Abstract
Human innate immunity against most African trypanosomes, including Trypanosoma brucei brucei, is mediated by a minor subclass of toxic serum HDL, called trypanosome lytic factor-1 (TLF-1). This HDL contains two primate specific proteins, apolipoprotein L-1 and haptoglobin (Hp)-related protein, as well as apolipoprotein A-1. These assembled proteins provide a powerful defense against trypanosome infection. Trypanosoma brucei rhodesiense causes human African sleeping sickness because it has evolved an inhibitor of TLF-1, serum resistance-associated (SRA) protein. Trypanosoma brucei gambiense lacks the SRA gene, yet it infects humans. As transfection of T. b. gambiense (group 1) is not possible, we initially used in vitro-selected TLF-1–resistant T. b. brucei to examine SRA-independent mechanisms of TLF-1 resistance. Here we show that TLF-1 resistance in T. b. brucei is caused by reduced expression of the Hp/Hb receptor gene (TbbHpHbR). Importantly, T. b. gambiense (group 1) also showed a marked reduction in uptake of TLF-1 and a corresponding decrease in expression of T. b. gambiense Hp/Hb receptor (TbgHpHbR). Ectopic expression of TbbHpHbR in TLF-1–resistant T. b. brucei rescued TLF-1 uptake, demonstrating that decreased TbbHpHbR expression conferred TLF-1 resistance. Ectopic expression of TbgHpHbR in TLF-1–resistant T. b. brucei failed to rescue TLF-1 killing, suggesting that coding sequence changes altered Hp/Hb receptor binding affinity for TLF-1. We propose that the combination of coding sequence mutations and decreased expression of TbgHpHbR directly contribute to parasite evasion of human innate immunity and infectivity of group 1 T. b. gambiense.
Keywords: African trypanosomes, haptoglobin/hemoglobin receptor, innate immunity, human serum resistance
The infectivity of African trypanosomes for humans is dependent on their ability to neutralize or avoid the cytotoxic activity of a powerful innate immune activity associated with a minor subclass of human serum HDLs, trypanosome lytic factor (TLF)-1 and a related protein complex, TLF-2 (1–3). It is likely that both TLF-1 and TLF-2 contribute to trypanosome killing, but TLF-2 is poorly characterized and its mechanism of killing is unknown. Trypanosoma brucei brucei causes a chronic wasting disease in cattle but is unable to infect humans because of its susceptibility to TLF. Trypanosomes causing human sleeping sickness are capable of circumventing the activity of TLF. Trypanosoma brucei rhodesiense has evolved a serum resistance-associated (SRA) protein that binds and neutralizes TLF-1, and expression of SRA in T. b. brucei is sufficient to confer resistance to TLF-1 (4, 5). Trypanosoma brucei gambiense also infects humans but lacks SRA and the mechanism of resistance to TLF-1 killing is unknown. T. b. gambiense consists of two genetically distinct groups. Group 1 is the dominant form, which has an invariant human serum resistance phenotype, whereas group 2 is less common and can lose its resistance to human serum during serial passage in rodents (6).
The major protein components of TLF-1 are apolipoprotein A-1 (apoA-1), and two primate specific proteins, apolipoprotein L-1 (apoL-1) and haptoglobin (Hp)-related protein (Hpr). Both Hpr and apoL-1 have been proposed to be toxic to trypanosomes (7–11). In addition, Hb is a necessary cofactor for maximal killing activity (12). ApoL-1 shares limited sequence similarity to bacterial colicins, and structural and functional similarities with members of the apoptotic Bcl2 family (13). In vitro studies have shown that apoL-1 interacts with membranes, leading to selective ion movement (10, 14). The mechanism of Trypanosoma brucei rhodesience resistance to TLF-1 is dependent on SRA binding to a C-terminal α-helical region of apoL-1 leading to inhibition of pore-forming activity (15). Based on this finding, it has been proposed that formation of the binary TLF-1/SRA complex prevents the interaction of apoL-1 with endosomal/lysosomal membranes, leading to human serum resistance (15). Hrp is highly similar (92%) to the human Hp, an abundant acute-phase Hb scavenger protein that is cleared from the circulation by a high-affinity receptor in mammals (16). Hrp also binds Hb, but is not an Hb scavenger protein as it does not bind the mammalian Hp/Hb receptor (12, 17–19). However, Hpr/Hb binds a T. b. brucei Hp/Hb receptor (TbbHpHbR) located in the flagellar pocket with high affinity (Kd of approximately 17 × 10−9 M), thus allowing TLF-1 uptake (18, 20). T. b. brucei killing requires trafficking of surface-bound TLF-1 to the acidic lysosome, where it is activated and associates with the lysosomal membrane, ultimately leading to parasite death (21, 22). Deletion of the TbbHpHbR gene by knockout or competition with an excess of Hp/Hb spared T. b. brucei from killing by TLF-1 (18, 23). TLF-1 resistance in T. b. rhodesiense is not the result of decreased expression of the Hp/Hb receptor gene, as both the affinity and copy number of the receptor are identical to that of T. b. brucei (20).
