Abstract
Müllerian-inhibiting substance (MIS), which is produced by fetal Sertoli cells shortly after commitment of the bipotential gonads to testicular differentiation, causes Müllerian duct (MD) regression. In the fetal female gonads, MIS is not expressed and the MDs will differentiate into the internal female reproductive tract. We have investigated whether dysregulated β-catenin activity affects MD regression by expressing a constitutively activated nuclear form of β-catenin in the MD mesenchyme. We show that constitutively activated (CA) β-catenin causes focal retention of MD tissue in the epididymides and vasa deferentia. In adult mutant mice, the retained MD tissues express α-smooth muscle actin and desmin, which are markers for uterine differentiation. MD retention inhibited the folding complexity of the developing epididymides and usually led to obstructive azoospermia by spermatoceles. The MDs of urogenital ridges from mutant female embryos showed less regression with added MIS in organ culture compared with control MDs when analyzed by whole mount in situ hybridization for Wnt7a as a marker for the MD epithelium. CA β-catenin did not appear to affect expression of either MIS in the embryonic testes or its type II receptor (AMHR2) in the MD mesenchyme nor did it inhibit pSmad1/5/8 nuclear accumulation, suggesting that dysregulated β-catenin must inhibit MD regression independently of MIS signaling. These studies suggest that dysregulated Wnt/β-catenin signaling in the MD mesenchyme might also be a contributing factor in persistent Müllerian duct syndrome, a form of male pseudohermaphroditism, and development of spermatoceles.
Keywords: anti-Müllerian hormone, epididymis, Mullerian inhibiting substance type II receptor (MISRII or MISR2), spermatocele, epididymis
Shortly after commitment of the bipotential embryonic mammalian gonadal ridge to male development, Sertoli cells differentiate in the testes and produce Müllerian-inhibiting substance [MIS; also known as anti-Müllerian hormone (AMH)], a TGFβ family member that causes Müllerian duct (MD) regression in males. MIS is not produced by the embryonic female gonads; thus, the MDs differentiate into the oviduct, uterus, cervix, and upper portion of the vagina (1, 2). Persistent Müllerian duct syndrome (PMDS) is a form of human male pseudohermaphroditism that is the result of retained female reproductive tract tissue. Homozygous deletion or knockdown of either MIS (3); its type I receptors, BMPR1A (Alk3) (4) and ACVR1 (Alk2) (5, 6); or type II receptor, AMHR2 (also known as MISRII) (7), in mice leads to retention of the MDs, and mutations in either MIS or MIS receptors are thought to account for 85% of patients with PMDS (8).
Proper MD differentiation in females requires Wnt signaling. For example, the uteri of mice with a deletion of Wnt7a are deficient in myometrial tissue, endometrial glands, and oviductal coiling (9, 10), and mice lacking Wnt5a have shortened uterine horns and defective development of cervices and vaginas (11). Wnts bind to their respective cell surface frizzled or low density lipoprotein-related protein (LRP) receptors and signal through two intracellular pathways, a canonical pathway induced by β-catenin stabilization or a Wnt/Ca2+ pathway (12–14). The stability of free β-catenin in the cytoplasm is regulated by a destruction complex formed by axin, adenomatosis polyposis complex (APC), casein kinase 1, and glycogen synthase kinase 3-β (GSK3β), which binds and phosphorylates β-catenin, making it a target for ubiquitination and degradation. During canonical Wnt signaling, β-catenin escapes this complex and translocates to the nucleus to activate the transcription of various targeted genes in concert with members of the TCF/LEF family of transcription factors. Nuclear β-catenin expression in the MD mesenchyme has been suggested as a mediator of MIS-induced MD regression (15). We have previously shown that expression of a tissue-specific, constitutively activated (CA) form of β-catenin (16) driven by Cre in the Amhr2 locus (AMHR2-Cre) did not result in MD regression in female mice (17), suggesting that β-catenin is not sufficient for regression.
We have previously reported that expression of CA β-catenin restricted to the uterine stroma and myometrium leads to myometrial smooth muscle cell hyperplasia and development of mesenchymal tumors in murine uteri (17). We and others have also shown that CA β-catenin causes sterility in males by inhibiting postnatal Sertoli maturation and spermatogenesis (18–20). Given the requirement for Wnt signaling in MD development (21) and the relative paucity of reports linking Wnt signaling and Wolffian duct (WD) differentiation, we examined whether dysregulated β-catenin activity in the adjacent MD mesenchyme affected normal WD development into the epididymis, vas deferens, and seminal vesicles (22, 23). We show that CA β-catenin expression in the MD mesenchyme results in focal MD retention and adjacent WD obstruction, which leads to azoospermia and infertility secondary to seminiferous tubule degeneration and defective spermatogenesis that develops later in the adult (18, 19).
