Abstract
Disturbance of neural activity by sedative drugs has been proposed to trigger a homeostatic response that resists unfavorable changes in net cellular excitability, leading to tolerance and dependence. The Drosophila slo gene encodes a BK-type Ca2+-activated K+ channel implicated in functional tolerance to alcohol and volatile anesthetics. We hypothesized that increased expression of BK channels induced by these drugs constitutes the homeostatic adaptation conferring resistance to sedative drugs. In contrast to the dogmatic view that BK channels act as neural depressants, we show that drug-induced slo expression enhances excitability by reducing the neuronal refractory period. Although this neuroadaptation directly counters some effects of anesthetics, it also causes long-lasting enhancement of seizure susceptibility, a common symptom of drug withdrawal. These data provide a possible mechanism for the long-standing counter-adaptive theory for drug tolerance in which homeostatic adaptations triggered by drug exposure to produce drug tolerance become counter-adaptive after drug clearance and result in symptoms of dependence.
Keywords: addiction, anesthesia, drug abuse, seizure, epilepsy
Conservation of the balance between excitation and inhibition of neural activity is critically important for the proper function of the nervous system. Many alcohols, anesthetics, and abused volatile solvents alter this balance, resulting in behavioral manifestations that range from hyperactivity to sedation. In 1968, W. R. Martin (1) proposed a counter-adaptive theory for drug tolerance. This theory states that drug exposure triggers adaptive homeostatic changes that oppose drug effects to produce drug tolerance, but that upon drug clearance these same changes become counter-adaptive and produce symptoms of dependence (2, 3). These ideas have been extended to account for the changes in motivation that underlie drug-seeking behavior as proposed by the opponent-process model (3, 4).
In Drosophila, rapid tolerance to the sedative effects of alcohols and anesthetics is mediated by an increase in the expression of the slo gene. A single exposure to a sedating dose of ethanol or the anesthetic benzyl alcohol induces transcription of slo. Consequentially, flies display a reduced response to a subsequent sedation with the same drug dose just 24 h after their first exposure. In these experiments, previously treated flies recover from sedation and resume normal behavior significantly earlier than naive flies. Transcription from the slo gene was shown to be both sufficient and necessary for the development of tolerance to the behavioral effects of benzyl alcohol, as a mutation that eliminates slo expression prevents tolerance, and expression from an inducible slo transgene mimics tolerance in naive animals (5, 6).
In flies, slo encodes the pore-forming subunit of the BK-type Ca2+-activated K+ channel (7). This channel responds both to increases in free Ca2+ and to changes in membrane potential and is thus believed to play a major role in shaping neuronal excitability and regulating synaptic transmission (8). Because of its central role as a regulator of excitability of nerve terminals, we have proposed that this channel is a likely contributor to the homeostatic mechanism that resists unfavorable changes in net cellular excitability and mediates tolerance to sedation (5). Here, we electrophysiologically test whether drug-induced slo expression acts as a neural excitant that could directly counter the depressant effects of the drug and if, after drug clearance, the change in slo expression generates a symptom of drug withdrawal.
Results
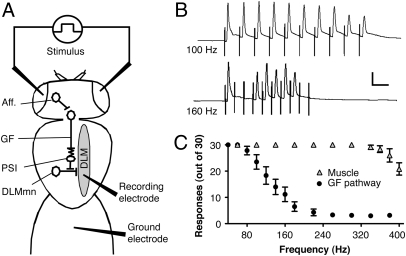
We used the giant fiber (GF) pathway of Drosophila to test if drug-induced BK channel expression enhances neural excitability and thereby helps the nervous system to resist subsequent sedation. In this pathway, the GF neurons conduct action potentials from the brain to the thoracic ganglion. Within the thoracic ganglion, the GF forms an electrical synapse with an interneuron that connects to a motor neuron innervating the dorsal-longitudinal flight muscles (DLM) (Fig. 1A) (9, 10). In the GF preparation, one stimulates the GF in the brain and records its response in the DLM. Each GF action potential gives rise to a single action potential in the DLM. We quantify the capacity of the GF pathway to fire repetitively as a biologically meaningful measure of the excitability of the pathway (11, 12).
Fig. 1.
The GF pathway. (A) GF neurons receive input from visual or other neural afferent pathways (Aff.) and are electrically connected to the peripheral synapsing interneuron (PSI) that synapses on the DLM motoneurons (DLMmn). The DLMmn synapses on the DLM. Stimulating potentials through electrodes on the eyes activate neural afferent pathways and trigger a response that can be recorded by an electrode in the DLM. (B) Representative traces of short-latency recordings from the DLM after 100-Hz and 160-Hz stimuli. Successful DLM responses closely follow each stimulus artifact at 100 Hz but fail at higher frequency stimulation. (Scale bars: 10 ms, 5 mV.) (C) Number of successful responses are plotted against frequency after GF stimulation (GF Pathway) or direct-muscle stimulation (Muscle) via electrodes in the thoracic ganglion. Note that the capacity of the muscle to follow high-frequency stimulations far exceeds that of the GF pathway. Error bars are SEM, n = 5 each.
