Abstract
T-cell interactions with antigen-presenting cells are important for CD8 T-cell effector or memory fate determination. The integrin leukocyte function-associated antigen-1 (LFA-1) mediates T-cell adhesion but the contribution of LFA-1–induced signaling pathways to T-cell responses is poorly understood. Here we demonstrate that proline-rich tyrosine kinase-2 (PYK2) deficiency impairs CD8 T-cell activation by synergistic LFA-1 and T-cell receptor stimulation. Furthermore, PYK2 is essential for LFA-1-mediated CD8 T-cell adhesion and LFA-1 costimulation of CD8 T-cell migration. During lymphocytic choriomeningitis virus infection in vivo, PYK2 deficiency results in a specific loss of short-lived effector CD8 T cells but does not affect memory-precursor CD8 T-cell development. Similarly, lack of LFA-1 primarily impairs the generation of short-lived effector cells. Thus, PYK2 facilitates LFA-1–dependent CD8 T-cell responses and promotes CD8 T-cell short-lived effector fate, suggesting that PYK2 may be an interesting therapeutic target to suppress exacerbated CD8 T-cell responses.
Keywords: integrin, costimulation, chemotaxis, lymphocytic choriomeningitis virus, memory
During the acute phase of immune responses to intracellular pathogens or allografts, CD8 T cells rapidly proliferate, induce cytokine expression, and acquire cytotoxic effector functions to eliminate the target cells (1). Although the majority of cytotoxic CD8 effector T cells are short-lived, a small fraction of the antigen-specific CD8 T cells, referred to as memory-precursor effector cells, survive long term and respond more vigorously to rechallenge with the same antigen. The signaling pathways that promote terminal CD8 T-cell differentiation are of great interest for intervention of allograft rejection, prevention of tissue damage during overly aggressive antiviral responses, and immunization against viruses and cancer.
CD8 T-cell short-lived effector or memory fate is directed by the cytokines IL-2, IL-7, IL-12, IL-15, and IFN-γ, and involves the transcription factors T-bet, eomesodermin, Runx3, and Blimp-1 (2, 3). Furthermore, signals that CD8 T cells receive during the priming by antigen presenting cells (APCs) can influence their fate. Initial antigen encounter can trigger both effector and memory differentiation programs in naive T cells (4–6). However, both the strength and the duration of the antigenic stimulus have been shown to influence the amplitude of the CD8 T-cell response or shift the ratio between effector and memory CD8 T-cell fate (7–11). Thus, prolonged CD8 T-cell interactions with APCs may facilitate the terminal differentiation of effector cells by extending antigen-mediated signals, and brief interactions may result in the maintenance of memory potential as a consequence of the early termination of signals (12). Furthermore, long-lived interactions of CD8 T cells with APCs throughout the first cell division were recently suggested to control effector and memory fate of CD8 T cells by affecting the asymmetric distribution of effector or memory fate determinants to the proximal or distal daughter cell, respectively (13). Access to APCs and cytokines largely depends on the ability of CD8 cells to migrate in response to chemokines, which is also important for appropriate CD8 T-cell differentiation (14–16). Therefore, the spatial and temporal regulation of T cell/APC interactions is critically important for short-lived effector versus memory fate decisions.
Both chemokine and antigen receptors induce signaling pathways that mediate the reorganization of the cellular cytoskeleton. However, T-cell polarity additionally requires directed interactions of T cells. The T-cell integrin, leukocyte function-associated antigen-1 (LFA-1), facilitates T-cell adhesion by binding to its ligand intercellular adhesion molecule-1 (ICAM-1), which is expressed on the surface of many cell types (17). In resting T cells, the extracellular domain of LFA-1 is in an inactive folded conformation that prevents binding to ICAM-1 and T-cell adhesion in the absence of antigen or chemokines. T-cell receptor (TCR) or chemokine receptor engagement triggers signaling pathways (inside-out signaling) that induce a conformation change and clustering of LFA-1, allowing it to bind ICAM-1 with high affinity. Consequently, signaling pathways downstream of LFA-1 (outside-in signaling) are activated that induce cytoskeletal rearrangements. Although some specific components of the inside-out signaling pathway that regulates LFA-1 activity have been identified in recent years, the role of LFA-1 outside-in signaling in T cells is less well-studied. Src family kinases, Syk kinases, the adaptor protein SLP-76, and Vav have been shown to be involved in integrin-mediated functions in neutrophils and platelets (18–22). The role of these signaling proteins in regulating LFA-1 function in T cells has been difficult to study because proximal TCR signaling also critically depends on them and their loss results in impaired T-cell development. Interestingly, SLP-76 binding to adhesion and degranulation-promoting adapter protein (ADAP) has recently been identified to be critical for LFA-1 outside-in signaling in T cells using mutant proteins (23, 24). However, the physiological importance of LFA-1 outside-in signaling for overall T-cell responses is unclear.
Proline-rich tyrosine kinase-2 (PYK2) is closely related to the nonreceptor tyrosine kinase focal adhesion kinase (FAK). Both kinases have been implicated in the regulation of the actin cytoskeleton (25). PYK2 is highly expressed in immune cells and activated in response to LFA-1, antigen receptor, or chemokine receptor stimulation (26–33). In macrophages and B cells, PYK2 has been shown to be important for chemokine-induced migration (34, 35). TCR-induced phosphorylation and activation of PYK2 is dependent on the Src family kinase FYN, but does not require LCK, which mediates ZAP-70 activation and the canonical antigen receptor signaling pathways leading to Ca2+/NFAT, NF-κB, and MAP kinase activation (28, 29). Normal T-cell development in PYK2-deficient mice suggested that PYK2 is not critical for TCR signaling pathways that mediate T-cell selection in the thymus (35). LFA-1 stimulation of T cells induces PYK2 colocalization with the microtubule organizing center and PYK2 relocalizes to the interface between T cells and APCs (36, 37). Nevertheless, whether PYK2 has a nonredundant function in T cells is not known.
