Abstract
Murine hematopoietic blast colony-forming cells (BL-CFCs) are able to generate up to 30,000 progeny blast cells within 10 d in agar cultures. Contained in these populations are large numbers of lineage-committed progenitor cells in the granulocytic and macrophage lineages. Sequential analyses of blast colonies revealed that self-generation of BL-CFCs occurs but is surprisingly late in clonal expansion, as is the emergence of progenitor cells committed to megakaryocytic and eosinophil lineages. Self-generating BL-CFCs were highly enriched in lineage− Kit+ Sca1+ CD34− Flt3R− populations, and colonies generated by such cells contained colony-forming units–spleen and formed erythroid and lymphoid progeny in vivo. The data suggest the existence of a hierarchical structure in BL-CFC populations with at least a subset being cells assayable as colony-forming units–spleen. Because BL-CFCs can self-generate and are able to generate lymphoid and myeloid populations, BL-CFCs appear to be ideal cells in which to analyze the processes of self-generation and lineage commitment in clonal in vitro cultures.
Keywords: colony-forming units–spleen, megakaryocytes, eosinophils
Because of the short lifespan of blood cells, there is a continuous need to generate massive numbers of multilineage maturing progeny. It is generally considered that hematopoiesis is initiated by a small population of long-term repopulating hematopoietic stem cells (LT-HSCs) (1). Their immediate progeny are multipotential cells capable of producing hematopoietic colonies in the spleen of irradiated mice (colony-forming units–spleen; CFU-S) (2, 3). It would seem logical that, at first, the clonogenic initiating cells would self-generate to produce large numbers of starting clonogenic cells and so reduce the overall burden of cell production by individual immature cells. Further, when committed progenitors in multiple lineages are to be generated, the commitment process into the various lineages, if at all competitive, might be expected to occur simultaneously.
Direct evidence regarding these questions is particularly difficult to obtain because studies have had to be restricted to following the fate of injected stem cells or CFU-S in whole animals. However, there was evidence from previous studies on the in vivo growth of CFU-S–initiated spleen colonies that a surprising delay occurs in self-generation during clonal expansion. The absolute number of self-generated CFU-S in expanding colonies was observed to increase progressively from day 7 to day 14, and similarly the number of more mature lineage-committed progenitors in such spleen colonies also increased progressively with increasing colony size (4).
To better understand and manipulate the basic processes of self-generation and lineage commitment in hematopoietic precursor cells, it is important to exploit the convenience of any in vitro model of hematopoiesis with suitable properties. Blast colonies are clones of blast cells formed in vitro by single blast colony-forming cells (BL-CFCs) (5, 6). Within individual blast colonies, multiple lineages of progenitor cells have been demonstrated, including granulocytic, macrophage, eosinophil, megakaryocyte, erythroid, dendritic, and T and B lymphocytes. In addition, when 7-d colonies were analyzed, low but definite numbers of BL-CFCs were present, indicating that at least some BL-CFCs had a capacity for self-generation (6). The multipotentiality of BL-CFCs and their capacity for self-generation means that blast colonies fulfill most of the requirements of a system that would potentially allow clonal analysis of the processes of self-generation and lineage commitment.
The present study in blast colonies grown from mouse bone marrow has documented a surprising delay in self-generation of BL-CFCs and in the formation of committed progenitor cells in the megakaryocytic and eosinophil lineages. It was shown that the marked heterogeneity among individual colonies in the production of these cells was linked to the phenotype of the BL-CFCs, and advantage was taken of this finding to demonstrate that a subset of BL-CFCs are in fact CFU-Ss, making it highly important to exploit the blast colony-forming system in future studies on blood cell formation.
Results
Self-Renewal of BL-CFCs.