Results
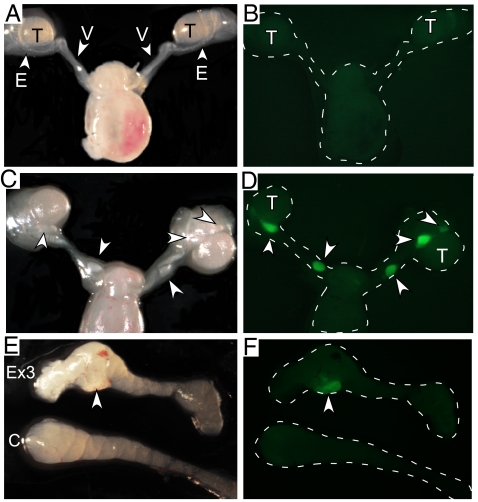
To investigate whether dysregulated CA β-catenin expression in the MD mesenchyme affects differentiation of the adjacent WD, we generated mutant mice that lack exon 3 of the β-catenin gene (Ctnnb1fl(ex3)), which would otherwise contain the serine/threonine residues normally phosphorylated by GSK3β before ubiquitination (16), by mating with mice that express Cre recombinase in the Amhr2 locus (4). Confirmation of nuclear β-catenin expression in the MD mesenchyme between the WD and MD of both male and female urogenital ridges of Amhr2-Cre;Ctnnb1Δ(ex3) mice is shown in Fig. 1. In the urogenital ridges of control Ctnnb1fl(ex3) mice, we observed membranous expression of β-catenin in the MD epithelium at embryonic day (E) 14.5 of both females (Fig. 1 A and C) and males (Fig. 1 E and G). In ridges from Amhr2-Cre;Ctnnb1Δ(ex3) mice, membranous β-catenin continued to be expressed in the MD epithelium and strong nuclear β-catenin expression was observed the MD mesenchyme (Fig. 1 B, D, F, and H). By P1, the MD has completely regressed in control male embryos (Fig. 2 A and B), but MD remnants are still observed in the epididymides and vasa deferentia of Amhr2-Cre;Ctnnb1Δ(ex3) males (Fig. 2 C and D), which persisted through adulthood (Fig. 2 E and F and Fig. S1).
Fig. 1.
Nuclear β-catenin expression in the MD mesenchyme. Membranous expression of β-catenin is observed by immunofluorescence (green) in E14.5 male and female urogenital ridges of control Amhr2-Cre/+ (A, C, E, G) in the epithelial cells but is largely nuclear in the MD mesenchyme of Amhr2-Cre/+;Ctnnb1Δ(ex3)/+ (B, D, F, H) embryos. DAPI staining is shown in blue to mark nuclei. Gonocytes cells are shown by mvh immunofluorescence (red). O, ovary; T, testis; MDM, Müllerian duct mesenchyme; MDE, Müllerian duct epithelium; WD, Wolffian duct. (Scale bars, 50 μm.)
Fig. 2.
Focal inhibition of Müllerian duct regression in mice with constitutive activated β-catenin in the MD mesenchyme. Gross analyses of male reproductive tracts of a control Amhr2-Cre/+;YFP mouse (A and B) show complete MD regression by P1 and that of an Amhr2-Cre/+;Ctnnb1Δ(ex3)/+;YFP mouse show bilateral focal retention (C and D, indicated with arrowheads). Müllerian duct tissue is visible along side the epididymides of PND20 mutant mice (E and F, indicated with arrowheads) but not in control epididymides. T, testis; E, epididymis; V, vasa deferentia; Ex3, Amhr2-Cre/+;Ctnnb1Δ(ex3)/+;YFP; C, control.
