Abstract
Maintenance of genomic integrity is an essential cellular function. We previously reported that the transcription factor and tumor suppressor CCAAT/enhancer binding protein δ (C/EBPδ, CEBPD; also known as “NFIL-6β”) promotes genomic stability. However, the molecular mechanism was not known. Here, we show that C/EBPδ is a DNA damage-induced gene, which supports survival of mouse bone marrow cells, mouse embryo fibroblasts (MEF), human fibroblasts, and breast tumor cells in response to the DNA cross-linking agent mitomycin C (MMC). Using gene knockout, protein depletion, and overexpression studies, we found that C/EBPδ promotes monoubiquitination of the Fanconi anemia complementation group D2 protein (FANCD2), which is necessary for its function in replication-associated DNA repair. C/EBPδ interacts with FANCD2 and importin 4 (IPO4, also known as “Imp4” and “RanBP4”) via separate domains, mediating FANCD2–IPO4 association and augmenting nuclear import of FANCD2, a prerequisite for its monoubiquitination. This study identifies a transcription-independent activity of C/EBPδ in the DNA damage response that may in part underlie its tumor suppressor function. Furthermore, we report a function of IPO4 and nuclear import in the Fanconi anemia pathway of DNA repair.
Keywords: Fanconi anemia, DNA repair, mitomycin C, importin 4, protein adaptor
Maintaining the integrity of the genome is pivotal to life. Therefore, a multitude of mechanisms have evolved to deal with damage to the genome. Depending on the type and timing of DNA damage, specific pathways are activated that sense the lesion, communicate the problem to checkpoint kinases that arrest the cell cycle, and initiate appropriate repair mechanisms (1). Several different checkpoint kinases phosphorylate the ubiquitous histone H2AX, which then binds to sites of DNA damage. The phosphorylated form of H2AX (termed “γH2AX”) is, therefore, a surrogate marker for DNA lesions and activation of check-point signaling (2, 3). Genetic defects in components of these pathways, if not lethal, can lead to specific syndromes. Fanconi anemia (FA) is an autosomal recessive disorder, characterized by developmental abnormalities, progressive bone marrow failure, acute myeloid leukemia, and susceptibility to cancer (4, 5). Cells derived from FA patients accumulate DNA damage at an increased rate and exhibit hypersensitivity to DNA cross-linking agents such as mitomycin C (MMC), resulting in a greater number of chromosomal abnormalities, including translocations and radial chromosomes (6). FA can be caused by mutation of any one of 13 genes identified to date. In response to DNA damage, the FA proteins Fanconi anemia complementation group (FANC)-A, -B, -C, -E, -F, -G, -L, and -M form a ubiquitin ligase core complex in the nucleus and monoubiquitinate FANCD2 and FANCI. Monoubiqutination of FANCD2 and FANCI is considered the essential step in the FA pathway that mediates replication-dependent removal of interstrand DNA cross-links (7–9).
In this report we describe the physical and functional association of FANCD2 with the CCAAT/enhancer binding protein δ (C/EBPδ) transcription factor. C/EBPδ is a leucine zipper (LZ) DNA-binding protein that usually is not highly expressed but is inducible by many different stimuli and is considered a stress-response gene (10). C/EBPδ has many tumor suppressor-like properties. For example, its expression is down-regulated in several types of cancer (11–14), and its expression in tumors has been associated with favorable prognosis (15, 16). Although C/EBPδ-knock-out (KO) mice are viable and fertile (17), primary C/EBPδ-null mouse embryo fibroblasts (MEFs) in vitro exhibit chromosomal instability, including triradial chromosomes (18), a lesion often seen in cells with defects in the FA pathway (4–6). This phenotype suggested that C/EBPδ plays a role in genome maintenance or DNA-repair pathways. Here, we report that C/EBPδ augments cell survival after DNA damage from interstrand cross-linkers by facilitating nuclear import of FANCD2.
Results
C/EBPδ Interacts with FANCD2.
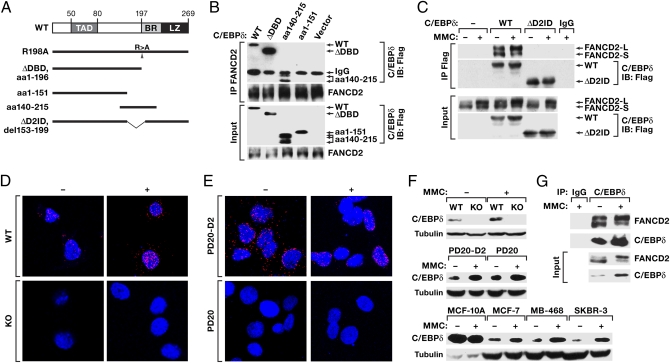
To understand the molecular functions of C/EBPδ, we explored which proteins it can interact with. Ectopic C/EBPδ was immunoprecipitated from 293T cells, and proteins to which it bound were identified using mass spectrometry (Table S1). This approach suggested that C/EBPδ interacts with FANCD2. Both these proteins are predominantly nuclear, and their expression is down-regulated in breast cancer (19–21). Furthermore, primary C/EBPδ-null MEFs exhibit genomic instability, including triradial chromosomes (18), which are a hallmark of FA-deficient cells (22). For these reasons we decided to pursue the significance of C/EBPδ interaction with FANCD2. Fig. 1A illustrates the various C/EBPδ expression constructs used in this study. The nuclear localization signal of C/EBPδ lies within its DNA-binding domain (23). Therefore, mutants lacking this domain do not enter the nucleus. However, the R198A point mutation, which inhibits sequence-specific DNA binding of C/EBPδ, does not interfere with nuclear localization (24, 25). First, we confirmed the interaction between C/EBPδ and FANCD2 in 293T cells by coimmunoprecipitation (co-IP) assay. As shown in Fig. 1B, endogenous FANCD2 interacted with wild type (WT) C/EBPδ, with a protein truncated at residue 196 (ΔDBD), and with an internal fragment comprising amino acids 140–215 but not with a mutant truncated at residue 151. A mutant with an internal deletion of amino acids 153–199 was unable to coprecipitate FANCD2 (Fig. 1C). We therefore termed this 47-aa region the FANCD2 interaction domain (D2ID). WT C/EBPδ interacted with both the unubiquitinated “S” form of FANCD2 (FANCD2-S) and the monoubiquitinated “L” form (FANCD2-L) in MMC-treated or untreated 293T cells (Fig. 1C), suggesting that potential modifications of either protein in response to DNA damage are not necessary for and do not interfere with their interaction.