T. b. gambiense lacks the SRA gene, and this led us to investigate SRA-independent defense mechanisms that trypanosomes might have evolved against TLF-1 (24). Here we show that both mutations to the T. b. gambiense Hp/Hb receptor (TbgHpHbR) coding sequence and decreased mRNA levels are conserved among group 1 T. b. gambiense isolates and that the lack of a functional Hp/Hb receptor directly influences the susceptibility of these parasites to human innate immunity.
Results
Susceptibility to TLF-1 Is Dependent on Expression of TbbHpHbR.
To study the mechanism of SRA-independent human serum resistance, we treated T. b. brucei Lister 427–221 [expressing variant specific glycoprotein (VSG) 221] with progressively higher concentrations of purified human HDL containing TLF-1. A highly TLF-1 resistant population, T. b. brucei 427–800R, was obtained (25). When cultivated in vitro for extended periods antigenic variants of T. b. brucei 427–800R expressing a new VSG were observed. By limiting dilution, a clonal line, T. b. brucei 427–060R, was obtained that retained resistance to TLF-1 (Fig. 1 A and B and Fig. S1 A and B). A striking characteristic of both T. b. brucei 427–800R and 060R was the complete loss of TLF-1 uptake as measured by fluorescence-activated cytometry (FAC; Fig. 1C) and fluorescence microscopy with Alexa Fluor-488 conjugated TLF-1.
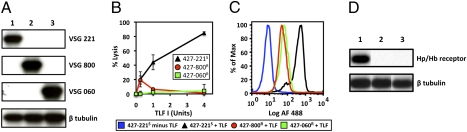
Fig. 1.
T. b. brucei resistance to TLF-1 correlates with decreased uptake and reduced levels of TbbHpHbR mRNA. (A) Northern blot hybridization analysis with specific probes for VSGs and β-tubulin. Lane 1, T. b. brucei 427–221S; lane 2, T. b. brucei 427–800R; lane 3, T. b. brucei 427–060R. β-Tubulin was used as a control. (B) In vitro susceptibility of the T. b. brucei cell lines to TLF-1. Two-hour lysis assays were carried out with increasing concentration of TLF-1 [expressed as units (2)]. T. b. brucei 427–221S is marked by a black triangle; T. b. brucei 427–800R a red circle; T. b. brucei 427–060R a green square. (C) Analysis of Alexa Fluor–488–conjugated TLF-1 uptake by FAC. T. b. brucei 427–221S minus TLF-1 is blue; T. b. brucei 427–221S plus TLF-1 is black; T. b. brucei 427–800R plus TLF-1 is red; and T. b. brucei 427–060R plus TLF is green. Each cell line was designated by the expressed VSG and its susceptibility to TLF-1. (D) Northern blot analysis of total RNA with probes specific for TbbHpHbR (Hp/Hb receptor) and β-tubulin mRNAs. Lanes are as in A.
The observed decrease in TLF-1 binding and uptake may reflect mutations within the coding region of the TbbHpHbR gene, resulting in loss of TLF-1 binding, or altered expression of the receptor. To examine these possibilities, the TbbHpHbR gene was PCR amplified, cloned, and sequenced from the TLF-1–resistant and –susceptible cell lines (Fig. S2). No changes in coding sequences were observed. In contrast, Northern blot analysis showed that TbbHpHbR mRNA was expressed in only the TLF-1–susceptible trypanosomes (Fig. 1D).
