Abstract
Background
Corticobasal degeneration (CBD) is a neurodegenerative disease characterized pathologically by neuronal loss, gliosis and tau deposition in neocortex, basal ganglia and brainstem. Typical clinical presentation is known as corticobasal syndrome (CBS) and involves the core features of progressive asymmetric rigidity and apraxia, accompanied by other signs of cortical and extrapyramidal dysfunction. Asymmetry is also emphasized on neuroimaging.
Objective
To describe a series of cases of CBD with symmetric clinical features and to compare clinical and imaging features of these symmetric CBD cases (S-CBD) to typical cases of CBS with CBD pathology.
Methods
All cases of pathologically confirmed CBD from the Mayo Clinic Rochester database were identified. Clinical records were reviewed and quantitative volumetric analysis of symmetric atrophy on head MRI using atlas based parcellation was performed. Subjects were classified as S-CBD if no differences had been observed between right- and left-sided cortical or extrapyramidal signs or symptoms. S-CBD cases were compared to 10 randomly selected typical CBS cases.
Results
Five cases (2 female) met criteria for S-CBD. None had limb dystonia, myoclonus, apraxia or alien limb phenomena. S-CBD cases had significantly less asymmetric atrophy when compared with CBS cases (p=0.009); they were also younger at onset (median 61 versus 66 years, p<0.05) and death (67 versus 73 years, p<0.05). Family history was present in 40% of S-CBD cases.
Conclusions
CBD can have a symmetric presentation, clinically and radiologically, in which typical features of CBS, such as limb apraxia, myoclonus, dystonia and alien limb phenomenon, may be absent.
Keywords: Corticobasal degeneration, Corticobasal syndrome, Symmetric CBD, Atlas Based Parcellation, Pathology
Introduction
Corticobasal degeneration is a neurodegenerative disease of late middle age, characterized pathologically by neuronal loss, gliosis and tau deposition in gray and white neocortex, basal ganglia and brainstem [1]. It was first described in three cases by Rebeiz et al in 1968 as ‘corticodentatonigral degeneration with neuronal achromasia [2]. The typical associated clinical presentation is known as corticobasal syndrome (CBS). The core clinical features of this syndrome are progressive asymmetric rigidity and apraxia accompanied by signs of cortical and extrapyramidal dysfunction. Typical cortical signs are alien limb phenomena, cortical sensory loss, myoclonus; extrapyramidal signs include dystonia, bradykinesia and tremor [3]. Impaired cortical and basal ganglia function can be supported by neuropsychological testing with attention, concentration, executive function, praxis, language and visuospatial domains affected [4,5]. Neuroimaging, structural and functional, supports the clinical diagnosis. Computed tomography and magnetic resonance imaging of the brain have demonstrated asymmetric cortical atrophy, especially frontoparietal, as well as basal ganglia, lateral ventricles and cerebral peduncles. Similarly, asymmetric hypoperfusion in single photon emission CT (SPECT) and hypometabolism on positron emission tomography (PET) of the parieto-frontal and/or basal ganglia have been reported [6–10]. Thus, the criteria for suspecting a clinical diagnosis of CBD focuses on asymmetry of key findings, clinically or radiologically.
Corticobasal syndrome accounts for approximately 50% of CBD case presentations [11]. There is significant clinical and pathological overlap in both CBS and CBD with other tauopathies, such as frontotemporal dementia with Parkinsonism linked to Chromosome 17 (FTDP-17), Pick’s disease with Pick bodies and progressive supranuclear palsy (PSP) [11,20]. CBS is the most common clinical presentation of CBD. Others reported in the literature include progressive non-fluent aphasia [12,13], apraxia of speech [14], behavioral variant frontotemporal dementia, PSP [11] and dementia not otherwise specified [15]. Grimes et al [15] reported 13 postmortem cases of CBD and noted four cases had an antemortem diagnosis of CBS, while most presented with dementia. Murray et al [16] reported 15 cases of autopsy-confirmed CBD, of which the antemortem diagnoses were CBS (six cases), FTD (six cases) and PSP (two cases). Cognitive difficulties were noted to be as prominent as motor deficits on presentation. Variable clinical presentations and symptom progression of CBD likely reflect the cerebral location of pathological lesions rather than the actual histopathology itself. However, there is no literature reporting specifically symmetric onset and progression of clinical features.
