It is what many of us dream of: to sleep for only a fraction of our usual sleep time and still feel so well rested and attentive that daytime productivity does not suffer and extra time is gained. Of those who have tried to achieve this dream, few if any have succeeded. After skipping most sleep for a single night, we notice an increased sleep pressure that compels us to soon make up for the lost sleep. Is there no way to escape the characteristic homeostatic buildup of sleep pressure with an increasing duration of wakefulness?
Three years ago, a PNAS report by Kim et al. (1) gave some hope. Their findings suggested that rats gradually lowered their homeostatic set point, so that they actually learned to do with less sleep if exposed to chronic sleep restriction for several days rather than acute sleep deprivation for a single day. The finding by Kim et al. triggered the following question: could we humans learn the same by not giving in to our sleep need after a brief night of sleep? Unfortunately, the answer seems “no.” Last year, Åkerstedt et al. (2) reported that humans exposed to repeated sleep restriction do not reset their homeostatic set point and preserve the need to recover, leaving us with a puzzling—and frustrating for those of us who dream of doing with less sleep—apparent discrepancy between the effects of chronic sleep restriction on homeostatic sleep regulation in rats versus humans.
In PNAS, Leemburg et al. (3) use more refined methodologies to investigate in detail whether rats indeed respond to chronic sleep restriction by resetting their sleep homeostat. Their findings lead to a different conclusion than the report of Kim et al. (1) and suggest that the methods of enforcing wakefulness and of assessing the effects of sleep deprivation that we have traditionally relied on in short-term protocols may not apply in chronic sleep restriction protocols.
As to the improved method for sleep deprivation, Leemburg et al. (3) carry out an unprecedented scrutiny in trying to keep the animals awake by placing them under observation for all 5 d of partial sleep deprivation, 24 h/d, and by taking action if behavioral signs suggested attempts to sleep. It was thus demonstrated that a chronic sleep restriction protocol requires an ever-increasing rate of perturbation of the rat to counteract its growing number of sleep attempts. Nonetheless, as in chronic “total” sleep deprivation protocols (4), it appeared to be impossible to keep rats awake for more than about 90–95% of the time.
As to the improved method for evaluating homeostatic sleep pressure, the authors carefully quantify these sleep intrusions into forced wakefulness. Moreover, they managed to quantify electroencephalographic (EEG) slow wave activity (SWA <4 Hz)—the accepted best readout of homeostasis—not only during sleep but also during 94% of the time awake—notably difficult because of the artifacts. As with chronic “total” sleep deprivation, SWA as well as low θ (5–7 Hz) activity started to “leak” into periods of forced wakefulness during which the animal could even be observed to show locomotor activity and have its eyes open.
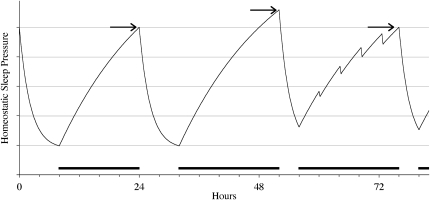
These brief intrusions of sleep and slow wave activity into awake epochs may not have received enough attention in previous deprivation studies. The sleep homeostasis model, discussed below, is usually interpreted with the implicit assumption that epochs of wakefulness increase sleep pressure, whereas epochs of non-rapid eye movement sleep dissipate sleep pressure. The work of Leemburg et al. (3) shows that this dichotomization does not account for the subtle sleep intrusions and SWA leakage that occur during repeatedly enforced prolonged wakefulness. Actually, awake epochs dissipated rather than accumulated sleep pressure when they contained SWA intrusions; the more leakage of SWA that occurred during enforced wakefulness, the less SWA increased in subsequent sleep. Even when the intrusions are brief, their sleep pressure dissipating effect may still be considerable because the strongest dissipation of sleep pressure occurs at the onset of SWA and subsequently declines exponentially (Fig. 1).
Fig. 1.
Schematic view of homeostatic buildup of sleep pressure. On baseline day 1, for as long as wakefulness is maintained (16 h on day 1 in this example) sleep pressure increases to a certain level (arrow on left), after which it dissipates during sleep. On day 2, representing short-term sleep restriction, wakefulness is forced to be maintained for 20 h, causing sleep pressure to build up to a higher level (arrow in center). Only 4 h of sleep is subsequently allowed. On day 3, representing long-term sleep restriction, sleep pressure builds up for another 20 h, but brief periods of dissipation due to intrusion of sleep and/or EEG slow wave activity result in a sleep pressure at sleep onset (arrow on right) that does not differ from baseline; sleep homeostasis is preserved, however. Black horizontal bars represent forced wakefulness.
