Abstract
The formation of primitive (embryonic) blood in vertebrates is mediated by spatio-temporally restricted signaling between different tissue layers. In Xenopus, in which primitive blood originates in the ventral blood island, this involves the secretion of bone morphogenetic protein (BMP) ligands by the ectoderm that signal to the underlying mesoderm during gastrulation. Using novel transgenic reporter lines, we report that the canonical Wnt/β-catenin pathway is also activated in the blood islands in Xenopus. Furthermore, Wnt-reporter activity was also detected in the blood islands of the mouse yolk sac. By using morpholino-mediated depletion in Xenopus, we identified Wnt4 as the ligand that is expressed in the mesoderm of the ventral blood island and is essential for the expression of hematopoietic and erythroid marker genes. Injection of an inducible Wnt-interfering construct further showed that, during gastrulation, Wnt/β-catenin signaling is required both in the mesoderm and in the overlying ectoderm for the formation of the ventral blood island. Using recombination assays with embryonic explants, we document that ectodermal BMP4 expression is dependent on Wnt4 signals from the mesoderm. Our results thus reveal a unique role for Wnt4-mediated canonical signaling in the formation and maintenance of the ventral blood island in Xenopus.
Keywords: Xenopus, ventral blood islands, Wnt reporter, bone morphogenetic protein, destabilized GFP
The development of the hematopoietic system involves multiple steps ranging from induction and patterning of mesoderm and specification of the earliest blood cell progenitors to completion of the full differentiation program (1). In Xenopus, the earliest hematopoietic cells of the embryo, known as primitive blood cells, form in the ventral blood island (VBI), which is the equivalent of the extraembryonic blood islands in the yolk sac of mammals. Definitive hematopoietic stem cells (HSCs) in Xenopus arise from the dorsal lateral plate (DLP). In mammals, these HSCs originate in the aorta gonad–mesonephros region and in the mature yolk sac (2, 3). Studies using ES cells and novel transplantation protocols have indicated that primitive progenitors can also contribute to definitive hematopoiesis (4, 5).
Intensive investigation of the signaling events required for blood development has exposed functions for several growth and differentiation factors (6). Recent studies using in vitro differentiation of ES cells and manipulation of zebrafish embryos point to a role for Wnt signaling in primitive blood development (7, 8). However, there is still no direct conclusive evidence for a role for Wnt signaling in the formation of primitive blood in vivo.
Here, we uncovered a role for Wnt/β-catenin signaling, and particularly the Wnt4 ligand, in the specification and maintenance of the primitive blood cells in Xenopus embryos. We developed a sensitive and dynamic fluorescent reporter to detect the spatiotemporal pattern of Wnt/β-catenin signaling in transgenic Xenopus tropicalis embryos and tadpoles. This reporter construct revealed Wnt activity in the VBI and the DLP and later in the circulating primitive blood cells. By using loss-of-function assays in whole embryos and explants, we found that Wnt4 is required in the mesoderm, and that it functions in a non–cell-autonomous manner by modulating bone morphogenetic protein (BMP) in the ectoderm during gastrulation stages to promote the hematopoietic fate of the mesodermal tissues. Furthermore, we show that targeted inhibition of Wnt/β-catenin signaling down-regulates the expression of VBI markers and that Wnt needs to signal both in the mesodermal and ectodermal tissues for proper induction and maintenance of the VBI.
Results
To uncover unknown roles of the canonical Wnt pathway during embryonic development and postembryonic growth, we designed and characterized a reporter construct consisting of a synthetic promoter containing eight copies of an optimal binding sequence for LEF/TCF factors and a minimal TATA box in front of a reporter gene (SI Appendix, Fig. S1). To reveal as clearly as possible the temporal dynamics of Wnt pathway activation, we used destabilized EGFP as a reporter gene and flanked it with chromosomal insulator sequences from the chicken β-globin gene to increase its expression (9). The reporter construct (pbin8Lef-dGFP) was integrated by transgenesis in X. tropicalis embryos.
Expression of GFP was analyzed throughout embryonic development and larval growth. Both fluorescent illumination and whole-mount in situ hybridization (WISH) with a GFP probe showed several recurrent patterns of GFP expression in the transgenic embryos and the growing tadpoles (SI Appendix, Figs. S2 and S3 and Table S1). A full description of these dynamic GFP expression patterns, and a description of the correlative expression domains of Wnt ligands and Frizzled receptors or of reported biological functions of the canonical Wnt pathway during early and late development, are provided (SI Appendix, Table S1 and SI Text). In summary, we obtained several transgenic lines that faithfully report the activation of the canonical Wnt pathway in both the embryonic and the juvenile stages.