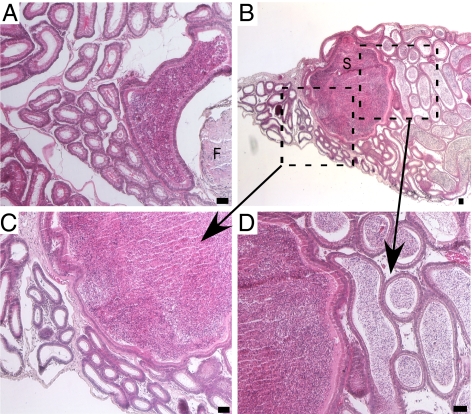
Whereas membranous β-catenin expression was observed in epididymal epithelia, predominantly nuclear β-catenin was observed in the cells surrounding the retained MD-derived tissue of Amhr2-Cre;Ctnnb1Δ(ex3) postnatal mice (Fig. 3 A and B). We also observed elevated expression of TCF1, LEF1, and cyclinD1, which have been shown to be up-regulated by Wnt/β-catenin signaling (24–28), in the P1 remnants of MD mesenchyme in the mutants compared with the normal surrounding tissue (Fig. S2), indicating that the nuclear β-catenin we observed was functionally active. The retained MD mesenchyme differentiates into an enlarged, simple tube-like structure resembling a uterus that expresses α-smooth muscle actin (αSMA) and desmin (Fig. 3 C and D), which are indicative of myometrium and endometrial stroma, respectively, but without endometrial glands and luminal folds, and is similarly observed in mice homozygously deleted for either MIS or one of its receptors (3, 4). Histological analyses showed that the remaining MD-derived tissue in Amhr2-Cre;Ctnnb1Δ(ex3) inhibited coiling and integrity of the ducts in the caput and corpus regions of the epididymides (Fig. 3 E–H). Analysis of cytokeratin expression also showed that the integrity of the pseudostratified columnar ductal epithelium in the cauda epididymides was compromised near the retained MD tissue in mutant mice (Fig. 3 I and J). Additionally, we observed fewer luminal epithelial folds in the vasa deferentia of adult Amhr2-Cre;Ctnnb1Δ(ex3) mice (Fig. 3 K and L). However, neither proliferation nor apoptosis in the mutant epididymal epithelia were remarkably different when compared with controls by immunofluorescence with a PCNA antibody and by TUNEL, respectively (Fig. S3), suggesting that the effects observed on epididymal morphology in the mutants might be due to mechanical forces. The retained MD tissue was often accompanied by fibrosis near the adjacent tubules, which had the deleterious effect of causing obstructive azoospermia by formation of enlarged epididymal cysts (Fig. 4) of impacted sperm that resemble spermatoceles in most of the mutant mice with what little spermatogenesis was occurring in their testes before seminiferous tubule degeneration begins in Amhr2-Cre;Ctnnb1Δ(ex3) mice when they are ∼4 wk old (18, 19).
Fig. 3.
Müllerian duct remnants in Amhr2-Cre/+;Ctnnb1Δ(ex3)/+ male mice adversely affect the development and function of the epididymides and vasa deferentia. Only membranous β-catenin immunofluorescence was observed in the control Ctnnb1Δ(ex3)/+ and mutant Amhr2-Cre/+;Ctnnb1Δ(ex3)/+ epididymal ductal epithelia of P1 mice (A and B). However, strong nuclear accumulation of the β-catenin was observed in the uterine mesenchyme of the mutant animals (B). Desmin and αSMA were limited to the stroma and periductal cells, respectively, of the control and mutant epididymides (C and D) and to the inner and outer areas of the retained MD tissue (D). DAPI staining is shown in blue to mark nuclei. Histology of the caput epididymus shows fewer ducts in the areas with retained MD tissue of mutant mice compared with the controls (E and F). The number and quality of the ducts (evaluated by histology) appeared normal in the corpus epididymus near the retained MD tissue (G and H). However, immunostaining for keratins showed that the integrity of the ductal epithelium was compromised in the mutants (I and J). In vasa deferentia, H&E staining showed that the presence of the vestigial uterine tube markedly diminishes the luminal folding in the neighboring vas deferens in the mutants compared with normal vas deferens in control animals (K and L). (Scale bars, 50 μm.) U, vestigial uterine remnant; E, epididymis; V, vas deferens.
Fig. 4.
Spermatocele formation in mutant mice. The epididymides of adult Amhr2-Cre/+;Ctnnb1Δ(ex3)/+ mice were analyzed by H&E. Obstructive azoospermia were observed upstream of retained fibrotic (F) MD tissue (A). (B) An epididymis from another mutant with a large spermatocele (S). The boxes show the areas enlarged in C and D, which show the absence of sperm in the duct downstream of the spermatocele and the presence of sperm in the duct upstream, respectively. (Scale bar = 50 μm.)