Sequential sampling of day 4 multicentric colonies initiated by stem cell factor (SCF) plus IL-6 in 1 mL cultures of 25,000 C57BL marrow cells gave a mean colony cell count of 200 ± 100 cells; that of day 7 multicentric colonies gave a mean count of 3,500 ± 820 cells per blast colony; and that of day 10 multicentric colonies gave 32,370 ± 24,520 cells per blast colony. Counts of multicentric blast colony numbers in stained cultures gave a mean number of 5 ± 1 for day 4 colonies, 6 ± 1 for day 7 colonies, and 6 ± 3 for day 10 cultures. At day 10, some blast colonies contained dying cells but the unchanged colony counts indicated that little selection of colonies could have occurred between these incubation time points. Furthermore, all secondary cultures were performed using the largest three or four colonies per culture and, because of this, the day 10 colonies sampled must have been present and available for sampling at day 7.
Reculture of cells from multicentric 7-d blast colonies revealed the presence of a high number of granulocyte/macrophage progenitor cells but a low and highly variable number of BL-CFCs and committed progenitors in the megakaryocytic and eosinophil lineages. When day 4 blast colonies were similarly recultured (Table 1), no BL-CFCs were detected, indicating an unexpected failure of initiating BL-CFCs to self-renew early in colony formation. In sharp contrast, reculture of day 10 or day 11 blast colony cells revealed not only greatly increased granulocyte/macrophage progenitor cell production but the presence of substantial numbers of BL-CFCs in 17% of colonies versus 7% of day 7 colonies. Similar elevated numbers of megakaryocytic progenitors were seen in 25% of colonies versus 7% of day 7 colonies and eosinophil progenitors in 39% of colonies versus 2% of day 7 colonies.
Table 1.
Progressively calculated total number of progenitor cells per colony with age
| GM-CSF | M-CSF | SCF + IL-3 + EPO | |||||||||||
| Colony age | G | GM | M | Eo | G | GM | M | Blast | G | GM | M | Eo | Meg |
| Day 4 (n = 30) | 10 ± 18 | 6 ± 12 | 18 ± 41 | 0 ± 0 | 2 ± 5 | 2 ± 3 | 28 ± 32 | 0 | 28 ± 34 | 9 ± 13 | 18 ± 29 | 0 ± 0 | 0.3 ± 1.5 |
| Day 7 (n = 44) | 240 ± 348 | 73 ± 91 | 308 ± 319 | 0.7 ± 2.3 | 13 ± 32 | 21 ± 37 | 845 ± 771 | 1 ± 4 | 341 ± 463 | 99 ± 176 | 94 ± 124 | 0.4 ± 2.4 | 0.9 ± 3.6 |
| Day 10 (n = 36) | 434 ± 901 | 305 ± 609 | 646 ± 873 | 11 ± 38 | 179 ± 408 | 98 ± 152 | 1,644 ± 1,698 | 6 ± 17 | 676 ± 997 | 556 ± 1,107 | 261 ± 504 | 8 ± 14 | 20 ± 79 |
All blast colonies were initiated in cultures of 25,000 C57BL marrow cells stimulated by 100 ng/mL stem cell factor plus 100 ng/mL IL-6. Individual blast colonies were resuspended and cultured in parallel in duplicate cultures stimulated by 10 ng/mL GM-CSF, 10 ng/mL M-CSF, or a mixture of 100 ng/mL stem cell factor, 10 ng/mL IL-3, and 4 IU erythropoietin. Secondary cultures were scored after 7 d of incubation. Blast, BL-CFCs; Eo , eosinophil; G, granulocyte, GM, granulocyte-macrophage; M, macrophage; Meg, megakaryocyte. Values presented are mean colony counts ± SD.
To determine whether the delayed onset of BL-CFC self-generation and appearance of committed megakaryocytic and eosinophil progenitors was a peculiarity of the C57BL cells used, marrow cells from five other strains of mice were cultured with SCF plus IL-6. The resulting blast colonies were analyzed after 7 d and 10 d of incubation. As shown in Table 2, somewhat variable results were obtained, reflecting the same wide individual blast colony heterogeneity as had been seen with C57BL cells. However, clear examples of increased frequencies of BL-CFC and committed progenitors of megakaryocytes and eosinophils were encountered, the elevated numbers being well in excess of the general rise in progenitor cell numbers.
Table 2.