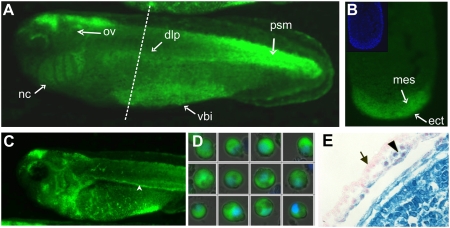
Aside from identifying signals that overlap with known patterns of Wnt ligand expression, we detected in embryos at stage 21–40 major GFP expression in a ventral zone in which the ventral blood island is located (Fig. 1 A–C and SI Appendix, Figs. S2 C and E–G′ and S3 G–J). A ventral view of these embryos shows a V-shaped GFP expression domain that is very similar to the pattern of the hematopoietic marker genes SCL, GATA1, and T3-globin (SI Appendix, Fig. S3 L and M). Transverse sectioning of a stage-32 embryo shows that the GFP signal is localized in both the ventral mesoderm and the overlying epidermis (Fig. 1B). WISH with a GFP probe was used to detect expression in the earlier embryonic stages. dGFP transcripts were detected throughout the ventral region of postneurulation embryos (SI Appendix, Fig. S3G) both before and after stage 15, at which the first hematopoietic stem cell marker SCL can be detected (10). The first GFP signal in the precursor of the VBI was detected in bisected gastrula stage embryos at about stage 12.5 in the leading edges of the anterior leading edge endomesoderm and the ventral mesoderm, which meet at this stage (SI Appendix, Fig. S4). In addition, the overlying ventral ectoderm (VE) also showed expression of GFP. This indicates that Wnt/β-catenin signaling is already activated within the mesoderm of the future VBI and in the VE during hematopoietic commitment and before formation of the hemangioblast. The ventral GFP signal disappeared in an anterior-to-posterior wave at the time when the embryonic heart starts to beat around stage 35. However, GFP-positive cells at this and later stages were seen to leave the ventral blood island through the lateral vittelline veins and to enter the blood circulation network (Fig. 1C and Movie S1). Microscopic analysis of blood collected from stage-37 embryos showed the presence of GFP-positive cells that were morphologically identified as erythrocytes (Fig. 1D). GFP transcript and protein were also detected at the dorsal lateral plate mesoderm (DLPM) from stage 22 onward (Fig. 1A and SI Appendix, Figs. S2C and S3 G and H). The DLPM is the mesodermal tissue that later differentiates to form the pronephric anlage, the pronephric duct, and the DLP, a source for definitive hematopoietic cells.
Fig. 1.
Wnt/β-catenin signaling is active in the ventral blood island of Xenopus and in the yolk sac mesoderm of mouse embryo. (A–D) Expression of the d2EGFP protein in transgenic embryos carrying the Wnt/β-catenin reporter construct. (A) GFP is expressed in the DLP mesoderm and the VBI, from which definitive and primitive blood cells originate, respectively. (B) Transverse section of a stage-32 embryo (dashed line in E) shows high levels of Wnt activity in the VBI (indicated by mes) and also in the overlying ectoderm (ect). (Inset) DAPI staining of the section. (C) Stage 37 embryos show GFP-expressing blood cells leaving the VBI through the vitelline veins and circulating in the posterior cardinal vein (arrowhead) and throughout the whole body. (D) GFP-expressing and DAPI-stained individual erythrocytes collected from stage-37 embryos and viewed with confocal microscope. (E) Detection of Wnt/β-catenin signaling in E8.5 embryo of BAT-Gal Wnt-reporter mouse. Strong β-gal activity is visible in the embryo proper (lower left half) and in the extraembryonic mesoderm that forms the blood islands in the yolk sac (arrowhead). No activity is seen in the surrounding visceral endoderm (arrow).
Prompted by the results in Xenopus embryos, we reinvestigated the expression of a Wnt-reporter in transgenic mice. Embryos were obtained from the BAT-GAL mouse (11) at different days postcoitum and stained for β-gal activity. The extraembryonic mesoderm that forms the blood islands in the yolk sac of E8.5 embryos showed strong activation of the Wnt-reporter gene (Fig. 1E). The analysis of transgenic Wnt-reporter frogs and mice show that the Wnt/β-catenin pathway is active at the site of primitive hematopoiesis in both Xenopus and mouse as well as at the site of definitive hematopoiesis in Xenopus.
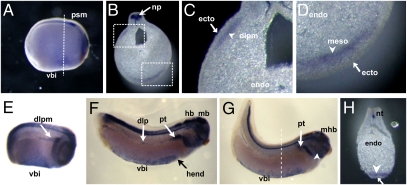
Having determined that canonical Wnt/β-catenin signaling is active in the VBI and overlaps with the expression of several hematopoietic marker genes, we next sought to identify the Wnt ligand responsible for the pathway's activation. Interestingly, a paper focusing on the role of Wnt4 in the formation of the pronephros also documented the presence of Wnt4 mRNA in a region probably corresponding to the VBI (12). Repeating the WISH with a Wnt4 probe in bisected gastrula stage embryos, we detected Wnt4 mRNA in the leading edge of the anterior endomesoderm, which migrates on the roof of the blastocoel to meet the ventral mesoderm to form the VBI (SI Appendix, Fig. S4). Wnt4 mRNA was further present in the VBI of stage 21 Xenopus laevis embryos in a region posterior to the cement gland and corresponding to the expression domain of SCL at this stage (Fig. 2A) and was detected at least until stage 34 (Fig. 2 E–G). Also in tailbud stage embryos, vibratome sections showed that Wnt4 mRNA is localized in the mesoderm but is absent from the overlying ectoderm (Fig. 2 B, D, and H). Wnt4 transcripts are also present in the DLP region (Fig. 2 B, C, and E).
Fig. 2.
WISH analysis of Wnt4 mRNA expression in the VBI and DLP in Xenopus embryos. (A–D) Wnt4 transcripts are observed in the neural plate and in the VBI and DLP mesoderm in stage-21 embryos. (B) Transverse section at site indicated by dashed line in A. (C) Closer view of dorsolateral and ventral regions (D). Wnt4 signal is clearly excluded from overlying ectoderm (C and D). Wnt4 transcripts in DLP are sustained in embryos at stage 25 (E) and restricted to pronephric region in embryos at stage 28 (F) and stage 34 (G). Wnt4 is clearly expressed in VBI (E–H) and specifically in the mesodermal layers (arrowhead in H), but not in overlying ectoderm (arrow in H). Wnt4 is also expressed in the brain, branchial arches, and lateral plate mesoderm (arrowhead in C). dlp, dorsal lateral plate, dlpm, dorsal lateral plate mesoderm; ecto, ectoderm; endo, endoderm; hb, hindbrain; hend, hepatic endoderm; mb, midbrain; mhb, midbrain–hindbrain boundary; meso, mesoderm; np, neural plate; nt, neural tube; pt, pronephric tubules; vbi, ventral blood island.
