Abstract
There is a long-standing controversy about the role of serotonin in sleep/wake control, with competing theories that it either promotes sleep or causes arousal. Here, we show that there is a marked increase in wakefulness when all serotonin neurons are genetically deleted in mice hemizygous for ePet1-Cre and homozygous for floxed Lmx1b (Lmx1bf/f/p). However, this only occurs at cool ambient temperatures and can be explained by a thermoregulatory defect that leads to an increase in motor activity to generate heat. Because some serotonin neurons are stimulated by CO2, and serotonin activates thalamocortical networks, we hypothesized that serotonin neurons cause arousal in response to hypercapnia. We found that Lmx1bf/f/p mice completely lacked any arousal response to inhalation of 10% CO2 (with 21% O2 in balance N2) but had normal arousal responses to hypoxia, sound, and air puff. We propose that serotonin neurons mediate the potentially life-saving arousal response to hypercapnia. Impairment of this response may contribute to sudden unexpected death in epilepsy, sudden infant death syndrome, and sleep apnea.
Keywords: chemoreception, hypercapnia, sleep, thermoregulation, Lmx1b
Serotonin [5-hydroxytryptamine (5-HT)] has long been implicated in the regulation of sleep and wakefulness. However, its specific role remains unclear and controversial (1). 5-HT is considered by some as a sleep-promoting agent, because both pharmacological depletion of 5-HT and chemical lesions of 5-HT neurons lead to insomnia in cats (1–3). However, there are others who consider 5-HT to be a wakefulness promoter (4). Consistent with this, the firing rate of 5-HT neurons is fastest during waking (W), is slower during nonrapid eye movement sleep (NREM) and nearly ceases during rapid eye movement sleep (REM) (5, 6). 5-HT neurons in the dorsal raphé nucleus project to thalamic, cortical, and other structures involved in sleep/wake transitions (7), and 5-HT can convert the firing patterns of thalamic reticular and thalamocortical neurons in slices from a bursting pattern seen in NREM to a tonic single-spiking pattern seen in W (8). 5-HT (along with norepinephrine, histamine, and acetylcholine) is considered by some to be part of the ascending arousal system (AAS), which regulates transitions from sleep to wakefulness (4). However, there is no direct evidence that 5-HT neurons are required for normal arousal, and there is some evidence that they promote NREM (1, 3). Given the complexity of the 5-HT system, it has been difficult to define the roles, either direct or indirect, of 5-HT in different aspects of sleep regulation.
In vitro studies have shown that a subset of 5-HT neurons in both the medullary and midbrain raphé increases firing rate in response to a rise in CO2 or decrease in pH (9). When studied in unanesthetized behaving mammals, similar results have been obtained in vivo by multiple groups (9). 5-HT neurons in both loci are also juxtaposed to large cerebral blood vessels, making them ideally situated to accurately monitor changes in arterial PCO2 (10, 11). Medullary 5-HT neurons project to and stimulate respiratory neurons (12, 13), and they have a large response to pH when they are isolated in vitro, with the firing rate increasing 3-fold on average when pH decreases from 7.4 to 7.2 (14). These and other observations have led to the proposal that medullary 5-HT neurons are central respiratory chemoreceptors that regulate respiratory motor output to maintain normal systemic CO2/pH (13, 15–17). There has been some controversy over this hypothesis, in part because it has been reported that some 5-HT neurons do not respond to hypercapnia in vivo (18). However, in contrast to the data described above for 5-HT neurons in the raphé nuclei in behaving unanesthetized animals, these nonresponsive 5-HT neurons were located in the ventrolateral medulla and were recorded under anesthesia, which has major effects on breathing. The bulk of the existing data is in favor of the hypothesis that 5-HT neurons are central chemoreceptors, as discussed in a recent review (9).
In contrast to medullary 5-HT neurons, the function subserved by chemosensitivity of midbrain 5-HT neurons is not readily apparent, given that they are not directly involved in control of breathing. Acute hypercapnia is a potent stimulus for arousal from sleep (19, 20) and can also activate limbic structures, leading to the experience of dyspnea (21) and panic (22). Functional MRI studies have shown that hypercapnia and hypoxia induced by brief apnea cause an increase in activity within the thalamus and cerebral cortex (23). There is recent evidence that acid sensing ion channels in the nucleus accumbens mediate a portion of the limbic response to hypercapnia (24), but the neurons and pathways responsible for the arousal response to hypercapnia have not been identified. Given the involvement of midbrain 5-HT neurons in sleep/wake regulation and their ability to sense pH changes, it has been proposed that midbrain 5-HT neurons initiate the arousal response to hypercapnia (11, 13, 25, 26).