Fig. 1.
Interaction of C/EBPδ and FANCD2 proteins. (A) Schematic representation of mutations in C/EBPδ constructs. R198A is the full-length protein with an R-to-A mutation at residue 198; ΔDBD is a deletion of the DNA-binding basic region (BR) and leucine zipper (LZ); ΔD2ID is a deletion of amino acids 153–199. TAD, transactivation domain. (B) FANCD2 co-IP analysis of 293T cells transfected with Flag-tagged C/EBPδ constructs. Immunoprecipitates (IP) and input samples were analyzed with anti-FANCD2 and anti-Flag antibodies. The multiple bands generated by amino acids 140–215 probably are caused by phosphorylation events because this region harbors several kinase recognition motifs. (C) C/EBPδ co-IP analysis from 293T cells as in B. (D) WT and KO MEFs were treated for 3 h with 5 μg/mL MMC. Fixed cells were incubated with antibodies against C/EBPδ and FANCD2 followed by OLink in situ PLA and fluorescence microscopy using appropriate filters (DAPI, blue; PLA, red). (Fig. S1 shows single-channel images.) (E) FANCD2-deficient cells (PD20) and cells reconstituted with FANCD2 (PD20-D2) were treated for 20 h with 500 ng/mL MMC before processing as described in D. (F) Western blot analysis of whole-cell lysates from WT and C/EBPδ-KO MEFs, PD20-D2 or PD20 cells, or the human breast epithelial cell lines MCF-10A, MCF-7, MDA-MB-468, and SKBR-3; treated for 20 h with 1 μg/mL MMC (100 ng/mL for PD20/-D2 cells). (G) Immunoprecipitation from MDA-MB-468 cells with anti-C/EBPδ antibody (Rockland) and input samples were analyzed with anti-FANCD2 and anti-C/EBPδ (BD Biosciences) antibodies.
To detect interaction of endogenous C/EBPδ and FANCD2, we used the DuoLink in situ proximity ligation assay (PLA). In this assay, two proteins are immunostained with opposite species-specific secondary antibodies that are linked to complementary oligonucleotides. When two different antibody molecules bind in close proximity, the linked DNA can be amplified and visualized with a fluorescent probe as distinct foci. Each signal spot may represent one molecule of each of two interacting proteins. In WT MEFs, the interaction between C/EBPδ and FANCD2 was clearly detectable in both the cytoplasm and the nucleus (Fig. 1D and Fig. S1A). No significant signal was generated in C/EBPδ-null (KO) MEFs because of the absence of C/EBPδ protein (Fig. 1D and Fig. S1A). To verify this interaction further, we obtained human FANCD2-null PD20 fibroblasts, which indeed generated no signal in the PLA assay (Fig. 1E and Fig. S1B). However, in cells reconstituted with FANCD2 (PD20-D2), an interaction with C/EBPδ was again detectable (Fig. 1E and Fig. S1B). Because this assay detects FANCD2 and C/EBPδ only when they are in complex, and C/EBPδ does not localize to DNA-damage foci (SI Results), this assay does not visualize FANCD2 in the DNA-damage foci. As a negative control, we assessed the combination of FANCA and C/EBPδ antibodies in PD20-D2 cells, which did not generate any signal in this assay (Fig. S1C). In summary, this approach demonstrated specific interaction of endogenous C/EBPδ and FANCD2.
In PD20-D2 cells, in particular, there also was a shift of signal from more cytoplasmic to nuclear localization in response to MMC. Moreover, we noted that in MEFs the PLA signal increased with MMC in both the cytoplasm and the nucleus. Given that the co-IP assays with overexpressed C/EBPδ did not indicate an effect of MMC on interaction per se (Fig. 1C), we asked whether expression of endogenous C/EBPδ changed upon DNA damage. Indeed, C/EBPδ protein levels were induced in several different cell types, such as MEFs, PD20/-D2, and human breast tumor cell lines (Fig. 1F). In contrast, MMC did not induce C/EBPδ expression in untransformed human MCF-10A breast epithelial cells, which have higher basal levels of C/EBPδ (25) (Fig. 1F). To assess further the interaction of endogenous C/EBPδ and FANCD2 proteins, we performed co-IP assays in MDA-MB-468 cells and confirmed that both FANCD2-S and FANCD2-L associate with C/EBPδ in the presence and absence of MMC treatment (Fig. 1G).
Taken together, induction of C/EBPδ expression in response to DNA damage and the interaction of C/EBPδ with FANCD2 observed in mouse and human fibroblasts and in human breast cancer cells suggest that C/EBPδ may play a role in the cellular response to DNA damage.
C/EBPδ Augments Cell Survival in Response to DNA Damage.