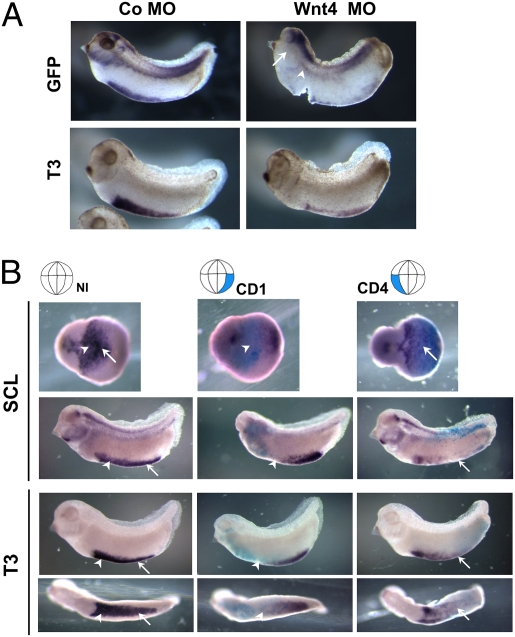
We assessed the effect of Wnt4 depletion on the formation of the VBI by using the morpholino (MO) that had been carefully characterized in the original paper by Saulnier et al. (12). Injection of Wnt4 MO induced the loss of dGFP expression in the region of the VBI in the Wnt-reporter embryos (Fig. 3A, Upper). Concomitantly, expression of the erythroid marker gene αT3-globin was lost (Fig. 3A, Lower). As Wnt4 expression is not restricted to the VBI, general Wnt4 depletion also affected the overall morphology of the embryo, especially the head region, where it is expressed abundantly in the brain (13). Hence we targeted the region of the embryo that contributes to the mesodermal compartment of the VBI, in which Wnt4 mRNA is expressed. In Xenopus this compartment has a dual origin. The anterior part (aVBI) is derived from the dorsal mesoderm and can be traced back to blastomeres C1 and D1 of 32-cell stage embryos (14). In 16-cell stage embryos, this region can be targeted by injecting the CD1 blastomere (also called the D2.1). In contrast, the posterior mesodermal compartment of the VBI (pVBI) originates in the ventral-most mesoderm and can be targeted by injecting the CD4 blastomere. Injecting the Wnt4 MO in the CD1 blastomeres resulted in loss of the marker genes SCL, LMO2, and αT3-globin, especially in the aVBI (Fig. 3B and SI Appendix, Fig. S5, arrowheads, and Table S2). In contrast, injection of the Wnt4 MO in the CD4 blastomeres affected marker gene expression most strongly in the pVBI (Fig. 3B and SI Appendix, Fig. S5, arrows). Furthermore, targeted knockdown of Wnt4 in V2 blastomeres of embryos at the eight-cell stage, which gives rise to the tissue generating the DLP (14) also resulted in loss of SCL and LMO2 expression at the DLP (SI Appendix, Fig. S6A, arrows).
Fig. 3.
Depletion of Wnt4 affects formation of VBI. (A) Wnt-reporter embryos were injected at two-cell stage with 10 ng Co MO or Wnt4 MO in marginal zones, cultured to stage 32, and hybridized in situ with probes for dGFP and blood marker T3-globin (T3). Wnt4 morphant lost most of the GFP and T3-globin signals at the VBI. GFP transcripts were totally abolished at the DLP in Wnt4-depleted embryos (Right, arrowhead). Note that the GFP signal in the morphant embryo was also abrogated in the branchial arches (Upper Right, arrows) and lateral plate mesoderm area, which normally show abundant Wnt4 expression (Fig. 2G, arrow and arrowhead). (B) Targeted depletion of Wnt4 at CD1 or CD4 blastomeres (Upper, highlighted) by injection of Wnt4 morpholino ablates expression of hematopoietic cell markers SCL and T3-globin (violet blue) in corresponding VBI compartments. Arrowheads and arrows indicate aVBI and pVBI, respectively. Cytoplasmic β-gal RNA was coinjected as a lineage tracer (cyan blue).
To evaluate the specificity of the MO and its effects on the VBI, we performed rescue experiments. Coinjection of the Wnt4 MO with a synthetic Wnt4 RNA (HA-Wnt4) that is insensitive to the MO (12) rescued the reduction in the expression of the hematopoietic markers SCL and T3-globin from 86% (n = 45) to 22% (n = 36) (SI Appendix, Fig. S6B). These data indicate that the defects in VBI formation seen with Wnt4 MO are specific. Together, the expression pattern and the functional data show that Wnt4 is the ligand required for formation of the VBI during Xenopus development. Importantly, in the VBI, Wnt4 appears to signal through the canonical Wnt/β-catenin pathway, and the signal originates from the mesoderm.