Age changes in the composition of blast colonies grown from different mouse strains
| Calculated colony-forming cells per blast colony | ||||||
| Strain | Colony age, d | Blast | Eosinophil | Megakaryocyte | Total | Fold change at 10 d |
| BALB/c | 7 (n = 35) | 7 ± 21 | 0 ± 0 | 0.7 ± 3.0 | 1,385 | 2.4 |
| 10 (n = 30) | 39 ± 67 | 23 ± 58 | 0.8 ± 2.4 | 3,352 | ||
| AKR | 7 (n = 15) | 2 ± 6 | 2 ± 6 | 1 ± 4 | 1,942 | 3.3 |
| 10 (n = 15) | 6 ± 11 | 46 ± 54 | 5 ± 12 | 6,419 | ||
| CBA | 7 (n = 15) | 0 ± 0 | 1 ± 3 | 1 ± 4 | 1,574 | 1.6 |
| 10 (n = 20) | 30 ± 56 | 8 ± 10 | 7 ± 23 | 2,562 | ||
| DBA/2 | 7 (n = 15) | 1 ± 3 | 0 ± 0 | 0 ± 0 | 1,408 | 2.2 |
| 10 (n = 20) | 21 ± 43 | 4 ± 10 | 4 ± 9 | 3,074 | ||
| SJL | 7 (n = 15) | 0.5 ± 2.1 | 0 ± 0 | 0 ± 0 | 2,417 | 3.5 |
| 10 (n = 10) | 21 ± 33 | 1 ± 3 | 6 ± 18 | 8,408 | ||
Primary cultures were prepared using 25,000 marrow cells stimulated by 100 ng/mL stem cell factor plus 100 ng/mL IL-6. Resuspended blast colonies were recultured for 7 d in secondary cultures stimulated by 100 ng/mL stem cell factor plus 10 ng/mL IL-3 and 4 IU/mL erythropoietin. Values presented as mean ± SD. “Total colonies” represents total colonies with SCF, IL-3, and EPO plus M colonies with M-CSF.
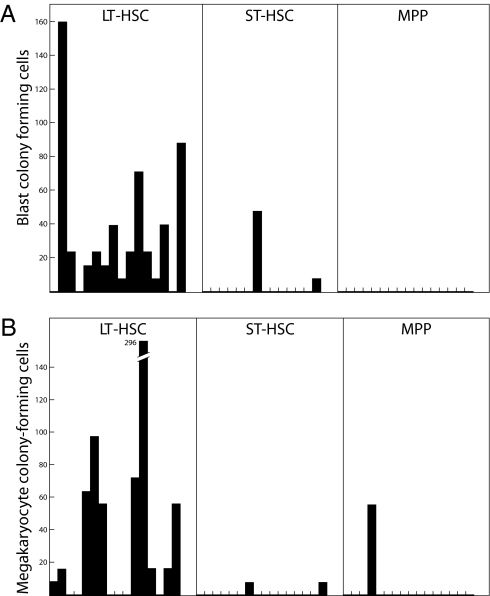
Most day 10 and day 11 blast colonies are still composed of an apparently uniform population of blast cells but they were strikingly heterogeneous in their content of clonogenic cells (Fig. S1). Previous studies had shown that, after FACS fractionation, lineage− Kit+ Sca1+ cells could be further fractionated using CD34 and FltR markers to give three populations: CD34− Flt3R− (LT-HSC fraction), CD34+ Flt3R− (ST-HSCs, the CFU-S–containing fraction), and CD34+ Flt3R+ [multipotential progenitor (MPP) cells (3)]. In previous studies, BL-CFCs were detected in all three fractions and the blast colonies generated appeared similar in appearance, but were at their highest frequency (approximately 30%) in the CFU-S–containing ST-HSC fractions (6). When day 7 blast colonies formed by all three fractions were recultured, little difference was noted in their content of progenitor cells. However, a remarkable difference became apparent in day 11 colonies (Table S1). As shown more clearly in Fig. 1, most day 11 blast colonies generated by LT-HSC cells contained numerous BL-CFCs, which was in marked contrast to virtual absence of such cells in secondary cultures of blast colonies derived from ST-HSC or MPP fractions. This was in notable contrast to the fact that ST-HSC fractions have the highest frequency of BL-CFCs in primary cultures. The results strongly suggest that the BL-CFCs in LT-HSC fractions are ancestral to BL-CFCs in the other two fractions, in keeping with the general view that LT-HSCs are ancestral to both ST-HSC and MPP cells (1, 3). A similar result was seen with megakaryocyte-committed progenitor cells, as shown in Fig. 1 and Table S1, and a comparable trend was noted with eosinophil progenitors (Table S1).