Although resistance to TLF-1 is a relatively stable characteristic of T. b. brucei 427–060R, we observed a small fraction of cells that spontaneously revert to being susceptible to TLF-1. To determine whether these cells had activated TbbHpHbR expression, we developed a FACS procedure. As Alexa Fluor-488 conjugation of TLF-1 results in the loss of killing activity with no affect on binding or uptake, we were able to sort a minor subpopulation of live trypanosomes that bound TLF-1. Trypanosomes that bound TLF-1 were sorted, grown in conditioned growth media, and further subjected to additional rounds of FACS, resulting in a population enriched for TLF-1 uptake (Fig. 2A). This population was used to derive a clonal line, designated as T. b. brucei 427–060S, that bound TLF-1 and was highly susceptible to TLF-1 killing (Fig. 2 B and C). Based on Northern blot and SDS/PAGE analysis, both T. b. brucei 427–060R and 427–060S express the VSG060 (Fig. 2D and Fig. S3). However, Northern blot and RT-PCR showed that only the T. b. brucei 427–060S line expresses the TbbHpHbR gene (Fig. 2D). Together these data suggest that TLF-1 resistance is independent of VSG expression, and differential expression of the TbbHpHbR gene may have a profound effect on TLF-1 susceptibility in T. b. brucei.
Fig. 2.
Spontaneous reexpression of the TbbHpHbR gene restores susceptibility to TLF-1 in susceptible T. b. brucei 427–060R. (A) Third round of FACS for T. b. brucei 427–060R cells that bound Alexa Fluor–488–conjugated TLF-1. Successive rounds of sorting enriched for a subpopulation of cells that bound TLF-1, T. b. brucei 427–060S. T. b. brucei 427–221S minus TLF-1 is blue; T. b. brucei 427–221S plus TLF-1 is black; T. b. brucei 427–060R plus TLF-1 is green; and T. b. brucei 427–060R (third FACS sort) plus TLF-1 is orange. SI Materials and Methods provides details of FACS. (B) Analysis of Alexa Fluor-488–conjugated TLF-1 uptake by FAC. T. b. brucei 427–221S plus TLF-1 is black; T. b. brucei 427–060R plus TLF-1 is green; clonal line from the TLF-1–positive subpopulation in the third round of FACS T. b. brucei 427–060S plus TLF-1 is red. (C) In vitro TLF-1 lysis assay: percentage of cells lysed following incubation with TLF for 2 h at 37 °C. T. b. brucei 427–221S is marked as a black triangle; T. b. brucei 427–060R a green square; T. b. brucei 427–060S a red circle. (D) Northern blot analysis of total RNA with probes specific for VSG 060, TbbHpHbR (Hp/Hb receptor), and β-tubulin mRNAs. Lane 1, T. b. brucei 427–060R; lane 2, T. b. brucei 427–060S.
TLF-1 Susceptibility Is Reduced in RNAi Knockdowns of TbbHpHbR.
The correlation between decreased expression of TbbHpHbR gene and TLF-1 resistance makes it tempting to propose that the resistance phenotype is caused solely by loss of expression of this receptor. To examine this possibility, a portion of the coding sequence from TbbHpHbR was cloned into an RNAi plasmid vector between opposing T7 RNA polymerase promoters. The construct was transfected into T. b. brucei Lister 427, bloodstream line 90–13 (26). Induction of RNAi with doxycycline resulted in slightly reduced growth (Fig. S4A). In addition, knockdown of the TbbHpHbR mRNA resulted in a reduction both in uptake and susceptibility to TLF (Fig. S4 B–D). The overall reduction in TLF killing (approximately 50% relative to T. b. brucei 427–221S) was consistent with the incomplete knockdown of TbbHpHbR mRNA in these cells and supports the possibility that decreased expression of TbbHpHbR confers TLF-1 resistance (Fig. S4B).
Reduced TLF-1 Uptake and TbgHpHbR Expression in Group 1 T. b. Gambiense.
Our findings and those of a previous study (18) with the TLF-1 resistant T. b. brucei lines showed that the reduced expression of the TbbHpHbR gene provided an SRA-independent mechanism of resistance to TLF-1. This led us to investigate whether the reduced expression of the TbgHpHbR gene also contributed to TLF-1 resistance in T. b. gambiense. The uptake of TLF-1 and the expression of the TbgHpHbR gene were examined in group 1 T. b. gambiense (Fig. 3 A–C). The binding and uptake of TLF-1 by T. b. brucei (STIB247), T. b. rhodesiense (Baganzi), and T. b. gambiense group 1 (Eliane) was compared using Alexa Fluor–488–conjugated TLF-1 by immunofluorescence microscopy (Fig. 3A). Both T. b. brucei and T. b. rhodesiense accumulate TLF-1 intracellularly within the lysosome, whereas T. b. gambiense group 1 (Eliane) failed to take up TLF-1. We also compared the uptake of TLF-1 by T. b. gambiense group 1 (Eliane) with T. b. brucei 427–221 and T. b. brucei 427–800R by FAC and observed a decrease in TLF-1 uptake in T. b. gambiense group 1 (Eliane), similar to the Hp/Hb receptor minus T. b. brucei 427–800R (Fig. 3C). Consistent with these results, we found that the abundance of TbgHpHbR mRNA was reduced approximately fivefold relative to TLF-1–susceptible T. b. brucei and SRA expressing T. b. rhodesiense (strain Baganzi; Fig. 3B). When T. b. gambiense group 1 (Eliane) was isolated from infected mice, the level of TbgHpHbR mRNA was reduced greater than 20-fold relative to TLF-1–susceptible T. b. brucei (Fig. 3B). To investigate whether reduced expression of TbgHpHbR was a general feature of group 1 T. b. gambiense, we examined five additional isolates from three different countries and found that all showed consistent reduction in TbgHpHbR mRNA abundance (Fig. 3B).