We tested this hypothesis using genetically modified mice in which deletion of Lmx1b in Pet1-expressing cells leads to a selective and near-complete reduction in the number of 5-HT neurons in the brain (27). As neonates, these mice hemizygous for ePet1-Cre and homozygous for floxed Lmx1b (Lmx1bf/f;e-Pet1-Cre/+ or Lmx1bf/f/p) have severely impaired breathing and a delayed growth rate compared with mice homozygous for floxed Lmx1b but lacking a ePet1-Cre allele (Lmx1bf/f or WT) mice (28). Surprisingly, some survive to adulthood. Those that do have an impaired ventilatory response to hypercapnia and markedly impaired thermoregulatory control as adults but are normal in many other respects (27, 29). The defect in temperature regulation is manifest as a severe drop in temperature in response to cold challenge (4 °C) but is not as apparent if animals are maintained at temperatures within the usual thermoneutral range for rodents (29). Given that these mice provide a unique opportunity to examine the role of 5-HT neurons in sleep/wake regulation and arousal, we determined sleep architecture in WT and Lmx1bf/f/p mice throughout the 24-h day at room temperature (∼23 °C) within the normal rodent thermoneutral range (∼30 °C) and at an elevated ambient temperature (∼33 °C) after having been housed for 10 d at 23 or 30 °C. We then analyzed vigilance state changes in response to hypercapnic challenges in WT and Lmx1bf/f/p mice as well as in response to hypoxic, auditory, and tactile challenges. Our findings do not support a major role for 5-HT in baseline sleep/wake regulation; instead, they demonstrate that it is essential for the robust arousal that normally occurs in response to hypercapnia.
Results
Lmx1bf/f/p Mice Have Normal Baseline Sleep/Wake Architecture Unless Cold-Stressed.
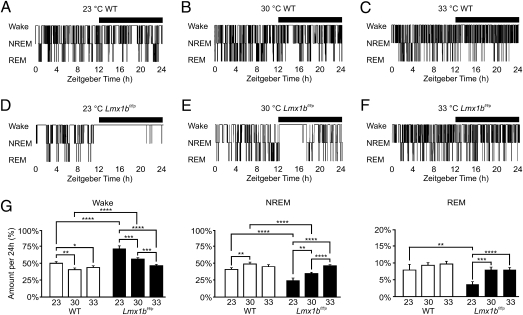
WT and Lmx1bf/f/p mice both cycled through W, NREM, and REM states throughout the 24-h day (Fig. 1 and Fig. S1). Both genotypes spent more time asleep during the light hours and more time awake during the dark hours (Fig. S2). At 23 °C (mean range: 22.8–23.4 °C), Lmx1bf/f/p mice spent a much greater percentage of time awake and less time in NREM and REM than WT mice (Fig. 1). To determine whether the difference in sleep architecture in Lmx1bf/f/p mice was simply a residual effect of sleep deprivation from being chronically housed at low ambient temperature, experiments were repeated at 23 °C after housing animals at 30 °C for at least 10 d. This had no effect on the percentage of time that mice of either genotype spent in W, NREM, or REM at 23 °C (Fig. S3).
Fig. 1.
Lmx1bf/f/p mice have decreased sleep at cool ambient temperatures. Representative 24-h sleep/wake histograms in male WT (Upper) and Lmx1bf/f/p (Lower) mice at 23 °C (A and D), 30 °C (B and E), and 33 °C (C and F) are shown. The horizontal black bars in A–F indicate lights-off. (G) Bar graphs depict the percentage of the 24-h recording period spent in each vigilance state for each genotype at 23 °C (n = 8 WT, n = 8 Lmx1bf/f/p), 30 °C (n = 9 WT, n = 8 Lmx1bf/f/p), and 33 °C (n = 10 WT, n = 10 Lmx1bf/f/p), as indicated. Four WT and four Lmx1bf/f/p mice were housed at 30 °C for 10 d before being studied at each of the three recording temperatures. The remaining mice were housed at 23 °C. These data are pooled because there was no significant effect of housing temperature on sleep architecture at any given recording temperature (Fig. S3). *P < 0.05; **P < 0.005; ***P < 0.001; ****P < 0.0001.
As previously demonstrated, Lmx1bf/f/p mice displayed normal light-entrained circadian rhythms of body temperature and locomotor activity in a 12-h light/12-h dark cycle (29). However, at an ambient temperature of 23 °C, body temperature dropped very low (35 °C) during periods of sleep and inactivity and increased to normal (37–38 °C) during periods of wakefulness and motor activity. One animal remained awake and active for over 9 h straight (Fig. 1), driving its body temperature up to ∼39 °C.