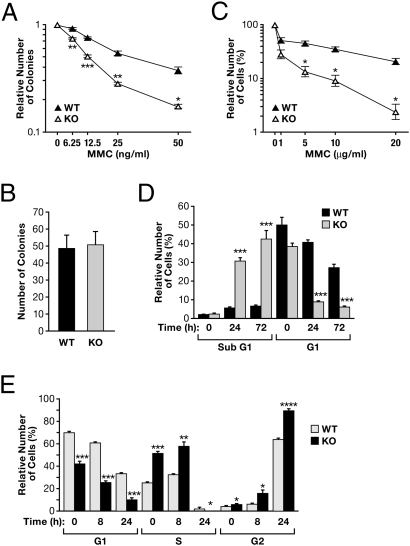
Because we had found that C/EBPδ interacts with FANCD2, we assessed the role of C/EBPδ in cell survival in response to MMC by comparing cells from WT and C/EBPδ-KO mice. The number of colonies formed by primary mouse bone marrow cells diminished when the cells were cultured in the presence of increasing concentrations of MMC (Fig. 2A). Depending on the MMC concentration, C/EBPδ-null bone marrow cells formed 19–54% fewer colonies than WT cells. In the absence of MMC, there was no difference in colony numbers between genotypes (Fig. 2B). This result shows that C/EBPδ promotes cell survival in the presence of MMC. To test the role of C/EBPδ in response to acute damage, MEFs were treated with MMC for 2 h, and cell numbers were evaluated 1 wk later. Loss of C/EBPδ significantly impaired cell survival after MMC at concentrations of 5 μg/mL or higher (Fig. 2C). Consistent with these observations, lack of C/EBPδ led to 5- to 6-fold increased cell death after 24 h or 72 h of MMC exposure (Fig. 2D). Concomitantly, KO cells were lost more rapidly from the G1 phase of the cell cycle (Fig. 2D). Profiling the distribution of living cells among cell-cycle phases showed that C/EBPδ-null cells were enriched at 4N DNA content (G2) under all conditions (Fig. 2E), a finding that is consistent with the arrest in late S-phase and G2 as seen in FA pathway-deficient cells (26, 27). Interestingly, C/EBPδ-null cells exhibited significantly more cells in S-phase, both untreated and after 8 h MMC treatment, perhaps because of the elevated levels of cyclin D1 in these cells (25). However, after 24 h of MMC exposure, virtually all living WT and KO cells were either in G1 or G2, with C/EBPδ-null cells being enriched at G2 (4N). Collectively, these data show that, like FA cells (5, 28), C/EBPδ-deficient cells are hypersensitive to MMC, although the phenotype is milder than in FA cells.
Fig. 2.
Analysis of cell survival in response to DNA damage. (A) Bone marrow cell colony formation assay. Primary total bone marrow cells from WT and C/EBPδ-KO mice were cultured in methylcellulose-based medium with MMC at the indicated concentrations. Colonies were counted 10–14 d later. Data are mean ± SEM of four mice per genotype; each experiment was performed in triplicate. Controls were set at 1. *P < 0.05; **P < 0.01; ***P < 0.001. (B) Average number of colonies (mean ± SEM) in dishes without MMC from the experiments in A. (C) Analysis of cell viability. Immortalized WT and KO MEFs were treated for 2 h with MMC as indicated. Medium was changed, and cell viability was assessed 1 wk later. Data are means ± SEM of three experiments; controls were set at 100%. *P < 0.05. (D) Analysis of cell death. Primary WT and KO MEFs were treated with 500 ng/mL MMC for the indicated times. Shown is the fraction of cells at G1 and at sub-G1 DNA content as a measure of cell death from three independent MEF isolates (mean ± SEM) analyzed by flow cytometry. ***P < 0.001. (E) Analysis of cell-cycle distribution in primary WT and KO MEFs. The cellular DNA content was determined by flow cytometry as a measure of cell-cycle phase in cells treated with 1 μg/mL MMC for the indicated times. Shown is the distribution of living cells (%) in the G1, S, and G2 phases from three independent MEF isolates for each genotype (mean ± SEM). *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.00001.
To investigate whether DNA-repair mechanisms are deficient in C/EBPδ-null cells, we assessed the amount of γH2AX, a marker for sites of DNA damage (2). As shown previously (25), untreated KO MEFs contained more γH2AX foci than controls (Fig. S2A). MMC treatment caused an increase in the number of γH2AX foci (Fig. S2A) and in γH2AX levels in cell lysates (Fig. S2B). This response was more pronounced in KO MEFs than in controls. These data are consistent with a DNA-repair defect in C/EBPδ-null cells and support the notion that C/EBPδ interaction with FANCD2 may be functionally relevant.
C/EBPδ Augments Monoubiquitination of FANCD2.
To investigate further the consequence(s) of C/EBPδ interaction with FANCD2, we compared FANCD2 protein in C/EBPδ WT and KO MEFs. There was no significant difference in the steady-state levels of FANCD2 (Fig. 3A). However, upon MMC treatment, KO MEFs exhibited less monoubiquitinated FANCD2-L. This phenotype could be partially rescued by ectopic C/EBPδ (Fig. 3A), suggesting a direct role for C/EBPδ in FANCD2 monoubiquitination. Similar results were obtained in a HEK293 cell line with a tetracycline-inducible C/EBPδ transgene (25) in which C/EBPδ augmented the shift of FANCD2-S to FANCD2-L by MMC (Fig. S3). To assess the functional significance of this observation, we compared the survival response of these cells. Indeed, tetracycline significantly enhanced the survival in the presence of MMC specifically of 293-C/EBPδ cells but not of the parental 293 cells (Fig. 3B). Furthermore, overexpression of WT C/EBPδ improved the survival of MMC-treated, FANCD2-reconstituted PD20-D2 cells by about 30%. However, C/EBPδ did not promote survival of FANCD2-deficient PD20 cells under these conditions (Fig. 3C). Importantly, the C/EBPδ mutant lacking the FANCD2 interaction domain (ΔD2ID) was unable to promote survival of PD20-D2 cells (Fig. 3C). Consistent with these results, FANCD2 monoubiquitination was augmented specifically by WT C/EBPδ but not by ΔD2ID-C/EBPδ in PD20-D2 cells (Fig. 3D). On the other hand, silencing of endogenous C/EBPδ expression reduced the ratio of FANCD2-L to -S isoforms in MMC-treated PD20-D2 cells (Fig. 3E). Taken together, these data demonstrate that C/EBPδ promotes monoubiquitination of FANCD2 and cell survival in response to MMC by interacting directly with FANCD2.