Fig. 3.
Group 1 T. b. gambiense shows reduced TLF-1 uptake and expression of the TbgHpHbR gene. (A) Uptake of TLF-1 examined by fluorescence microscopy. Images are typical examples showing phase contrast, DAPI staining of the nucleus and kinetoplast, uptake of Alexa Fluor–488–conjugated TLF-1, and identification of the lysosome by lysotracker. (1) T. b. brucei, strain STIB247; (2) T. b. rhodesiense, strain Baganzi; (3) group 1 T. b. gambiense, strain Eliane. (B) Relative expression of Hp/Hb receptor genes was determined using real-time RT-PCR. T. b. brucei STIB247 black; T. b. rhodesiense, strain Baganzi, is green; and the following are all red: T. b. gambiense A1 (group 1 T. b. gambiense strain Eliane, in vitro; Côte d'Ivoire), T. b. gambiense A2 (group 1 T. b. gambiense strain Eliane from mice; Côte d'Ivoire), T. b. gambiense B (group 1 T. b. gambiense strain from mice, “Tobo”; Côte d'Ivoire), T. b. gambiense C (group 1 T. b. gambiense strain “Isti”; from mice, Côte d'Ivoire), T. b. gambiense D (group 1 T. b. gambiense strain “Bim”; from mice, Cameroon), T. b. gambiense E (group 1 T. b. gambiense strain “Mos”; from mice, Cameroon), T. b. gambiense F (group 1 T. b. gambiense strain “Pa”; from mice, Democratic Republic of Congo; ± 1 SD). (C) Analysis of Alexa Fluor-488–conjugated TLF-1 uptake by FAC. T. b. brucei, strain STIB247 minus TLF-1 is orange; T. b. brucei, strain STIB247 plus TLF-1 is black; T. b. brucei 427–800R plus TLF-1 is blue; T. b. rhodesiense KETRI2482 plus TLF-1 is green; and T. b. gambiense group 1, strain Eliane, plus TLF-1 is red.
Ectopic Expression of the TbbHpHbR, but Not TbgHpHbR, Rescues TLF-1 Susceptibility.
To determine whether TbbHpHbR expression was sufficient to restore susceptibility to TLF-1 in resistant cell lines, we expressed TbbHpHbR ectopically in T. b. brucei 427–060R (Fig. 4). The regulated expression of trypanosome genes is most commonly posttranscriptional, and is often associated with differences in mRNA degradation determined by 3′UTRs (27). To prevent the possible degradation of ectopically expressed TbbHpHbR mRNA in T. b. brucei 427–060R, we replaced the endogenous UTRs with sequences from housekeeping genes (5′ actin A and 3′ α-tubulin UTRs; Fig. 4A). The entire TbbHpHbR ORF was cloned into the pTub-phleo vector (28). T. b. brucei 427–060R was transfected with this construct, which integrates into the tubulin locus. Transfectants were cloned and expression of the endogenous TbbHpHbR gene was selectively examined by a nested RT-PCR with two TbbHpHbR ORF-specific primers and a primer for the 3′UTR of TbbHpHbR. Expression of both the endogenous and ectopic TbbHpHbR genes was analyzed by a nested RT-PCR with ORF-specific primers (Fig. 4B). The predicted RT-PCR products from endogenous and ectopic genes were detected in T. b. brucei 427–221S and the T. b. brucei 427–060_rescueS, respectively, whereas no TbbHpHbR mRNA was detected in T. b. brucei 427–060R (Fig. 4B). The ability of the T. b. brucei 427–060_rescueS to take-up TLF-1 was examined by FAC, and susceptibility to TLF-1 killing determined by in vitro lysis assays (Fig. 4 C and D). Ectopic expression of TbbHpHbR results in a rescue of TLF-1 uptake and susceptibility, indicating that loss of the Hp/Hb receptor expression is the mechanism of TLF-1 resistance in T. b. brucei-060R.