Fig. 1.
FACS fractionation enriches the LT-HSC for BL-CFC able to self-renew and form megakaryocyte-committed progenitor cells. (A) Total number of BL-CFCs in 15 sequential blast colonies grown from LT-HSCs compared with a low number or no BL-CFCs produced in blast colonies formed by ST-HSCs or MPP cells. (B) Similar elevated production of megakaryocyte-committed progenitor cells in blast colonies formed by LT-HSCs compared with those in colonies formed by ST-HSCs or MPP cells.
Identity of CFU-S and BL-CFCs.
The preceding data indicated the likely presence of a hierarchial stratification within BL-CFC populations. From previous studies, FACS fractionation data from ST-HSCs showed that CFU-Ss and BL-CFCs were equivalently enriched, with each comprising approximately 30% to 40% of total cells in these fractions (6). Because ST-HSC populations are currently the most enriched (i.e., purified) population of both cell types, the implication was that the two populations may be identical. CFU-Ss cannot be cultured in vitro because this would involve the use of ST-HSC cells and be precisely the same experiment as is used in the culture of BL-CFC cells. However, CFU-Ss have been shown in previous studies to have a delayed capacity for self-generation (4), and if BL-CFCs are indeed CFU-Ss, the later progeny of blast colony-forming should include CFU-Ss, particularly if the initiating cells were from LT-HSC fractions.
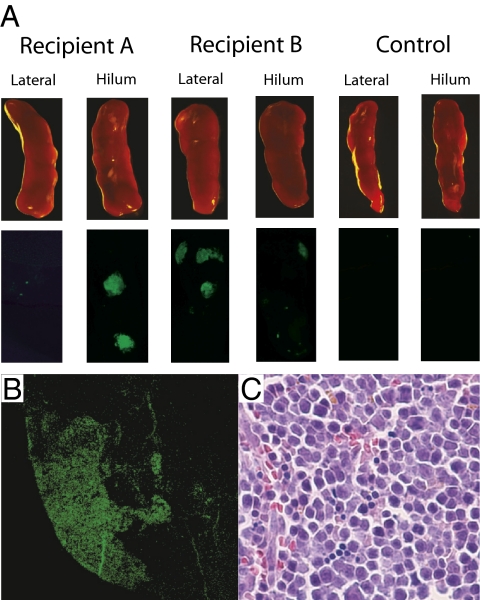
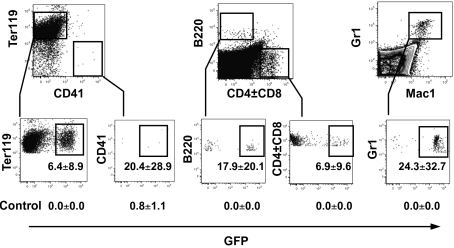
To determine whether this was so, marrow from GFP-positive C57BL mice was FACS-fractionated and the LSK subpopulation that was CD34− FltR− was cultured with SCF plus IL-6. At day 10 (chosen to maximize the frequency of BL-CFCs within the colonies), blast colonies were pooled, washed three times, then filtered to remove residual agar. Then, 105 pooled colony cells were injected into C57BL mice following two 5.5-Gy irradiations. Included in the inoculum were 105 filler cells from C57BL marrow populations that had been fractionated to produce lineage− Kit+ but Sca1− cells. After 12 to 14 d, the spleen and marrow cells were harvested. Examination of the spleens was undertaken for typical “superficial” spleen colonies as well as confirmatory analysis of spleen frozen sections for GFP-expressing colonies (Fig. 2). Adjacent sections from spleen stained with H&E showed that the fluorescent colonies were composed of typical erythroid populations (Fig. 2). Single-cell suspensions of a portion of each spleen and bone marrow were also subjected to FACS analysis, which revealed GFP-positive populations of Ter119-positive erythroid cells, B220-positive B-lymphoid cells, and CD4-/CD8-positive T-lymphoid cells (Fig. 3).