Lmx1bf/f/p mice can sense a decrease in temperature and activate normal heat conservation mechanisms but are unable to generate heat from shivering or brown fat metabolism in response to a cold stress (29). We hypothesized that room temperature was perceived as cold to Lmx1bf/f/p mice and that the animals increased their motor activity to generate heat. We tested this hypothesis by performing 24-h sleep/wake recordings at 30 °C (mean range: 29.6–30.7 °C), a temperature within the thermoneutral range for normal rodents (30). Lmx1bf/f/p mice spent much less time awake than they did at 23 °C but still slightly more time awake than WT mice at 30 °C and less time in NREM (Fig. 1). The differences were greater during the dark hours compared with the light hours (Fig. S2) despite a constantly maintained ambient temperature. Lmx1bf/f/p and WT mice spent similar amounts of time in REM in total, in dark, and in light. Lmx1bf/f/p mice displayed fewer vigilance state transitions at both 23 and 30 °C, regardless of previous housing temperature, with a reduced number of individual vigilance state bouts and longer duration wakeful bouts, especially during the early portion of the dark period (Fig. S4). As an extreme example, one Lmx1bf/f/p animal had a continuous wakefulness bout of over 9 h at 23 °C and another had a bout of over 6.5 h at 30 °C. WT mice spent slightly more time awake and less time in NREM at 23 °C compared with 30 °C with no difference in the percentage of time spent in REM, but this difference was much less profound than that in Lmx1bf/f/p mice (Fig. 1). Compared with their WT counterparts, female Lmx1bf/f/p mice (n = 4 per genotype) housed at 23 °C and recorded at 30 °C spent slightly more time awake and less time in NREM, with a similar amount of time spent in REM (Table S1). Similarly, compared with male Lmx1bf/f/p mice, female Lmx1bf/f/p mice spent slightly more time awake and less time in NREM, again spending a similar amount of time in REM (Table S1). Sex differences in baseline sleep were not studied further because the remaining the 24-h recordings were conducted in male mice.
We postulated that Lmx1bf/f/p mice were still cold at 30 °C, even though this is at the lower end of the thermoneutral range for normal mice (30). To test this hypothesis, we repeated 24-h data collections at ∼33 °C (mean range: 33.3–33.8 °C). In contrast to the differences in the percentage of time spent in W and NREM between WT and Lmx1bf/f/p mice at 23 and 30 °C, there was no significant difference in the percentage of time spent in W and NREM at 33 °C (Fig. 1 and Fig. S2). Again, there was no significant difference in the percentage of time spent in REM for Lmx1bf/f/p mice compared with WT mice. At 33 °C compared with 23 or 30 °C, Lmx1bf/f/p mice spent significantly less time in W and more time in NREM but a similar amount of time in REM. There was no significant difference in the amount of time WT mice spent in any given vigilance state at 33 °C compared with 23 or 30 °C. Furthermore, at 33 °C, Lmx1bf/f/p mice did not display any long-duration activity bouts at the onset of dark, as seen at 23 and 30 °C (Fig. 1 and Fig. S4). As at 23 °C, the temperature at which mice were housed before the experiment had no effect on the percentage of time that WT or Lmx1bf/f/p mice spent in W, NREM, or REM at 30 or 33 °C (Fig. S3).
Lmx1bf/f/p Mice Do Not Arouse in Response to Hypercapnia.
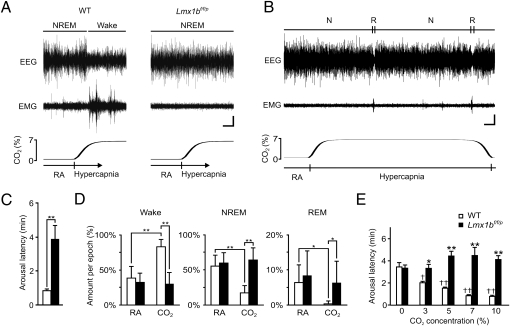
We then tested the hypothesis that 5-HT neurons are required for arousal to hypercapnia. Animals were placed into a chamber, and the gas composition was altered from 21% O2/0% CO2 [room air (RA)] to 21% O2/7% CO2 (both with balance N2). This was repeated four times for each animal for 10 min per trial, with each trial initiated shortly after mice fell asleep, as determined by real-time observation of behavior and EEG data. WT and Lmx1bf/f/p mice were asleep for similar lengths of time before initiation of each hypercapnic challenge (1.16 ± 1.02 min for WT vs. 1.22 ± 1.43 min for Lmx1bf/f/p). Robust arousal was reliably induced by hypercapnic challenge in all WT mice, as evidenced by a decrease in EEG amplitude, increase in EEG frequency, increase in electromyographic (EMG) power (Fig. 2A), and opening of the eyes (Fig. S5). In