Meanwhile, CBS can have different underlying pathological substrates [17]. Boeve and colleagues in 1999 published autopsy findings in 13 cases of CBS. The pathological diagnosis was CBD in seven, Alzheimer’s disease (AD) in two; and one for each of Pick’s disease; PSP, non-specific degenerative changes and Creutzfeld-Jakob disease. Shelley et al [18] reported 12 cases of CBS in life and of these fifty percent had CBD pathology and fifty percent had Alzheimer’s pathology. Also reported are motor neuron inclusion body dementia [19] and dementia with Lewy bodies [20]. Interestingly, the sensitivity of diagnosing CBD correctly, even when the classic CBS clinical features are present, is low. Litvan et al showed a sensitivity of 33% in a simulated diagnostic study [21].
A neuropathological diagnostic criterion for CBD was updated in 2002 by a working group formed by Dickson and colleagues [1]. This emphasized a specific clinical phenotype is not required because of the known diverse clinical presentations. The pathological criteria require cortical and striatal tau-positive neuronal and glial lesions, astrocytic plaques and thread-like lesions in white and grey matter with neuronal loss in focal cortical regions and substantia nigra. Cortical atrophy, ballooned neurons and degeneration of substantia nigra are typically also present. Asymmetric atrophy is recognized but not essential for diagnosis. The clinical symptoms and signs are believed to reflect the distribution and pattern of brain pathology which may have variable sites. Relatively less basal ganglia involvement but higher frontoparietal deposition will therefore have minimal extrapyramidal features while behavioral and frontal domains are preferentially affected. This is illustrated by Hu et al who compared patients with autopsy-proven AD and CBD pathology presenting as CBS clinically. AD patients with clinical CBS had similar characteristics to CBD patients, including core features and disease duration. Functional brain imaging could differentiate the two however, with parietal hypoperfusion in AD cases, and frontotemporal hypoperfusion in CBD cases [22].
Ultimately, asymmetric clinical signs and symptoms are emphasized in pathologically confirmed CBD. Symmetric clinical presentations including presence of symmetrical imaging findings, has not been emphasized or well described in the literature. We report five cases of historically symmetric cases of autopsy-confirmed CBD and contrast these with ten randomly selected cases of typical clinically asymmetric CBD.
Methods
Case Ascertainment
We identified all cases of pathologically confirmed CBD from the Mayo Clinic Rochester database between January 1993 and December 2007. All cases had been evaluated at least twice in the movement disorder and behavioral neurology subspecialty clinics. At death, all cases proceeded to post-mortem evaluation of a single hemisphere per autopsy protocol, at Mayo Clinic, and met established neuropathologic diagnostic criteria for CBD [1].
Clinical Assessment
The clinical records of all cases were retrospectively reviewed by two authors (AH, KAJ). All cases were examined at least twice (range two to ten visits, median four visits) between January 1991 and December 2007. Demographic and clinic features from time of initial presentation to final evaluation before death were abstracted. Gender, age at clinical onset, age at death, disease duration, clinical signs and symptoms, neuroimaging findings, and family history of neurodegenerative disease were reviewed. Disease duration was defined as the difference between age at onset and age at death. One or more first-degree relatives with a history of dementia and/or parkinsonism and/or motor neuron disease was considered positive for a family history of neurodegenerative disease.
Inclusion criterion for symmetric CBD (Box 1) was as follows: autopsy diagnosis of CBD based on current neuropathological criteria; no difference observed at any time in the disease course between right- and left-sided cortical or extrapyramidal signs or symptoms by the evaluating physician. Five cases met these criteria. These were compared to ten randomly selected typical CBS cases.