Fig. 2.
Pooled blast colony cells contain CFU-Ss. (A) Spleens from two irradiated recipients of GFP-labeled pooled blast colony cells at day 12 show typical GFP-positive spleen colonies. (B) Frozen sections of such spleens show typical GFP-positive spleen colonies. (C) Adjacent frozen sections stained with H&E show that the GFP-positive colonies are composed of erythroid cells.
Fig. 3.
FACS analysis of the spleens from irradiated mice injected 12 d previously with pooled GFP-positive blast colony cells show GFP-positive Ter119-positive (erythroid) cells, B220-positive (B-lymphoid) cells, and CD4CD8-positive (T-lymphoid) cells. Spleens from mice injected with control material contained virtually no GFP-positive cells.
A remote possibility was that CFU-Ss and BL-CFCs might indeed be separate cells and that the few CFU-Ss present in LT-HSC fractions (3) might have persisted without division in the agar cultures. Some of these cells might then have become accidentally incorporated into expanding blast colonies. To test this, volumes of agar were collected from intercolony areas and from areas containing likely granulocytic or macrophage colonies. The volumes taken were twice those used in the removal of the blast colonies. This material was also triple-washed and filtered, then injected into recipient irradiated C57BL mice together with 105 filler cells.
An average of 4 ± 2 superficial (P = 0.0006, two-tailed t test, vs. colony-free agar controls) and 4 ± 1 histologically determined (P = 0.00157, two-tailed t test, vs. colony-free agar controls) GFP-positive spleen colonies were observed in 10 recipients of pooled blast colony cells. No GFP-positive spleen colonies were noted in four recipients of colony-free agar and only 1 ± 1 superficial (P = 0.008, two tailed t test, vs. colony-free agar controls) and 1 ± 1 histological (P = 0.178, two tailed t test, vs. colony-free agar controls) GFP-positive colonies were noted in two recipients of presumptively nonblast colony cells.
Some dispersion of blast colony cells does occur into the surrounding agar during culture and colony removal, and this could have been the origin of the occasional CFU-S detected in control nonblast colony preparations. Overall, the data indicated that CFU-Ss with typical erythroid-generating properties were generated in blast colonies formed by LT-HSC fractions. The simplest conclusion is that BL-CFCs in LT-HSC fractions are CFU-Ss and that CFU-Ss detected in blast colonies represent self-renewal by these BL-CFCs.
Although typical day 10 to 12 spleen colonies are in no way comparable with blast colonies of a similar age, because of the extreme bias in typical spleen colonies to erythropoiesis (4) (Fig. 2), it was of interest that 8 of 31 day 10 to 12 C57BL spleen colonies tested were found to contain BL-CFCs, providing some symmetry to the observations described here.
Discussion
Analysis of the key developmental features of hematopoiesis, namely self-renewal and lineage commitment, in initiating stem cells and their immediate CFU-S progeny, has been difficult because both cell types have needed to be assayed in whole animals. In contrast, blast colonies formed in culture by BL-CFCs are multipotential and can allow the analysis of self-renewal and committed progenitor cell formation (5, 6). In an expanding clone of differentiating hematopoietic cells, the expectation would be that, initially, there would be an increase in the numbers of initiating cells by self-generation to allow the subsequent easier production of large numbers of maturing nonclonogenic cells. Contrary to this expectation, in blast colonies generated by BL-CFCs, BL-CFCs were not present in early day 4 colonies but began to appear at day 7 and were clearly more numerous at day 11. The population size of day 4 colonies was, of course, much smaller than the sizes of day 7 and day 11 colonies. However, in each case, one fourth of the entire colony was being assayed in the relevant secondary cultures, making a comparison of the data valid on a colony-to-colony basis.