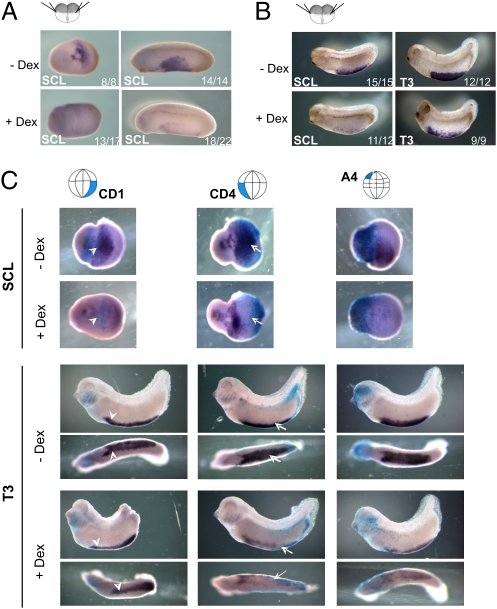
Having determined that Wnt4 inactivation disrupts Wnt/β-catenin signaling in a region corresponding to the VBI and interferes with the expression of hematopoietic marker genes, we wanted to investigate whether we could disrupt VBI formation by using the dexamethasone (Dex)-inducible Wnt-interfering construct EnR-LEFΔN-GR (further called EnR) (15). The addition of Dex at stage 13 resulted in prominent reduction of the hematopoietic marker gene SCL (Fig. 4A, Lower). Even when the Wnt-interfering construct was activated at later stages (stage 20), the erythrocyte markers SCL and T3-globin were still reduced (Fig. 4B). To identify the tissue in which activation of the canonical Wnt signaling is required, EnR RNA was injected in specific blastomeres, and Dex was added to the embryos at stage 11.5. Embryos injected in the CD1 blastomeres showed loss of hematopoietic marker expression in the aVBI (Fig. 4C and SI Appendix, Fig. S7, Left, arrowheads, and Table S3). Likewise, targeting the CD4 blastomeres reduced the expression of these markers in the pVBI (Fig. 4C and SI Appendix, Fig. S7, Middle, arrows). This shows that canonical Wnt signaling is required in the mesodermal compartment of the VBI, i.e., where the Wnt4 ligand is expressed. In addition, we found that injection of EnR RNA in A4 blastomeres also resulted in reduced expression of the hematopoietic markers. Note that most of the signal of the aVBI disappeared but only the anterior part of the pVBI was affected. Fate-mapping experiments have shown that the A4 blastomeres contribute to the epidermis that, during gastrulation, overlies the blood-forming mesoderm of the entire aVBI and part of pVBI (16). Combining the data on the Wnt-reporter with the results of the targeted injection of the inducible Wnt-interfering construct indicates that hematopoietic commitment requires Wnt/β-catenin signaling during gastrulation both in the mesoderm of the future VBI and in the overlying ectoderm. In addition, at later embryonic stages, Wnt/β-catenin signaling is also required for erythrocyte differentiation or survival.
Fig. 4.
Wnt/β-catenin signaling is required in both ectoderm and mesoderm for formation of VBI. (A) Global inhibition of Wnt/β-catenin signaling reduced expression of the hematopoietic marker gene SCL in VBI. Embryos were injected at the two-cell stage with 0.5 ng RNA of Wnt-inhibiting construct EnR and cultured in the absence or presence of Dex from stage 13 onward, and then assayed for SCL expression by WISH at stage 20 or 24. Ratio in lower right corner indicates proportion of embryos that had the phenotype shown in the picture. (B) Global inhibition of Wnt signaling just before differentiation of erythrocytes still reduces erythrocyte differentiation or survival. Embryos were injected as above and cultured in the absence or presence of Dex from stage 20 onward. Embryos were assayed by WISH for SCL and T3-globin (T3) at stages 25 and 32, respectively. (C) Embryos were injected at the 16-cell stage in CD1, CD4, or A4 blastomeres (as indicated on top) and with 100 pg RNA of EnR and 10 pg RNA of cytoplasmic β-gal as lineage tracer. Embryos were treated with Dex during gastrulation (stage 11.5). SCL was completely abrogated in Dex-treated embryos, regardless of injection site. CD4-injected embryos lost T3 expression completely at pVBI (arrows), whereas CD1-injected embryos show only partial loss of T3 gene expression in aVBI (arrowheads). Domain of T3 down-regulation in both cases was extended to neighboring compartment, i.e., beyond β-gal boundary. Inhibition of Wnt signaling in ventral ectoderm (A4 descendants) during gastrulation resulted in overall down-regulation of T3 expression.
We wondered whether endothelial development was also dependent on Wnt4-mediated signaling. R-spondin 3 (Rspo3), a secreted ligand that can also activate the Wnt/β-catenin pathway, was recently shown to be required for endothelial differentiation, actually at the expense of erythrocyte differentiation (17). Expression of Rspo3 and the endothelial marker Xfli1 was slightly reduced in Wnt4 morphant embryos (SI Appendix, Fig. S8). This indicates that Wnt4 signaling is also required for vascular development.
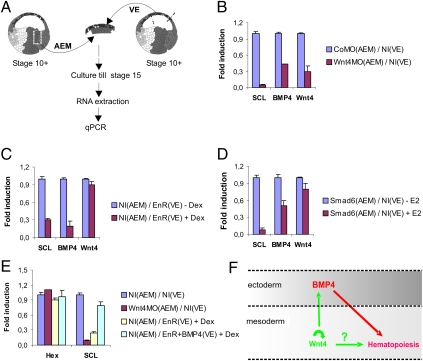
Examining the data on the Wnt-reporter together with the results of the functional studies using our inducible Wnt-interfering construct led us to think that Wnt/β-catenin signaling could play a role both in the mesoderm of the VBI and in the overlying ectoderm. Several reports have documented the involvement of BMP expression in the ectoderm for the induction of the VBI (18–20). Hence, we wondered whether, during the stages of hematopoietic commitment, crosstalk exists between the Wnt4 expressed in the mesoderm and the BMP expressed in the overlying ectoderm. We injected CD1 blastomeres, which contribute to the anterior endomesoderm (AEM), with Wnt4 or control (Co) MO. At the onset of gastrulation (stage 10+), we explanted the zone of AEM and recombined the tissue with noninjected (NI) VE, which later in development normally overlies the AEM during VBI formation (Fig. 5A). Next, we analyzed gene expression by quantitative PCR (qPCR) at stage 15 or by WISH at stage 32 (SI Appendix, Fig. S9). Compared with CoMO(AEM)/NI(VE) recombinants, expression of BMP4 was strongly diminished in Wnt4 MO(AEM)/NI(VE) recombinants, concomitant with a reduction of the hematopoietic markers SCL and T3-globin (Fig. 5B and SI Appendix, Fig. S9). This indicates that, during gastrulation, BMP4 expression in the ectoderm is dependent on Wnt4 secreted from the mesoderm. Strikingly, Wnt4 transcript levels were also significantly reduced in the explant recombinants, suggesting that Wnt4 maintains itself in an autocrine or paracrine loop.