Here we study PYK2-deficient T cells to determine whether PYK2 is essential for T-cell responses in vitro or in vivo. Our findings demonstrate that PYK2 facilitates LFA-1 costimulation of CD8 T-cell activation and migration by regulating T-cell polarity. Furthermore, this signaling pathway is critically important for the generation of short-lived effector but not memory-precursor effector CD8 T cells during lymphocytic choriomeningitis virus (LCMV) infection in vivo.
Results
PYK2-Deficient CD8 T Cells Are Impaired in Activation by Synergistic TCR and LFA-1 Stimulation.
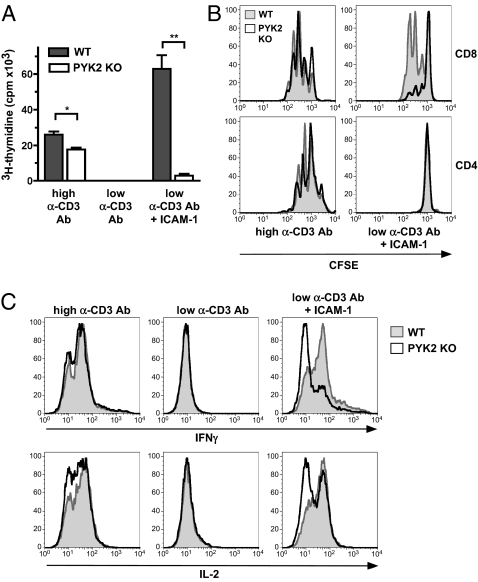
Because the tyrosine kinase PYK2 is activated in response to TCR stimulation, we first analyzed whether PYK2 deficiency affects T-cell activation in response to stimulation solely with anti-CD3 antibody in vitro. However, only a small defect in the proliferation of PYK2-deficient T cells was observed (Fig. 1 A and B). Simultaneous TCR and integrin stimulation has been reported to result in enhanced PYK2 activation, indicating that PYK2 might integrate signaling pathways downstream of these receptors (38). Therefore, we also stimulated PYK2-deficient T cells simultaneously through both TCR and LFA-1 under conditions in which these two stimuli synergize. To achieve such synergy with TCR stimulation, we titrated the anti-CD3 antibody down to limiting concentrations, which alone were insufficient to induce T-cell proliferation, but additional LFA-1 stimulation by ICAM-1 synergistically facilitated proliferation of wild-type T cells. This synergistic response was severely impaired in PYK2-deficient T cells. Interestingly, and consistent with previous reports, only wild-type CD8 but not wild-type CD4 T cells responded to LFA-1 costimulation (Fig. 1B) (39). IL-2 and IFN-γ production in response to TCR and LFA-1 costimulation was also reduced in PYK2 deficient CD8 T cells (Fig. 1C). These data indicate that PYK2 facilitates synergy between LFA-1 and TCR signaling during CD8 T-cell activation under limiting TCR-stimulation conditions.
Fig. 1.
Impaired activation of PYK2-deficient CD8 T cells in response to synergistic TCR and LFA-1 stimulation in vitro. WT or PYK2-deficient T cells were stimulated with plate-bound anti-CD3 antibody at standard concentrations (0.5 μg per well) or at limiting concentrations (0.1 μg per well) in the presence or absence of 0.3 μg per well plate-bound ICAM-1. (A) Proliferation was analyzed by 3H-thymidine uptake at 72 h. Graph shows average signal ± SD: *0.01 < P < 0.05; **0.001 < P < 0.01 (unpaired two-tailed Student's t test). (B) Proliferation was analyzed by CFSE labeling and FACS analysis. (C) IFN-γ and IL-2 expression in CD8 T cells was determined at 40 h by intracellular FACS staining after 5-h Brefeldin A treatment. (A–C) Data are representative of three or more experiments.
PYK2 Is Essential for LFA-1–Induced T-Cell Polarity.
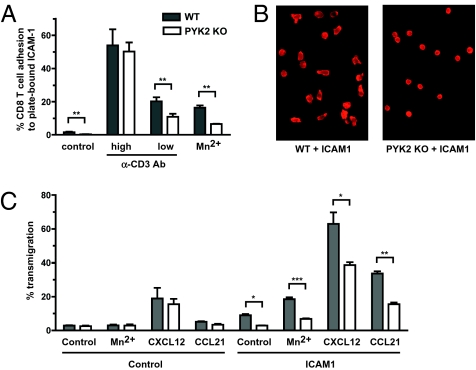
We then investigated how PYK2 might regulate LFA-1 costimulation of T-cell responses. An important function of LFA-1 is to facilitate T-cell adhesion by binding to its ligand ICAM-1 and to induce the reorganization of the cellular cytoskeleton, thereby promoting the ability of T cells to polarize. To test whether PYK2 is important for LFA-1–mediated T-cell adhesion, the ability of PYK2-deficient CD8 T cells to adhere to plate-bound ICAM-1 was tested (Fig. 2A). In these experiments, standard doses of anti-CD3 antibody were able to induce normal binding of PYK2-deficient CD8 T cells to ICAM-coated plastic plates, suggesting that inside-out signaling leading to the induction of active LFA-1 is intact in these cells. However, basal adhesion and adhesion in response to low-dose anti-CD3 antibody stimulation was significantly decreased in PYK2-deficient CD8 T cells. Moreover, after the addition of MnCl2, which bypasses inside-out signaling by artificially inducing a constitutively active confirmation of LFA-1, adhesion of PYK2-deficient CD8 T cells to ICAM-1 was also impaired. These data indicate that PYK2 contributes to the adhesion of CD8 T cells to ICAM-1–coated surfaces, but this function is at least partially downstream of LFA-1 and can be bypassed by strong TCR stimulation.