Box 1: Clinicopathologic criteria for S-CBD
Pathological diagnosis of CBD based on current neuropathological criteria
Absence of asymmetric signs and symptoms at presentation and during the disease course
Relative absence of asymmetric frontoparietal atrophy on head MRI
Relative absence of asymmetric frontoparietal hypometabolism or hypoperfusion on PET and SPECT
Radiographic Assessment
Volumetric head MRI scans were available in all five cases of symmetric CBD (age = 60±11, 40% female) and in five of the ten randomly selected typical CBS cases (age = 70±4, 100% female). An atlas-based parcellation technique was employed using SPM5 and the automated anatomic labeling (AAL) atlas in order to generate left and right grey matter volumes for the following regions of the brain for each subject: entire supratentorial hemisphere, central regions (including precentral gyrus, postcentral gyrus and paracentral lobule), frontal lobe, temporal lobe, parietal lobe, insula and striatum. An asymmetry score was calculated for each region for each subject using the following formula: (left volume – right volume)*2/ left volume plus right volume. Asymmetry scores were also calculated for a control cohort of 30 healthy subjects (age=61±12, 63% female).
Neuropathologic Assessment
Tissue obtained at the time of autopsy was examined as per neuropathological protocol. In all cases, pathology diagnosis of CBD was made by one of two neuropathologists (JEP, DWD). All cases were also re-reviewed in 2009 to ensure that older cases met recent published criteria for CBD, (1) by one neuropathologist (DWD). The neuropathological diagnosis of CBD was made using criteria that emphasized tau-immunoreactive lesions in cortical and subcortical regions in a characteristic distribution. This includes tau-positive neuronal and glial lesions, astrocytic plaques and extensive tau-immunoreactive lesions in cortical, subcortical and white matter regions [1]. (Fig. 1)
Figure 1.
Symmetric-CBD neuropathology
Statistical Analysis
Statistical analyses were performed utilizing the JMP computer software (JMP Software, version 6.0.0; SAS Institute Inc, Cary, NC) with α set at 0.05. All binary data were compared across symmetric CBD and CBS groups with Chi-square test (Fishers Exact test was used for cells with small numbers), while Mann-Whitney U test was used to compare continuous data across both groups.
Results
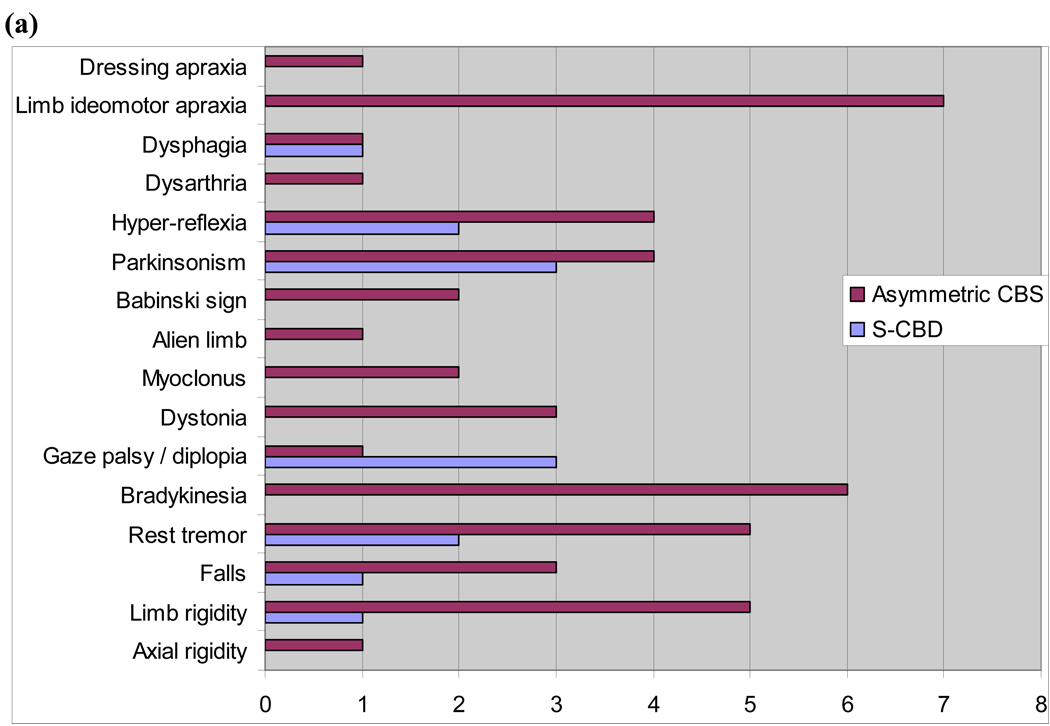
For the years 1993 to 2007, we found 31 cases that met the pathological diagnosis of CBD. Of these, five cases (16%) met criteria for symmetric CBD. (Table 1). Two of the five cases were female. The antemortem clinical diagnoses were behavioral variant frontotemporal dementia in three cases, and one case each of frontotemporal dementia with Parkinsonism and atypical AD. The presence of clinical symptoms and signs were contrasted between both groups, independent of whether the feature was symmetric or asymmetric. Grouping the signs into motor, behavioral, apraxia, cognitive, language and sleep highlighted the differences. Behavioral changes were prominent in symmetric CBD and absent in CBS. On the contrary, language abnormalities, limb apraxia and several motor features (axial rigidity, bradykinesia, myoclonus, alien limb, Babinski sign) were prominent in CBS and absent in symmetric CBD cases. Both groups had motor findings of limb rigidity, falls, parkinsonism, rest tremor, gaze palsy, hyper-reflexia, dysphagia and cognitive changes of memory impairment and acalculia. (Table 2, Fig. 2). Motor features when present in symmetric CBD were symmetric affecting both sides equally.