Fig. 3.
C/EBPδ augments FANCD2 monoubiquitination and cell survival in response to MMC. (A) C/EBPδ-KO MEFs were transfected with Flag-tagged WT mouse C/EBPδ or vector control for 16 h before treatment with 500 ng/mL MMC for 20 h. Whole-cell lysates were analyzed for expression of C/EBPδ and FANCD2 in comparison with WT MEFs. Tubulin was used as loading control. L/S, quantification of the FANCD2-L/S ratio in lysates of MMC-treated cells. (B) 293-C/EBPδ and 293P cells were seeded in 96-well plates and 16 h later were treated with tetracycline (Tet; 500 ng/mL) and/or MMC (1 μg/mL) for the indicated times before relative quantification of cell numbers. Data represent mean ± SEM of two experiments, each done in six wells per data point, shown relative to untreated controls (set at 1 for each time point). **P < 0.01 comparing MMC-treated cells with or without C/EBPδ induction by tetracycline. (C) PD20-D2 and PD20 cells were transfected with Flag-tagged WT C/EBPδ, the ΔD2ID mutant, or vector control for 8 h before being placed in 96-well dishes at 5,000 cells/well. The next day, MMC (100 ng/mL) was added, and cell viability was assessed 48 h later. Data are mean ± SEM of three experiments, each performed in triplicate, and relative to cells without MMC (set at 100%). **P < 0.01 relative to vector and ΔD2ID transfected cells. (D) Western blot analysis of FANCD2 and C/EBPδ in PD20-D2 and PD20 cells treated as in C. C/EBPδ was detected by anti-Flag antibodies. (E) PD20-D2 cells were transfected with shRNA constructs against C/EBPδ (+) or GFP (-) as control and 24 h later were treated with 500 ng/mL MMC for another 24 h. Whole-cell lysates were analyzed for FANCD2 and C/EBPδ expression.
C/EBPδ Augments Nuclear Import of FANCD2.
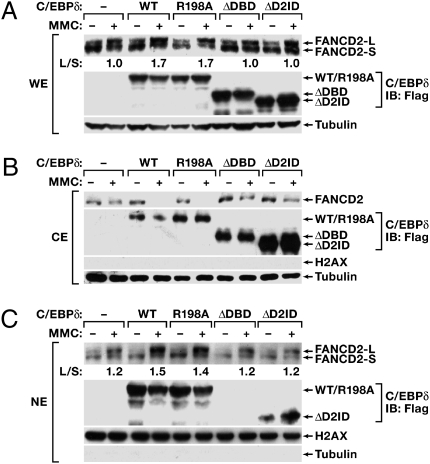
To investigate the mechanism by which C/EBPδ promotes FANCD2 monoubiquitination, we introduced various C/EBPδ constructs into HEK293 cells. MMC-induced monoubiquitination was promoted by WT C/EBPδ and the R198A mutant but not by the ΔDBD or ΔD2ID mutants (Fig. 4A). Because monoubiquitination occurs in the nucleus, and our previous results suggested that MMC may cause a shift of C/EBPδ-FANCD2 complexes from the cytoplasm to the nucleus (Fig. 1 D and E), we analyzed the subcellular localization of these proteins by cell fractionation. Although most FANCD2 protein was in the nucleus, MMC triggered further translocation of cytoplasmic FANCD2 to the nucleus (Fig. 4 B and C). Interestingly, WT C/EBPδ and the R198A mutant promoted MMC-induced nuclear translocation of FANCD2, as seen by the loss of cytoplasmic FANCD2-S (Fig. 4B) and increased nuclear FANCD2-L (Fig. 4C). In contrast, C/EBPδ mutants that did not promote monoubiquitination also failed to augment nuclear translocation and were deficient in either nuclear localization (ΔDBD) or FANCD2 interaction (ΔD2ID). These observations suggested that C/EBPδ facilitates the nuclear import of FANCD2 by direct protein interaction. In agreement with this conclusion, analysis of cell fractions revealed reduced FANCD2 levels in the nuclear protein extracts of C/EBPδ-null MEFs (Fig. S4). Additional experiments with FANCA-deficient cells show that FANCD2 monoubiquitination is not required for its nuclear import and that induction of C/EBPδ expression by MMC does not require the FA pathway (SI Results and Fig. S5).
Fig. 4.
C/EBPδ augments nuclear import of FANCD2. HEK293 cells were transfected with Flag-tagged C/EBPδ expression constructs and treated with MMC (1 μg/mL) for 24 h. (A) Whole-cell (WE), (B) cytoplasmic (CE), and (C) nuclear (NE) extracts were analyzed for expression of FANCD2 and C/EBPδ (anti-Flag). Tubulin and H2AX are shown as loading controls. The purity of cytoplasmic versus nuclear fractions also is shown in Fig. S7.