Fig. 4.
Ectopic expression of the TbgHpHbR gene fails to rescue TLF-1 susceptibility to T. b. brucei 427–060R. (A) Schematic showing the predicted mRNAs from the endogenous TbbHpHbR gene and the complete ORF for either the TbbHpHbR or TbgHpHbR genes ectopically expressed from the tubulin locus with actin A 5′UTR and α-tubulin 3′UTR. (B) RT-PCR analysis of mRNAs from endogenous TbbHpHbR (Top) or ectopically expressed TbbHpHbR or TbgHpHbR (Middle). Lane 1, T. b. brucei 427–221S; lane 2, T. b. brucei 427–060R; lane 3, T. b. brucei 427–060_T. b. brucei rescueS; lane 4, T. b. brucei 427–060_T. b. gambiense rescueR. Enolase expression was analyzed as a loading control (Bottom). (C) In vitro TLF lysis assay: percentage of cells lysed following incubation with TLF-1 for 2 h at 37 °C. T. b. brucei 427–221S is shown as a black triangle; T. b. brucei 427–060R a blue triangle; T. b. brucei 427–060_T. b. brucei rescueS a red triangle; and T. b. brucei 427–060_T. b. gambiense rescueR a green triangle. (D) Analysis of Alexa Fluor-488–conjugated TLF uptake by FACS. Samples are as in C.
Next we attempted to rescue the TLF-1 susceptibility in T. b. brucei 427–060R by ectopic expression of the TbgHpHbR coding sequence (Fig. 4 A–D). Transfection of T. b. brucei 427–060R with TbgHpHbR failed to restore uptake of or susceptibility to TLF-1 despite expression of the TbgHpHbR mRNA (Fig. 4 B–D). Analysis of the TbgHpHbR sequence from four group 1 T. b. gambiense isolates (Eliane, DAL 972, BIM, and PA) revealed a high degree of similarity to the TbbHpHbR gene, with only eight nonsynonymous polymorphisms (Fig. S5). Based on the inability of the TbgHpHbR gene to rescue TLF-1 binding and susceptibility in T. b. brucei-060R, it is likely that one or more of these changes resulted in a reduction in the affinity of the receptor for TLF-1. Together these findings suggest that both mutations within the TbgHpHbR coding sequence and decreased expression of the TbgHpHbR mRNA contribute to the resistance of group 1 T. b. gambiense to TLF-1.
Discussion
A great deal is known about the resistance of T. b. rhodesiense to human innate immunity. These parasites express a truncated member of the VSG superfamily, SRA, which has a high affinity for the apoL-1 component of TLF-1 (9). Formation of the SRA/TLF-1 complex prevents killing and allows parasite growth in humans. On the contrary, little is known about the factors that allow human infection by T. b. gambiense (24). These parasites lack the SRA gene, indicating that SRA-independent mechanisms of TLF-1 resistance must exist. This raises the possibility that TLF-1 resistance in T. b. gambiense might be caused by the evolution of another TLF-1 neutralizing factor or that resistance could result from evasion of TLF-1 uptake. Group 1 T. b. gambiense has proven to be largely intractable to genetic manipulation leading us to develop a model for SRA-independent resistance to TLF-1 in T. b. brucei (25). Results presented here show that the loss of expression of the Hp/Hb receptor, encoded by the TbbHpHbR gene, can be selected for in T. b. brucei. Loss of binding allows these parasites to evade TLF-1 killing. These findings are directly relevant to human disease, as we found that several group 1 T. b. gambiense isolates also showed decreased TLF-1 uptake and reduced expression of TbgHpHbR mRNA. In addition, we showed that the TbgHpHbR was unable to rescue TLF-1 uptake and susceptibility in TLF-1–resistant T. b. brucei 427–060R, suggesting that coding sequence mutations altered TLF-1 binding by the T. b. gambiense Hp/Hb receptor.
The results presented here show that TLF-1 resistance in group 1 T. b. gambiense is a consequence of reduced expression and mutations to the TbgHpHbR gene. The combination of loss-of-function mutations, coupled with reduced abundance of the mRNA, may be necessary to provide complete protection from TLF-1 killing. Alternatively, evolution of TLF-1 resistance may have initially involved down-regulation in TbgHpHbR expression that provided only partial protection against TLF-1. Repeated inoculation of these parasites into primates could have selected for TbgHpHbR mutations that lead to a loss of TLF-1 binding and complete protection. Experiments currently under way will define the TbgHpHbR sequence changes that influence TLF-1 affinity.