Fig. 5.
Mesodermal Wnt4 regulates primitive hematopoiesis by modulating BMP4 in ectoderm during gastrulation. (A) Schematic representation of tissue recombinations with anterior endomesoderm (AEM) and ventral ectoderm (VE) used in the quantitative RT-PCR (qPCR) experiments. Embryos were injected at the two-cell stage and explant combinations were carried out at stage 10+ and qPCR assay at stage 15. (B) Mesodermal Wnt4 is required for BMP4 expression in the AEM explant. AEM of embryos injected with Wnt4 MO or Co MO were recombined with VE of embryos injected with Co MO and assayed for SCL, BMP4 and Wnt4 expression by qPCR. All three genes were down-regulated following Wnt4 depletion. (C) Blocking Wnt/β-catenin signaling in the VE down-regulates ectodermal BMP4. Embryos were injected with 0.5 ng RNA of EnR in animal area. VE was excised and combined with AEM of NI embryos, and the combined explants were cultured from stage 11 onward in the presence (+Dex) or absence (-Dex) of Dex. Both BMP4 and SCL were down-regulated upon inhibition of Wnt signaling in the ectoderm. (D) Wnt4 is not a target of BMP signaling. Embryos were injected in marginal zones at two-cell stage with 2 ng RNA of ER-Xsmad6 (Smad6). AEM of injected embryos were combined with VE of NI embryos, and combined explants were cultured with E2 (+E2) or without E2 (-E2) from stage 11. SCL and BMP4 were suppressed upon Smad6 activation, but Wnt4 expression was unaffected. (E) Resubstitution of BMP4 rescues expression of hematopoietic marker SCL in Wnt-repressed embryos. Embryos were injected with Wnt4 MO or EnR, and explant combinations were carried out as in B and C. For rescue experiment, EnR was coinjected with 3 ng BMP4 RNA in animal region of embryos at two-cell stage. Combined explants were cultured in the presence of Dex from stage 11 onward. Expression of SCL was rescued by ectopic BMP4 almost to level of control untreated explants. Hex expression was assayed as an internal control for the amount of endo-mesodermal tissue being explanted. All values in B–E are averages of triplicate reactions normalized to expression of housekeeping genes EF1α and ornithine decarboxylase (ODC). Error bars represent SD. (F) Model showing communication between mesodermal Wnt4 and ectodermal BMP4 during gastrulation leading to induction of hematopoietic program in VBI (detailed in Discussion). Indicated in red are interactions involved in induction of primitive blood that have already been documented in Xenopus. In green are previously uncharacterized factors and interactions that were identified in our study. Question mark refers to requirement for Wnt signaling in mesoderm (Fig. 4); we do not know whether this interaction is direct.
Because the formation of the VBI required Wnt/β-catenin signaling both in the mesoderm and in the ectoderm (Fig. 4C), we wondered whether inhibiting Wnt signaling in the ectoderm affects the expression of BMP4. We combined the VE of embryos injected with the Wnt-interfering construct EnR with the AEM of noninjected (NI) embryos, treated the combined explants with Dex at stage 11+, and harvested them for RNA extraction and qPCR at stage 15. Dex treatment strongly reduced BMP4 expression in the explants (Fig. 5C). Therefore, as Wnt signaling activity is required in the ectoderm proper, this implies that Wnt secreted from the mesoderm signals directly to the overlying ectoderm and does not act indirectly, e.g., via the secretion of another paracrine factor from the mesoderm. We wondered whether the BMP4 gene could be a direct target of the Wnt/β-catenin signaling pathway. So we injected embryos with a Dex-inducible Wnt-mimicking chimeric construct, LEFΔN-VP-16-GR, which we previously characterized (21). Embryos were treated with Dex to activate the construct and cultured for 2 h in the absence or presence of the translation-blocker, cycloheximide (CHX). Quantitative RT-PCR analysis of RNA extracted from these embryos showed that expression of BMP4 and Msx1, the latter of which is a direct Wnt-target gene, were both induced by the Wnt-mimicking construct. However, Msx1 was induced in the presence of CHX, but BMP4 was not (SI Appendix, Fig. S10). These data show that BMP4 is not a direct target gene of the Wnt/β-catenin pathway.
To delineate the window for VBI induction during which the Wnt4-expressing mesoderm needs to interact with the ectoderm it encounters during late gastrulation, we made combined explants of AEM and VE at stage 10.5 but separated the tissues at stage 11.5 or 12.5 (SI Appendix, Fig. S11). The brief coculture of AEM and VE (from stage 10.5 until stage 11.5) was sufficient to induce expression of BMP4 in the VE explants and SCL in the AEM. Neither gene was activated when expression of the Wnt4 MO was targeted to the AEM or when EnR RNA was expressed in the VE and activated at stage 10.5.
At the onset of zygotic transcription in Xenopus, Wnt/β-catenin signaling is known to inhibit the activity of the Spemann Organizer, which flanks the AEM. Hence, in our explant assays, loss of Wnt signaling in morpholino-injected AEM explants might have resulted in a stronger organizer activity leading to increased inhibition of BMP4 expression in the VE. Therefore, we analyzed the expression of organizer genes Goosecoid, Nodal-related 3, and Chordin in AEM injected with Co MO or Wnt4 MO. The expression of none of these three marker genes was increased upon the depletion of Wnt4 in the AEM (SI Appendix, Fig. S12), which makes it unlikely that the decrease of BMP4 expression in the Wnt4 knockdown is an indirect result of stronger organizer activity.