Fig. 2.
PYK2-deficient CD8 T cells are defective in LFA-1–mediated adhesion, polarity, and transmigration. (A) WT or PYK2-deficient T cells were stimulated as indicated in ICAM-1–coated microwell plates and adherent CD8 T cells were quantified by FACS. (B) WT or PYK2-deficient CD8 T-cell blasts were allowed to adhere to ICAM-1–coated cover slides. F-actin was stained with Alexa488-phalloidin and analyzed by fluorescence microscopy. Data are representative of an average view field from more than three experiments. (C) Migration of WT or PYK2-deficient CD8 T cells in the absence or presence of the indicated chemokines was measured using control or ICAM-1–coated transwell chambers. Migratory activity is presented as a percentage of migrating CD8 T-cell number divided by input CD8 T-cell number. (A and C) Graphs show average cell numbers from three independent experiments ± SEM: *0.01 < P < 0.05; **0.001 < P < 0.01; ***P < 0.001 (unpaired two-tailed Student's t test).
In addition to LFA-1 affinity, the ability of the cells to reorganize their cytoskeleton and spread on the surface contributes to the overall capacity of the cell to bind plate-bound ligands. To investigate whether LFA-1 induced T-cell spreading is affected by PYK2 deficiency, CD8 T-cell blasts were adhered to plate-bound ICAM-1 and imaged by immunofluorescence microcopy after intracellular staining for polymerized f-actin. Whereas wild-type CD8 T-cell blasts had the ability to acquire a polarized morphology in response to LFA-1 ligation and displayed polymerized actin at the leading edge and the uropod of migrating cells, PYK2-deficient CD8 T-cell blasts were markedly impaired in this response (Fig. 2B). These data indicate that PYK2 regulates an LFA-1 signaling pathway that contributes to the ability of CD8 T cells to spread and polarize.
PYK2 Facilitates LFA-1–Dependent CD8 T-Cell Migration.
LFA-1–mediated cell contacts and cytoskeletal polarity are also important for T-cell chemotaxis. PYK2 is activated by chemokine receptors and mediates chemokine-induced migration in macrophages and B cells (27, 32, 34, 35). Therefore, we examined whether PYK2 is essential for chemokine-induced transmigration of CD8 T cells in vitro (Fig. 2C). Interestingly, transmigration induced by CXCL12 or CCL21 alone was not impaired in PYK2-deficient CD8 T cells. However, coating the transmigration barrier with the LFA-1 ligand, ICAM-1, resulted in a synergistic increase in transmigration efficiency in wild-type CD8 T cells, but was significantly lower in PYK2-deficient CD8 T cells. These data show that although PYK2 is not required for LFA-1–independent chemotaxis of CD8 T cells, it is essential for LFA-1–dependent CD8 T-cell chemotaxis. Together, these results indicate that PYK2 plays a critical role in the synergistic induction of CD8 T-cell polarity in response to simultaneous LFA-1 and antigen or chemokine receptor triggering, which allows full CD8 T-cell activation and migration.
PYK2 Is Critical for the Expansion of CD8 T Cells in Response to LCMV.
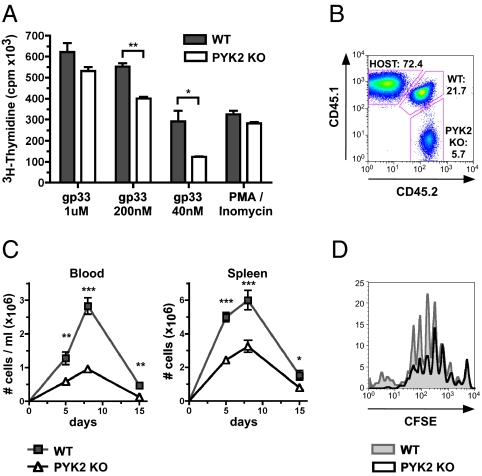
LFA-1 function is critical for the activation of T cells by APCs in vitro. To investigate whether PYK2 is essential for the activation of CD8 T cells by APCs, PYK2-deficient CD8 T cells expressing the transgenic P14 TCR, which recognizes the gp33-41 epitope of LCMV, were stimulated with syngeneic dendritic cells pulsed with various doses of gp33-41 peptide in vitro. PYK2 deficiency impaired P14 T-cell proliferation in response to stimulation, particularly at low doses of peptide; high doses of gp33-41 peptide were able to bypass the requirement for PYK2 (Fig. 3A).
Fig. 3.
PYK2 deficiency impairs the expansion of CD8 T cells in response to LCMV. (A) WT or PYK2-deficient P14 CD8 T cells were stimulated with dendritic cells and the indicated concentrations of LCMV gp33-41 peptide in vitro. Proliferation was analyzed by 3H-thymidine incorporation. Graph shows average counts ± SD. (B–D) WT (CD45.1+/CD45.2+) and PYK2-deficient (CD45.2+/CD45.2+) P14 CD8 T cells were mixed at a 1:1 ratio and adoptively transferred into host mice (CD45.1+/CD45.1+). The next day, mice were infected with LCMV Armstrong and P14 cells were analyzed at the indicated times. (B) Representative FACS plot of the analysis of congenic markers of P14 CD8 T cells from blood at day 8; numbers shown represent frequencies of the indicated population within CD8 T cells. (C) Graph shows average total P14 CD8 T-cell numbers in blood and spleen from three or more experiments (day 5: n = 10 mice; day 8: n = 20 mice; day 15: n = 6 mice) ± SEM. (D) WT and PYK2-deficient P14 transgenic CD8 T cells that were CFSE-labeled before adoptive transfer were analyzed by FACS after LCMV infection. (A and D) Data are representative of three experiments. (A and C) *0.01 < P < 0.05; **0.001 < P < 0.01; ***P < 0.001 (unpaired two-tailed Student's t test).