Table 1.
Comparison of clinical features of symmetric and typical (asymmetric) CBS cases.
| Case | Gender | Clinical diagnosis |
Onset (yrs) |
Death (yrs) |
Duration (yrs) |
Family history1 |
|---|---|---|---|---|---|---|
| 1 | F | Atypical AD | 69 | 73 | 4 | Negative |
| 2 | M | bvFTD | 52 | 57 | 5 | Positive |
| 3 | M | bvFTD | 61 | 67 | 6 | Negative |
| 4 | F | bvFTD | 40 | 47 | 7 | Negative |
| 5 | M | FTDP | 63 | 67 | 4 | Positive |
| 6 | F | CBS | 65 | 73 | 8 | Negative |
| 7 | F | CBS | 69 | 75 | 6 | Negative |
| 8 | F | CBS | 73 | 80 | 7 | Negative |
| 9 | M | CBS | 72 | 77 | 5 | Negative |
| 10 | M | CBS | 66 | 70 | 4 | Negative |
| 11 | M | CBS | 63 | 67 | 4 | Negative |
| 12 | M | CBS | 65 | 72 | 7 | Positive |
| 13 | F | CBS | 61 | 67 | 6 | Negative |
| 14 | M | CBS | 70 | 76 | 6 | Negative |
| 15 | F | CBS | 63 | 69 | 6 | Negative |
AD Alzheimer’s disease
bvFTD Behavioral variant frontotemporal dementia
FTDP Frontotemporal dementia with parkinsonism
CBS Corticobasal degeneration
Positive family history defined as one or more first-degree relatives with a history of dementia and/or parkinsonism and/ or motor neuro disease
Table 2.
Comparison of frequency of symptoms and signs in CBS versus S-CBD cases
| Symptoms /signs | S-CBD cases (n=5) |
Asymmetric CBS cases (n=10) |
|---|---|---|
| Motor | ||
| Axial rigidity | 0 | 1 |
| Limb rigidity | 1 | 5 |
| Falls | 1 | 3 |
| Rest tremor | 2 | 5 |
| Bradykinesia | 0 | 6 |
| Gaze palsy / diplopia | 3 | 1 |
| Dystonia | 0 | 3 |
| Myoclonus | 0 | 2 |
| Alien limb | 0 | 1 |
| Babinski sign | 0 | 2 |
| Parkinsonism | 3 | 4 |
| Hyper-reflexia | 2 | 4 |
| Dysarthria | 0 | 1 |
| Dysphagia | 1 | 1 |
| Apraxia | ||
| Limb ideomotor apraxia | 0 | 7 |
| Dressing apraxia | 0 | 1 |
| Cognitive | ||
| Visuospatial difficulty | 1 | 0 |
| L/R discrimination difficulty | 0 | 1 |
| Acalculia | 2 | 1 |
| Memory impairment | 1 | 1 |
| Language | ||
| Effortful speech | 0 | 5 |
| Word-finding difficulty | 0 | 2 |
| Echolalia | 2 | 0 |
| Behavior | ||
| Frontal lobe signs | 2 | 0 |
| Behavior/personality change | 3 | 0 |
| Withdrawn | 1 | 0 |
| Apathy | 2 | 0 |
| Executive dysfunction | 1 | 0 |
| Dishibition | 2 | 0 |
| Sleep | ||
| Dream enactment behavior | 2 | 0 |
Figure 2.