This dissipation may have gone unnoticed in the work of Kim et al. (1), although it may have been crucial in the reported gradual lowering of SWA during sleep over subsequent days of sleep restriction, which Kim et al. interpreted as an allostatic response. The careful recording and analysis of mostly artifact-free EEGs obtained throughout wakefulness and sleep during the entire 8-d period of the experiment allowed Leemburg et al. (3) to integrate all SWA and demonstrate that its net accumulation and dissipation were unaltered during chronic sleep restriction, indicative of the preservation of sleep homeostasis.
What makes the report of Leemburg et al. (3) important? First, the model of homeostatic regulation of sleep and its electrophysiological correlate of SWA is the best window that we have on the mechanisms and functions of sleep. The homeostatic regulation of sleep pressure has become a cornerstone in our quest to understand the mechanisms and functions of sleep. In the early 1980s, excellent scientists, including Borbély, Daan, Beersma, Åkerstedt, and others, all contributed to a model that combined the homeostatic buildup and circadian regulation of sleep (5–7). Many others subsequently added important findings that defined the parameters and interactions of the model (e.g., 8–10), which has continued to prove extremely valuable. As mentioned, slow oscillations in the EEG have continued to prove the best readout of homeostasis and have inspired many to build strong hypotheses of their functional relevance, ranging from a default oscillation that emerges intrinsically as soon as neuronal networks are formed (see ref. 11) to an indispensable process guarding the homeostasis of their synaptic density (e.g., 12, 13). Numerous studies have since demonstrated the specific functional relevance of SWA for health, brain function, and cognition (e.g., 14, 15). Thus, 30 y of studies of rodents and humans have shown the consistency and validity of the sleep homeostasis model across these species. There would be significant consequences if it was determined that a model of such wide-ranging influence to sleep neuroscience applied only to short-term deprivation studies, and not to long-term sleep restriction, or if it were concluded that rats and humans differ in a pronounced way in their sleep homeostat response to chronic sleep restriction. The work of Leemburg et al. (3) indicates that there is no reason to abandon either the model of sleep homeostasis or the use of the rat as a valuable model for human sleep regulation. Of course, there will be species differences. When exposed to chronic sleep restriction, rats may be somewhat more likely to show intrusions of sleep and slow oscillations simply because they are used to polyphasic sleep; humans might be more likely to show lapses of attention and θ oscillations because fast state switching is not a normal property of our biphasic sleep habit.
Second, only very few previous human studies and only one previous rat study have pushed the homeostatic sleep drive to its limits by applying chronic sleep restriction rather than acute total sleep deprivation. The report of Leemburg et al. (3) shows that valuable information can be obtained by doing so. For example, it led to the insight of the topography-dependent differential cortical effects of chronic sleep restriction. Although the frontal and parietal cortex responded with a boost in slow wave activity primarily during the 4-h time window that allowed for sleep, the occipital cortex responded with a boost in slow wave activity primarily during the 20-h window of enforced wakefulness. Also, the number of traveling slow waves originating in the frontal cortex increased during chronic sleep restriction but decreased in the occipital cortex. Furthermore, the careful recording of artifact-free EEGs showed that, during chronic sleep restriction, the brain tries to dissipate sleep pressure, as quantified by EEG SWA, in every possible way, even during wakefulness.
Finally, the work of Leemburg et al. (3) has consequences for the methodology that we use to evaluate sleep homeostasis. First, the study clearly shows that automated sleep deprivation methods that apply a fixed rate of perturbation fail to compensate for the increase in brief sleep attempts that occurs with repeated sleep restriction. It would make sense to develop automated sleep deprivation devices that gradually increase the rate of perturbation, which we are presently developing and validating in our laboratory. In addition to a gradually increasing rate of mechanical perturbation, it can be evaluated whether the expression of SWA during wakefulness may be suppressed by other manipulations of the environment, including its brightness and temperature (16, 17). Second, a strict discrimination of wakefulness—during which sleep pressure increases—and of sleep—during which sleep pressure decreases—does not seem to be an appropriate way to evaluate the homeostasis of sleep pressure in protocols that apply repeated sleep restriction, especially not if EEG is scored over larger intervals of several seconds. With the increasing possibility of recording from arrays of electrodes rather than from a few electrodes, the golden standard of power spectral density analyses may be supported by methods such as template matching and independent component analysis to eliminate movement artifacts in awake EEGs and to be able to better quantify slow wave activity during wakefulness.