contrast, Lmx1bf/f/p mice did not demonstrate this robust arousal response (Fig. 2A). In fact, several Lmx1bf/f/p mice slept through an entire hypercapnic challenge (Fig. 2B), whereas almost all WT mice remained awake throughout every CO2 challenge. The latency to arousal in WT mice was 0.84 ± 0.15 min (n = 9) (Fig. 2C) resulting in a significant reduction in the average NREM bout length from 2.95 ± 1.32 min to 2.02 ± 0.36 min (P = 0.008). On average, Lmx1bf/f/p mice did arouse after some latency (3.61 ± 1.93 min, n = 10) from the onset of CO2 challenge (Fig. 2C). However, the probability of arousal was no different from that during the RA acclimation period, as evidenced by similar NREM bout lengths in the absence or presence of CO2 (3.40 ± 1.05 min in RA vs. 4.97 ± 3.36 min in CO2; P = 0.21), indicating that arousal occurred as random chance rather than being caused by CO2. During the hypercapnic challenge, WT mice spent the majority of the time awake (80.3 ± 12.6% in CO2 vs. 45.7 ± 13.6% in RA; P < 0.0001), much less time in NREM (18.8 ± 12.4% vs. 48.9 ± 13.1%; P < 0.0001), and essentially no time in REM (0.4 ± 1.0% vs. 5.2 ± 4.9%; P = 0.0014) compared with RA (Fig. 2D). Remarkably, there was no difference in the amount of time Lmx1bf/f/p mice spent in each vigilance state in CO2 vs. RA (W: 33.5 ± 13.3% in CO2 vs. 33.9 ± 13.4% in RA, P = 0.38; NREM: 60.1 ± 16.8% vs. 57.5 ± 17.4%, P = 0.29; REM: 6.0 ± 4.8% vs. 8.6 ± 4.8%, P = 0.24) (Fig. 2D). There was no effect when the gas was changed from one RA tank to another (0% CO2 in Fig. 2E). There was no sex difference in the CO2 response in either genotype (Fig. S6).
Fig. 2.
Genetic deletion of 5-HT neurons prevents arousal to CO2. (A) Four-minute EEG (Top), EMG (Middle), and PCO2 (Bottom) traces from WT and Lmx1bf/f/p mice showing response to 7% CO2. Arousal in the WT mouse is indicated by a decrease in EEG amplitude (and a corresponding increase in EEG frequency), with a concomitant increase in EMG amplitude. The O2 level is 21% (balance N2) throughout the traces. (Horizontal scale bar, 30 s; vertical scale bar, 5 μV.) (B) Thirteen-minute EEG (Top), EMG (Middle), and PCO2 (Bottom) traces from an Lmx1bf/f/p mouse that remained asleep throughout 7% CO2 exposure. N, NREM; R, REM. (Horizontal scale bar, 60 s; vertical scale bar, 5 μV.) (C) Latency to arousal following gas change from RA to 7% CO2 in WT (n = 9) and Lmx1bf/f/p (n = 10) mice. **P < 0.0001. Arousal latency includes the delay from the gas change to gas arrival in the chamber. (D) Percentage of time spent in Wake, NREM, and REM during RA and 7% CO2 exposure for WT (n = 9) and Lmx1bf/f/p (n = 10) mice. *P < 0.01; **P < 0.0001. There was no significant difference between male and female mice (Fig. S6); thus, data have been pooled. (E) Hypercapnia induced arousal in a dose-dependent manner in WT mice, but Lmx1bf/f/p mice were indifferent to every PCO2 level (n = 6 of each genotype). Shown is arousal latency following RA (0% CO2) and 3, 5, 7, and 10% CO2. Arousal latency determined as in C. *P < 0.005; **P < 0.0001 between genotypes. †P < 0.005; ††P < 0.0001 among WT mice compared with RA (0% CO2). There was no significant difference among Lmx1bf/f/p mice at any CO2 concentration compared with RA.
To quantify the difference in sensitivity of the hypercapnia-induced arousal response better, male WT and Lmx1bf/f/p mice were subjected to graded hypercapnic challenges (10 min of 0, 3, 5, 7, or 10% CO2 in 21% O2/balance N2, alternating with at least 20 min RA) and arousal latency was assessed. WT mice displayed a dose-dependent decrease in arousal latency with increasing concentrations of inspired CO2, waking up to as little as 3% CO2 (Fig. 2E). In contrast, there was no significant difference in arousal latency following challenge of Lmx1bf/f/p mice with any concentration of inspired CO2, even as high as 10% (Fig. 2E).
There Was Not a Generalized Defect in Arousal in Lmx1bf/f/p Mice.
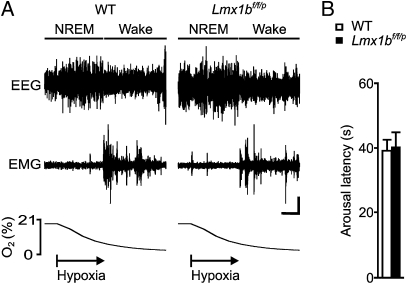
Robust arousal was induced equally well in both genotypes when the O2 concentration in the recording chamber was reduced to a level of ∼8–9% (Fig. 3A). The latency to arousal in WT and Lmx1bf/f/p mice during the hypoxia protocol was nearly identical (Fig. 3B). There was no significant sex difference.