We next investigated the significance of PYK2 for CD8 T-cell responses in vivo. To determine whether PYK2 is essential for the expansion of CD8 T cells in response to LCMV infection, PYK2-deficient P14 CD8 T cells were adoptively transferred together with wild-type P14 CD8 T cells at a 1:1 ratio into host mice. Five and 8 d after LCMV Armstrong challenge, the populations of transferred PYK2-deficient and wild-type P14 CD8 T cells in blood and spleen were determined by FACS analysis of congenic markers (Fig. 3 B and C). At the peak of the response on day 8, PYK2-deficient P14 CD8 T-cell numbers in the blood were approximately three times lower than wild-type P14 CD8 T cells. PYK2-deficient P14 CD8 T-cell numbers in the spleen were similarly reduced, suggesting that the defect is a consequence of impaired expansion rather than altered distribution of the cells because of a migration defect. A difference between PYK2-deficient and wild-type P14 CD8 T-cell number was noted as early as day 5 after LCMV infection. CFSE-labeling of P14 CD8 T cells before adoptive transfer as above demonstrated that although all transferred PYK2-deficient P14 CD8 T cells entered cell division, their numbers were reduced compared with wild-type P14 CD8 T cells after four to five cell divisions (Fig. 3D). However, CD25 up-regulation on P14 CD8 T cells 24 or 36 h after LCMV challenge was not affected by PYK2-deficiency, further suggesting that the impaired expansion of PYK2 knockout P14 CD8 T cells is not a consequence of an impact on proximal TCR signaling or the receipt of IL-2 signals (Fig. S1). Furthermore, the small population of PYK2-deficient P14 CD8 T cells that was present during the acute expansion phase did not display any differences from wild-type P14 CD8 T cells in expression of the activation markers glycosylated CD43 (1B11), CD44, and CD62L, or IFN-γ, and TNF up-regulation upon restimulation with gp33-41 peptide in vitro (Fig. S2 A–C). This finding suggests the PYK2-deficient CD8 T cells were activated normally but failed to fully expand during the acute phase of their response to LCMV.
CD8 T-Cell Intrinsic PYK2 Deficiency Results in a Loss of Short-Lived Effector Cells.
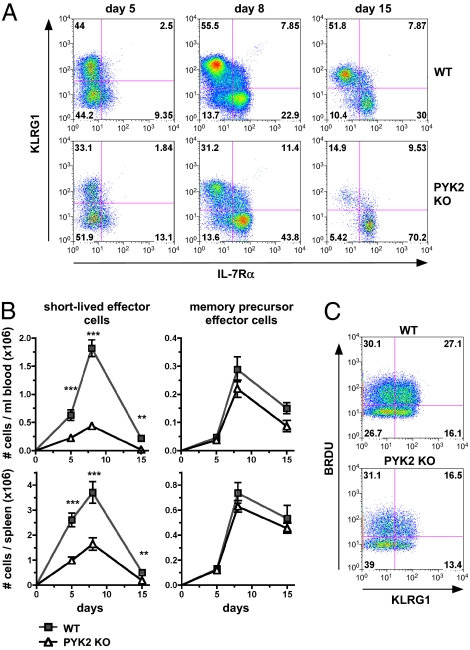
Rapid expansion is an attribute that is specifically associated with short-lived effector CD8 cells. To investigate whether PYK2-deficient P14 CD8 T cells have specific defects in the generation of short-lived effector or memory-precursor effector cell populations, expression of the specific markers IL-7Rα and killer cell lectin-like receptor G1 (KLRG1) was analyzed by FACS. This finding revealed that in both blood and spleen, the frequency of IL-7Rαlow/KLRG1high short-lived effector cells was markedly reduced and that of IL-7Rαhigh/KLRG1low memory-precursor effector cells increased in PYK2-deficient CD8 T cells (Fig. 4A). However, this reflected a decrease in total numbers of PYK2-deficient short-lived effector cells, whereas PYK2-deficient memory-precursor effector cells were generated at similar numbers as wild type (Fig. 4B). In vivo BrdU labeling on day 5 after infection demonstrated that a smaller fraction KLRG1+ PYK2 P14 CD8 T cells underwent cell divisions compared with KLRG1+ wild-type P14 CD8 T cells (Fig. 4C). PYK2 knockout and wild-type P14 CD8 T cells remaining at day 50 after LCMV infection expressed equivalent levels of TNF and IFN-γ upon restimulation, suggesting that PYK2-deficient memory cells are functionally competent in regard to cytokine expression (Fig. S2D). These data indicate that PYK2 is essential for the generation of short-lived effector CD8 T cells, but memory-precursor T cells develop independently of PYK2.
Fig. 4.
PYK2 deficiency results in a loss of short-lived effector cells but does not affect memory precursor cell generation. (A–C) WT and PYK2-deficient P14 CD8 T cells were adoptively transferred and mice were infected with LCMV Armstrong as in Fig. 3. (A) Representative FACS analysis of IL-7Rαlow/KLRG1high short-lived effector versus IL-7Rαhigh/KLRG1low memory precursor cells at day 8 after infection. (B) Graph shows average total cell numbers of short-lived effector cells or memory precursor cells from three or more experiments (day 5: n = 10 mice; day 8: n = 20 mice; day 15: n = 6 mice) ± SEM: **0.001 < P < 0.01; ***P < 0.001 (unpaired two-tailed Student's t test). (C) FACS analysis of BrdU incorporation on day 5 after LCMV infection. Data are representative of three experiments.