The counterintuitive delay in self-generation by BL-CFCs parallels an earlier observation in developing hematopoietic spleen colonies whereby CFU-Ss did demonstrate a capacity for self-generation but the absolute numbers of CFU-Ss in developing colonies increased progressively as the colonies aged between 7 d and 11 d (4).
Similar considerations applied to the delayed appearance of committed progenitor cells in developing blast colonies. Committed progenitor cells in eosinophil and megakaryocytic lineages were essentially absent from day 7 colonies but had appeared by day 10 or 11 of incubation. Lineage-committed progenitor cells in other lineages such as granulocytic or macrophage had long since appeared in such blast colonies, and it is curious why corresponding cells in the eosinophil and megakaryocytic lineages should have had such a delayed appearance. Although most observations were made on C57BL colonies, analysis of blast colonies from other strains showed a similar phenomenon, which therefore appears to be a common characteristic of developing murine hematopoietic colonies.
The mechanisms responsible for the late emergence of BL-CFCs or certain committed progenitor cells in expanding, very large, colonies can only be speculated upon. Such events might be triggered by anoxia or lack of metabolites within such colonies. Alternatively, some colony cells might have an inductive influence on particular transcription events in other colony cells.
As previously noted, striking heterogeneity was observed in the BL-CFC and progenitor cell content of individual blast colonies (6). Some otherwise unremarkable blast colonies contained high numbers of BL-CFCs and might even have represented a special subset of BL-CFCs designed to sustain BL-CFC numbers whereas most BL-CFCs had little or no capacity for self-generation. A similar comment could be made regarding subsets of BL-CFCs forming megakaryocytic- or eosinophil-committed progenitor cells.
Analysis of day 11 blast colonies formed by FACS-fractionated populations showed that BL-CFCs from LT-HSC fractions uniformly produced high numbers of daughter BL-CFCs in contrast to the virtual lack of BL-CFCs in blast colonies formed by ST-HSCs or MPP fractions. This suggests that BL-CFCs in LT-HSC fractions may be ancestral to those in other populations.
With this lead, the possible identity of CFU-Ss and BL-CFCs was reexplored. Both are cycling cells (7, 8) and both are multipotential and capable of self-renewal (4, 6). Unfortunately, the most enriched FACS fractions for both populations are the same ST-HSC population, wherein the frequency of both cell types is 30% to 40% (3, 6). Cross-assay of either population in vivo or in vitro is pointless because the same starting cell population is involved.
An alternative approach was to explore the progeny of BL-CFCs for the presence of CFU-Ss, which would document self-renewal of BL-CFCs/CFU-Ss. This revealed the presence of CFU-Ss in blast colonies formed by BL-CFCs in LT-HSC fractions at a frequency of four per 105 blast colony cells. With correction for seeding efficiency (4), this would represent 40 per 105 cells: a number not too dissimilar from the estimates of 100 CFU-Ss per 105 bone marrow cells. With seeding efficiency correction, the figures would indicate that approximately 15 CFU-Ss were present in the average day 10 blast colony. The data indicate that at least the subset of BL-CFCs in LT-HSC population is capable of producing CFU-Ss, indicating either that such BL-CFCs are CFU-Ss or are ancestral to them.
These data extend earlier observations of CFU-Ss in multipotential or blast colonies grown from fetal liver cells (9) or marrow cells after selection by hydroxyurea (10) or initial culture in suspension cultures (11, 12). In two of these studies, the in vitro colony origin of the CFU-Ss was certified by the use of the T6 marker chromosome.
The situation with ST-HSC populations that contain equal numbers of BL-CFCs and CFU-Ss, with these cells being at a higher frequency than in any other marrow fraction (6), requires further exploration. BL-CFCs in this fraction had little capacity for self-generation and may prove to have little capacity to form new CFU-Ss. It is possible that BL-CFCs and CFU-Ss in ST-HSC populations may both be the progeny of the less numerous BL-CFCs in LT-HSC fractions.