Differentiation of the WD into the epididymides and vasa deferentia requires testicular testosterone. Although we observed the retention of the MD tissue well before any testicular phenotype was observed in those studies, to rule out a contribution of the mutant testis to the retained MD phenotype, we analyzed the histology and expression of testicular markers in fetal testes for comparison with control mice (Fig. S4). The histology of the mutant testes appeared normal and expression nuclear of β-catenin was not readily observed at this age. Markers for Sertoli cells, germ cells, and Leydig cells also appeared normal in the mutants. Most importantly, expression of MIS, the hormone required for normal MD regression, appeared normal (Fig. S4 G and H). These results suggest that the retained MD phenotype is not due to dysregulated testicular development or function and that MD differentiates into postnatal uterine tissues independently of gonadal sex.
To ensure that the action of CA nuclear β-catenin in the MD mesenchyme is directly related to the inhibition of MIS signaling during MD regression, we performed organ culture assays with E13.5 female urogenital ridges from control and Amhr2-Cre;Ctnnb1Δ(ex3) mice. Addition of MIS to the control female urogenital ridges causes nearly complete MD regression as evidenced by the lack of expression of Wnt7a, a MD epithelial marker, in whole mount in situ hybridization (Fig. 5A). In contrast, we observed varying degrees of MD retention of Wnt7a expression in the mutant urogenital ridges (Fig. 5B), as predicted. Qualitative analyses showed that MD regression in organ culture was inhibited to varying degrees in the Amhr2-Cre;Ctnnb1Δ(ex3) urogenital ridges (Table 1). We next examined whether AMHR2 expression was inhibited in the MD mesenchyme of the mutant urogenital ridges by CA β-catenin in the focal areas of retention. No expression of AMHR2 mRNA was observed by whole mount in situ hybridization in control male reproductive tracts at E16.5 when MD regression is completed (Fig. 5C). However, as shown in Fig. 5D, AMHR2 is expressed in the mutant urogenital ridges, presumably in the areas of MD retention, suggesting that the phenotype is not due to CA β-catenin–mediated inhibition of AMHR2 expression. Lastly, to determine whether CA β-catenin was inhibiting canonical MIS signaling during MD regression, we analyzed the expression of phosphorylated Smad1/5/8 (pSmad1/5/8) in the MD mesenchyme of control and mutant E14.5 urogenital ridges. Nuclear pSMAD1/5/8 was observed in control and Amhr2-Cre;Ctnnb1Δ(ex3) MD mesenchyme (Fig. 5 E and F), indicating that MIS signaling is not perturbed by CA β-catenin. Additionally, ectopic Wnt7a expression was not detected in CA β-catenin mutant male urogenital ridges at E13.5, indicating that dysregulated β-catenin was not causing development of more than one MD epithelium that later fails to regress (Fig. S5A).
Fig. 5.
Müllerian duct regression in mutant urogenital ridges is inhibited in organ culture. Urogenital ridges from E13.5 control and mutant female fetuses were isolated, incubated for 48 h with 5 μg/mL MIS, and subjected to whole mount in situ hybridization with Wnt7a riboprobe detected by BM purple. (A) There is no Wnt7a mRNA expression in control female urogenital ridge with MIS, indicating complete regression of MD epithelium. (Inset) Wnt7a expression in a control female urogenital without added MIS (indicated with a white arrowhead). (B) Complete MD retention (indicated with a white arrowhead) with an Amhr2-Cre/+;Ctnnb1Δ(ex3)/+ female urogenital ridge similarly incubated with MIS. In situ hybridization for Amhr2 mRNA in control (C) and Amhr2-Cre/+;Ctnnb1Δ(ex3)/+ (D) E16.5 male reproductive tract shows expression in the retained MD tissue in the mutant (indicated with black arrowheads in the epididymis and a black arrow in the vas deferens). pSmad1/5/8 expression was detected by immunofluorescence in control (E) and mutant (F) E14.5 male urogenital ridges. DAPI staining in blue indicates nuclei. O, ovary; T, testis; E, epididymis; MDM, Müllerian duct mesenchyme; MDE, Müllerian duct epithelium; WD, Wolffian duct. (Scale bars, 50 μm.)
Table 1.