Fig. 3.
Genetic deletion of 5-HT neurons does not affect the arousal response to hypoxia. (A) Ninety-second EEG (Top), EMG (Middle), and PO2 (Bottom) traces from WT and Lmx1bf/f/p mice indicating arousal response to hypoxia (∼8% O2). Arousal is indicated as in Fig. 2. (Horizontal scale bar, 15 s; vertical scale bar, 5 μV.) (B) Latency to arousal following gas change from RA to ∼8% O2 [P = 0.36; n = 9 WT (5 female, 4 male) mice, n = 8 Lmx1bf/f/p (4 female, 4 male) mice]. There was no difference between male and female mice; thus, data were pooled.
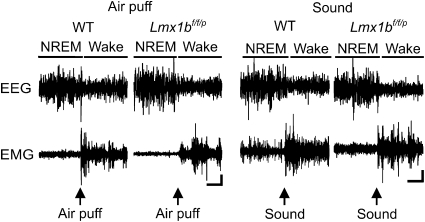
WT and Lmx1bf/f/p mice also aroused equally well to auditory (45–90 dB, 500 ms) and tactile (air puff applied to the rump, 100 ms) stimuli (Fig. 4). The threshold for arousal from the auditory stimulus was 59.44 ± 2.36 dB for WT and 60.83 ± 2.57 dB for Lmx1bf/f/p mice, which was not significantly different (P = 0.06; n = 8 each). Arousal to tactile stimulation occurred at an approximately equal pressure (8.0 ± 1.3 psi for WT, 8.33 ± 0.83 psi for Lmx1bf/f/p; P = 0.30; n = 8 each), with stimulus duration, tubing diameter, and distance from the animal kept constant. Directing the air puff away from the body did not induce arousal, indicating that arousal to air puff was specific to the tactile component and not the auditory component of the stimulus. All animals demonstrated immediate (<1 s) arousal to both stimuli once threshold was reached.
Fig. 4.
Genetic deletion of 5-HT neurons does not affect the arousal response to air puff or sound. Thirty-second EEG (Upper) and EMG (Lower) traces from WT and Lmx1bf/f/p mice indicate arousal response to air puff (Left) and sound (Right) stimuli. Arrows indicate stimulus presentation. Arousal is indicated as in Fig. 2. (Horizontal scale bar, 5 s; vertical scale bar, 5 μV.)
Discussion
The role of serotonin in control of sleep/wake states is controversial. Some investigators believe that 5-HT promotes sleep (1, 3), whereas others believe the opposite—that 5-HT neurons are a component of the AAS along with neurons that produce norepinephrine, histamine, acetylcholine, and possibly dopamine (31). The data presented here support the latter conclusion and provide a clear explanation for the confusing and contradictory findings reported in classic studies in which insomnia occurred after disruption of the 5-HT system (2, 32).
Lmx1bf/f/p mice have a severe defect in thermoregulation (29). They are able to sense ambient temperature normally and initiate heat conservation mechanisms, but they have an impaired ability to generate heat by shivering or brown fat metabolism (29). As in humans who do not sleep well in the cold (33), Lmx1bf/f/p mice may spend more time awake when the ambient temperature is low because they are uncomfortable. In response to cold, animals have been shown to move to warmer areas, to perform a learned task to receive a “thermal reinforcement” (34), and to increase motor activity to generate heat (35). Our data demonstrate that when Lmx1bf/f/p mice fall asleep at 23 °C, their body temperature drops precipitously. They then wake up and increase their locomotor activity, and this is followed by a return to baseline body temperature. This pattern was improved at 30 °C, which is within the thermoneutral temperature range for normal mice (30), but it was necessary to raise the temperature to 33 °C to eliminate the differences in sleep time between WT and Lmx1bf/f/p mice completely. There is no indication that a defect in thermoregulation was controlled for in classic studies on sleep after 5-HT lesions (2, 32); yet, such a defect was probably present. Thus, it is likely that the insomnia observed in those studies was attributable to animals being cold.