Most proteins require active, selective transport mechanisms to enter the nucleus (29). A number of nuclear import complexes have been identified, consisting of either importin α or importin β cargo-recognition molecules. Usually, substrate recognition and binding occurs through nuclear localization signals (NLSs) that are composed of basic residues and reside within DNA- or RNA-binding domains (30). The mass spectrometry results suggested that importin 4 (IPO4) interacts with C/EBPδ (Table S1). Co-IP studies demonstrated that all C/EBPδ variants that contained the NLS (Fig. 1A) could interact with IPO4 in HEK293 cells, whereas the ΔDBD mutant did not (Fig. 5 A and B), consistent with its exclusively cytoplasmic localization (Fig. 4 B and C). As shown previously (Fig. 1 B and C), all mutants except ΔD2ID interacted with FANCD2. On the other hand, IPO4 did not interact with FANCD2 unless WT- or R198A-C/EBPδ was present, as determined by co-IP of either IPO4 (Fig. 5B) or FANCD2 (Fig. 5C). In the presence of ΔDBD or ΔD2ID mutants, IPO4 did not interact with FANCD2. The ΔDBD mutant interacted only with FANCD2, whereas the ΔD2ID mutant interacted only with IPO4. Taken together, these interaction studies show that C/EBPδ mediates the indirect association of FANCD2 with IPO4 through its interaction with both of these proteins via separate domains (Fig. 5D). Although none of the interactions was altered in the presence or absence of MMC, the data suggest that they are a prerequisite for the MMC-induced nuclear translocation of FANCD2 in the presence of C/EBPδ.
Fig. 5.
C/EBPδ mediates FANCD2 interaction with IPO4. HEK293 cells were transfected with Flag-tagged C/EBPδ constructs and treated with MMC as indicated. Lysates were immunoprecipitated (IP) with (A) anti-Flag antibody for C/EBPδ, (B) anti-IPO4 antibody, or (C) anti-FANCD2 antibody or respective IgG control. LE, long exposure; SE, short exposure. Western blots of input (shown in A) and immunoprecipitates were analyzed for expression of the indicated proteins. In this experiment, C/EBPδ was detected by anti-C/EBPδ antibody (BD69319; BD Biosciences). (D) Schematic (not drawn to scale) indicating how FANCD2 and IPO4 interact with different domains of C/EBPδ but not with each other (see Fig. 1A for designation of C/EBPδ domains).
IPO4 Participates in the DNA-Damage Response.
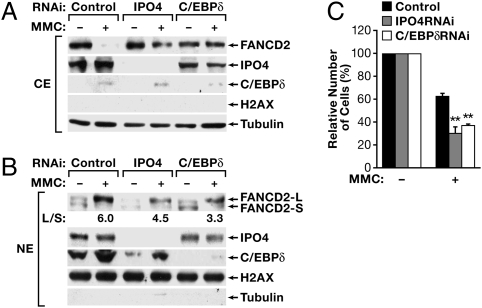
To assess directly the role of IPO4 in nuclear import of FANCD2 and C/EBPδ, we silenced IPO4 in MDA-MB-468 cells. These cells were chosen because of the expression levels of endogenous C/EBPδ, FANCD2, and IPO4 and because of the efficient nuclear translocation of FANCD2 in response to MMC (Fig. 6 A and B). Indeed, IPO4 depletion reduced nuclear localization of both FANCD2 and C/EBPδ both before and after MMC treatment (Fig. 6B), and cytoplasmic FANCD2 still was detectable upon MMC treatment (Fig. 6A). Similar results were observed after silencing of C/EBPδ (Fig. 6 and Fig. S5). Although these data confirm the role of endogenous C/EBPδ in nuclear translocation of FANCD2, they also identify an important role of IPO4 in nuclear import of both of these proteins.
Fig. 6.
IPO4 augments nuclear localization of FANCD2 and cell survival in response to MMC. MDA-MB-468 cells were transiently transfected with siRNA against IPO4 or C/EBPδ or with scrambled oligos as control. MMC (500 ng/mL) was added 24 h later, and cells were incubated for another 20 h before preparation of (A) cytoplasmic (CE) or (B) nuclear (NE) cell extracts, followed by Western blot analysis of protein expression as indicated. The purity of cytoplasmic versus nuclear fractions also is shown in Fig. S7. (C) MDA-MB-468 cells were transfected as above and were placed in 96-well dishes at 5,000 cells/well 8 h later. The next day, MMC (500 ng/mL) was added, and cell viability was assessed 48 h later. Data are mean ± SEM of three experiments, each performed in triplicate, and relative to cells before treatment (set at 100%). **P < 0.01 relative to control siRNA.
Last, we addressed the functional relevance of IPO4 and C/EBPδ in this system by assessing cell survival in response to MMC. Indeed, silencing of either IPO4 or C/EBPδ significantly enhanced the cytotoxicity of MMC on MDA-MB-468 cells (Fig. 6C), demonstrating that, like C/EBPδ, IPO4 promotes cell survival in response to DNA damage. Because numerous reports have documented the role of nuclear FANCD2 in cellular survival in response to MMC (7–9), we suggest that the reduced cell survival after silencing of C/EBPδ or IPO4 is the result, at least in part, of their role in augmenting nuclear import of FANCD2.
Discussion
In this study we identified a function of the transcription factor C/EBPδ and the nuclear import factor IPO4 in the DNA damage response. C/EBPδ mediates interaction of the DNA-repair protein FANCD2 with IPO4 and as a result facilitates nuclear import of FANCD2, which is essential for the FA DNA-repair pathway. This activity of C/EBPδ is independent of its functions as a transcription factor. Using silencing strategies or cells deficient in either FANCD2 or C/EBPδ and through the overexpression of WT C/EBPδ or mutants that cannot interact with either IPO4 or FANCD2, we show that both C/EBPδ and IPO4 play a significant role in nuclear import of FANCD2 and cell survival in response to MMC. The interaction of C/EBPδ with FANCD2 and IPO4 explains in part its role in cell survival in response to DNA damage by cross-linking agents. Furthermore, this study identifies IPO4 and nuclear import as players in the FA DNA damage-response pathway.