(a) Frequency of motor signs/symtpoms and apraxia in CBS versus S-CBD. (b) Frequency of behavioral, cognitive and language symptoms / signs in CBS versus S-CBD.
Age of onset was 61 (median) (range 40–69) for symmetric and 66 (median) (61–73 range) for asymmetric cases. Age at death was 67 (range 47–69) and 72.5 (range 67–80) respectively. Compared to typical cases of CBS, those with symmetric CBD were younger at onset (median 61 versus 66 years, p<0.05) and younger at death (67 versus 73 years, p<0.05). However, both groups had similar disease duration (5 versus 6 years, p=0.20). There was no gender preference; 40% of symmetric and 50% of typical CBD cases were female. (Table 3).
Table 3.
Comparison of demographics in symmetric CBD (S-CBD) and typical asymmetric CBD (CBS) cases.
| Symmetric CBD (n=5) | Asymmetric CBD (CBS) (n=10) |
P value | |
|---|---|---|---|
| Age of onset (median) | 61 (range 40–69) | 66 (range 61–73) | (p<0.05) |
| Age at death (median) | 67 (range 47–69) | 73 (range 67–80) | (p<0.05) |
| Disease duration (yrs) | 5 | 6 | (p=0.20) |
| Gender | 2 (40%) female | 5 (50%) female | |
| Family history of neurodegenerative disease |
2 (40%) | 1 (10%) | (p=0.24) |
Family history of neurodegenerative disease was different between both groups; 40% in symmetric cases and 10% in asymmetric cases although this did not reach significance. (p=0.24). In the symmetric CBD group, one case had a maternal grandfather with Pick’s disease (genetic testing was not performed in this case). A second case had a paternal uncle with a parietal lobe syndrome, and interestingly another paternal uncle with Creutzfeldt Jakob disease. MAPT genetic testing in this patient was negative.
All symmetric CBD cases had symmetric atrophy on visual inspection of their head MRI brain scans. (Table 4). A typical imaging example is demonstrated in Fig 3. Using atlas based parcellation, the asymmetric CBD group had a significantly greater hemispheric asymmetry score than the symmetric CBD group (p=0.009) (Table 5). The asymmetric CBD group showed significantly greater asymmetry in regions of the brain surrounding the central sulcus (p=0.004) and the striatum (p=0.03), with a trend for greater asymmetry in the temporal lobe (p=0.06), compared to the symmetric CBD group. Asymmetry scores in the symmetric CBD group were not significantly different from controls. There was a slight trend for increased asymmetry in the frontal lobe (p=0.09), primarily in the medial frontal lobe compared to controls.
Table 4.
Comparison of radiological features of symmetric S-CBD and typical (asymmetric) CBS cases
| Case | MRI FINDINGS | PET/SPECT FINDINGS |
|---|---|---|
| 1 | Mild generalized atrophy | NP |
| 2 | Mild bifrontal atrophy | NP |
| 3 | Mild generalized atrophy | Hypometabolism right frontal (PET) |
| 4 | Bifrontotemporal atrophy | Bifrontal reduced perfusion (SPECT) |
| 5 | Bifrontal atrophy | Bifrontal reduced perfusion (SPECT) |
| 6 | Minimal atrophy | Left temporoparietal reduced perfusion (SPECT) |
| 7 | Normal | Right temporoparietal reduced perfusion (SPECT) |
| 8 (PET) |
Bitemporal atrophy, R>L | Hypometabolism left frontoparietal, BG, cerebellum |
| 9 | N/A | Left temporal, frontoparietal reduced perf (SPECT) |
| 10 | N/A | Right >left parietotemporal reduced perf (SPECT) |
| 11 | Generalized atrophy (N/A) | NP |
| 12 | Generalized atrophy (N/A) | N/A |
| 13 | Asymmetric atrophy | NP |
| 14 | Mild generalized atrophy | NP |
| 15 | Mild generalized atrophy | N/A |
PET Positron emission tomography
SPECT Single photon emission CT
N/A Not available
NP Not performed
L/R Left/Right
Figure 3.