We propose that reduced expression and coding sequence mutations to the TbgHpHbR were strongly selected for in group 1 T. b. gambiense, allowing these parasites to evade TLF-1 killing and to survive in the human host. However, reduced Hp/Hb uptake may also have a fitness cost to the parasite. Long-term growth of TLF-1–resistant T. b. brucei lines, in the absence of the selective pressure of TLF-1, resulted in the spontaneous outgrowth of cells that expressed the TbbHpHbR. Thus, we propose that, although the Hp/Hb receptor is not essential in trypanosomes, its expression may influence growth rate and the virulence of the parasite in the mammalian host. Previous studies indicated that the Hp/Hb receptor is used by T. b. brucei for heme uptake and was necessary for optimal growth and contributed to resistance to oxidative burst by host macrophages (18). It is possible that other mechanisms for heme uptake are present in group 1 T. b. gambiense. The studies presented here show that SRA independent resistance to TLF-1 can be caused by the loss of expression of a functional Hp/Hp receptor and suggests a unique strategy for evasion of human innate immunity by T. b. gambiense.
Materials and Methods
In Vitro Growth, Generation, and Transfection of T. b. brucei Cell Lines.
Bloodstream form T. b. brucei Lister 427 (MiTat 1.2) were used in these studies. TLF-1–resistant T. b. brucei 427–800R cells were described previously (25). Prolonged culturing in the absence of TLF-1 resulted in subpopulations of TLF-1–resistant cells (T. b. brucei 427–060R). Before subsequent experiments, cells were cloned by limiting dilution. Transfections were performed using the Amaxa electroporation system (Human T Cell Nucleofactor Kit; program X-001). TLF-1–sensitive T. b. brucei 427–060S cells were obtained after FACS with Alexa Fluor–488–conjugated TLF-1. Alexa Fluor-488 conjugation resulted in the loss of TLF-1 killing but not binding, thus allowing us to sort live cells that bound TLF-1 (SI Materials and Methods provides further details).
TLF-1 Purification, Labeling, Lysis Assays, and Flow Cytometry.
TLF-1 purification, labeling, and lysis assays were performed as described previously (2, 8). FACS analysis was performed on samples with 3 μg /mL Alexa Fluor-488–conjugated TLF-1. Cells were incubated for 1 h at 37 °C, washed three times with ice-cold 1× PSG buffer (50 mM NaPi, 45 mM NaCl, 55 mM glucose, pH 8.0) and analyzed with a Cyan cytometer (Dako).
SDS/PAGE and Northern Blot Analysis.
Total cell protein from 2 × 106 trypanosomes was run on 10% SDS/PAGE and stained with Coomassie brilliant blue. For Northern blot analysis, radiolabeled probes containing entire ORFswere generated (Prime-It random primer labeling kit; Stratagene) and hybridized in a 40% (vol/vol) formamide hybridization mix with the addition of 10% (wt/vol) dextran sulfate. Final washes were performed with 0.1× SSC (150 mM NaCl, 15 mM sodium citrate, pH 7.4)/0.1% SDS at 65 °C for 20 min.
RT-PCR of the Expressed VSGs and Hp/Hb Receptor.
Total RNA was isolated with Tripure isolation reagent (Roche) or with the RNeasy mini kit (real-time PCR; Qiagen) and DNase I treated (Invitrogen). cDNA was generated in a reverse transcription reaction (Promega) or with the Omniscript RT kit (real-time PCR; Qiagen). SI Materials and Methods includes a description of quantitative PCR methodology and Table S1 shows all primers used. Control reactions were performed with enolase and GPI8 primers (real-time PCR), as well as reactions without added reverse transcription. Four replicates were performed for each parasite line. For cloning and sequencing, PCR products were generated with Platinum High Fidelity Taq Polymerase (Invitrogen), gel-purified, and cloned into the PCR 2.1 vector (Invitrogen). Both strands were sequenced with M13 forward and reverse primers. VSG and Hp/Hb receptor sequences were compared with the T. b. brucei TREU 927 data set (GeneDB; www.genedb.org).
Ectopic Expression of the Hp/Hb Receptor.
The Hp/Hb receptor ORF was PCR amplified from T. b. brucei 427–221S and group 1 T. b. gambiense (Eliane strain) and cloned into the pTub-phleo construct (28) after sequence verification, using Sbf I and Asc I linkers (underlined). Hp/Hb receptor (Act/Tub_UTR) was transfected in T. b. brucei 427–060R cells.