This study, together with two recent reports (25, 31), underscores a multifaceted impact of C/EBPδ on the DNA damage response. By promoting cyclin D1 degradation (25), C/EBPδ may augment growth arrest to allow DNA repair to proceed. Furthermore, C/EBPδ induces superoxide dismutase 1 (SOD1) expression, which reduces reactive oxygen species and supports cell survival in response to cisplatin compounds (31). These, and possibly other, activities of C/EBPδ also may contribute to cell survival in response to MMC. However, our data showing that C/EBPδ-augmented cell survival requires its FANCD2 interaction domain and the presence of endogenous FANCD2 strongly suggest that interaction with FANCD2 plays a significant part in the role of C/EBPδ in the MMC response. However, C/EBPδ-null cells display only a mild FA phenotype, consistent with nuclear FANCD2-L still being observed in C/EBPδ null cells, albeit at reduced levels.
FA is a cancer-susceptibility syndrome (5). Interestingly, a very recent immunohistochemical study showed that a significant proportion of malignant breast tumors had lost expression of nuclear FANCD2 but retained cytoplasmic expression, whereas benign lesions retained both nuclear and cytoplasmic staining (32). These observations are consistent with FANCD2 nuclear import serving as a tumor-suppressing mechanism. Hence, the functional interaction of C/EBPδ with FANCD2 may be part of its tumor suppressor activity and its role in maintaining genomic stability.
FANCD2 is a large protein that requires an active nuclear import mechanism to participate in DNA repair. How FANCD2 is transported to the nucleus has been unknown to date. Using several relevant software programs, we were not able to identify an NLS in FANCD2 itself. Therefore, FANCD2 association with IPO4 is achieved through an adaptor mechanism using the transcription factor C/EBPδ. Interestingly, TNF-α signaling was reported to promote nuclear translocation of FANCD2 in HEK293 cells (33). Because C/EBPδ expression can be induced by TNF-α (10), it may play a role in this pathway also, although the physiological significance is unknown. We also could speculate that FANCD2-C/EBPδ complexes may serve functions other than nuclear import of FANCD2. One of the most prominent features of FANCD2 is its localization to sites of DNA damage, which with FANCD2 staining can be visualized as discrete nuclear foci in cells with DNA lesions (34). We were unable to produce evidence of significant colocalization of C/EBPδ with FANCD2 foci (Fig. S6). However, because other C/EBP factors have been shown to associate tightly with chromatin, and because C/EBPδ, among C/EBP family proteins, has the lowest stringency for DNA sequence recognition to bind DNA (35, 36), it is conceivable that C/EBPδ may shuttle FANCD2 to the chromatin but not remain associated within DNA damage foci.
IPO4 is a monomeric import receptor that also plays a role in ribosomal RNA processing (37). Only a few proteins are known to be substrates of IPO4 (30, 38–40). Interestingly, one study suggests that the IPO4 ortholog Imp4 of Schistosoma mansoni, Escherichia coli, and yeast promotes cell survival in response to alkylating agents (41). IPO4 has an RNA-binding domain (37) and therefore also may bind single-stranded DNA. Because prokaryotes do not have nuclear membranes, one can speculate that IPO4 may have a direct role in DNA-repair pathways that potentially could extend to its association with FANCD2 and precede the evolution of its function as a nuclear import protein.
IPO4 depletion efficiently reduced nuclear FANCD2 even in the absence of DNA damage, demonstrating that IPO4 is responsible for translocation of a significant fraction of FANCD2 to the nucleus. However, silencing of C/EBPδ or IPO4 did not completely block FANCD2 nuclear translocation. Most likely, FANCD2 also may use other adaptor proteins and importin molecules to enter the nucleus. The interaction of ectopic C/EBPδ with FANCD2 and IPO4 in cell lysates was not modulated by MMC, indicating that C/EBPδ can bind both proteins even in the absence of activated DNA damage-response pathways. However, the in situ DuoLink assay and cell fractionations revealed that nuclear translocation of FANCD2 is stimulated by MMC in some cell types, such as PD20-D2 and MDA-MB-468. This stimulation may be, in part, the result of MMC-induced C/EBPδ expression. However, the active nuclear import process also may be stimulated by DNA damage-induced signals, although the association of C/EBPδ, IPO4, and FANCD2 is independent of such signals. In any case, the nuclear import function described here is a prerequisite for FANCD2 monoubiquitination and for any subsequent functional relevance of FANCD2-C/EBPδ-IPO4 complexes in the nucleus. This study revealed nuclear import of FANCD2 as a regulated step in the DNA damage response. Furthermore, we identified C/EBPδ and IPO4 as two molecules that play important roles in the nuclear import of FANCD2.
Materials and Methods
Cells.
All cell lines have been described previously (SI Text). Cells were treated with MMC at 1 μg/mL for 24 h, unless indicated otherwise. Culture, transfection, survival, and cell cycle assays according to standard procedures are described in SI Materials and Methods.
Western Analysis, Immunoprecipitation, Immunocytochemistry, and RNAi.
Western analysis, immunoprecipitation, immunocytochemistry, and RNAi were performed according to standard procedures. Details are given in SI Materials and Methods.
DuoLink in Situ Proximity Ligation Assay.