It has previously been shown that 5-HT neurons are required for a normal ventilatory response to hypercapnia (9, 13, 29, 30). The results presented here indicate that 5-HT neurons are also required for a normal arousal response to hypercapnia. These data are not consistent with Lmx1bf/f/p mice simply having global cortical depression, because baseline sleep was normal and there was not a defect in arousal to the other stimuli tested (hypoxia, sound, and air puff). Instead, our data support the hypothesis that a subset of 5-HT neurons plays the specific role of inducing arousal in response to hypercapnia. Hypercapnia directly activates midbrain 5-HT neurons (11, 36). 5-HT release within the thalamus leads to a change in thalamocortical rhythms from a bursting pattern to a tonic pattern (8, 37). This coincides with a change from high-voltage low-frequency synchronized activity in the cortical electroencephalogram consistent with slow wave sleep to low-voltage high-frequency desynchronized activity consistent with W (8). REM is also suppressed by 5-HT (38), possibly via projections to “REM On” neurons in the pons (39). Thus, 5-HT neurons may mediate the arousal response to hypercapnia via their projections to the thalamus, cortex, and pons as well as to other sleep/wake regulatory sites such as hypothalamic and other brainstem nuclei (4). Our data do not prove that 5-HT neurons are the sensors of pH themselves, but that conclusion is consistent with other data in the literature. However, if there are other arousal chemoreceptors (40, 41), 5-HT neurons may be needed to allow them to cause arousal in response to hypercapnia.
A rise in blood CO2 can be life threatening because it causes acidosis, and also because it is often accompanied by a decrease in O2. Changes in respiratory motor output are critical for CO2 control. However, when an individual is asleep with the face covered by a blanket or pillow, the hypercapnic arousal response can be just as important as the hypercapnic ventilatory response, because waking up is necessary to relieve the airflow obstruction. A role for midbrain 5-HT neurons as “arousal chemoreceptors” would then ultimately serve the same purpose as medullary 5-HT neurons that are respiratory chemoreceptors (13, 15–17), in that both would effect changes to prevent severe hypercapnia and hypoxia.
Hypercapnia is a significant factor in a number of diseases with high morbidity and mortality. Among these, serotonergic mechanisms have been proposed to play a role in sudden unexplained death in epilepsy (SUDEP), sudden infant death syndrome (SIDS), and sleep apnea. SUDEP is the leading cause of death in people with refractory epilepsy (42). Among the proposed etiologies for SUDEP is respiratory dysfunction (42, 43), and this may be attributable to 5-HT system abnormalities (44, 45). Most patients with SUDEP are found prone in bed (42), which could occur if depressed breathing and defective arousal to hypercapnia both contributed to death. SIDS is the leading cause of death in children between the ages of 1 mo and 1 y (46). Infants with SIDS are also often found prone (46), and it has been proposed that a defect in the response to CO2 and an impaired ability to arouse from sleep both contribute to the death of these infants (46, 47). Multiple abnormalities in the brainstem 5-HT system have been identified in human infants with SIDS (46, 47). In obstructive sleep apnea, a reduction of 5-HT tone occurs during sleep and has been proposed to contribute to the upper airway collapse that results in apnea (48), although the relative contribution of an effect on motor neurons has been debated (49). Apnea episodes are often associated with brief arousals, which result, in part, from CO2 elevation, and these arousals are likely very important for the resumption of breathing.
Here, we show that (i) decreased serotonergic output can lead to insomnia but that this is indirectly attributable to thermoregulatory dysfunction; (ii) there are no clear differences in baseline sleep in the complete absence of 5-HT neurons when thermoregulation is controlled for; and (iii) 5-HT neurons are essential for waking up in response to hypercapnia, consistent with the hypothesis that they are “arousal chemoreceptors.” These results can explain the link between 5-HT system abnormalities identified in SIDS and SUDEP and the prone position at the time of death. Defining serotonergic mechanisms involved in the response to hypercapnia may lead to improved methods to identify patients at highest risk for SUDEP and SIDS and ways to prevent their death.
Materials and Methods
Experimental Animals.
All procedures and protocols were approved by the Institutional Animal Care and Use Committee at Yale University. Generation (27), breeding, and genotyping (28) of Lmx1bf/f/p mice have been previously described. Mice (adult male and female weighing 24–42 g) were bred and housed using approved procedures (29). Our breeding strategy (29) led to litters with 50% Lmx1bf/f (WT) mice and 50% Lmx1bf/f/p mice. Age-matched littermates were used for all experiments. Detailed information is provided in SI Materials and Methods.
Sleep/Wake Analyses.
Mice were implanted with EEG/EMG electrodes and temperature/activity telemeters using methods similar to those previously described (29). EEG, EMG, body temperature, locomotor activity, and gas concentration data were collected and analyzed as previously described (29). Vigilance state was assigned using a standard approach (50) based on the EEG/EMG frequency characteristics (SI Materials and Methods). Twenty-four-hour sleep/wake recordings were conducted at 23, 30, or 33 °C after housing animals at 23 or 30 °C for at least 10 d. Temperatures within housing and recording chambers were maintained with a thermoelectric solid-state heating/cooling air conditioner (AHP-150FFHC; TECA Corp.). Ambient temperatures were measured and recorded continuously with a temperature probe inserted within the recording chamber (BAT-12 microprobe; Physitemp Instruments, Inc.). Detailed information is provided in SI Materials and Methods.
Stimulus-Induced Arousal Studies.