Symmetric atrophy in a symmetric-CBD case.
T1 MRI brain coronal slices demonstrating symmetric cerebral atrophy in a symmetric-CBD case.
Table 5.
Absolute asymmetry scores for the symmetric and asymmetric CBD groups and controls
| Region | Controls | Symmetric CBD (S-CBD) |
Asymmetric CBD (CBS) |
P value: Symmetric CBD vs. controls |
P value: Asymmetric CBD vs. controls |
P value: Asymmetric CBD vs. symmetric CBD |
|---|---|---|---|---|---|---|
| Supratentorial hemisphere |
0.01 (0.01) | 0.03 (0.03) | 0.10 (0.03) | 0.12 | 0.004 | 0.009 |
| Central regions | 0.04 (0.04) | 0.03 (0.02) | 0.17 (0.06) | 0.33 | 0.01 | 0.004 |
| Striatum | 0.04 (0.03) | 0.03 (0.03) | 0.16 (0.09) | 0.40 | 0.046 | 0.03 |
| Insula | 0.05 (0.03) | 0.07 (0.03) | 0.16 (0.14) | 0.29 | 0.16 | 0.23 |
| Frontal lobe | 0.03 (0.01) | 0.09 (0.06) | 0.09 (0.05) | 0.09 | 0.056 | 0.88 |
| Temporal lobe | 0.06 (0.03) | 0.06 (0.01) | 0.13 (0.06) | 0.98 | 0.056 | 0.06 |
| Parietal lobe | 0.09 (0.04) | 0.08 (0.05) | 0.07 (0.04) | 0.71 | 0.34 | 0.69 |
Pathological re-examination of all 15 CBD cases showed features typical of CBD with one S-CBD case (case 2; negative MAPT gene testing) showing severe thalamic pathology.
Conclusions
Corticobasal degeneration is traditionally suspected on the basis of asymmetric signs and symptoms in life. However the five cases we describe illustrate the fact that CBD can present as a symmetric syndrome. In these cases core features of CBS such as limb apraxia, myoclonus, dystonia and alien limb phenomenon may not be present. In addition, MRI neuroimaging demonstrated symmetric hemispheric atrophy in all five cases which was significantly different from typical CBS cases with quantitative analysis.
Symmetric presentations of CBD have earlier disease onset in this sample group, and earlier age of death, with similar disease duration to typical asymmetric subjects. Interestingly, a positive family history of neurodegenerative disease was higher in the S-CBD cases. CBD is traditionally considered a sporadic disorder, thus there may be a genetic predilection to development of symmetric degeneration. The reason for the differences between the two groups is unclear but suggests that symmetric CBD may be different from the more typical asymmetric variant presenting as CBS. Similar to PSP where it has been suggested that asymmetric PSP presenting as Parkinson’s disease should be designated PSP-P [23] we suggest that symmetric CBD be designated S-CBD to differentiate and separate the symmetric variants. This designation would allow future studies to determine whether S-CBD is a genetically or pathologically unique clinico-pathologic-genetic entity. One patient did have testing for mutation in the tau gene and was negative suggesting that S-CBD is simply not FTLD-17.
The quantitative MRI analysis was validation that the S-CBD group had significantly less asymmetry than the CBS group in regions of the brain that are typically associated with typical CBD [24], namely regions surrounding the central sulcus (precentral gyrus, postcentral gyrus and paracentral lobule) and the striatum. No significant difference was observed between the S-CBD group and controls although there was a slight trend for differences in the frontal lobe in the S-CBD subjects compared to controls which can be explained by the fact that the majority of S-CBD subjects had a clinical diagnosis of behavioral variant FTD which would be expected to target the frontal lobes.