Short-Lived Effector CD8 T-Cell Generation Is LFA-1–Dependent.
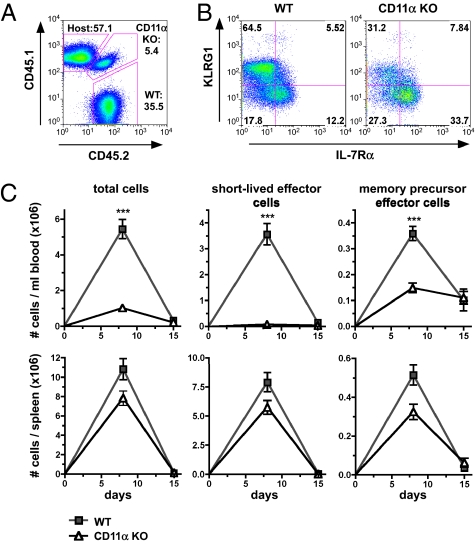
CD8 T-cell responses against LCMV were previously reported to occur independently of LFA-1 (40, 41). However, if the defect in short-lived effector cell expansion in PYK2-deficient P14 CD8 T cells is related to the role of PYK2 in regulating LFA-1 costimulation, then a similar phenotype should be caused by LFA-1 deficiency. To test this hypothesis, we adoptively transferred P14 CD8 T cells deficient in the CD11α subunit of LFA-1 together with the same number of wild-type P14 CD8 T cells before LCMV Armstrong infection of host mice. Analyses of congenic CD45 markers revealed impaired expansion of CD11α-deficient P14 CD8 T cells in the blood at day 8 after infection (Fig. 5 A and C). There was also a shift in the frequencies of IL-7Rαlow/KLRG1high short-lived effector versus IL-7Rαhigh/KLRG1low memory-precursor in CD11α-deficient P14 CD8 T cells compared with wild type P14 CD8 T cells (Fig. 5B). CD11α deficiency primarily affected total numbers of short-lived effector P14 CD8 T cell but memory-precursor P14 CD8 T-cell numbers were also reduced to some degree (Fig. 5C). The decrease CD11α-deficient P14 CD8 T-cell numbers in spleens was comparably smaller. This result could be because of the accumulation of LFA-1–deficient P14 CD8 T cells in the spleen as a consequence of impaired transmigration from the spleen to the blood. Therefore, LFA-1 is critical for short-lived effector CD8 T-cell generation similar to PYK2, but has additional functions during CD8 T-cell responses that are probably attributed to its more extensive role in T-cell adhesion.
Fig. 5.
CD11α deficiency primarily affects the generation of short-lived effector cells during LCMV infection. (A–C) WT (CD45.2+/CD45.2+) or CD11α-deficient (CD45.1+/CD45.2+) P14 CD8 T cells were mixed at a 1:1 ratio and adoptively transferred into host mice (CD45.1+/CD45.1+). The next day, chimeric mice were infected with LCMV Armstrong. (A) Representative FACS analysis of congenic markers on blood cells at day 8 after infection. Numbers shown represent frequencies of the indicated population within CD8 T cells. (B) Representative FACS analysis of IL-7Rαlow/KLRG1high short-lived effector versus IL-7Rαhigh/KLRG1low memory precursor cells in the blood at day 8 after infection. (C) Graph shows average total cell numbers from two experiments (day 8: n = 10 mice; day 15: n = 10 mice) ± SEM: *0.01 < P < 0.05; **0.001 < P < 0.01; ***P < 0.001 (unpaired two-tailed Student's t test).
Discussion
This study reveals that PYK2 plays a critical role in integrating LFA-1 and TCR or chemokine signaling pathways to facilitate the synergistic induction of cell polarity during CD8 T-cell activation and migration. Furthermore, we demonstrate that PYK2 and LFA-1 are important for the generation of short-lived effector CD8 T cells, suggesting that cell polarity may be a critical determinant of CD8 T-cell fate.
Although TCR and chemokine receptor stimulation induces PYK2 phosphorylation, neither TCR-mediated activation nor chemokine receptor-induced migration of T cells was critically dependent on PYK2. However, LFA-1 coligation by ICAM-1 revealed that CD8 T cells require PYK2 for LFA-1–dependent activation and migration. In line with the observation that TCR and integrin costimulation induces synergistic PYK2 phosphorylation, LFA-1 could provide an additional signal for PYK2 activation (38). Therefore, PYK2 may integrate proximal signaling pathways downstream of LFA-1 and TCR or chemokine receptors to fully induce the reorganization of the cytoskeleton. This synergistic increase in cytoskeletal reorganization and spreading may contribute to CD8 T-cell activation and migration in the presence of physiologic concentrations of antigen and chemokines. It may constitute an additional safeguard mechanism to allow full responses of CD8 T cells only when both integrins and antigen or chemokine receptors are engaged.
Interestingly, PYK2 function only seemed to be important for CD8 but not CD4 T-cell activation. CD8 T cells may be more dependent on the contribution of cytoskeletal reorganization to overall activation by surface-bound ligands than CD4 T cells because they rapidly reorient and spread. CD4 T cells may also be more dependent on additional costimulatory signals, such as CD28 (42).