For initial assessments of arousal response to hypercapnia and hypoxia, animals were subjected to four challenges (10 min at 7% CO2 or 2 min at ∼8% O2) alternating with 20 min of RA (21% O2/balance N2) or longer if needed for animals to fall back to sleep. Similarly, the dose effect of CO2 was assessed by challenging mice with 0, 3, 5, 7, or 10% CO2 (with 21% O2 in balance N2). The thresholds for auditory and air puff challenges were determined using four brief stimuli of equal strength separated by at least 10 min, and this protocol was repeated at increasing stimulus intensities. All challenge experiments were conducted at ∼30 °C (mean range: 29.6–30.7 °C). Temperatures within the recording chamber were maintained with an external heat source. For gas challenge experiments, latencies were determined from the onset of gas delivery to the chamber (i.e., the time gas was turned on minus the dead space time constant of 8 s). In all cases, gas concentrations in ambient air are expressed as volume per volume (vol/vol). For example, at sea level with a total pressure of 760 mm Hg, 10% CO2 is equivalent to a partial pressure for CO2 of 76 mm Hg. Detailed information is provided in SI Materials and Methods.
Statistics.
Interactions of genotype, sex, gas composition, recording temperature, housing temperature, and vigilance state were analyzed for all physiological variables using two-way ANOVA, a paired t test, or a two-tailed t test assuming unequal variance as appropriate. The significance threshold was P < 0.05 for all conditions. Analyses were accomplished using Microsoft Excel and OriginPro 8.0 (OriginLab Corp.).
Supplementary Material
Acknowledgments
We thank John Sayward for developing custom programs for data acquisition and analysis and Drs. Matthew Hodges and Christopher Ransom for helpful discussions about the data and manuscript. This study was supported by the National Institute of Child Health and Human Development, the Veterans Affairs Medical Center, the Bumpus Foundation, and Jazz Pharmaceuticals.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004587107/-/DCSupplemental.
References
- 1.Jouvet M. Sleep and serotonin: An unfinished story. Neuropsychopharmacology. 1999;21(Suppl):24S–27S. doi: 10.1016/S0893-133X(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 2.Jouvet M. Biogenic amines and the states of sleep. Science. 1969;163:32–41. doi: 10.1126/science.163.3862.32. [DOI] [PubMed] [Google Scholar]
- 3.Jones BE. In: Principles and Practice of Sleep Medicine. Kryger MH, Roth T, Dement WC, editors. Philadelphia: Elsevier Saunders; 2005. pp. 136–153. [Google Scholar]
- 4.Saper CB, Chou TC, Scammell TE. The sleep switch: Hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 5.McGinty DJ, Harper RM. Dorsal raphe neurons: Depression of firing during sleep in cats. Brain Res. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 6.Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: Correlation with level of behavioral arousal. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- 7.Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- 8.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 9.Corcoran AE, et al. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol. 2009;168:49–58. doi: 10.1016/j.resp.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley SR, et al. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci. 2002;5:401–402. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- 11.Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- 12.Ptak K, et al. Raphé neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol. 1998;511:433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001;90:1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- 16.Hodges MR, et al. Effects on breathing in awake and sleeping goats of focal acidosis in the medullary raphe. J Appl Physiol. 2004;96:1815–1824. doi: 10.1152/japplphysiol.00992.2003. [DOI] [PubMed] [Google Scholar]
- 17.Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol. 1995;73:933–944. doi: 10.1152/jn.1995.73.3.933. [DOI] [PubMed] [Google Scholar]
- 18.Mulkey DK, et al. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- 19.Phillipson EA, Kozar LF, Rebuck AS, Murphy E. Ventilatory and waking responses to CO2 in sleeping dogs. Am Rev Respir Dis. 1977;115:251–259. doi: 10.1164/arrd.1977.115.2.251. [DOI] [PubMed] [Google Scholar]
- 20.Berthon-Jones M, Sullivan CE. Ventilation and arousal responses to hypercapnia in normal sleeping humans. J Appl Physiol. 1984;57:59–67. doi: 10.1152/jappl.1984.57.1.59. [DOI] [PubMed] [Google Scholar]
- 21.von Leupoldt A, Dahme B. Cortical substrates for the perception of dyspnea. Chest. 2005;128:345–354. doi: 10.1378/chest.128.1.345. [DOI] [PubMed] [Google Scholar]
- 22.Gorman JM, et al. Anxiogenic effects of CO2 and hyperventilation in patients with panic disorder. Am J Psychiatry. 1994;151:547–553. doi: 10.1176/ajp.151.4.547. [DOI] [PubMed] [Google Scholar]