In conclusion, although previous studies in rats were less successful in obtaining continuous artifact-free awake EEGs, Leemburg et al. (3) succeed and show that sleep homeostasis is preserved during chronic sleep restriction, in part by intrusion of sleep and SWA into wakefulness. The excellent work of many dedicated researchers will ultimately bring us closer to understanding the mechanisms and brain substrates of homeostatic sleep regulation and its involvement in maintaining health and the level of alertness during wakefulness. Will it bring us closer to understanding the function of sleep? If, of course, this is a valid question at all; likewise, what would be the function of wakefulness? Perhaps the states of sleep and wakefulness reflect mainly an organizational principle, evolved to separate processes, ranging from the molecular to the behavioral, that would, if taking place simultaneously or close in time, be detrimental to the organism? The fact that chronic sleep restriction is detrimental to health and causes cumulative neurobehavioral deficits (18), despite the adaptive processes that reroute part of the homeostatic sleep pressure into wakefulness, supports this view. Time will tell whether timing is key. For now, doing with less sleep remains a dream.
Footnotes
The author declares no conflict of interest.
See companion article on page 15939 in issue 36 of volume 107.
References
- 1.Kim Y, Laposky AD, Bergmann BM, Turek FW. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc Natl Acad Sci USA. 2007;104:10697–10702. doi: 10.1073/pnas.0610351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Åkerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J. Sleep homeostasis during repeated sleep restriction and recovery: Support from EEG dynamics. Sleep. 2009;32:217–222. doi: 10.1093/sleep/32.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leemburg S, et al. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci USA. 2010;107:15939–15944. doi: 10.1073/pnas.1002570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: An update of the 1989 paper. Sleep. 2002;25:18–24. doi: 10.1093/sleep/25.1.18. [DOI] [PubMed] [Google Scholar]
- 5.Åkerstedt T, Gillberg M. The circadian variation of experimentally displaced sleep. Sleep. 1981;4:159–169. doi: 10.1093/sleep/4.2.159. [DOI] [PubMed] [Google Scholar]
- 6.Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: Effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–495. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 7.Daan S, Beersma DG, Borbély AA. Timing of human sleep: Recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–R183. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 8.Achermann P, Dijk DJ, Brunner DP, Borbély AA. A model of human sleep homeostasis based on EEG slow-wave activity: Quantitative comparison of data and simulations. Brain Res Bull. 1993;31:97–113. doi: 10.1016/0361-9230(93)90016-5. [DOI] [PubMed] [Google Scholar]
- 9.Bes F, Jobert M, Schulz H. Modeling napping, post-lunch dip, and other variations in human sleep propensity. Sleep. 2009;32:392–398. doi: 10.1093/sleep/32.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borg-Graham LJ. Systems neuroscience: The slowly sleeping slab and slice. Curr Biol. 2001;11:R140–R143. doi: 10.1016/s0960-9822(01)00062-8. [DOI] [PubMed] [Google Scholar]
- 12.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Der Werf YD, et al. Sleep benefits subsequent hippocampal functioning. Nat Neurosci. 2009;12:122–123. doi: 10.1038/nn.2253. [DOI] [PubMed] [Google Scholar]
- 16.Raymann RJEM, Swaab DF, Van Someren EJW. Skin deep: Enhanced sleep depth by cutaneous temperature manipulation. Brain. 2008;131:500–513. doi: 10.1093/brain/awm315. [DOI] [PubMed] [Google Scholar]
- 17.Trachsel L, Tobler I, Borbély AA. Sleep regulation in rats: Effects of sleep deprivation, light, and circadian phase. Am J Physiol. 1986;251:R1037–R1044. doi: 10.1152/ajpregu.1986.251.6.R1037. [DOI] [PubMed] [Google Scholar]
- 18.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]