To determine the following-frequency profile of the GF pathway, three trains of 10 stimuli were delivered and the number of responses was counted. The frequency of the stimulus trains was gradually increased, and as the refractory period of the neurons was exceeded, the incidence of failures increased (Fig. 1 B and C). A failure is the absence of an action potential in the muscle in response to an evoked stimulation. In order for this method to accurately report the following frequency of the GF pathway neurons, the following frequency of the DLM must substantially exceed that of the neurons. At stimulation frequencies higher than 80 Hz, the GF circuit becomes refractory and fewer responses are detected. However, using direct muscle simulation we show that the muscle will fire successfully at stimulus frequencies that exceed 300 Hz (Fig. 1C). These results indicate that the failures recorded from the GF pathway are neuronal rather than muscular in nature.
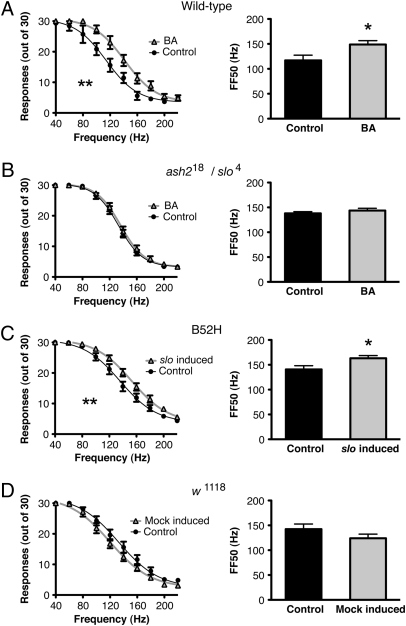
If increased slo expression enhances neural excitability by reducing the refractory period, then we should observe a higher following frequency in animals in which expression of the slo gene was induced. We first determined if slo induction caused by prior benzyl alcohol sedation enhances the following frequency of the GF pathway and whether this change could be attributed exclusively to increased slo expression. A single benzyl alcohol sedation, 24 h prior, which substantially increases slo expression (5), increases the following frequency of the GF pathway. This increase is shown by a significant rightward shift in the frequency-response curve and by an increase in the FF50 (following frequency with a 50% response rate) in animals previously treated with benzyl alcohol (Fig. 2A).
Fig. 2.
Sedation enhances the following frequency of the GF pathway and is dependent on a functional slo gene. Frequency-response curves (Left) and the FF50 plots (Right) for (A) wild-type flies that were sedated with benzyl alcohol (BA) 24 h earlier and for the nonsedated controls (Control), (B) ash218/slo4 transheterozygotes that do not express slo in the nervous system and that were sedated with benzyl alcohol (BA) 24 h earlier and for the nonsedated controls (Control), (C) B52H flies carrying a heat-shock inducible slo cDNA transgene that were heat-shocked (slo induced) for 30 min 24 h earlier and for the nonheat-shocked controls (Control), and (D) for the parental non transgenic stock (w1118) that were heat-shocked (Mock-induced) for 30 min 24 h earlier and for the nonheat-shocked controls (Control). Error bars are SEM, n > 7 each; **P < 0.05 by repeated measures ANOVA; *P < 0.05 by Student's t test.
Because a loss of slo expression prevents acquisition of sedation-induced tolerance (5), we used mutant analysis to test whether slo expression is also required for the electrophysiological adaptation to drug sedation. Because elimination of slo expression in muscles causes broadening in DLM action potentials and interferes with this assay, we used the ash218/slo4 transheterozygous double-mutant to eliminate slo expression in neurons but not in muscles. The intrinsic electrophysiological properties of DLM in ash218/slo4 transheterozygotes appear normal (13). Interestingly, in these transheterozygous mutants, prior sedation fails to produce an increase in following frequency (Fig. 2B), indicating that slo expression in the nervous system is required for the sedation-induced reduction in the neural refractory period.
To determine if an increase in slo expression would phenocopy the electrophysiological effect of sedation, we used a transgenic fly carrying a heat-inducible copy of a slo cDNA in a slo4 background (B52H). We do not observe wide muscle-action potentials in the B52H; slo4 double homozygotes indicating that basal expression from the B52H transgene complements the broadened muscle action potentials phenotype of the slo4 lesion (5). Heat-shock induction produces slo mRNA expression levels that mimic those induced by benzyl alcohol and also phenocopies behavioral tolerance to the drug (5). In B52H flies, a single 30-min heat pulse 24 h before testing rightward-shifted the frequency-response curve and increased the FF50 by 15% over the noninduced animals (Fig. 2C). As a control, nontransgenic animals (w1118) were also subjected to the same heat pulse and did not show an increase but rather a nonsignificant decrease in following frequency (Fig. 2D). This finding is in agreement with the previous observation that heat shock alone causes a state of hyperactivity that decreases endogenous slo expression (5).
An increase in following frequency induced by both the anesthetic and the transgenic slo gene induction clearly supports the idea of a slo-mediated homeostatic neuroadaptation. Because this adaptation is evident after the removal of the drug, it can be properly regarded as a type of hyperexcitability-related withdrawal symptom. Interestingly, the presence of both tolerance and symptoms of withdrawal are part of the current clinical diagnostic criteria for physiological drug dependence (14).