Müllerian duct regression in organ culture
| Genotype of embryos | No. | Complete regression (%) | Partial regression (%) | No regression (%) |
| Amhr2-Cre/+* | 5 | 0 (0) | 0 (0) | 5 (100) |
| Amhr2-Cre/+* +MIS | 7 | 3 (42.8) | 3 (42.8) | 1 (14) |
| Amhr2-Cre/+;Ctnnb1Δ(ex3)/+† +MIS | 8 | 0 (0) | 2 (25) | 6 (75) |
*Control.
†Mutant.
Discussion
We have shown that dysregulated Wnt/β-catenin signaling in the MD mesenchyme can inhibit MD regression in embryonic urogenital ridges both in vivo and in organ culture. These results suggest that there might be another mechanism for PMDS in humans that is independent of normal MIS and AMHR2 expression and function or, more likely, that dysregulated β-catenin activity is interfering with MIS-mediated MD regression. For example, in Wnt7a knockout mice, males have retained MDs due to suppressed AMHR2 expression by an unknown mechanism (10). Alternatively, loss of MIS expression has also been shown in mice with fetal Sertoli cell-specific expression of CA β-catenin (20). However, we have shown that AMHR2 (Fig. 5) and MIS (Fig. S4) are still expressed in the retained MD tissue of male embryos and in fetal male Sertoli cells, respectively, in the Amhr2-Cre CA β-catenin mutant mice. Similar to bone morphogenetic proteins, MIS signaling occurs through phosphorylation of cytoplasmic Smads 1, 5, and 8 that translocate to the nucleus as heterodimers with Smad4 to activate transcriptional target genes (29). A requirement for active Smad signaling has been linked to normal fate patterning during early development due to Wnt/β-catenin activity (30). Conversely, canonical Wnt signaling has also been shown to limit the duration of transcriptionally active pSmad1 by inducing its ubiquitination via GSK3β (31). We have shown that pSMAD1/5/8 levels do not appear to be inhibited in the MD mesenchyme, suggesting another mechanism responsible for MD retention in the CA β-catenin mice. These studies have highlighted the importance of interplay between Wnt/β-catenin and MIS signaling pathways and our future efforts will be directed at pinpointing the molecular mechanisms involved in their integration.
Müllerian duct regression is an exquisitely time-sensitive phenomenon because adding MIS to female rat urogenital ridges later than E15.5 (the equivalent of E14.5 in the mouse) will not result in complete regression (32–34). This led us to speculate that the focal MD retention in mutant mice could result from delayed expression of CA β-catenin. Amhr2-Cre is expressed from a knockin mutation of the Amhr2 locus and its expression occurs only when the endogenous AMHR2 is expressed (4, 35). However, Cre-mediated excision of exon 3 from the floxed allele of β-catenin is likely to occur later, perhaps after MD regression has already started. Expression from the AMHR2 locus begins at E13 (35), and our preliminary results show that the recombined Ctnnb1Δ(ex3) allele is only detected at E13.5 (Fig. S5B). Focal retention of the MD could occur if expression and accumulation of CA β-catenin is delayed until after E13.5 because removing MIS-producing testes from the organ culture assay after only 1 d of incubation still results in MD regression (33). Delayed expression of Amhr2-Cre could also explain the discrepancy between the previously reported requirement for nuclear β-catenin during MD regression (15) and normal differentiation of reproductive tract tissues in female mice with CA β-catenin expression in the MD mesenchyme (17). This leads us to speculate whether induced expression of CA β-catenin earlier than that which we achieved by Cre expression in the Amhr2 locus might result in greater MD retention.
Dysregulated Wnt/β-catenin signaling is commonly found in a variety of naturally occurring tumors, largely by mutations in APC or, less frequently, in β-catenin itself (36). In human patients, because surgical removal of the MD derivatives is often carried out when surgery in boys is also necessary for cryptorchid, nonpalpable testes and the vast majority of PMDS patients have mutations in MIS signaling components (8), later consequences of MD retention due to dysregulated Wnt/β-catenin signaling will be difficult to discern (37, 38). However, there is one reported case of uterine adenosarcoma (39) and another of clear cell adenocarcinoma (40) in human patients with PMDS. We did not observe evidence of neoplastic transformation in the retained MD tissue of these mice by 3 mo. Long term studies will be needed to determine whether these mice with retained MD remnants develop cancer.