- 23.Kannurpatti SS, Biswal BB, Hudetz AG. Regional dynamics of the fMRI-BOLD signal response to hypoxia-hypercapnia in the rat brain. J Magn Reson Imaging. 2003;17:641–647. doi: 10.1002/jmri.10311. [DOI] [PubMed] [Google Scholar]
- 24.Ziemann AE, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchanan GF, Hodges MR, Richerson GB. In: Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Monti JM, Pandi-Perumal SR, Jacobs BL, Nutt DJ, editors. Basel: Birkhauser; 2008. pp. 529–554. [Google Scholar]
- 26.Washburn CP, Sirois JE, Talley EM, Guyenet PG, Bayliss DA. Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH- and halothane-sensitive K+ conductance. J Neurosci. 2002;22:1256–1265. doi: 10.1523/JNEUROSCI.22-04-01256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao ZQ, et al. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci. 2009;29:10341–10349. doi: 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodges MR, et al. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon CJ. Relationship between autonomic and behavioral thermoregulation in the mouse. Physiol Behav. 1985;34:687–690. doi: 10.1016/0031-9384(85)90365-8. [DOI] [PubMed] [Google Scholar]
- 31.Saper CB, Cano G, Scammell TE. Homeostatic, circadian, and emotional regulation of sleep. J Comp Neurol. 2005;493:92–98. doi: 10.1002/cne.20770. [DOI] [PubMed] [Google Scholar]
- 32.Mouret J, Bobillier P, Jouvet M. Insomnia following parachlorophenylalanine in the rat. Eur J Pharmacol. 1968;5:17–22. doi: 10.1016/0014-2999(68)90151-9. [DOI] [PubMed] [Google Scholar]
- 33.Haskell EH, Palca JW, Walker JM, Berger RJ, Heller HC. The effects of high and low ambient temperatures on human sleep stages. Electroencephalogr Clin Neurophysiol. 1981;51:494–501. doi: 10.1016/0013-4694(81)90226-1. [DOI] [PubMed] [Google Scholar]
- 34.Baldwin BA, Ingram DL. The effects of food intake and acclimatization to temperature on behavioral thermoregulation in pigs and mice. Physiol Behav. 1968;3:395–400. [Google Scholar]
- 35.Dauncey MJ, Brown D. Role of activity-induced thermogenesis in twenty-four hour energy expenditure of lean and genetically obese (ob/ob) mice. Q J Exp Physiol. 1987;72:549–559. doi: 10.1113/expphysiol.1987.sp003096. [DOI] [PubMed] [Google Scholar]
- 36.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic dorsal raphe neurons to specific motor challenges in freely moving cats. Neuroscience. 1997;79:161–169. doi: 10.1016/s0306-4522(96)00673-2. [DOI] [PubMed] [Google Scholar]
- 37.Monckton JE, McCormick DA. Neuromodulatory role of serotonin in the ferret thalamus. J Neurophysiol. 2002;87:2124–2136. doi: 10.1152/jn.00650.2001. [DOI] [PubMed] [Google Scholar]
- 38.Slater IH, Jones GT, Moore RA. Inhibition of REM sleep by fluoxetine, a specific inhibitor of serotonin uptake. Neuropharmacology. 1978;17:383–389. doi: 10.1016/0028-3908(78)90010-2. [DOI] [PubMed] [Google Scholar]
- 39.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 40.Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience. 1997;77:723–743. doi: 10.1016/s0306-4522(96)00485-x. [DOI] [PubMed] [Google Scholar]
- 41.Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci USA. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomson T, Nashef L, Ryvlin P. Sudden unexpected death in epilepsy: Current knowledge and future directions. Lancet Neurol. 2008;7:1021–1031. doi: 10.1016/S1474-4422(08)70202-3. [DOI] [PubMed] [Google Scholar]
- 43.Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: Analysis of incidence, severity and risk factors. Brain. 2008;131:3239–3245. doi: 10.1093/brain/awn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tupal S, Faingold CL. Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia. 2006;47:21–26. doi: 10.1111/j.1528-1167.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- 45.Brennan TJ, Seeley WW, Kilgard M, Schreiner CE, Tecott LH. Sound-induced seizures in serotonin 5-HT2c receptor mutant mice. Nat Genet. 1997;16:387–390. doi: 10.1038/ng0897-387. [DOI] [PubMed] [Google Scholar]
- 46.Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol. 2009;4:517–550. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duncan JR, et al. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kubin L, Davies RO, Pack AI. Control of upper airway motoneurons during REM sleep. News Physiol Sci. 1998;13:91–97. doi: 10.1152/physiologyonline.1998.13.2.91. [DOI] [PubMed] [Google Scholar]
- 49.Horner RL. Neuromodulation of hypoglossal motoneurons during sleep. Respir Physiol Neurobiol. 2008;164:179–196. doi: 10.1016/j.resp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 50.Franken P, Malafosse A, Tafti M. Genetic variation in EEG activity during sleep in inbred mice. Am J Physiol. 1998;275:R1127–R1137. doi: 10.1152/ajpregu.1998.275.4.R1127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.