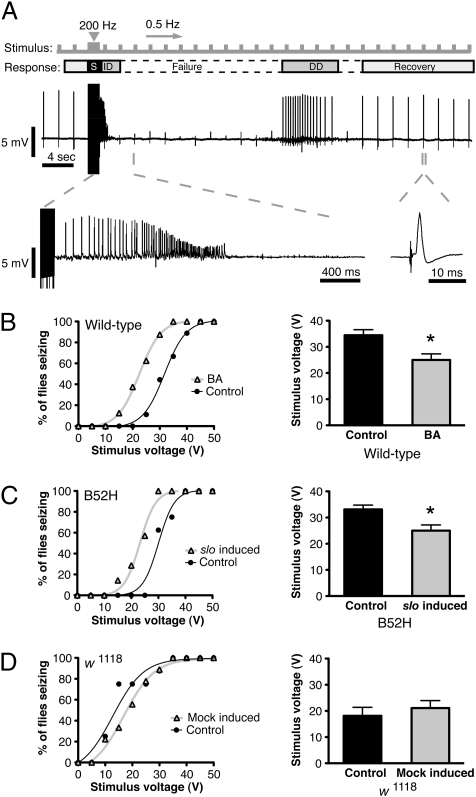
In mammals, withdrawal of sedative drugs has been linked to increased neural excitability, and symptoms can include the presence of seizures (15). Because slo4 mutants had previously been shown to decrease overall seizure susceptibility in flies (16), we hypothesized that the increased excitability caused by induction of slo might increase the probability of seizures. For this reason, we tested the effects of prior benzyl alcohol sedation and slo induction on susceptibility to seizures. In flies, seizures can be evoked by stimulation with high-frequency electroconvulsive shock (ECS). In this assay, a stimulation train in the fly brain triggers a stereotypical seizure repertoire that can be effectively recorded at the DLM. The seizure consists of a short, high-frequency, spontaneous initial discharge followed by a period of response failure and a subsequent secondary delayed discharge just before recovery of low-frequency evoked responses (Fig. 3A) (17, 18). Because seizure occurrence is an all-or-nothing event and is highly dependent on ECS voltage, we used the voltage threshold for seizure occurrence as a measure of seizure susceptibility.
Fig. 3.
Sedation and increased slo expression increase seizure susceptibility. Electroconvulsive stimuli (S) of varying voltages were applied to determine seizure threshold of flies after different treatments. A constant low-frequency stimulus was applied throughout the duration of the recording to assess the responsiveness of the GF pathway. (A) A typical seizure repertoire consisting of a high-frequency initial discharge (ID) followed by a period of evoked response failures (Failure), a delayed discharge (DD), and recovery from response failures. Seizure threshold curves (Left) and average seizure threshold (Right) are shown for (B) wild-type flies that were sedated with benzyl alcohol (BA) 24 h earlier and for the nonsedated controls (Control), (C) B52H flies carrying a heat-shock inducible slo cDNA transgene that were heat-shocked (slo induced) 24 h earlier and for the nonheat-shocked controls (Control), and (D) for the parental non transgenic stock (w1118) that were heat-shocked (Mock-induced) 24 h earlier and for the nonheat-shocked controls (Control). Error bars are SEM, n > 9 each; *P < 0.05 by Student's t test.
Flies were subjected to a series of electroconvulsive shocks of varying voltages that range from 5 to 50 V in 5-V increments, and the seizure threshold was calculated. In Fig. 3B, the seizure-response curves show the percentage of flies having seizures at any given voltage. Wild-type flies previously treated with benzyl alcohol were significantly more susceptible to seizures than the untreated controls were. This susceptibility was manifested by a leftward shift in the seizure-response curves and a significantly lower average-voltage threshold (Fig. 3B).
To determine whether these changes are a phenotype of increased slo expression, slo expression was manipulated with the B52H transgene. When slo expression was induced, we observed a leftward shift in the seizure-response curve and a lower average-voltage threshold for seizure induction (Fig. 3C). The protocol used to induce the B52H transgene did not cause a significant change in seizure susceptibility of nontransgenic animals (Fig. 3D). These results indicate that increased slo expression is sufficient to enhance seizure susceptibility in flies.
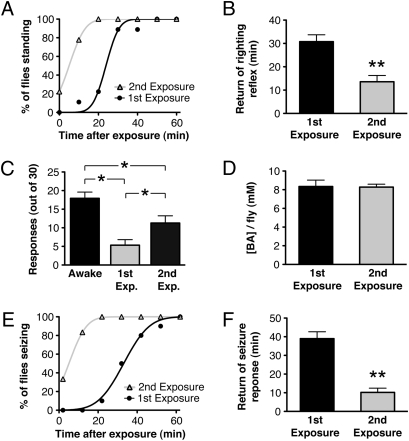
In the preceding portions of this article, we have not directly assayed tolerance but have inferred that it has developed based on previously published observations (5) and based on the appearance of increased neural excitability after drug exposure. Here, we assay tolerance in individual animals by using behavioral and electrophysiological methods (Fig. 4). Flies were sedated in a vapor chamber for 20 min. Some of the animals were immediately tested for behavioral recovery and others were assayed for seizure susceptibility and the capacity to follow high-frequency stimulation. Tolerance can be detected with all three measures by comparing responses of the animals as they recover from their first and second sedations.