Although sperm production in mice with homozygous deletion of either the MIS or Amhr2 genes is normal, most males are infertile because of blocked sperm transmission due to the retained MD-derived tissues (3, 7). Sperm transmission was similarly blocked in the epididymides of Amhr2-Cre;Ctnnb1Δ(ex3) mutants, all of which developed spermatoceles. Although thought to be present in 30% of men who are usually asymptomatic (41), these spermatoceles might account for about 4% of IVF patients with obstructive azoospermia (42). Spermatocele pathogenesis is not well understood; future studies of Wnt/β-catenin and MIS expression in surgical specimens from patients with spermatoceles might indicate previously unappreciated MD remnant involvement.
In conclusion, we have shown that activation of Wnt/β-catenin signaling partially inhibits MD regression and adversely affects WD morphogenesis; the molecular mechanisms of Wnt signaling and Wnt antagonists involvement in MIS-mediated MD regression or retention remain to be investigated.
Materials and Methods
Mouse Genetics and Husbandry.
The mice used in this study were housed under standard animal housing conditions and all protocols involving animal experimentation were approved by the Institutional Animal Care and Use Committee at Massachusetts General Hospital. All of the mice used in this study were maintained on C57BL/6;129/SvEv mixed genetic background. The parental mice alleles Ctnnb1tm1Mmt (16), Gt(ROSA)26Sortm1(EYFP)Cos (43), and Amhr2tm3(cre)Bhr (4) were used in the crosses and are hereafter called Ctnnb1fl(ex3) or Ctnnb1Δ(ex3), YFP, and Amhr2-Cre, respectively. Genotyping was performed on DNA from tail biopsies using standard PCR protocols. The age of embryos was determined by the day a vaginal plug was detected, which was considered as E0.5. PCR was performed on genomic DNA collected from the gonadal ridges of 11.5, 12.5-13, and 13.5 dpc mutant embryos to demonstrate the wild-type and recombined knock-in alleles of β-catenin using following primers (β-catGF2: GGTAGGTGAAGCTCAGCGCAGAGC; β-catAS5: ACGTGTGGCAAGTTCCGCGTCATCC). Gross analyses were photographed using a Nikon SMZ1500 microscope with an attached Spot digital camera (Diagnostic Instruments).
Histology Analysis, Immunofluorescence, Immunohistochemistry, and TUNEL Staining.
Tissues were fixed overnight at 4 °C in Bouins fluid for histological analysis and in 4% paraformaldehyde for all other analyses. Methods used have been previously described in detail (17) using the following primary and secondary antibodies: anti-β-catenin (1:250; BD Transduction Laboratories); αSMA (1:1,000; Sigma Chemical); desmin (1:250; Neomarkers); cytokeratin (1:50; Neomarkers); anti-mouse vasa homolog (mvh) (1:250; Abcam); anti-3βHSD, anti-MIS (1:100; Santa Cruz Biotechnology); anti-PCNA (ready to use; Zymed); anti-cyclinD1 (ready to use; Neomarkers); TCF-1, LEF-1, and pSmad1/5/8 (1:250; Cell Signaling Technology); AlexaFluor secondary antibodies (1:500; Invitrogen); and Biotinylated donkey anti-mouse or anti-rabbit antibody Fab (1:1,000; Jackson ImmunoResearch Laboratories). TUNEL staining was performed according to the instructions provided with the Cell Death Detection kit (Roche). Photomicrographs were taken with a Nikon TS2000 microscope equipped with a Spot digital camera (Diagnostic Instruments).
Embryonic Urogenital Ridge Organ Culture.
The method of culturing the embryonic gonadal ridge was previously described in detail (29). Briefly, E13.5 female urogenital ridges were collected from the appropriate genotypes and cultured in CMRL1066 medium (Invitrogen) supplemented with 10% female FBS. Cultures were carried out in the presence or absence of recombinant human MIS (44). At the end of the experiments, the urogenital ridges were fixed overnight in 4% paraformaldehyde at 4 °C and used to perform whole mount in situ hybridization as described elsewhere (29, 45). Briefly, Amhr2 (46) and Wnt7a (10) riboprobes were prepared using a MaxiScript kit from Ambion with digoxigenin and detected with antidigoxigenin F(ab)2 fragments and BM purple (all from Roche). Wnt7a staining was considered evidence of retained MD epithelium and scored as either partial or complete if the length of MD epithelium was equal to or greater than the length of the gonad.