The present observations in blast colonies make it even more important to analyze further the processes of self-renewal and lineage determination that occur in convenient form in vitro. Note must be taken, however, of the marked heterogeneity evident in blast colony populations and the delay in self-generation and some types of lineage commitment, making the cells involved a small minority in the more numerous blast colony cells. Here the preliminary enrichment by FACS sorting of BL-CFCs able to self-renew will be of some assistance.
Materials and Methods
Mice.
All mice were produced in the animal facility of the Walter and Eliza Hall Institute and all studies were approved by the Animal Ethics Committee of this institute. Mice used were of C57BL, DBA/2, CBA, BALB/c, SJL, or AKR strains or C57BL mice transgenic for GFP with an ubiquitin promoter (13).
Culture.
Marrow (25,000) cells were cultured in 35-mm Petri dishes containing 1 mL of DMEM containing 20% modified newborn calf serum and 0.3% agar. Blast colony formation was stimulated by the use of a final concentration of 100 ng/mL murine SCF plus 100 ng/mL murine IL-6. After 1 wk of incubation at 37 °C in a fully humidified atmosphere of 10% CO2 in air, routine cultures were scored at 35× magnifications, then fixed with 1 mL of 2.5% glutaraldehyde. After 4 h, cultures were floated intact onto glass slides, allowed to dry, stained for acetylcholinesterase and then with Luxol fast blue and hematoxylin. Final colony counts and typing were then performed on the entire cultures at 50× or 100× magnifications (14).
Reculture of Blast Colonies.
Individual blast colonies after 4, 7, 10, or 11 d of incubation were removed using a fine sterile pipette, resuspended in 8 mL of agar medium, then cultured in duplicate secondary cultures for a further 7 d using 10 ng/mL of murine GM-CSF, 10 ng/mL of murine macrophage colony-stimulating factor (M-CSF) or 100 ng/mL of murine SCF plus 10 ng/mL of murine IL-3 and 4 IU erythropoietin (EPO). Secondary colonies were processed and scored as described earlier.
Mass Harvesting of Blast Colonies.
Blast colonies at 10 d were removed using a fine sterile pipette and pooled in 7-mL volumes of 5% serum saline solution. Colony cells were resuspended by repeated pipetting and washed three times by centrifugation at 500 × g. To remove remaining agar fragments, each cell suspension was passed through a sterile 5-mL polystyrene cell strainer (BD Falcon).
Cell Sorting and Staining.
For agar culture experiments using 7- to 12-wk C57BL bone marrow or GFP expressing C57BL bone marrow (Jackson Laboratories) (13), single suspension bone marrow cells were stained with rat anti-mouse antibodies specific for lineage markers CD4 (GK1.5), CD8 (53-6.7), B220 (682), Gr-1 (8C5), TER119, and Mac1 (M1/70), and lineage-depleted using goat anti-rat microbeads in an LS MACS column (Miltenyi Biotec). The lineage negative population was fractionated using fluorochrome-conjugated antibodies against cKit (2B8), Sca1 (D7), CD34 (RAM34), and Flt3R (CD135 A2F10.1), and the CD34− and CD34+ LSK fractions sorted by flow cytometry on a FACSAria (BD Biosciences) for primary cultures. Lineage staining was performed with fluorochrome conjugated antibodies against Ter119, Gr1, Mac1, B220, CD4, and CD8 for analysis of single cell suspensions of spleen and bone marrow compartments of recipients injected with GFP-expressing blast-colony cells and analyzed on the LSR II instrument (BD Biosciences) and FlowJo version 8.7 software (TreeStar).
Colony-Forming Unit Spleen Assays.