During the in vivo response to LCMV, ablation of PYK2 resulted in a specific loss of short-lived effector CD8 T cells, but memory-precursor effector CD8 T-cell generation was normal. Because PYK2 was important for LFA-1–induced spreading but not for LFA-1 activity, LFA-1–dependent interactions of PYK2-deficient CD8 T cells with APCs may be sufficient to facilitate memory-precursor cell development. Alternatively, memory-precursor effector cell development may be independent of LFA-1. CD11α deficiency also primarily affected short-lived effector cell generation, but there was some impact on memory-precursor effector cell numbers. Therefore, we propose that LFA-1 activation of PYK2, rather than LFA-1–mediated T-cell contacts per se, sets a threshold for short-lived effector versus memory-precursor effector CD8 T-cell differentiation.
PYK2-mediated T-cell polarity could be important for CD8 T cell short-lived effector fate by regulating the kinetics or quality of the CD8 T cell APC interaction. This process may indirectly impact on antigen triggering and exposure to cytokines. In line with the report by Chang et al., failure to polarize in the absence of PYK2 could also result in the miss-localization of effector-fate determinants from the APC proximal daughter cell during CD8 T-cell division (13). Furthermore, the loss of LFA-1 signaling via PYK2 may impact on the formation or molecular makeup of signaling microclusters (43). The molecular mechanism behind the role of PYK2 in short-lived effector fate is very exciting, but also difficult to study. Because we have demonstrated that PYK2 is only critical for CD8 T cells under physiological levels of TCR or chemokine receptor stimulation in the presence of appropriate LFA-1 costimulation, the molecular mechanisms would be best studied in vivo, but this exceeds the scope of the present study and will be subject to further investigation.
Mice deficient in the LFA-1–specific CD11α subunit fail to reject immunogenic tumors but were surprisingly reported to mount normal antiviral responses (40, 41, 44, 45). However, in the present study we revealed a defect in the generation of LCMV-specific short-lived effector cells as a consequence of both PYK2 and CD11α deficiency. This result was achieved using simultaneous adoptive transfer of wild-type and knockout P14 TCR transgenic CD8 T cells, which directly compared their competitive fitness. Previous studies showed that after LCMV priming, CD11α-deficient cytotoxic T lymphocytes lysed target cells normally. However, this result is expected because the cytolytic activity of short-lived effector and memory-precursor effector cells is similar (46). Short-lived effector and memory-precursor CD8 T-cell populations were not specifically analyzed previously.
Interestingly, it has also been reported that ICAM-1 is dispensable for short-lived, but essential for long-lasting, T cell/dendritic cell interactions (47). Using in vivo transfer of ICAM-1–deficient dendritic cells and subsequent immunization, the authors also observed that after initial normal onset of the CD8 T-cell response, CD8 T-cell numbers were decreased after 7 to 14 d. However, this finding was interpreted as a defect in the memory phase of the CD8 T-cell response. Characterization of short-lived effector versus memory-precursor CD8 T-cell populations in this system would shed light into whether the report is consistent with the data presented here. Furthermore, Zehn et al. demonstrated curtailed OT1 CD8 T-cell responses to low-affinity variants of the SIINFEKL peptide (11). Interestingly, the low-affinity TCR stimulation allowed early egress of CD8 T cells from lymph nodes, indicating that altered T-cell interactions with APCs could contribute to this phenomenon.
Our studies reveal that PYK2 has not only a quantitative contribution to the overall CD8 T-cell response, but a very specific qualitative impact on the differentiation of short-lived effector versus memory-precursor effector CD8 T cells. This result may open a very interesting window of opportunity for therapeutic intervention by targeting PYK2. PYK2 may be an interesting candidate for the reduction of acute CD8 T-cell responses without completely ablating CD8 T cell-mediated surveillance of acute viral infections and dormant retroviruses. Prevention of allograft rejection after transplantation by targeting PYK2 may be problematic because memory-precursor effector cells that develop independently of PYK2 may cause acute or chronic rejection. However, PYK2 inhibition may be useful to reduce acute pathogenic CD8 T-cell responses in the context of viral infections, which cause severe tissue damage leading to death (i.e., fulminant hepatitis or influenza), although maintaining the beneficial anti-viral response.
Materials and Methods
Mice and Reagents.
PYK2 and CD11α knockout mice (34, 48), BoyJ (CD45.1+), and P14 TCR transgenic mice bearing the DbGP33-specific TCR were fully backcrossed to C57BL/6. All animals were housed in specific pathogen-free facility at the University of California, San Francisco, according to University and National Institutes of Health guidelines. Antibodies were purchased from BD Biosciences [CD3 (2C11), CD8, CD4, IFN-γ, IL-2, IL-7Rα, TNF, CD44, CD62L] or Biolegend (CD45.1, CD45.2, KLRG1). Recombinant mouse ICAM-1-FC, CXCL12, and CCL21 was purchased from R&D Biosciences.
Cell Isolation and in Vitro T-Cell Activation.
T cells were purified from spleens or lymph nodes by MACS (MiltenYi Biotec) according to the manufacturers protocol (purity >95%) and cultured in DMEM containing 10% FCS, 10 mM Hepes, penicillin, streptomycin, 2 nM glutamate, 1 mM sodium pyruvate, 1× nonessential amino acids, and 50 mM 2-mercaptoethanol at 37 °C in the presence of 5% CO2. Next, 2 × 105 T cells were stimulated in a 96-well plate with 0.5 or 0.1 μg/100 μL plate-bound anti-CD3 antibody per well in the presence or absence of 0.3 μg /100 μL plate-bound ICAM-1-FC per well for 72 h. P14 TCR transgenic CD8 T cells were stimulated in vitro with 1 μM to 40 nM LCMV gp33-41 peptide and 2 × 104 CD11c MACS enriched syngeneic splenic dendritic cells for 48 h. Proliferation was assessed by liquid scintillation of 3H-thymidine uptake during the last 6 h of the culture or by FACS analysis of CFSE labelled cells 72 h after stimulation. Cytokine expression was determined at 40 h after stimulation by treating cells with 10 μg/mL Brefeldin A for 5 h, followed by intracellular staining and FACS analysis.