To analyze whether the expression of Wnt4 in the mesoderm is dependent on a BMP signal from the ectoderm, we made explant recombinations in which the mesoderm was injected with RNA encoding an inducible BMP-interfering ER-Xsmad6 construct. This construct is inactive in the absence of hormone, but its activation by estrogen enables it to interfere with BMP signaling (19). We found that SCL expression was reduced when BMP signaling was impaired in the mesoderm but that expression of Wnt4 was not affected (Fig. 5D). This indicates that the expression of Wnt4 in the mesoderm is not dependent on BMP signals from the ectoderm.
Together, our results show that, during hematopoietic commitment, Wnt4 ligands from the mesoderm signal directly to the overlying ectoderm to induce the expression of BMP4. In addition, Wnt/β-catenin signaling is also required in the mesoderm to maintain Wnt4 expression. Finally, Wnt4 expression in the mesoderm is not dependent on BMP signaling. One of the implications of our data is that the impairment of hematopoietic commitment observed upon Wnt interference could be rescued by ectopic expression of BMP4 in the ectoderm overlying the mesoderm of the VBI. Indeed we showed, in combined explant cultures, that the reduced expression of SCL observed upon ectodermal expression of the Wnt-interfering EnR construct is rescued when BMP4 is coexpressed in the ectoderm (Fig. 5E).
Discussion
We designed a Wnt-reporter construct based on multiple Lef1/TCF binding sites combined with a minimal promoter TATA box and destabilized GFP as the reporter. Insulator elements were incorporated to overcome mosaic expression due to variation and variegation as well as to enhance expression of the reporter gene. We showed that transgenic Xenopus tropicalis embryos that integrated the bin8Lef-dGFP construct reflect all known domains of Wnt/β-catenin signaling activity during early development of Xenopus (SI Appendix, Figs. S2 and S3 and Table S1). A detailed description and discussion of the different GFP patterns is given in SI Appendix, SI Text. The Wnt-reporter X. tropicalis embryos revealed active Wnt/β-catenin signaling in the VBI and in the primitive erythrocytes. We identified Wnt4 as the ligand that is expressed in the VBI of developing embryos and determined that it is required for its formation. Importantly, within the VBI, Wnt4 seems to act through canonical Wnt signaling because its knockdown suppresses the GFP signal in the VBI of Wnt/β-catenin reporter Xenopus embryos. Although we focused our study on the role of Wnt4 in the development of the VBI, we noticed that Wnt4 is also expressed in the dorsal lateral plate mesoderm (DLPM) and that Wnt4 depletion in the presumptive DLPM abolished the expression of SCL and LMO2. The DLPM is the common precursor of the pronephric duct and the dorsal lateral plate (DLP), which is the source of the first definitive blood cells that colonize the tadpole liver (2). Therefore, Wnt4 appears to be important not only for regulating primitive hematopoiesis but also for definitive hematopoiesis in Xenopus embryos.
We explored further the spatial and temporal requirements for Wnt/β-catenin signaling in regulating the process of VBI induction and its development. By using an inducible Wnt-interfering construct (15), we demonstrated that Wnt/β-catenin signals are required throughout the development of the VBI. We provide evidence that Wnt/β-catenin signaling is needed for the commitment and differentiation of hematopoietic cells after the specification of the mesoderm toward the hematopoietic cell fate. It has been shown that aVBI and pVBI originate from dorsal and ventral lateral mesoderm (14), respectively, and that during gastrulation the two compartments require BMP signaling in the ectoderm and in the mesoderm itself for their induction and maintenance (18–20). The expression of blood markers was down-regulated throughout the whole VBI when Wnt/β-catenin signaling was inhibited in the ectoderm at gastrulation, indicating that Wnt4 delivers signals from the mesoderm to the ectoderm during gastrulation to induce formation of the ventral blood island. However, Wnt/β-catenin signaling is also required in the mesoderm proper for the differentiation or maintenance of the hematopoietic population in the VBI. The latter data are in line with the continuous presence of a GFP signal in the VBI and in the mature circulating primitive blood cells in Wnt/β-catenin reporter embryos. In addition, we found that Wnt4 positively regulates its own expression and thereby forms a positive induction loop in the mesoderm. A model summarizing these results is presented in Fig. 5F.
Different lines of evidence have demonstrated the role of ectodermally expressed BMP in regulating hematopoeisis in Xenopus embryos (22). Using explant combinations, we here show that BMP4 expression in the ectoderm is dependent on Wnt4 expression in the mesoderm. Furthermore, inhibiting Wnt/β-catenin signaling in the ectoderm also resulted in BMP4 down-regulation, and the concomitant reduced expression of SCL could be rescued by ectopic ectodermal expression of BMP4. These data suggest that Wnt/β-catenin signaling regulates hematopoietic commitment by directly and non–cell-autonomously modulating BMP4 in the ectoderm (Fig. 5F). Our data agree with recent evidence obtained from murine ES cell cultures modeling primitive and definitive hematopoietic programs (7). In these studies, β-catenin, BMP, and Activin signaling synergized to regulate the formation of Flk1+ mesoderm, which has hematopoietic potential, and β-catenin signaling was required for specification of the primitive erythroid lineage (7). However, our data conflict with a similar report by Lengerke et al., who also found a role for both Wnt and BMP signaling in primitive blood formation but put Wnt signaling downstream of BMP signaling (8). In any case, our study provides conclusive direct evidence for the involvement of Wnt/β-catenin signaling in the induction and maintenance of the primitive hematopoietic program in vivo.