Fig. 4.
Behavioral and electrophysiological manifestation of tolerance. Flies were sedated with benzyl alcohol in a vapor chamber for 20 min and immediately tested for behavioral recovery, following frequency, and seizure susceptibility. (A) Behavioral recovery plot for flies recovering from the first and second exposure (first exposure was 24 h prior) to benzyl alcohol in the vapor chamber. (B) Average time for return of righting reflex after benzyl alcohol exposure. (C) Capacity of the GF to follow a 200-Hz stimulation, 2 min after the 20-min sedation period. Number of successful responses from 30 stimuli were counted in awake, wild-type flies, in flies recovering from their first exposure, and in flies recovering from their second exposure. (D) Benzyl alcohol concentration per fly immediately after the first and second exposures. (E) Seizure activity plots of flies after the first and second exposure to benzyl alcohol in the vapor chamber. Seizures were evoked by a 50 V ECS every 10 min, starting after a 2-min mounting delay after removal from the vapor chamber. (F) Average time for return of seizure response. n = 9 each; **P < 0.01 by Student's t test, *P < 0.05 by one-way ANOVA, Newman-Keuls multiple comparison test. All error bars are SEM.
We first measured functional behavioral tolerance by measuring the time required for animals to regain postural control after drug sedation (Fig. 4 A and B). Following the first drug exposure, flies required an average of 25 ± 4 min to recover from sedation. However, recovery from the second bout of drug sedation was substantially accelerated with an average recovery time of 13 ± 3 min.
Because we wished to directly correlate behavioral and electrophysiological measures of tolerance, we could not determine the following frequency and seizure threshold in the standard manner (stimulation at a variety of test frequencies or potentials interspersed with rest periods) because the duration of the standard protocol far exceeds the period required for recovery from sedation. Instead, we measured the capacity of the GF to follow a single high-frequency stimulus train immediately after the 20-min period of sedation in the vapor chamber. Similarly, to measure susceptibility for seizures, we used a single stimulation potential: the minimal stimulation potential that produced seizures in 100% of the wild-type control animals. In this manner, the capacity to follow a high-frequency stimulation and the seizure potential could be recorded in individual animals during the sedation recovery period. This approach permits direct comparisons of the behavioral and electrophysiological attributes.
The capacity of benzyl alcohol to inhibit the activity of the GF pathway also showed functional tolerance. Fig. 4C shows that the capacity of the GF to follow a 200-Hz stimulation was reduced by sedation. We counted the number of responses to 30 stimuli delivered at 200 Hz, 2 min after the benzyl alcohol exposure period. Awake, drug-naive, wild-type animals responded to an average of 17.9 ± 1.7 of 30 stimulations, whereas flies recovering from their first sedation respond to only 5.3 ± 1.6 of 30 stimulations. This finding demonstrates that benzyl alcohol substantially enhances the refractory period of the GF pathway. In response to the second benzyl alcohol sedation, the GF pathway shows a markedly improved capacity to follow the stimulation train and responds to 11.3 ± 2 of 30 stimulations. Here, therefore, tolerance is manifest as a reduction in the capacity of the drug to inhibit the response of the GF. As shown in Fig. 4D, the tolerance observed in this unique assay is not the product of differential drug loading during the first and second rounds of drug exposure.
In the same way, seizure activity was probed in sedated flies immediately after removal from the vapor chamber. A 50-V ECS was used to evoke seizures every 10 min after the 20-min sedation period. Although this voltage is sufficient to elicit seizures in 100% of awake flies (naive or previously sedated) (Fig. 3B), seizures in flies under the effects of anesthesia are significantly inhibited (Fig. 4E). Notice that the capacity of the anesthetic to inhibit seizure is substantially reduced in animals recovering from their second round of sedation. Two minutes after the sedation protocol, none of the flies experiencing their first exposure exhibited seizures, but 33% of flies recovering from their second sedation produced evoked seizures. As the effects of the anesthetic wear off, evoked-seizure activity is regained in an average of 39 ± 3.8 min after the first exposure and 10 ± 2.4 min after the second exposure (Fig. 4 E and F).
