number of patients with broadened clusters is shown for each BIRADS category, and their percentage is calculated for BIRADS categories 1–4 (negative, normal, benign or probably benign; 25%) and 5 and 6 (probable or known cancer; 79%), cumulatively. This difference is statistically significant (p = 0.0003). The DCEMRI kinetics data were available for 24 patients. There were no patients with a slow initial uptake, and there were no patients with broadened clusters among 4 cases of medium initial uptake (1 DCIS, 3 benign). Only patients with rapid uptake, 13 of 20 (12/16 IDC, 1/2 DCIS, 0/2 benign) showed broadened clusters. Of these, 1 of 3 patients with persistent uptake (1/2 IDC, 0/1 DCIS), 3 of 5 patients with plateau (3/3 IDC, 0/2 benign) and 9 of 12 patients with washout delayed kinetics (8/11 IDC, 1/1 DCIS), showed broadened clusters.
Table 2.
The statistics for number of patients and patients with broadened clusters in BIRADS categories of standard clinical exam
| BIRADS category | Np | total Np | Np with BCs | percent |
|---|---|---|---|---|
| 0 | 1 | 0 | ||
| 1 | 2 | 0 | ||
| 2 | 1 | 1 | ||
| 3 | 0 | 0 | ||
| 4 | 9 | 2 | ||
| 1,2,3,4 total | 12 | 3 | 25% | |
| 5 | 11 | 8 | ||
| 6 | 8 | 7 | ||
| 5,6 total | 19 | 15 | 79% | |
| Statistical significance | p = 0.0003 |
Np: number of patients
BC: broadened cluster
DISCUSSION
Strong off-peak signal in water resonances, producing broadened clusters, was detected with significantly higher frequency in malignant lesions, compared to benign lesions, and was typically detected within lesions, rather than the surrounding parenchyma. Thus broadened clusters can be understood as markers for malignancy. This is meaningful even when they are detected in the parenchyma neighboring the lesion, as they could correlate to pre-cancerous changes in the parenchyma, or blood vessels recruited by the tumor.
Broadened clusters highlight a spatially extended region in which significant inhomogeneous broadening of the water resonance occurs. It is not clear though, what the broadened clusters correlate to at the microscopic level. Off-resonance water signal is the result of local microscopic (sub-voxel) variations in magnetic susceptibility – which could be produced at the boundaries of blood vessels, microcalcifications, and other anatomic boundaries. (25) Previously published data (26) suggests that regions with high vascular density, particularly deoxygenated blood vessels that are common in tumors, give rise to off-resonance features. The non-random frequency offset of the off-resonance signal (being most frequent at 10 Hz) further supports its physiological origin. Animal model studies that seek to correlate HiSS-derived information, including broadened clusters, to histology, as well as phantom studies seeking to characterize the effect of calcifications on water resonance structure are currently underway. (27) (28) If broadened clusters are markers for sub-voxel anatomy and physiology, HiSS MRI could image information not currently accessible with other MR imaging methods, potentially leading to higher specificity in breast MR diagnostics and screening. The further study of off-resonance water signal – either in the current image-processing context intuitively familiar to Radiologists – or in a more rigorous spectral analysis context, is warranted. Two potential directions that are currently explored are sensitive studies of water peak asymmetry, and modeling the water volume with a two compartment model, and thus the water resonance as a sum of two Lorentzian peaks, to determine whether this increases sensitivity to cancers.
It is important to note that the strong peak splitting or asymmetry observed in many voxels, and by extension broadened clusters, cannot be explained as artifacts. Improper shimming and time-dependent eddy currents were discounted with phantom experiments, as described in the Methods and Materials section. Motion artifacts would distribute signal along the phase encoding direction, and resulting off-resonance components would be of low intensity, random frequency offset, and would occur along the phase encoding direction. Chemical shift artifacts could not produce broadened clusters, as water and fat resonances are extremely well resolved. The observed peak splitting and asymmetry (FCI10 producing features more frequently than FCI-10), speak against any type of homogenous broadening as source of broadened clusters.