Supplementary Material
Acknowledgments
We thank Dr. Richard Behringer (MD Anderson, Houston, TX) for supplying us with the Amhr2-Cre mice and Dr. Barry Hinton for his help with the analysis of the epididymides. These studies were supported in part by National Institutes of Health Grant HD057201 (to J.M.T.) and by Vincent Memorial research funds.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011606107/-/DCSupplemental.
References
- 1.Teixeira J, Maheswaran S, Donahoe PK. Müllerian inhibiting substance: An instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr Rev. 2001;22:657–674. doi: 10.1210/edrv.22.5.0445. [DOI] [PubMed] [Google Scholar]
- 2.Josso N, Picard JY, Rey R, di Clemente N. Testicular anti-Müllerian hormone: History, genetics, regulation and clinical applications. Pediatr Endocrinol Rev. 2006;3:347–358. [PubMed] [Google Scholar]
- 3.Behringer RR, Finegold MJ, Cate RL. Müllerian-inhibiting substance function during mammalian sexual development. Cell. 1994;79:415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 4.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat Genet. 2002;32:408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 5.Clarke TR, et al. Mullerian inhibiting substance signaling uses a BMP-like pathway mediated by ALK2 and induces Smad6 expression. Mol Endocrinol. 2001;15:946–959. doi: 10.1210/mend.15.6.0664. [DOI] [PubMed] [Google Scholar]
- 6.Visser JA, et al. The serine/threonine transmembrane receptor ALK2 mediates Müllerian inhibiting substance signaling. Mol Endocrinol. 2001;15:936–945. doi: 10.1210/mend.15.6.0645. [DOI] [PubMed] [Google Scholar]
- 7.Mishina Y, et al. Genetic analysis of the Müllerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 1996;10:2577–2587. doi: 10.1101/gad.10.20.2577. [DOI] [PubMed] [Google Scholar]
- 8.Josso N, Belville C, di Clemente N, Picard JY. AMH and AMH receptor defects in persistent Müllerian duct syndrome. Hum Reprod Update. 2005;11:351–356. doi: 10.1093/humupd/dmi014. [DOI] [PubMed] [Google Scholar]
- 9.Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development. 1998;125:3201–3211. doi: 10.1242/dev.125.16.3201. [DOI] [PubMed] [Google Scholar]
- 10.Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature. 1998;395:707–710. doi: 10.1038/27221. [DOI] [PubMed] [Google Scholar]
- 11.Mericskay M, Kitajewski J, Sassoon D. Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development. 2004;131:2061–2072. doi: 10.1242/dev.01090. [DOI] [PubMed] [Google Scholar]
- 12.Mikels AJ, Nusse R. Wnts as ligands: Processing, secretion and reception. Oncogene. 2006;25:7461–7468. doi: 10.1038/sj.onc.1210053. [DOI] [PubMed] [Google Scholar]
- 13.Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- 14.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 15.Allard S, et al. Molecular mechanisms of hormone-mediated Müllerian duct regression: Involvement of beta-catenin. Development. 2000;127:3349–3360. doi: 10.1242/dev.127.15.3349. [DOI] [PubMed] [Google Scholar]
- 16.Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanwar PS, et al. Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod. 2009;81:545–552. doi: 10.1095/biolreprod.108.075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanwar PS, et al. Constitutive WNT/beta-catenin signaling in murine Sertoli cells disrupts their differentiation and ability to support spermatogenesis. Biol Reprod. 2010;82:422–432. doi: 10.1095/biolreprod.109.079335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyer A, Hermo L, Paquet M, Robaire B, Boerboom D. Seminiferous tubule degeneration and infertility in mice with sustained activation of WNT/CTNNB1 signaling in sertoli cells. Biol Reprod. 2008;79:475–485. doi: 10.1095/biolreprod.108.068627. [DOI] [PubMed] [Google Scholar]
- 20.Chang H, et al. Wt1 negatively regulates beta-catenin signaling during testis development. Development. 2008;135:1875–1885. doi: 10.1242/dev.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet. 2003;4:969–980. doi: 10.1038/nrg1225. [DOI] [PubMed] [Google Scholar]