T. b. gambiense, T. b. brucei, and T. b. rhodesiense Cell Lines.
STIB247 T. b. brucei, T. b. gambiense group 1 (Eliane strain MHOM/CI/52/ITMAP 2188) have been described previously (29). The T. b. rhodesiense (Baganzi strain) contains the SRA gene and was isolated from a human in southeastern Uganda in 1990. The T. b. rhodesiense strain KETRI 2482 contains the SRA gene and was isolated from a human in Lumino, Uganda. Other T. b. gambiense group 1 strains isolated from infected individual were MHOM/CM/75/ITMAP1789/BIM and MHOM/CM/74/ITMAP1787/Mos from Cameroon, MHOM/CG/80/ITMAP1843/PA from the Democratic Republic of Congo, and MHOM/CI/83/DAL596/TOBO and MHOM/CI/83/DAL607/ISTI from Côte d'Ivoire. These isolates were grown in imprinting control region mice and analyzed ex vivo. All animal procedures were carried out in accordance with the Animals (Scientific Procedures) Act of 1986, and ethical permission was granted by the University of Glasgow.
Fluorescence Microscopy of TLF Uptake.
Trypanosomes were resuspended in serum-free HMI9 media at a concentration of 106 cells/mL and incubated in 10 μg/mL of Lysotracker (Invitrogen) and 5 μg/mL of Alexa Fluor-488–conjugated TLF. Details of the methods used for imaging TLF uptake are provided (SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank Sara Faulkner, James Chappel, April Shiflett, Justin Widener, David Seidman, Natalie Stephens, John Harrington, Torsten Ochsenreiter (University of Bern, Switzerland), Bob Sabatini (University of Georgia), Laura Cliffe (University of Georgia), and Jay Bangs (University of Wisconsin) for assistance; Julie Nelson in the Flow Cytometry Laboratory (University of Georgia) for assistance; and Jayne Raper (New York University School of Medicine) for the kind gift of purified human HDL used in some of the initial experiments. The work on TLF in the S.H. laboratory was supported by National Institutes of Health Grant AI039033. C.M.R.T. and A.M. thank the Wellcome Trust for financial support. A.M. is a Wellcome Trust Research Career Development Fellow. P.C. is a UK Biotechnology and Biological Sciences Research Council research student.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007074107/-/DCSupplemental.
References
- 1.Rifkin MR. Identification of the trypanocidal factor in normal human serum: High density lipoprotein. Proc Natl Acad Sci USA. 1978;75:3450–3454. doi: 10.1073/pnas.75.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajduk SL, et al. Lysis of Trypanosoma brucei by a toxic subspecies of human high density lipoprotein. J Biol Chem. 1989;264:5210–5217. [PubMed] [Google Scholar]
- 3.Raper J, Fung R, Ghiso J, Nussenzweig V, Tomlinson S. Characterization of a novel trypanosome lytic factor from human serum. Infect Immun. 1999;67:1910–1916. doi: 10.1128/iai.67.4.1910-1916.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xong HV, et al. A VSG expression site-associated gene confers resistance to human serum in Trypanosoma rhodesiense. Cell. 1998;95:839–846. doi: 10.1016/s0092-8674(00)81706-7. [DOI] [PubMed] [Google Scholar]
- 5.Oli MW, Cotlin LF, Shiflett AM, Hajduk SL. Serum resistance-associated protein blocks lysosomal targeting of trypanosome lytic factor in Trypanosoma brucei. Eukaryot Cell. 2006;5:132–139. doi: 10.1128/EC.5.1.132-139.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson WC. Will the real Trypanosoma b. gambiense please stand up. Parasitol Today. 1986;2:255–257. doi: 10.1016/0169-4758(86)90011-6. [DOI] [PubMed] [Google Scholar]
- 7.Smith AB, Esko JD, Hajduk SL. Killing of trypanosomes by the human haptoglobin-related protein. Science. 1995;268:284–286. doi: 10.1126/science.7716520. [DOI] [PubMed] [Google Scholar]
- 8.Shiflett AM, Bishop JR, Pahwa A, Hajduk SL. Human high density lipoproteins are platforms for the assembly of multi-component innate immune complexes. J Biol Chem. 2005;280:32578–32585. doi: 10.1074/jbc.M503510200. [DOI] [PubMed] [Google Scholar]
- 9.Vanhamme L, et al. Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature. 2003;422:83–87. doi: 10.1038/nature01461. [DOI] [PubMed] [Google Scholar]