Although we here describe the activation of a Wnt/β-catenin reporter in the yolk sac mesoderm of E8.5 mouse embryos, there is currently no evidence to support a role for the Wnt4 ligand in the formation of the primitive blood in mammals. Although Wnt4 transcripts are expressed in the yolk sac (23), the Wnt4 knockout mouse does not manifest a hematopoietic phenotypic abnormality indicating the involvement of another Wnt ligand (24, 25). As for the interplay between Wnt and BMP signaling during the induction of hematopoiesis, both pathways might interact in the mouse as well. However, in contrast to Xenopus, there is no ectodermal layer overlying the yolk sac mesoderm, but BMP2 and BMP4 are expressed in the yolk sac mesoderm proper (26). The visceral endoderm overlies the mesoderm, but it neither expresses BMPs nor shows Wnt-reporter activity (our current results). Hence, whereas primitive blood formation in Xenopus requires paracrine Wnt- and BMP-based signaling between adjacent tissues, in the mouse this signaling might act within the yolk sac mesoderm proper.
Although we found a biphasic role for Wnt/β-catenin signaling during blood formation in Xenopus, i.e., an early role for hematopoietic commitment during gastrulation and a role in erythroid differentiation or maintenance after the formation of the hemangioblast, the situation in the mouse might be different. The Wnt reporter was induced continuously in Xenopus until the stage of circulating blood cells, but the window of reporter activation in the blood islands in the yolk sac of the BAT-GAL mouse was much narrower (between E7.5 and E8.5). A similar narrow window of Wnt activation was reported in early mouse embryos stained with an antibody recognizing activated β-catenin, and the use of ES cell differentiation protocols has also indicated that Wnt/β-catenin is required for hemangioblast formation but needs to be inhibited for erythroid differentiation (27). Hence, we believe that the role of Wnt/β-catenin signaling in the mouse might be restricted to hematopoietic commitment and hemangioblast formation. It was also reported recently that in Xenopus, R-spondin 3, which is an activator of Wnt/β-catenin signaling, is expressed in the VBI and induces VEGF; this situation favors endothelial differentiation while repressing erythroid differentiation (17). Thus far, we cannot reconcile these data with our results. One possible explanation is that Wnt acts as a morphogen in the VBI, and that specific levels of Wnt/β-catenin signaling activity are required for blood versus endothelial differentiation. Hence, Rspo3 could act synergistically with the Wnt4 protein to increase signaling to a level that might be required for endothelial differentiation in the VBI. However, this is pure speculation, and further studies are needed to clarify this apparent discrepancy.
In summary, our study provides unique evidence for the involvement of Wnt/β-catenin signaling in the induction and maintenance of the primitive hematopoietic program in Xenopus embryos. We identified Wnt4 as the ligand expressed in the mesodermal compartment and regulating these processes by modulating BMP4 expression in the overlying ectoderm in a non–cell-autonomous manner. In addition, we report that Wnt/β-catenin expression is required in the mesoderm to maintain Wnt4 expression in a positive induction loop.
Materials and Methods
RNA/Morpholino Injection and Transgenesis.
Eggs from X. laevis were collected, fertilized, and injected as described elsewhere (15). X. tropicalis embryos were collected from natural mating couples and dejellied in 3% cystein in 1× MMR buffer. Embryos were injected while submerged in 0.1× MMR/6% Ficol and cultured at 22 °C. Wnt4 MO (as described by Saulnier et al. (12), and control MO (Gene Tools) were injected at the doses indicated in the figure legends (Fig. 3). Capped RNA encoding pEnR (15), Wnt4-HA (12), and ER-Smad6 (19) was synthesized by using SP6 RNA polymerase. Transgenesis was performed as described by Sekkali et al. (9).
WISH.
Embryos were grown to the desired stages, fixed in 1× MEMFA (0.1 M Mops, pH 7.4, 2 mM EGTA, 1 mM MgSO4, and 3.7% formaldehyde) for 1 h at room temperature and processed for β-gal staining. The embryos were washed several times in 1× PBS and histochemically stained at 37 °C in 1× PBS containing 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 2 mM MgCl2, and 1 mg/mL X-gal. The embryos were then were washed in 1× PBS, refixed for 1 h in 1× MEMFA, dehydrated in 100% methanol, and either stored at –20 °C or processed immediately for WISH. XWnt4 (12), αT3-globin, SCL, and LMO2 (28) probes were generated, and WISH was performed as described.
Additional details on materials and methods are provided in SI Appendix, SI Text.
Supplementary Material
Acknowledgments
We thank Stefano Piccollo (Padua, Italy) for use of the BAT-Gal Wnt reporter mouse, and we are grateful to Jody Haigh and Steven Goossens (VIB, Ghent, Belgium) for providing data on this mouse (Fig. 1E) and for critical reading of the manuscript. We thank Andre Brändli (Munchen, Germany), Todd Evans (New York, NY), Paul Mead (Memphis, TN), Jan Christian (Portland, OR), Roger Patient (Oxford, UK), Hans Clevers (Utrecht, The Netherlands), Barry Gumbiner (Charlottesville, VA), Christof Niehrs (Heidelberg, Germany), and Randall Moon for sharing probes and plasmids. We are also indebted to Christophe De Beule for technical assistance and Amin Bredan for editing the manuscript. This work was supported by grants from the Research Foundation—Flanders (FWO) and the Belgian Federation against Cancer.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007725107/-/DCSupplemental.