Recipient mice 7 to 12 wk of age were administered 11 Gy γ-irradiation using a 137Cs source (Atomic Energy) at a dose rate of 30 cGy/min split over two doses as much as 4 h apart. Ten-day pooled blast colonies from the GFP-expressing CD34− Flt3R− LSK fractions enriched for long-term HSCs were derived as described earlier, and 105 blast colony cells together with 105 filler cells were injected into each irradiated recipient. Recipient mice were maintained on oral antibiotic after transplantation (neomycin sulfate; Sigma). Spleens were harvested from recipients at 12 to 14 d following transplantation and fixed in 4% paraformaldehyde after macroscopic colonies were counted. GFP-expressing colonies were identified using Argon laser 488-nm excitation and images acquired from harvested spleens at 40× magnification (Axioplan-2 microscope, 40×/0.75 NA objective with AxioCam Hrc and Axiovision Version 3.1 image-acquisition software; Zeiss) or from paraformaldehyde-fixed spleens that had been cryosectioned and mounted with fluorescent mounting media (DakoCytomation), with images acquired using a TCS SP2 scanning confocal microscope (Leica) using 488-nm Argon laser excitation and Leica image acquisition software at 40× magnification.
Supplementary Material
Acknowledgments
This work was supported by the Carden Fellowship Fund of the Cancer Council (Victoria, Australia); Program 461219 of the National Health and the Medical Research Council (Canberra, Australia); National Health and Medical Research Council Independent Research Institutes Infrastructure Support Scheme Grant 361646; and a Victorian State Government Operational Infrastructure Support grant. A.N. was a recipient of an Australian Postgraduate Award.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011881107/-/DCSupplemental.
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 3.Yang L, et al. Identification of Lin(-)Sca1(+)kit(+)CD34(+)Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- 4.Metcalf D, Moore MAS. Haemopoietic Cells. Amsterdam: North-Holland; 1971. [Google Scholar]
- 5.Metcalf D, et al. Two distinct types of murine blast colony-forming cells are multipotential hematopoietic precursors. Proc Natl Acad Sci USA. 2008;105:18501–18506. doi: 10.1073/pnas.0810072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metcalf D, et al. Murine hematopoietic blast colony-forming cells and their progeny have distinctive membrane marker profiles. Proc Natl Acad Sci USA. 2009;106:19102–19107. doi: 10.1073/pnas.0910354106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Necas E, Znojil V. CFU-S content and cycling rate in several strains of mice. Exp Hematol. 1987;15:759–764. [PubMed] [Google Scholar]
- 8.Metcalf D, Laâbi Y. Lineage commitment and maturation induction in normal and leukemic preprogenitor cells. Ann N Y Acad Sci. 2001;938:278–291. doi: 10.1111/j.1749-6632.2001.tb03597.x. [DOI] [PubMed] [Google Scholar]
- 9.Metcalf D, Johnson GR, Mandel TE. Colony formation in agar by multipotential hemopoietic cells. J Cell Physiol. 1979;98:401–420. doi: 10.1002/jcp.1040980216. [DOI] [PubMed] [Google Scholar]
- 10.Keller GM, Phillips RA. Detection in vitro of a unique, multipotent hemopoietic progenitor. J Cell Physiol Suppl. 1982;1(suppl):31–36. doi: 10.1002/jcp.1041130408. [DOI] [PubMed] [Google Scholar]
- 11.Humphries RK, Jacky PB, Dill FJ, Eaves AC, Eaves CJ. CFU-S in individual erythroid colonies derived in vitro from adult mouse marrow. Nature. 1979;279:718–720. doi: 10.1038/279718a0. [DOI] [PubMed] [Google Scholar]
- 12.Humphries RK, Eaves AC, Eaves CJ. Self-renewal of hemopoietic stem cells during mixed colony formation in vitro. Proc Natl Acad Sci USA. 1981;78:3629–3633. doi: 10.1073/pnas.78.6.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. Observation of antigen-dependent CD8+ T-cell/ dendritic cell interactions in vivo. Cell Immunol. 2001;214:110–122. doi: 10.1006/cimm.2001.1895. [DOI] [PubMed] [Google Scholar]
- 14.Metcalf D. Blood Lines: An Introduction to Characterizing Blood Diseases of the Post-Genomic Mouse. Durham, NC: AlphaMed Press; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.