Discussion
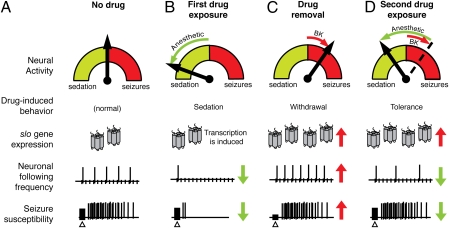
The increase in seizure susceptibility after benzyl alcohol sedation represents a remarkable parallel to drug-withdrawal symptoms observed in humans and in other mammalian models of this condition (15). Interestingly, as with drug tolerance, increased seizure susceptibility can be provoked merely by induction of the slo gene. Fig. 5 is a schematic model of how drug-induced modulation of a single gene can produce an adaptation that underlies both tolerance and withdrawal-like symptoms. Previously, Lin and Nash (11) demonstrated that volatile-solvent anesthetics produce a dose-dependent reduction in the following frequency of this neural pathway in concordance with our own data. These data suggest that anesthetics act, at least in part, by increasing the neuronal refractory period, which in turn results in neuronal failure and ultimately sedation (Fig. 5 A and B). Accordingly, here we show that prior sedation of flies results in a significant increase in the following frequency of the GF pathway, indicative of an adaptive, opposing response to the acute effect of volatile-solvent anesthesia (Fig. 5 C and D). This adaptation is slo-dependent in that mutations in slo block it. Most important, this adaptation can also be produced by the induction of slo expression from a transgene. These results unambiguously indicate that an increase in slo neural expression is sufficient to reduce the refractory period of the pathway.
Fig. 5.
Schematic model of a slo-mediated homeostatic response underlying drug tolerance and a withdrawal phenotype. (A) In a “no drug” state, a balance between excitation and inhibition of neural activity in the brain prevails, allowing for normal physiological and behavioral activity, including average seizure susceptibility to electroconvulsive shock (Δ). (B) During exposure to a sedative drug, this balance is pushed toward a more inhibited state characterized by sedation, decreased following frequency, and suppression of seizures. Drug exposure also produces an increase in slo expression. (C) After drug withdrawal, the increase in slo expression is unmasked, resulting in an excited neural state and characterized by increased neuronal following frequency and higher seizure susceptibility. (D) When drug exposure is repeated, the slo-mediated compensation resists the effect of the drug, resulting in a milder reduction in following frequency and reduced seizure suppression. The electrophysiological traces shown here are schematic representations of hypothetical data, not real traces.
This model is consistent with the idea that increased neural expression of slo can act as a neural excitant. The role of neural excitant is an unusual one to postulate for a K+ channel, as these channels are commonly associated with cell repolarization and suppression of neural activity. Certainly, in some preparations, increased BK channel activity reduces neural excitability (19, 20). However, BK channel activity has long been positively correlated with neural excitability (21–23).
Recently, increased BK channel activity has also been linked to epilepsy. Mice lacking the β-4 BK channel auxiliary subunit show increased BK channel activity that augments high-frequency firing and leads to temporal lobe seizures (24). In humans, a gain-of-function mutation in human slo (KCNMA1) that increases BK channel open probability has been shown to be a cause of coexistent generalized epilepsy and paroxysmal dyskinesia (25). In this instance, treatment with paxilline—a specific blocker of BK channels—is associated with reduced neuronal activity. Interestingly, some individuals carrying a gain-of-function mutation are sensitive to alcohol-induced dyskinesias (25).
It has been proposed that although an increase in BK channel activity can limit the instantaneous response of the neuron, the increase enhances the capacity for repetitive neural activity by reducing the neural refractory period (21, 22). There is strong evidence that increased BK channel activity can reduce the refractory period by repolarizing the synapse before the activation of other classes of K+ channels can occur (26). Activation of these K+ channels would lead to a long-lasting hyperpolarization that would slow the firing rate. Additionally, in posterior pituitary nerve terminals, BK channel-induced after hyperpolarization has been implicated in enhancing neuronal firing frequency by speeding the recovery of Na+ channels from inactivation (27).
Neural pharmacodynamic tolerance to any drug is likely to involve many components (28–31). However, the slo gene is uniquely positioned to be a key part of such a homeostatic response. Because the encoded channel has the highest conductance of any neural ion channel, small changes in its density can have a large influence on membrane excitability. This influence allows for a fast, low-cost adaptation that can influence a myriad of neural components, including calcium channel kinetics, action potential propagation, and neurotransmitter release. In fact, modulation in slo expression may be a generalized homeostatic strategy in response to sudden changes in neural activity. Increased slo expression is triggered by a myriad of sedative compounds, whereas treatments predicted to increase neural excitability reduce slo expression (5).
Intriguingly, the BK channel has been implicated in the production of ethanol tolerance in mammals (32). Recent studies to investigate the physiological mechanism of ethanol tolerance in the rat hypothalamic neurohypophysial explant model system show evidence of reduced BK channel expression following ethanol exposure. In these studies, the explant is chronically exposed to ethanol, which causes a reduction in BK channel density through the internalization of membrane channels and the down-regulation of ethanol-sensitive BK isoforms caused by microRNA degradation of selected mRNA splice variants (33, 34). These results contrast with the alcohol/anesthetic-induced increase in BK channel expression that we observe in Drosophila (5, 6). There are many potential sources that could account for this difference and the meaning of the difference will not be resolved here. One possible source of the discrepancy is that our data reflects the net change in the entire brain and it is possible that this change is the product of a decrease in expression in one neural structure (as in the mammalian explant) that is masked by increases in other regions of the Drosophila brain. Alternatively, the root of the difference could be accounted for by differences in the treatment protocol (continuous 24 h ethanol exposure vs. a single 15- to 20-min exposure in flies), which may generate different molecular outcomes. Our treatment paradigm was developed to reliably produce tolerance that could be assayed in a simple behavioral assay. The benzyl alcohol and ethanol treatments consisted of sedating doses leading to whole-animal paralysis, whereas in the mammalian studies, ethanol treatments consisted of doses that would not induce rapid sedation in whole animals. Rather than inducing sedation, the lower dose used in the mammalian studies may be excitatory for some neural circuits (35–37) and in humans are not immediately sedating and can cause euphoria and behavioral disinhibition (38). Interestingly, in flies, nonsedating doses of benzyl alcohol that induce behavioral hyperexcitability also evoke a reduction in slo gene expression and produce sensitization to subsequent sedation. The response of the fly to a reduced dose of benzyl alcohol has only been studied cursorily (5).