There are limitations to our study: the normalization of FCIs is currently dependent on the local anatomy (e.g. number of lesion vs. parenchyma voxels), and although this is an accepted ‘edge sharpening’ technique, an independent normalization method would be desirable. Also, the nature of the broadened clusters detected outside the lesion remains to be determined. It is tempting to attribute them to noise, based on positive findings in benign patients, but there are strong reasons not to do so. The statistical significance of broadened clusters is very high, and therefore they are not attributable to noise. Further, about a third of detected malignant lesions were only flagged due to broadened clusters present in nearby parenchyma. Thus broadened clusters could be sensitive to early pre-cancerous changes in the parenchyma, or to the additional blood vessels recruited by the tumor. Another limitation of this study is the single-slice coverage of the lesion, chosen in order to minimize impact on the clinical imaging protocol. After utility of FCIs is established, a stronger rationale for multi-slice FCI can be made, and a higher coverage of the lesion can be achieved. Finally, a larger study sizes of benign and malignant lesions are matched would strengthen the statistical findings. The present study does not include the analysis of post-contrast FCI images, as the contrast agent broadens the water resonance, potentially making water resonance FCI analysis more difficult. Instead the present study focuses on the use of pre-contrast HiSS to identify and stage tumors. Nevertheless, use of HiSS to detect effects of contrast agents, based on spectrally inhomogeneous effects of contrast agents (10) are of interest, and may be clinically useful. Effects of contrast agents will be the subject of later reports.
The present results are encouraging. First, they demonstrate a highly statistically significant correlation between contrast in FCI images and the BIRADS category determined from the standard clinical exam. Second, the ROC AUC value of 0.83 is very promising and suggests that FCI analysis may have diagnostic utility. A large multivariate study of currently used breast MRI diagnostic features found ROC AUCs for individual features to range between 0.54–0.78. (29) For example, the best-performing feature in focal mass enhanced lesions was the lesion margin with ROC AUC = 0.76; the best-performing feature derived from dynamic imaging was the qualitative description of contrast agent kinetics, with ROC AUC = 0.66. Both of these features are widely used and considered good predictors of tumor grade. Thus FCI analysis has a lot of potential to increase diagnostic accurace, especially as part of a multi-variate model. It should be noted that a large number of additional parameters, e.g. the frequency offset, or imaging sequence parameters, have not been optimized yet, and thus an even better ROC performance is possible.
With that in mind, it is important to consider the correlation of broadened clusters with other diagnostic features, and especially to parameters derived from DCEMRI, since data on animal models of cancer demonstrate such a correlation. (26) The present report demonstrates that patients who exhibit ‘malignant-like’ contrast kinetics are more likely to demonstrate broadened clusters, and a more detailed study with improved spatial correlation is in progress. However, correlation does not mean that broadened clusters and DCEMRI signal carry the same information. For example, DCEMRI parameters depend on perfusion and vascular permeability, while water resonance broadening could depend on the volume of deoxygenated blood – thus independent new information could be gained from HiSS data. On the other hand, even if water resonance broadening and DCEMRI signal carry largely the same information, HiSS imaging could become a valuable tool to substitute for DCEMRI in patients for whom contrast agent administration is a health risk. HiSS pre-contrast images could also be used to guide prescription of subsequent high temporal resolution DCEMRI scans.
CONCLUSIONS
In conclusion, we show that reproducible FCIs and broadened clusters can be generated from HiSS datasets in human breast, and perform well in the task of separating the malignant and benign lesions. Different FCIs show markedly different inherent contrast due to the fact that the water resonance is highly spatially heterogeneous, and the voxels containing off-resonance shoulders and/or partially resolved components of the water signal that differ in amplitude and frequency. These structures, when selected at a high statistical level of significance, occur significantly more frequently in and around malignant lesions. They are also likely to have anatomical or physiological correlates. A high ROC AUC for separation of benign and malignant lesions is obtained through analysis of contrast in Fourier component images, and the separation was achieved with a high statistical significance. FCI imaging can enhance breast MRI specificity by providing means of direct imaging of sub-voxelar environments, and thus can potentially aid MR breast cancer diagnostics and/or screening,. This novel source of MRI contrast is derived from the inhomogeneous broadening of the water resonance, and likely cannot be duplicated using conventional methods.
Acknowledgments
Grant Support: This work was funded by the NCI (1RO1CA78803, and 1P50CA125183-01), NIBIB (5RO1EB003108-03), and the Paul C. Hodges Society.
This work was funded by the NCI (1RO1CA78803, and 1P50CA125183-01), NIBIB (5RO1EB003108-03), and the Paul C. Hodges Society. The authors thank Dr. Alan Koretsky and Dr. Charles Springer for their helpful advice, and the American Cancer Society volunteers of Will and Grundy Counties, in Illinois, for their enthusiastic support.