T-Cell Adhesion.
For T-cell adhesion, 1 × 106 T cells were plated in 96-well plates coated with 0.3 μg/100 μL plate-bound ICAM-1-FC per well on ice and stimulated with 0.5 μg/mL or 5 μg/mL soluble anti-CD3 antibody cross-linked with secondary antibody for 10 min at 37 °C. Nonadherent cells were washed off before adherent cells were eluted and counted by FACS. CD8 T-cell blast were generated by stimulating MACS-enriched CD8 T cells with 10 ng/mL PMA and 200 ng/mL Ionomycin for 18 h and culturing them in the presence of 50 U/mL IL-2 for 5 to 7 d. For immunofluorescent imaging of CD8 T-cell blasts, cells were plated on ICAM-1-FC–coated glass coverslips for 10 min at 37 °C and, after washing, F-actin was stained using Alexa Fluor 488 phalloidin (Invitrogen) according to the manufacture's protocol. Images were acquired using a Zeiss microscope.
Transwell Migration Assay.
The migratory ability of T cells was measured using 5-μm pore size Transwell plates (Corning Costar Corp.), as described previously (49). Cells were collected, stained with anti-CD4, -CD8, or -CD3 mAb, and quantified using flow cytometry. Transwell assays were performed in duplicates for each different chemokine (CXCL12, 400 ng/mL and CCL21, 1 μg/mL). For transwell migration assays using filters coated with ICAM-1, 5-μm polycarbonate transwell filters were coated in 100 μL of PBS with ICAM-1 (R&D Systems, 3 μg/mL) overnight at 4 °C. All filters were washed three times with PBS and blocked with 2% BSA for 1 h at 37 °C. Filters were rinsed with PBS and dried. Coated filters were checked for leakage, then used for transwell migration assays.
T-Cell Adoptive Transfer and in Vivo Analyses.
Wild-type and PYK2- or CD11α-deficient P14 CD8 T cells were mixed at a 1:1 ratio and transferred at 2 × 105 cell/mice by i.v. tail-vein injection. Then, 24 to 48 h later mice were infected i.p. with 2 × 105 PFU per mouse LCMV Armstrong and P14 CD8 T cell were characterized by FACS 5, 8, or 15 d after infection. Proliferation in vivo was investigated by CFSE labeling of P14 CD8 T cells before adoptively transferring 1 × 106 cells per mice. FACS analysis of CFSE dilution was performed 66 h after LCMV Armstrong infection. BrdU incorporation analysis was performed by i.p. injection of 10 μg BrdU per mouse at day 5 after LCMV Armstrong infection and FACS analysis of splenic P14 CD8 T cells 1.5 h after the BrdU pulse.
Supplementary Material
Acknowledgments
We thank Al Roque and Kristin Doan for help with animal maintenance and Andre Limnander and Byron Au-Yeung for inspiring discussions, support, and critically reading the manuscript. This study was supported in part by a long-term fellowship from the Human Frontier Science Foundation (to S.B.) and a Leukemia and Lymphoma Society Special Fellow award (to H.P.); J.M.C. is a recipient of a National Science Foundation scholarship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011556107/-/DCSupplemental.
References
- 1.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 2.Arens R, Schoenberger SP. Plasticity in programming of effector and memory CD8 T-cell formation. Immunol Rev. 2010;235:190–205. doi: 10.1111/j.0105-2896.2010.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutishauser RL, Kaech SM. Generating diversity: Transcriptional regulation of effector and memory CD8 T-cell differentiation. Immunol Rev. 2010;235:219–233. doi: 10.1111/j.0105-2896.2010.00901.x. [DOI] [PubMed] [Google Scholar]
- 4.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: Initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naïve CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 6.Mercado R, et al. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 7.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 8.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 9.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Usharauli D, Kamala T. Brief antigenic stimulation generates effector CD8 T cells with low cytotoxic activity and high IL-2 production. J Immunol. 2008;180:4507–4513. doi: 10.4049/jimmunol.180.7.4507. [DOI] [PubMed] [Google Scholar]
- 11.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: Intermediates, effectors, and memory cells. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 13.Chang JT, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 14.Castellino F, Germain RN. Chemokine-guided CD4+ T cell help enhances generation of IL-6RalphahighIL-7Ralpha high prememory CD8+ T cells. J Immunol. 2007;178:778–787. doi: 10.4049/jimmunol.178.2.778. [DOI] [PubMed] [Google Scholar]
- 15.Castellino F, et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 16.Molon B, et al. T cell costimulation by chemokine receptors. Nat Immunol. 2005;6:465–471. doi: 10.1038/ni1191. [DOI] [PubMed] [Google Scholar]
- 17.Evans R, et al. Integrins in immunity. J Cell Sci. 2009;122:215–225. doi: 10.1242/jcs.019117. [DOI] [PubMed] [Google Scholar]
- 18.Lowell CA, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J Cell Biol. 1996;133:895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mócsai A, Ligeti E, Lowell CA, Berton G. Adhesion-dependent degranulation of neutrophils requires the Src family kinases Fgr and Hck. J Immunol. 1999;162:1120–1126. [PubMed] [Google Scholar]
- 20.Obergfell A, et al. Coordinate interactions of Csk, Src, and Syk kinases with [alpha]IIb[beta]3 initiate integrin signaling to the cytoskeleton. J Cell Biol. 2002;157:265–275. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gakidis MA, et al. Vav GEFs are required for beta2 integrin-dependent functions of neutrophils. J Cell Biol. 2004;166:273–282. doi: 10.1083/jcb.200404166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newbrough SA, et al. SLP-76 regulates Fcgamma receptor and integrin signaling in neutrophils. Immunity. 2003;19:761–769. doi: 10.1016/s1074-7613(03)00305-4. [DOI] [PubMed] [Google Scholar]