These results support the counter-adaptive theory for drug tolerance, in which withdrawal-like symptoms are a by-product of a homeostatic neuroadaptive process, and they provide a well-founded mechanism for the phenomenon of dependence. In the current study, slo induction represents a feed-forward neuroadaptive process that underlies drug tolerance. In the absence of the drug, the increased expression of slo leads to an allostatic state that causes a symptom of withdrawal—an increase in seizure susceptibility—and demonstrates a common origin for tolerance and withdrawal hyperexcitability in Drosophila.
Materials and Methods
Fly Stocks.
Drosophila stocks used were Canton S (wild type), B52H (w1118; B52H; slo4), w1118, and the ash218/slo4 transheterozygote. Stocks were raised on standard cornmeal agar medium in a 12/12 h light/dark cycle. Newly eclosed flies were collected over a 1- to 2-d interval and studied 3 to 4 d after eclosion. The B52H transgene (5) expresses a slo cDNA from an hsp70 promoter. B52H was induced at 37 °C for 30 min in a humidified incubator. To account for the nonspecific effects of temperature, a mock induction was performed using w1118 flies. The ash218 mutation is a chromosomal deletion that removes the neural promoters from the slo gene and extends into the neighboring ash2 gene. The ash218 allele is recessive lethal. Previously, we have shown that ash218/slo4 transheterozygotes do not show neural expression but have normal muscle expression of the slo gene. Furthermore, flight muscles in ash218/slo4 transheterozygotes have normal electrical properties (13).
Drug Treatment.
Preexposure to benzyl alcohol was performed using 30-mL glass vials coated with benzyl alcohol as described in detail in SI Materials and Methods and in Ghezzi et al. (5). One group of 10- to 15 age-matched female flies was placed in a benzyl alcohol vial and exposed until sedation (10–15 min, approximately). A second group was placed in a benzyl alcohol-free control vial for the same time and did not sedate. Both groups were returned to food vials and allowed to recover for 24 h before testing. For the tolerance assays, single flies were exposed to benzyl alcohol using a custom-built benzyl alcohol vapor chamber described in SI Materials and Methods and Fig. S1. Single age-matched female flies were placed in the chamber and allowed to sedate during a 20-min benzyl alcohol exposure. After this period, flies were immediately removed from the chamber and tested for behavioral recovery, seizure susceptibility, and the ability to follow a high frequency stimulus.
Electrophysiological Assay.
Individual adult female flies were set up for electrophysiological recordings as described in detail in SI Materials and Methods. Using micromanipulators (Narishige), a 200-μM diameter uninsulated tungsten-wire electrode (FHC Inc.) electrolytically sharpened to approximately 5 μm was placed on each of the compound eyes. A 75-μm diameter recording electrode electrolytically sharpened to approximately 5 μm was inserted through the dorsal cuticle into the right-uppermost DLM that lies just beneath the cuticle. Finally, a 200-μm diameter reference electrode, electrolytically sharpened to approximately 5 μm, was inserted into the abdomen (11). Stimulating potentials were generated using a S48 square pulse stimulator, isolated with a SIU5 Stimulus Isolation Unit (Grass-Telefactor). Responses from the DLM muscles were amplified with a Microelectrode Amplifier Model 1800 (A-M Systems, Inc.), digitized by a DigiData 1200 (Axon Instruments) and recorded for analysis on a PC using FETCHEX, pCLAMP 6 software (Axon Instruments). To determine the threshold potential to efficiently stimulate the GF pathway, single 0.1-ms pulses of increasing voltages were delivered to the eyes at 5-s intervals. Once the threshold for a muscle response was reached, the latency to the response after each stimulus was measured from the beginning of the stimulus artifact to the beginning of the evoked muscle response. The amplitude of the stimulus was increased until a response with a constant short-latency of ≈1.4 ms was detected (typically around 20–30 V).
Determination of Neuronal Following Frequency.