Anti-mouse PLA probe plus, anti-rabbit PLA probe minus, and detection kit 563 were purchased from OLink Bioscience. Formalin-fixed cells were permeabilized using 0.3% Triton X-100, blocked with 3% BSA, and incubated with primary antibody for C/EBPδ (BD69319; BD Biosciences Pharmingen), FANCD2 (Novus), or FANCA (R6512; Fanconi Anemia Research Fund) at 1:600 each for 1 h. PLA probes were diluted 1:15 in blocking solution, and all other steps were performed according to the manufacturer's instructions. Detection of the PLA signals was carried out with an LSM 510 META confocal fluorescence microscope (Zeiss).
Supplementary Material
Acknowledgments
BD69319 mouse monoclonal antibody against C/EBPδ was provided by BD Biosciences Pharmingen through an Antibody Co-development Collaboration between the National Cancer Institute and BD Bioscience. The cell lines PD20, PD20-D2, PD220, and PD220RV and FANCD2 (9700) and FANCA (R6512) antibodies were kindly provided by the Fanconi Anemia Research Fund (Eugene, OR). We thank Dr. J. Keller for advice on bone marrow cell colony assays; Drs. Stephen Lockett and Thomas Turbyville for kind help with confocal microscopy; and Glenn Summers, Jennifer Beachley, and Linda Miller for outstanding assistance with mouse work. We thank Drs. Alan D'Andrea (Dana-Faber Cancer Institute) and Shyam Sharan (National Cancer Institute) for reagents and discussion. We are indebted to Dr. Nigel Jones for expert advice. We thank Drs. Shyam Sharan, Nigel Jones, Ira Daar, Kuppusamy Balamurugan, Tom Misteli, Stephen Lockett, and Thomas Turbyville for constructive comments on the manuscript and Jiro Wada for preparation of the figures. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and in part with federal funds under Contract HHSN261200800001E.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002603107/-/DCSupplemental.
References
- 1.Lazzaro F, et al. Checkpoint mechanisms at the intersection between DNA damage and repair. DNA Repair (Amst) 2009;8:1055–1067. doi: 10.1016/j.dnarep.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Bonner WM, et al. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mah LJ, El-Osta A, Karagiannis TC. gammaH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–686. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- 4.Bagby GC., Jr Genetic basis of Fanconi anemia. Curr Opin Hematol. 2003;10:68–76. doi: 10.1097/00062752-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 5.D'Andrea AD, Grompe M. The Fanconi anaemia/BRCA pathway. Nat Rev Cancer. 2003;3:23–34. doi: 10.1038/nrc970. [DOI] [PubMed] [Google Scholar]
- 6.Green AM, Kupfer GM. Fanconi anemia. Hematol Oncol Clin North Am. 2009;23:193–214. doi: 10.1016/j.hoc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moldovan GL, D'Andrea AD. How the Fanconi anemia pathway guards the genome. Annu Rev Genet. 2009;43:223–249. doi: 10.1146/annurev-genet-102108-134222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson LH, Hinz JM. Cellular and molecular consequences of defective Fanconi anemia proteins in replication-coupled DNA repair: Mechanistic insights. Mutat Res. 2009;668:54–72. doi: 10.1016/j.mrfmmm.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knipscheer P, et al. The Fanconi anemia pathway promotes replication-dependent DNA interstrand cross-link repair. Science. 2009;326:1698–1701. doi: 10.1126/science.1182372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: Structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal S, et al. The C/EBPdelta tumor suppressor is silenced by hypermethylation in acute myeloid leukemia. Blood. 2007;109:3895–3905. doi: 10.1182/blood-2006-08-040147. [DOI] [PubMed] [Google Scholar]
- 12.Tang D, Sivko GS, DeWille JW. Promoter methylation reduces C/EBPdelta (CEBPD) gene expression in the SUM-52PE human breast cancer cell line and in primary breast tumors. Breast Cancer Res Treat. 2006;95:161–170. doi: 10.1007/s10549-005-9061-3. [DOI] [PubMed] [Google Scholar]
- 13.Porter D, et al. Molecular markers in ductal carcinoma in situ of the breast. Mol Cancer Res. 2003;1:362–375. [PubMed] [Google Scholar]
- 14.Ko CY, Hsu HC, Shen MR, Chang WC, Wang JM. Epigenetic silencing of CCAAT/enhancer-binding protein delta activity by YY1/polycomb group/DNA methyltransferase complex. J Biol Chem. 2008;283:30919–30932. doi: 10.1074/jbc.M804029200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naderi A, et al. A gene-expression signature to predict survival in breast cancer across independent data sets. Oncogene. 2007;26:1507–1516. doi: 10.1038/sj.onc.1209920. [DOI] [PubMed] [Google Scholar]
- 16.Barresi V, Vitarelli E, Cerasoli S, Barresi G. The cell growth inhibitory transcription factor C/EBPdelta is expressed in human meningiomas in association with low histological grade and proliferation index. J Neurooncol. 2009;97:233–240. doi: 10.1007/s11060-009-0024-0. [DOI] [PubMed] [Google Scholar]
- 17.Sterneck E, et al. Selectively enhanced contextual fear conditioning in mice lacking the transcriptional regulator CCAAT/enhancer binding protein delta. Proc Natl Acad Sci USA. 1998;95:10908–10913. doi: 10.1073/pnas.95.18.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang AM, et al. Loss of CCAAT/enhancer binding protein δ promotes chromosomal instability. Oncogene. 2004;23:1549–1557. doi: 10.1038/sj.onc.1207285. [DOI] [PubMed] [Google Scholar]