- 23.Baker RG, et al. The adapter protein SLP-76 mediates “outside-in” integrin signaling and function in T cells. Mol Cell Biol. 2009;29:5578–5589. doi: 10.1128/MCB.00283-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Wei B, Bismuth G, Rudd CE. SLP-76-ADAP adaptor module regulates LFA-1 mediated costimulation and T cell motility. Proc Natl Acad Sci USA. 2009;106:12436–12441. doi: 10.1073/pnas.0900510106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: In command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 26.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 27.Dikic I, Dikic I, Schlessinger J. Identification of a new Pyk2 isoform implicated in chemokine and antigen receptor signaling. J Biol Chem. 1998;273:14301–14308. doi: 10.1074/jbc.273.23.14301. [DOI] [PubMed] [Google Scholar]
- 28.Qian D, et al. Tyrosine phosphorylation of Pyk2 is selectively regulated by Fyn during TCR signaling. J Exp Med. 1997;185:1253–1259. doi: 10.1084/jem.185.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berg NN, Ostergaard HL. T cell receptor engagement induces tyrosine phosphorylation of FAK and Pyk2 and their association with Lck. J Immunol. 1997;159:1753–1757. [PubMed] [Google Scholar]
- 30.Ganju RK, et al. RAFTK, a novel member of the focal adhesion kinase family, is phosphorylated and associates with signaling molecules upon activation of mature T lymphocytes. J Exp Med. 1997;185:1055–1063. doi: 10.1084/jem.185.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Astier A, et al. The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after beta1-integrin stimulation in B cells and binds to p130cas. J Biol Chem. 1997;272:228–232. doi: 10.1074/jbc.272.1.228. [DOI] [PubMed] [Google Scholar]
- 32.Ganju RK, et al. Beta-chemokine receptor CCR5 signals via the novel tyrosine kinase RAFTK. Blood. 1998;91:791–797. [PubMed] [Google Scholar]
- 33.Dutt P, Wang JF, Groopman JE. Stromal cell-derived factor-1 alpha and stem cell factor/kit ligand share signaling pathways in hemopoietic progenitors: A potential mechanism for cooperative induction of chemotaxis. J Immunol. 1998;161:3652–3658. [PubMed] [Google Scholar]
- 34.Okigaki M, et al. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc Natl Acad Sci USA. 2003;100:10740–10745. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez-Fernández JL, et al. The interaction of activated integrin lymphocyte function-associated antigen 1 with ligand intercellular adhesion molecule 1 induces activation and redistribution of focal adhesion kinase and proline-rich tyrosine kinase 2 in T lymphocytes. Mol Biol Cell. 1999;10:1891–1907. doi: 10.1091/mbc.10.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sancho D, et al. TCR engagement induces proline-rich tyrosine kinase-2 (Pyk2) translocation to the T cell-APC interface independently of Pyk2 activity and in an immunoreceptor tyrosine-based activation motif-mediated fashion. J Immunol. 2002;169:292–300. doi: 10.4049/jimmunol.169.1.292. [DOI] [PubMed] [Google Scholar]
- 38.van Seventer GA, Mullen MM, van Seventer JM. Pyk2 is differentially regulated by beta1 integrin- and CD28-mediated co-stimulation in human CD4+ T lymphocytes. Eur J Immunol. 1998;28:3867–3877. doi: 10.1002/(SICI)1521-4141(199811)28:11<3867::AID-IMMU3867>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 39.Chen T, et al. ICAM-1 co-stimulation has differential effects on the activation of CD4+ and CD8+ T cells. Eur J Immunol. 1999;29:809–814. doi: 10.1002/(SICI)1521-4141(199903)29:03<809::AID-IMMU809>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 40.Bachmann MF, et al. Distinct roles for LFA-1 and CD28 during activation of naive T cells: Adhesion versus costimulation. Immunity. 1997;7:549–557. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- 41.Schmits R, et al. LFA-1-deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J Exp Med. 1996;183:1415–1426. doi: 10.1084/jem.183.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deeths MJ, Mescher MF. ICAM-1 and B7-1 provide similar but distinct costimulation for CD8+ T cells, while CD4+ T cells are poorly costimulated by ICAM-1. Eur J Immunol. 1999;29:45–53. doi: 10.1002/(SICI)1521-4141(199901)29:01<45::AID-IMMU45>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen K, Sylvain NR, Bunnell SC. T cell costimulation via the integrin VLA-4 inhibits the actin-dependent centralization of signaling microclusters containing the adaptor SLP-76. Immunity. 2008;28:810–821. doi: 10.1016/j.immuni.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 44.Shier P, et al. Impaired immune responses toward alloantigens and tumor cells but normal thymic selection in mice deficient in the beta2 integrin leukocyte function-associated antigen-1. J Immunol. 1996;157:5375–5386. [PubMed] [Google Scholar]
- 45.Shier P, Ngo K, Fung-Leung WP. Defective CD8+ T cell activation and cytolytic function in the absence of LFA-1 cannot be restored by increased TCR signaling. J Immunol. 1999;163:4826–4832. [PubMed] [Google Scholar]
- 46.Sarkar S, et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scholer A, Hugues S, Boissonnas A, Fetler L, Amigorena S. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity. 2008;28:258–270. doi: 10.1016/j.immuni.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 48.Ding ZM, et al. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- 49.Phee H, et al. Regulation of thymocyte positive selection and motility by GIT2. Nat Immunol. 2010;11:503–511. doi: 10.1038/ni.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.