References
- 1.Heywang-Köbrunner SH, Bick U, Bradley WG, Jr, et al. International investigation of breast MRI: results of a multicentre study (11 sites) concerning diagnostic parameters for contrast-enhanced MRI based on 519 histopathologically correlated lesions. Eur Radiol. 2001;11(4):531–546. doi: 10.1007/s003300000745. [DOI] [PubMed] [Google Scholar]
- 2.Kvistad KA, Rydland J, Vainio J, et al. Breast lesions: evaluation with dynamic contrast-enhanced T1-weighted MR imaging and with T2*-weighted first-pass perfusion MR imaging. Radiology. 2000;216(2):545–553. doi: 10.1148/radiology.216.2.r00au36545. [DOI] [PubMed] [Google Scholar]
- 3.Sinha S, Sinha U. Functional magnetic resonance of human breast tumors: diffusion and perfusion imaging. Ann N Y Acad Sci. 2002;980:95–115. doi: 10.1111/j.1749-6632.2002.tb04891.x. [DOI] [PubMed] [Google Scholar]
- 4.McKnight AL, Kugel JL, Rossman PJ, Manduca A, Hartmann LC, Ehman RL. MR elastography of breast cancer: preliminary results. AJR Am J Roentgenol. 2002;178(6):1411–1417. doi: 10.2214/ajr.178.6.1781411. [DOI] [PubMed] [Google Scholar]
- 5.Yuen S, Yamada K, Kinosada Y, et al. Equivalent cross-relaxation rate imaging of breast cancer. J Magn Reson Imaging. 2004;20(1):56–65. doi: 10.1002/jmri.20088. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hallaq HA, Fan X, Zamora M, River JN, Moulder JE, Karczmar GS. Spectrally inhomogeneous BOLD contrast changes detected in rodent tumors with high spectral and spatial resolution MRI. NMR Biomed. 2002;15(1):28–36. doi: 10.1002/nbm.728. [DOI] [PubMed] [Google Scholar]
- 7.Karczmar GS, Fan X, Al-Hallaq HA, et al. Uptake of a superparamagnetic contrast agent imaged by MR with high spectral and spatial resolution. Magn Reson Med. 2000;43:633–639. doi: 10.1002/(sici)1522-2594(200005)43:5<633::aid-mrm3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Karczmar GS, Fan X, Al-Hallaq H, et al. Functional and anatomic imaging of tumor vasculature: high-resolution MR spectroscopic imaging combined with a superparamagnetic contrast agent. Acad Radiol. 2002;9 (Suppl 1):S115–118. doi: 10.1016/s1076-6332(03)80414-2. [DOI] [PubMed] [Google Scholar]
- 9.Karczmar GS, Du W, Medved M, et al. Spectrally inhomogeneous effects of contrast agents in breast lesion detected by high spectral and spatial resolution MRI. Acad Radiol. 2002;9 (Suppl 2):S352–354. doi: 10.1016/s1076-6332(03)80227-1. [DOI] [PubMed] [Google Scholar]
- 10.Du W, Du YP, Bick U, et al. Breast MR Imaging with High Spectral and Spatial Resolutions: Preliminary Experience. Radiology. 2002;224(2):577–585. doi: 10.1148/radiol.2242011022. [DOI] [PubMed] [Google Scholar]
- 11.Foxley S, Fan X, Mustafi D, et al. Quantitative analysis of water proton spectral lineshape: a novel source of contrast in MRI. Phys Med Biol. 2008;53(17):4509–4522. doi: 10.1088/0031-9155/53/17/003. [DOI] [PubMed] [Google Scholar]
- 12.Zhong K, Li X, Shachar-Hill Y, Picart F, Wishnia A, Springer CSJ. Magnetic susceptibility shift selected imaging (MESSI) and localized (1)H(2)O spectroscopy in living plant tissues. NMR Biomed. 2000;13(7):392–397. doi: 10.1002/1099-1492(200011)13:7<392::aid-nbm659>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Al-Hallaq HA, Zamora M, Fish BL, Farrell A, Moulder JE, Karczmar GS. MRI measurements correctly predict the relative effect of tumor oxygenating agents on hypoxic fraction in rodent BA1112 tumors. Int J Radiat Oncol Biol Phys. 2000;47:481–488. doi: 10.1016/s0360-3016(00)00445-4. [DOI] [PubMed] [Google Scholar]
- 14.Reichenbach JR, Jonetz-Mentzel L, Fitzek C, et al. High-resolution blood oxygen-level dependent MR venography (HRBV): a new technique. Neuroradiology. 2001;43(5):364–369. doi: 10.1007/s002340000503. [DOI] [PubMed] [Google Scholar]