- 19.Barroso E, et al. FANCD2 associated with sporadic breast cancer risk. Carcinogenesis. 2006;27:1930–1937. doi: 10.1093/carcin/bgl062. [DOI] [PubMed] [Google Scholar]
- 20.Naderi A, et al. BEX2 is overexpressed in a subset of primary breast cancers and mediates nerve growth factor/nuclear factor-kappaB inhibition of apoptosis in breast cancer cell lines. Cancer Res. 2007;67:6725–6736. doi: 10.1158/0008-5472.CAN-06-4394. [DOI] [PubMed] [Google Scholar]
- 21.Porter DA, et al. A SAGE (serial analysis of gene expression) view of breast tumor progression. Cancer Res. 2001;61:5697–5702. [PubMed] [Google Scholar]
- 22.Niedernhofer LJ, Lalai AS, Hoeijmakers JH. Fanconi anemia (cross)linked to DNA repair. Cell. 2005;123:1191–1198. doi: 10.1016/j.cell.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Williams SC, Angerer ND, Johnson PF. C/EBP proteins contain nuclear localization signals imbedded in their basic regions. Gene Expr. 1997;6:371–385. [PMC free article] [PubMed] [Google Scholar]
- 24.Miller M, Shuman JD, Sebastian T, Dauter Z, Johnson PF. Structural basis for DNA recognition by the basic region leucine zipper transcription factor CCAAT/enhancer-binding protein alpha. J Biol Chem. 2003;278:15178–15184. doi: 10.1074/jbc.M300417200. [DOI] [PubMed] [Google Scholar]
- 25.Pawar SA, et al. C/EBPδ targets cyclin D1 for proteasome-mediated degradation via induction of CDC27/APC3 expression. Proc Natl Acad Sci USA. 2010;107:9210–9215. doi: 10.1073/pnas.0913813107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiser TN, et al. Flow cytometric characterization of the response of Fanconi's anemia cells to mitomycin C treatment. Cytometry. 1982;2:291–297. doi: 10.1002/cyto.990020505. [DOI] [PubMed] [Google Scholar]
- 27.Akkari YM, et al. The 4N cell cycle delay in Fanconi anemia reflects growth arrest in late S phase. Mol Genet Metab. 2001;74:403–412. doi: 10.1006/mgme.2001.3259. [DOI] [PubMed] [Google Scholar]
- 28.Bogliolo M, et al. The Fanconi anaemia genome stability and tumour suppressor network. Mutagenesis. 2002;17:529–538. doi: 10.1093/mutage/17.6.529. [DOI] [PubMed] [Google Scholar]
- 29.Wagstaff KM, Jans DA. Importins and beyond: Non-conventional nuclear transport mechanisms. Traffic. 2009;10:1188–1198. doi: 10.1111/j.1600-0854.2009.00937.x. [DOI] [PubMed] [Google Scholar]
- 30.Jäkel S, Mingot JM, Schwarzmaier P, Hartmann E, Görlich D. Importins fulfill a dual function as nuclear import receptors and cytoplasmic chaperones for exposed basic domains. EMBO J. 2002;21:377–386. doi: 10.1093/emboj/21.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hour TC, et al. Transcriptional up-regulation of SOD1 by CEBPD: A potential target for cisplatin resistant human urothelial carcinoma cells. Biochem Pharmacol. 2010;80:325–334. doi: 10.1016/j.bcp.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudland PS, et al. Significance of the Fanconi anemia FANCD2 protein in sporadic and metastatic human breast cancer. Am J Pathol. 2010;176:2935–2947. doi: 10.2353/ajpath.2010.090779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma DJ, et al. Temporal and spatial profiling of nuclei-associated proteins upon TNF-alpha/NF-kappaB signaling. Cell Res. 2009;19:651–664. doi: 10.1038/cr.2009.46. [DOI] [PubMed] [Google Scholar]
- 34.Gregory RC, Taniguchi T, D’Andrea AD. Regulation of the Fanconi anemia pathway by monoubiquitination. Semin Cancer Biol. 2003;13:77–82. doi: 10.1016/s1044-579x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 35.Osada S, Yamamoto H, Nishihara T, Imagawa M. DNA binding specificity of the CCAAT/enhancer-binding protein transcription factor family. J Biol Chem. 1996;271:3891–3896. doi: 10.1074/jbc.271.7.3891. [DOI] [PubMed] [Google Scholar]
- 36.Baer M, Johnson PF. Generation of truncated C/EBPbeta isoforms by in vitro proteolysis. J Biol Chem. 2000;275:26582–26590. doi: 10.1074/jbc.M004268200. [DOI] [PubMed] [Google Scholar]
- 37.Lee SJ, Baserga SJ. Imp3p and Imp4p, two specific components of the U3 small nucleolar ribonucleoprotein that are essential for pre-18S rRNA processing. Mol Cell Biol. 1999;19:5441–5452. doi: 10.1128/mcb.19.8.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyauchi Y, et al. Importin 4 is responsible for ligand-independent nuclear translocation of vitamin D receptor. J Biol Chem. 2005;280:40901–40908. doi: 10.1074/jbc.M509347200. [DOI] [PubMed] [Google Scholar]
- 39.Chachami G, et al. Transport of hypoxia-inducible factor HIF-1alpha into the nucleus involves importins 4 and 7. Biochem Biophys Res Commun. 2009;390:235–240. doi: 10.1016/j.bbrc.2009.09.093. [DOI] [PubMed] [Google Scholar]
- 40.Pradeepa MM, Manjunatha S, Sathish V, Agrawal S, Rao MR. Involvement of importin-4 in the transport of transition protein 2 into the spermatid nucleus. Mol Cell Biol. 2008;28:4331–4341. doi: 10.1128/MCB.00519-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furtado C, et al. Schistosoma mansoni: The IMP4 gene is involved in DNA repair/tolerance after treatment with alkylating agent methyl methane sulfonate. Exp Parasitol. 2007;116:25–34. doi: 10.1016/j.exppara.2006.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.