- 22.Joseph A, Yao H, Hinton BT. Development and morphogenesis of the Wolffian/epididymal duct, more twists and turns. Dev Biol. 2009;325:6–14. doi: 10.1016/j.ydbio.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Archambeault DR, Tomaszewski J, Joseph A, Hinton BT, Yao HH. Epithelial-mesenchymal crosstalk in Wolffian duct and fetal testis cord development. Genesis. 2009;47:40–48. doi: 10.1002/dvg.20453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shtutman M, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 26.Roose J, et al. Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science. 1999;285:1923–1926. doi: 10.1126/science.285.5435.1923. [DOI] [PubMed] [Google Scholar]
- 27.Hovanes K, et al. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28:53–57. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- 28.Filali M, Cheng N, Abbott D, Leontiev V, Engelhardt JF. Wnt-3A/beta-catenin signaling induces transcription from the LEF-1 promoter. J Biol Chem. 2002;277:33398–33410. doi: 10.1074/jbc.M107977200. [DOI] [PubMed] [Google Scholar]
- 29.Zhan Y, et al. Müllerian inhibiting substance regulates its receptor/SMAD signaling and causes mesenchymal transition of the coelomic epithelial cells early in Müllerian duct regression. Development. 2006;133:2359–2369. doi: 10.1242/dev.02383. [DOI] [PubMed] [Google Scholar]
- 30.Eivers E, Fuentealba LC, De Robertis EM. Integrating positional information at the level of Smad1/5/8. Curr Opin Genet Dev. 2008;18:304–310. doi: 10.1016/j.gde.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuentealba LC, et al. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Josso N, Picard JY, Tran D. The anti-Müllerian hormone. Birth Defects Orig Artic Ser. 1977;13:59–84. [PubMed] [Google Scholar]
- 33.Donahoe PK, Ito Y, Hendren WH., 3rd A graded organ culture assay for the detection of Mullerian inhibiting substance. J Surg Res. 1977;23:141–148. doi: 10.1016/0022-4804(77)90202-5. [DOI] [PubMed] [Google Scholar]
- 34.Picon R. Action of the fetal testis on the development in vitro of the Müllerian ducts in the rat. Arch Anat Microsc Morphol Exp. 1969;58:1–19. [PubMed] [Google Scholar]
- 35.Arango NA, et al. A mesenchymal perspective of Müllerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev. 2008;75:1154–1162. doi: 10.1002/mrd.20858. [DOI] [PubMed] [Google Scholar]
- 36.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Donahoe PK, Crawford JD, Hendren WH. Management of neonates and children with male pseudohermaphroditism. J Pediatr Surg. 1977;12:1045–1057. doi: 10.1016/0022-3468(77)90617-0. [DOI] [PubMed] [Google Scholar]
- 38.Donahoe PK. Neoseminal vesicle created from retained mullerian duct to preserve the vas in male infants. J Pediatr Surg. 1988;23:272–274. doi: 10.1016/s0022-3468(88)80739-5. [DOI] [PubMed] [Google Scholar]
- 39.Thiel DD, Erhard MJ. Uterine adenosarcoma in a boy with persistent müllerian duct syndrome: First reported case. J Pediatr Surg. 2005;40:e29–e31. doi: 10.1016/j.jpedsurg.2005.05.071. [DOI] [PubMed] [Google Scholar]
- 40.Shinmura Y, Yokoi T, Tsutsui Y. A case of clear cell adenocarcinoma of the müllerian duct in persistent müllerian duct syndrome: The first reported case. Am J Surg Pathol. 2002;26:1231–1234. doi: 10.1097/00000478-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Rubenstein RA, Dogra VS, Seftel AD, Resnick MI. Benign intrascrotal lesions. J Urol. 2004;171:1765–1772. doi: 10.1097/01.ju.0000123083.98845.88. [DOI] [PubMed] [Google Scholar]
- 42.Hirsh AV, Dean NL, Mohan PJ, Shaker AG, Bekir JS. Natural spermatoceles in irreversible obstructive azoospermia—reservoirs of viable spermatozoa for assisted conception. Hum Reprod. 1996;11:1919–1922. doi: 10.1093/oxfordjournals.humrep.a019517. [DOI] [PubMed] [Google Scholar]
- 43.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorenzo HK, et al. New approaches for high-yield purification of Müllerian inhibiting substance improve its bioactivity. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;766:89–98. doi: 10.1016/s0378-4347(01)00436-4. [DOI] [PubMed] [Google Scholar]
- 45.Arango NA, et al. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol. 2005;288:276–283. doi: 10.1016/j.ydbio.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 46.Teixeira J, et al. Developmental expression of a candidate müllerian inhibiting substance type II receptor. Endocrinology. 1996;137:160–165. doi: 10.1210/endo.137.1.8536608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.