References
- 1.Zon LI. Developmental biology of hematopoiesis. Blood. 1995;86:2876–2891. [PubMed] [Google Scholar]
- 2.Turpen JB. Induction and early development of the hematopoietic and immune systems in Xenopus. Dev Comp Immunol. 1998;22:265–278. doi: 10.1016/s0145-305x(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 3.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 4.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 5.Turpen JB, Kelley CM, Mead PE, Zon LI. Bipotential primitive-definitive hematopoietic progenitors in the vertebrate embryo. Immunity. 1997;7:325–334. doi: 10.1016/s1074-7613(00)80354-4. [DOI] [PubMed] [Google Scholar]
- 6.Dzierzak E, Speck NA. Of lineage and legacy: The development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nostro MC, Cheng X, Keller GM, Gadue P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lengerke C, et al. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell. 2008;2:72–82. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Sekkali B, et al. Chicken beta-globin insulator overcomes variegation of transgenes in Xenopus embryos. FASEB J. 2008;22:2534–2540. doi: 10.1096/fj.07-098111. [DOI] [PubMed] [Google Scholar]
- 10.Mead PE, Kelley CM, Hahn PS, Piedad O, Zon LI. SCL specifies hematopoietic mesoderm in Xenopus embryos. Development. 1998;125:2611–2620. doi: 10.1242/dev.125.14.2611. [DOI] [PubMed] [Google Scholar]
- 11.Maretto S, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saulnier DM, Ghanbari H, Brändli AW. Essential function of Wnt-4 for tubulogenesis in the Xenopus pronephric kidney. Dev Biol. 2002;248:13–28. doi: 10.1006/dbio.2002.0712. [DOI] [PubMed] [Google Scholar]
- 13.McGrew LL, Otte AP, Moon RT. Analysis of Xwnt-4 in embryos of Xenopus laevis: A Wnt family member expressed in the brain and floor plate. Development. 1992;115:463–473. doi: 10.1242/dev.115.2.463. [DOI] [PubMed] [Google Scholar]
- 14.Ciau-Uitz A, Walmsley M, Patient R. Distinct origins of adult and embryonic blood in Xenopus. Cell. 2000;102:787–796. doi: 10.1016/s0092-8674(00)00067-2. [DOI] [PubMed] [Google Scholar]
- 15.Deroo T, Denayer T, Van Roy F, Vleminckx K. Global inhibition of Lef1/Tcf-dependent Wnt signaling at its nuclear end point abrogates development in transgenic Xenopus embryos. J Biol Chem. 2004;279:50670–50675. doi: 10.1074/jbc.M408969200. [DOI] [PubMed] [Google Scholar]
- 16.Bauer DV, Huang S, Moody SA. The cleavage stage origin of Spemann's Organizer: Analysis of the movements of blastomere clones before and during gastrulation in Xenopus. Development. 1994;120:1179–1189. doi: 10.1242/dev.120.5.1179. [DOI] [PubMed] [Google Scholar]
- 17.Kazanskaya O, et al. The Wnt signaling regulator R-spondin 3 promotes angioblast and vascular development. Development. 2008;135:3655–3664. doi: 10.1242/dev.027284. [DOI] [PubMed] [Google Scholar]
- 18.Maéno M, et al. Regulation of primary erythropoiesis in the ventral mesoderm of Xenopus gastrula embryo: Evidence for the expression of a stimulatory factor(s) in animal pole tissue. Dev Biol. 1994;161:522–529. doi: 10.1006/dbio.1994.1050. [DOI] [PubMed] [Google Scholar]
- 19.Schmerer M, Evans T. Primitive erythropoiesis is regulated by Smad-dependent signaling in postgastrulation mesoderm. Blood. 2003;102:3196–3205. doi: 10.1182/blood-2003-04-1094. [DOI] [PubMed] [Google Scholar]
- 20.Walmsley M, Ciau-Uitz A, Patient R. Adult and embryonic blood and endothelium derive from distinct precursor populations which are differentially programmed by BMP in Xenopus. Development. 2002;129:5683–5695. doi: 10.1242/dev.00169. [DOI] [PubMed] [Google Scholar]
- 21.Denayer T, et al. Canonical Wnt signaling controls proliferation of retinal stem/progenitor cells in postembryonic Xenopus eyes. Stem Cells. 2008;26:2063–2074. doi: 10.1634/stemcells.2007-0900. [DOI] [PubMed] [Google Scholar]
- 22.Maéno M. Regulatory signals and tissue interactions in the early hematopoietic cell differentiation in Xenopus laevis embryo. Zoolog Sci. 2003;20:939–946. doi: 10.2108/zsj.20.939. [DOI] [PubMed] [Google Scholar]
- 23.Corrigan PM, Dobbin E, Freeburn RW, Wheadon H. Patterns of Wnt/Fzd/LRP gene expression during embryonic hematopoiesis. Stem Cells Dev. 2009;18:759–772. doi: 10.1089/scd.2008.0270. [DOI] [PubMed] [Google Scholar]
- 24.Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 25.Vainio S, Heikkilä M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- 26.Farrington SM, Belaoussoff M, Baron MH. Winged-helix, Hedgehog and Bmp genes are differentially expressed in distinct cell layers of the murine yolk sac. Mech Dev. 1997;62:197–211. doi: 10.1016/s0925-4773(97)00664-3. [DOI] [PubMed] [Google Scholar]
- 27.Cheng X, Huber TL, Chen VC, Gadue P, Keller GM. Numb mediates the interaction between Wnt and Notch to modulate primitive erythropoietic specification from the hemangioblast. Development. 2008;135:3447–3458. doi: 10.1242/dev.025916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mead PE, Deconinck AE, Huber TL, Orkin SH, Zon LI. Primitive erythropoiesis in the Xenopus embryo: The synergistic role of LMO-2, SCL and GATA-binding proteins. Development. 2001;128:2301–2308. doi: 10.1242/dev.128.12.2301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.