- 10.Molina-Portela MdelP, Lugli EB, Recio-Pinto E, Raper J. Trypanosome lytic factor, a subclass of high-density lipoprotein, forms cation-selective pores in membranes. Mol Biochem Parasitol. 2005;144:218–226. doi: 10.1016/j.molbiopara.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Molina-Portela MP, Samanovic M, Raper J. Distinct roles of apolipoprotein components within the trypanosome lytic factor complex revealed in a novel transgenic mouse model. J Exp Med. 2008;205:1721–1728. doi: 10.1084/jem.20071463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Widener J, Nielsen MJ, Shiflett A, Moestrup SK, Hajduk S. Hemoglobin is a co-factor of human trypanosome lytic factor. PLoS Pathog. 2007;3:1250–1261. doi: 10.1371/journal.ppat.0030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan G, et al. Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. J Biol Chem. 2008;283:21540–21549. doi: 10.1074/jbc.M800214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pérez-Morga D, et al. Apolipoprotein L-I promotes trypanosome lysis by forming pores in lysosomal membranes. Science. 2005;309:469–472. doi: 10.1126/science.1114566. [DOI] [PubMed] [Google Scholar]
- 15.Lecordier L, et al. C-terminal mutants of apolipoprotein L-I efficiently kill both Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense. PLoS Pathog. 2009;5:e1000685. doi: 10.1371/journal.ppat.1000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristiansen M, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen MJ, et al. Haptoglobin-related protein is a high-affinity hemoglobin-binding plasma protein. Blood. 2006;108:2846–2849. doi: 10.1182/blood-2006-05-022327. [DOI] [PubMed] [Google Scholar]
- 18.Vanhollebeke B, et al. A haptoglobin-hemoglobin receptor conveys innate immunity to Trypanosoma brucei in humans. Science. 2008;320:677–681. doi: 10.1126/science.1156296. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen MJ, Moestrup SK. Receptor targeting of hemoglobin mediated by the haptoglobins: Roles beyond heme scavenging. Blood. 2009;114:764–771. doi: 10.1182/blood-2009-01-198309. [DOI] [PubMed] [Google Scholar]
- 20.Drain J, Bishop JR, Hajduk SL. Haptoglobin-related protein mediates trypanosome lytic factor binding to trypanosomes. J Biol Chem. 2001;276:30254–30260. doi: 10.1074/jbc.M010198200. [DOI] [PubMed] [Google Scholar]
- 21.Hager KM, et al. Endocytosis of a cytotoxic human high density lipoprotein results in disruption of acidic intracellular vesicles and subsequent killing of African trypanosomes. J Cell Biol. 1994;126:155–167. doi: 10.1083/jcb.126.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrington JM, Howell S, Hajduk SL. Membrane permeabilization by trypanosome lytic factor, a cytolytic human high density lipoprotein. J Biol Chem. 2009;284:13505–13512. doi: 10.1074/jbc.M900151200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith AB, Hajduk SL. Identification of haptoglobin as a natural inhibitor of trypanocidal activity in human serum. Proc Natl Acad Sci USA. 1995;92:10262–10266. doi: 10.1073/pnas.92.22.10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berberof M, Pérez-Morga D, Pays E. A receptor-like flagellar pocket glycoprotein specific to Trypanosoma brucei gambiense. Mol Biochem Parasitol. 2001;113:127–138. doi: 10.1016/s0166-6851(01)00208-0. [DOI] [PubMed] [Google Scholar]
- 25.Faulkner SD, et al. In vitro generation of human high-density-lipoprotein-resistant Trypanosoma brucei brucei. Eukaryot Cell. 2006;5:1276–1286. doi: 10.1128/EC.00116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wirtz E, Leal S, Ochatt C, Cross GA. A tightly regulated inducible expression system for conditional gene knock-out genetics in Typanosoma brucei. Mol Biochem Parasitol. 1999;99:89–101. doi: 10.1016/s0166-6851(99)00002-x. [DOI] [PubMed] [Google Scholar]
- 27.Clayton CE. Life without transcriptional control? From fly to man and back again. EMBO J. 2002;21:1881–1888. doi: 10.1093/emboj/21.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudenko G, Blundell PA, Taylor MC, Kieft R, Borst P. VSG gene expression site control in insect form Trypanosoma brucei. EMBO J. 1994;13:5470–5482. doi: 10.1002/j.1460-2075.1994.tb06882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner CMR, et al. Human infectivity trait in Trypanosoma brucei: Stability, heritability and relation to SRA expression. Parasitol. 2004;129:445–454. doi: 10.1017/s0031182004005906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.