Unfortunately, we are unable to determine whether S-CBD is also symmetric pathologically due to the universal protocol of examining one hemisphere only at autopsy. Typical CBD pathology is reported to be asymmetric but the exact prevalence is unknown. However, if MRI volumetric analysis is used as a surrogate for macroscopic pathological atrophy, it would appear that this too is symmetric, although we cannot comment on microscopic lesion deposition.
Limitations of our study are that it is a retrospective analysis of small numbers of patients evaluated over many years. There may be a referral bias to our institution for diagnostically complex cases of neurodegenerative disease. Thus symmetric case may be rarer than they appear in this review. Conversely, there may in fact be a more common symmetric presentation of CBD but the diagnosis is not considered due to the emphasis on asymmetrical findings in CBD.
The strengths of the study are that all cases were clinically evaluated at one institution, all cases were evaluated by specialists in movement disorders and behavioral neurology, all cases had multiple clinical evaluations, all cases were re-reviewed by an expert neuropathologist, and the application of an unbiased quantitative method, atlas based parcellation, to validate our clinical MRI findings.
It is therefore important to again highlight that not all cases of CBD will meet clinical criteria for CBS. Although the diagnosis is typically made when the clinical presentation is relatively classic, there is still low sensitivity. The best predictors are limb dystonia, alien limb, asymmetric Parkinsonism, ideomotor apraxia and absence of gait or balance disorder in one study reported by Litvan et al, and supported by asymmetry on neuroimaging [21]. However at best this still misses a significant proportion of antemortem CBD diagnoses. Emphasizing the presence of asymmetric features may sway the clinician from considering CBD as a diagnosis. Predicting underlying CBD pathology in cases not presenting as CBS can be difficult, but we suggest it is more likely to occur in patients presenting less than age 65 years old, and may be more likely in those with a positive family history of neurodegenerative disease. At present, the gold standard for CBD diagnosis remains neuropathological. Hence the continuing importance of post-mortem analysis of neurodegenerative diseases to further refines the phenotype and frequency of antemortem clinical presentations. Separating S-CBD could lead to important genetic discoveries.
Acknowledgement
KAJ is supported by the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (K12/NICHD)-HD49078 and the Dana Foundation. Co-authors on this study are also supported by NIH grants P50-AG16574, U01-AG06786, R01-AG11378, P50-NS40256 and the generous support of the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer´s Disease Research Program of the Mayo Foundation.
We thank all patients and their families who participated in neuroscience research and ADRC research program staff at Mayo Clinic Rochester.
Footnotes
Disclosures:
Dr BF Boeve received grant support from Myriad Pharmaceuticals and Honorarium from GE Healthcare. The remaining authors have nothing to disclose.
References
- 1.Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Houropian D, Ikeda K, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–946. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- 2.Rebeiz JJ, Kolodny EH, Richardson EP. Corticodentatonigral Degeneration with Neuronal Achromasia. Arch Neurol. 1968;18:20–33. doi: 10.1001/archneur.1968.00470310034003. [DOI] [PubMed] [Google Scholar]
- 3.Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol. 54:S15–S19. doi: 10.1002/ana.10570. 200. [DOI] [PubMed] [Google Scholar]
- 4.Pillon B, Blin J, Viadailet M, Deweer B, Sirigu A, Dubois B, Agid Y. The neuropsychological pattern of corticobasal degeneration. Neurol. 1995;45:1477–1483. doi: 10.1212/wnl.45.8.1477. [DOI] [PubMed] [Google Scholar]
- 5.Massman PJ, Kreiter KT, Jankovic J, Doody RS. Neuropsychological functioning in cortico-basal ganglionic degeneration: Differentiation from Alzheimer’s disease. Neurol. 1996;46:20–726. doi: 10.1212/wnl.46.3.720. [DOI] [PubMed] [Google Scholar]