The following frequency of the GF pathway was determined using a stimulus protocol that consisted of sets of three trains of 10 suprathreshold stimuli (5 V over the short-latency threshold) at increasing frequencies. The stimulus frequency was increased by 20 Hz after each set, starting at 40 Hz up to 220 Hz. The animals were allowed to rest for 5 s between each stimulus train and 15 s between each three-train set to prevent neuronal exhaustion. For direct muscle stimulation, stimulating electrodes were inserted laterally (one from each side of the fly) into the thoracic-abdominal ganglion and stimulated at 5 V. To determine the following frequency of the muscle, a 40- to 400-Hz frequency range was used instead. The number of stimulus responses detected in the muscle (of 30 possible) were counted for each frequency. Averaged responses were plotted against frequency to generate a frequency-response curve. Two-way repeated measures ANOVA was used to determine significance. Following frequency with 50% response was independently calculated for each fly from the linear phase of the frequency-response curves by linear interpolation of the frequency that elicited 15 responses (12). Significance was determined by Student's t test. All recordings were performed around the same time of day (Zeitgeber Time 6 ± 2 h).
Determination of Seizure Susceptibility.
To determine seizure susceptibility, the same electrophysiology paradigm was used with minor modifications (see SI Materials and Methods for details). Seizure threshold was determined using a 1.5 s long, high-frequency ECS consisting of 0.1-ms pulses delivered at 200 Hz. Single ECS were applied at increasing voltages until a seizure was detected by the recording electrode in the DLM. A seizure consists of a high-frequency initial discharge, followed by a prolonged period of evoked response failures (Failure), followed by a delayed discharge, and ended with a return of normal capacity to respond to evoked stimuli. The stimulation potential was increased from 10 to 50 V in increments of 5 V, with an 8-min rest period between each ECS. The minimum voltage that induced a complete seizure repertoire was recorded as the voltage threshold for that fly. For each fly, low-frequency stimulation (0.5 Hz, 30 V, 0.1-ms pulses) was applied before and after each ECS to asses the health of the GF pathway, and to determine the duration of the failure period induced by each seizure episode (17, 18).
Behavioral and Electrophysiological Tolerance Assay.
For behavioral recovery, individually sedated flies were transferred from the vapor chamber to small plastic Petri dishes and allowed to recover. For each fly, recovery time was the period between removal from the sedation chamber and the return of the righting reflex as indicated by a return to a natural standing position. For the seizure susceptibility and following frequency assays, benzyl alcohol-sedated flies were immediately set up for electrophysiology. Seizure susceptibility was probed every 10 min using a 50-V, 1.5-s, electroconvulsive shock of 200 Hz, 0.1-ms pulses, for 60 min, starting at 2 min after removal from the vapor chamber (because of a 2-min mounting delay). In the same flies, following frequency was determined by counting the successful DLM responses to the first 30 stimuli of the first ECS (delivered 2 min after removal of benzyl alcohol).
Measurement of Benzyl Alcohol Concentrations.
Benzyl alcohol concentration was measured in the sedated flies immediately after removal from the vapor chamber using liquid-phase gas chromatography, as described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Adron Harris, Richard Aldrich, Yazan Al-Hasan, Harish Krishnan, and Rudi Bohm for insightful comments and criticisms, Jane Kirschman for copyediting, and Harold Zakon for sharing laboratory space. This work was supported by National Institute on Drug Abuse Grant DA022219 (to N.S.A.) and Graduate Fellowships from the Waggoner Center for Alcohol Addiction Research (to J.B.P and Y.W.)
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005439107/-/DCSupplemental.
References
- 1.Martin WR. XVI. A homeostatic and redundancy theory of tolerance to and dependence on narcotic analgesics. Res Publ Assoc Res Nerv Ment Dis. 1968;46:206–225. [PubMed] [Google Scholar]
- 2.Eddy NB, Halbach H, Isbell H, Seevers MH. Drug dependence: Its significance and characteristics. Bull World Health Organ. 1965;32:721–733. [PMC free article] [PubMed] [Google Scholar]
- 3.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 4.Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- 5.Ghezzi A, Al-Hasan YM, Larios LE, Bohm RA, Atkinson NS. slo K(+) channel gene regulation mediates rapid drug tolerance. Proc Natl Acad Sci USA. 2004;101:17276–17281. doi: 10.1073/pnas.0405584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowmeadow RB, et al. Ethanol tolerance caused by slowpoke induction in Drosophila. Alcohol Clin Exp Res. 2006;30:745–753. doi: 10.1111/j.1530-0277.2006.00087.x. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. doi: 10.1126/science.1857984. [DOI] [PubMed] [Google Scholar]
- 8.Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- 9.Tanouye MA, Wyman RJ. Motor outputs of giant nerve fiber in Drosophila. J Neurophysiol. 1980;44:405–421. doi: 10.1152/jn.1980.44.2.405. [DOI] [PubMed] [Google Scholar]
- 10.King DG, Wyman RJ. Anatomy of the giant fibre pathway in Drosophila. I. Three thoracic components of the pathway. J Neurocytol. 1980;9:753–770. doi: 10.1007/BF01205017. [DOI] [PubMed] [Google Scholar]