- 15.Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997;204(1):272–277. doi: 10.1148/radiology.204.1.9205259. [DOI] [PubMed] [Google Scholar]
- 16.Naritomi H, Kanashiro M, Sasaki M, Kuribayashi Y, Sawada T. In vivo measurements of intra- and extracellular Na+ and water in the brain and muscle by nuclear magnetic resonance spectroscopy with shift reagent. Biophys J. 1987;52(4):611–616. doi: 10.1016/S0006-3495(87)83251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medved M, Du W, Zamora MA, et al. The effect of varying spectral resolution on the quality of high spectral and spatial resolution magnetic resonance images of the breast. J Magn Reson Imaging. 2003;18(4):442–448. doi: 10.1002/jmri.10378. [DOI] [PubMed] [Google Scholar]
- 18.Medved M, Newstead GM, Fan X, et al. Fourier components of inhomogeneously broadened water resonances in breast: a new source of MRI contrast. Magn Reson Med. 2004;52(1):193–196. doi: 10.1002/mrm.20115. [DOI] [PubMed] [Google Scholar]
- 19.Mansfield P. Spatial mapping of the chemical shift in NMR. Magn Reson Med. 1984;1:370–386. doi: 10.1002/mrm.1910010308. [DOI] [PubMed] [Google Scholar]
- 20.Kovar DA, Al-Hallaq HA, Zamora MA, River JN, Karczmar GS. Fast spectroscopic imaging of water and fat resonances to improve the quality of MR images. Acad Radiol. 1998;5(4):269–275. doi: 10.1016/s1076-6332(98)80226-2. [DOI] [PubMed] [Google Scholar]
- 21.Al-Hallaq HA, Zamora M, River JN, Karczmar GS. MR correctly predicts the relative effect of two tumor oxygenating agents on hypoxic fraction in rodent BA1112 tumors. Philadelphia: International Society for Magnetic Resonance in Medicine; 1999. [DOI] [PubMed] [Google Scholar]
- 22.Oikawa H, Al-Hallaq HA, Lewis MZ, River JN, Kovar DA, Karczmar GS. Spectroscopic imaging of the water resonance with short repetition time to study tumor response to hyperoxia. Magn Reson Med. 1997;38(1):27–32. doi: 10.1002/mrm.1910380106. [DOI] [PubMed] [Google Scholar]
- 23.Fan X, Du W, MacEneaney P, Zamora M, Karczmar G. Structure of the water resonance in small voxels in rat brain detected with high spectral and spatial resolution MRI. J Magn Reson Imaging. 2002;16(5):547–552. doi: 10.1002/jmri.10193. [DOI] [PubMed] [Google Scholar]
- 24.Kovar DA, Karczmar GS. Fast spectroscopic imaging of water and fat proton resonances improves image contrast and signal-to-noise ratio. Vancouver, Canada: 1997. [Google Scholar]
- 25.Medved M, Newstead G, Abe H. Water resonance structure obtained in high spectral and spatial resonance MRI of human breast lesions reveals two predominant components. South Beach, Miami, Florida: International Society for Magnetic Resonance in Medicine; 2005. [Google Scholar]
- 26.Foxley S, Fan X, Mustafi D, et al. Sensitivity to tumor micro-vasculature without contrast agents in high spectral and spatial resolution MR images. Magnetic Resonance in Medicine. doi: 10.1002/mrm.21801. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haney CR, Pelizzari CA, Foxley S, et al. Validation of EPSI Angiography by Image Co-registration. Berlin, Germany: 2007. [Google Scholar]
- 28.Mustafi D, River JN, Foxley S, Jansen SA, Newstead GM, Karczmar GS. In Vitro MRI Identification and Characterization of Small Calcium Crystals for Probing Micro-calcifications in Breast Cancer. Chicago, IL: 2008. [Google Scholar]
- 29.Schnall MD, Blume J, Bluemke DA, et al. Diagnostic architectural and dynamic features at breast MR imaging: multicenter study. Radiology. 2006;238(1):42–53. doi: 10.1148/radiol.2381042117. [DOI] [PubMed] [Google Scholar]