- 6.Josephs KA, Tang-Wai DF, Edland SD, et al. Correlation between antemortem magnetic resonance imaging findings and pathologically confirmed corticobasal degeneration. Arch Neurol. 2004;61:1881–1884. doi: 10.1001/archneur.61.12.1881. [DOI] [PubMed] [Google Scholar]
- 7.Huang KJ, Lu MK, Kao A, Tsai CH. Clinical, Imaging and Electrophysiological Studies of Corticobasal Degeneration. Acta Neurologica Taiwanica. 2007;16:13–21. [PubMed] [Google Scholar]
- 8.Koyama M, Yagishita A, Nakata Y, Hayashi M, Bandoh M, Mizutani T. Imaging of corticobasal degeneration syndrome. Neuroradiology. 2007;49:905–912. doi: 10.1007/s00234-007-0265-6. [DOI] [PubMed] [Google Scholar]
- 9.Boxer AL, Geschwind MD, Belfor N, Gorno-Tempini ML, Schauer GF, Miller BL, et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol. 2006;63:81–86. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- 10.Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66:41–48. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- 11.Boeve BF. Links between frontotemporal lobar degeneration, progressive supranuclear palsy, and amyotrophic lateral sclerosis. Alzheimer Dis Assoc. Disord. 2007;21:S31–S38. doi: 10.1097/WAD.0b013e31815bf454. [DOI] [PubMed] [Google Scholar]
- 12.Kimura N, Kumamoto T, Hanaoka T, Hazama Y, Nakamura K, Arakawa R. Corticobasal degeneration presenting with progressive conduction aphasia. Journal of the Neurological Sciences. 2008;269:163–168. doi: 10.1016/j.jns.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Takao M, Tsuchiya K, Mimura M, Momoshima S, Kondo H, Akiyama H, et al. Corticobasal degeneration as a cause of progressive non-fluent aphasia: Clinical, radiological and pathological study of an autopsy case. Neuropath. 2006;26:569–578. doi: 10.1111/j.1440-1789.2006.00731.x. [DOI] [PubMed] [Google Scholar]
- 14.Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimes DA, Lang AE, Bergeron CB. Dementia as most common presentation of cortical-basal ganglionic degeneration. Neurol. 1999;53:1969–1974. doi: 10.1212/wnl.53.9.1969. [DOI] [PubMed] [Google Scholar]
- 16.Murray R, Neumann M, Forman MS, Farmer J, Massimo L, Rice A, et al. Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurol. 2007;68:1274–1283. doi: 10.1212/01.wnl.0000259519.78480.c3. [DOI] [PubMed] [Google Scholar]
- 17.Boeve BF, Maraganore DM, Parisi JE, Ahlskog JE, Graff-Radford N, Caselli RJ, et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurol. 1999;53:795–800. doi: 10.1212/wnl.53.4.795. [DOI] [PubMed] [Google Scholar]
- 18.Shelley BP, Hodges JR, Kipps CM, Xuereb JH, Bak TH. Is the pathology of corticobasal syndrome predictable in life? Movement Disord. doi: 10.1002/mds.22558. published online 16 June 2009, DOI: 10.1002/mds.22558. [DOI] [PubMed] [Google Scholar]
- 19.Grimes DA, Bergeron CB, Lang AE. Motor neuron disease- inclusion dementia presenting as cortico-basal ganglionic degeneration. Mov Disord. 1999;14:674–680. doi: 10.1002/1531-8257(199907)14:4<674::aid-mds1019>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 20.Schneider JA, Watts RL, Gearing M, Brewer RP, Mirra SS. Corticobasal degeneration: Neuropathologic and clinical heterogeneity. Neurol. 1997;48:959–969. doi: 10.1212/wnl.48.4.959. [DOI] [PubMed] [Google Scholar]
- 21.Litvan I, Agid Y, Goetz C, Jankovic J, Wenning GK, Brandel JP, et al. Accuracy of the clinical diagnosis of corticobasal degeneration: A clinicopathologic study. Neurol. 1997;48:119–125. doi: 10.1212/wnl.48.1.119. [DOI] [PubMed] [Google Scholar]
- 22.Hu WT, Rippon GW, Boeve BF, Knopman DS, Petersen RC, Parisi JE, et al. Alzheimer’s disease and corticobasal degeneration presenting as corticobasal syndrome. Mov Disord. 2009;24:1375–1379. doi: 10.1002/mds.22574. [DOI] [PubMed] [Google Scholar]
- 23.Williams DR, de Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, et al. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain. 2005;128:1247–1258. doi: 10.1093/brain/awh488. [DOI] [PubMed] [Google Scholar]
- 24.Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiology of Aging. 2008;29:280–289. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]