- 11.Lin M, Nash HA. Influence of general anesthetics on a specific neural pathway in Drosophila melanogaster. Proc Natl Acad Sci USA. 1996;93:10446–10451. doi: 10.1073/pnas.93.19.10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engel JE, Wu C-F. Interactions of membrane excitability mutations affecting potassium and sodium currents in the flight and giant fiber escape systems of Drosophila. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1992;171:93–104. doi: 10.1007/BF00195964. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson NS, et al. Molecular separation of two behavioral phenotypes by a mutation affecting the promoters of a Ca-activated K channel. J Neurosci. 2000;20:2988–2993. doi: 10.1523/JNEUROSCI.20-08-02988.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 15.Hughes JR. Alcohol withdrawal seizures. Epilepsy Behav. 2009;15:92–97. doi: 10.1016/j.yebeh.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 16.Kuebler D, Zhang H, Ren X, Tanouye MA. Genetic suppression of seizure susceptibility in Drosophila. J Neurophysiol. 2001;86:1211–1225. doi: 10.1152/jn.2001.86.3.1211. [DOI] [PubMed] [Google Scholar]
- 17.Kuebler D, Tanouye MA. Modifications of seizure susceptibility in Drosophila. J Neurophysiol. 2000;83:998–1009. doi: 10.1152/jn.2000.83.2.998. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Wu CF. Electroconvulsive seizure behavior in Drosophila: Analysis of the physiological repertoire underlying a stereotyped action pattern in bang-sensitive mutants. J Neurosci. 2002;22:11065–11079. doi: 10.1523/JNEUROSCI.22-24-11065.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Q, Dale N. Developmental changes in expression of ion currents accompany maturation of locomotor pattern in frog tadpoles. J Physiol. 1998;507:257–264. doi: 10.1111/j.1469-7793.1998.257bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel beta-subunits. News Physiol Sci. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- 21.Lovell PV, McCobb DP. Pituitary control of BK potassium channel function and intrinsic firing properties of adrenal chromaffin cells. J Neurosci. 2001;21:3429–3442. doi: 10.1523/JNEUROSCI.21-10-03429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warbington L, Hillman T, Adams C, Stern M. Reduced transmitter release conferred by mutations in the slowpoke-encoded Ca2(+)-activated K+ channel gene of Drosophila. Invert Neurosci. 1996;2:51–60. doi: 10.1007/BF02336660. [DOI] [PubMed] [Google Scholar]
- 23.Jin W, Sugaya A, Tsuda T, Ohguchi H, Sugaya E. Relationship between large conductance calcium-activated potassium channel and bursting activity. Brain Res. 2000;860:21–28. doi: 10.1016/s0006-8993(00)01943-0. [DOI] [PubMed] [Google Scholar]
- 24.Brenner R, et al. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- 25.Du W, et al. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet. 2005;37:733–738. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- 26.Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol. 2007;580:859–882. doi: 10.1113/jphysiol.2006.126367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klyachko VA, Ahern GP, Jackson MB. cGMP-mediated facilitation in nerve terminals by enhancement of the spike after hyperpolarization. Neuron. 2001;31:1015–1025. doi: 10.1016/s0896-6273(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 28.Wen T, Parrish CA, Xu D, Wu Q, Shen P. Drosophila neuropeptide F and its receptor, NPFR1, define a signaling pathway that acutely modulates alcohol sensitivity. Proc Natl Acad Sci USA. 2005;102:2141–2146. doi: 10.1073/pnas.0406814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger KH, Heberlein U, Moore MS. Rapid and chronic: Two distinct forms of ethanol tolerance in Drosophila. Alcohol Clin Exp Res. 2004;28:1469–1480. doi: 10.1097/01.alc.0000141817.15993.98. [DOI] [PubMed] [Google Scholar]
- 30.Dzitoyeva S, Dimitrijevic N, Manev H. Identification of a novel Drosophila gene, beltless, using injectable embryonic and adult RNA interference (RNAi) BMC Genomics. 2003;4:33. doi: 10.1186/1471-2164-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SK, Sedore SA, Cronmiller C, Hirsh J. Type II cAMP-dependent protein kinase-deficient Drosophila are viable but show developmental, circadian, and drug response phenotypes. J Biol Chem. 2000;275:20588–20596. doi: 10.1074/jbc.M002460200. [DOI] [PubMed] [Google Scholar]
- 32.Knott TK, Dopico AM, Dayanithi G, Lemos J, Treistman SN. Integrated channel plasticity contributes to alcohol tolerance in neurohypophysial terminals. Mol Pharmacol. 2002;62:135–142. doi: 10.1124/mol.62.1.135. [DOI] [PubMed] [Google Scholar]
- 33.Pietrzykowski AZ, et al. Alcohol tolerance in large-conductance, calcium-activated potassium channels of CNS terminals is intrinsic and includes two components: Decreased ethanol potentiation and decreased channel density. J Neurosci. 2004;24:8322–8332. doi: 10.1523/JNEUROSCI.1536-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pietrzykowski AZ, et al. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59:274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- 36.Roberto M, et al. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: An in vitro and in vivo analysis. J Neurosci. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Julien RM. A Primer of Drug Action: A Concise, Nontechnical Guide to the Actions, Uses, and Side Effects of Psychoactive Drugs. New York, NY: Worth Publishers Inc; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.