Abstract
Dopamine release associated with motivational arousal is thought to drive goal-directed learning and consolidation of acquired memories. This dopamine hypothesis of learning and motivation directly suggests that dopamine is necessary for modifications of excitatory synapses in dopamine terminal fields, including the prefrontal cortex (PFC), to “stamp in” posttrial memory traces. It is unknown how such enabling occurs in native circuits tightly controlled by GABAergic inhibitory tone. Here we report that dopamine, via both D1-class receptors (D1Rs) and D2-class receptors (D2Rs), enables the induction of spike timing–dependent long-term potentiation (t-LTP) in layer V PFC pyramidal neurons over a “window” of more than 30 ms that is otherwise closed under intact inhibitory constraint. Dopamine acts at D2Rs in local GABAergic interneurons to suppress inhibitory transmission, gating the induction of t-LTP. Moreover, dopamine activates postsynaptic D1Rs in excitatory synapses to allow t-LTP induction at a substantially extended, normally ineffective, timing interval (+30 ms), thus increasing the associability of prepost coincident stimuli. Although the D2R-mediated disinhibition alone is sufficient to gate t-LTP at a normal timing (+10 ms), t-LTP at +30 ms requires concurrent activation of both D1Rs and D2Rs. Our results illustrate a previously unrecognized circuit-level mechanism by which dopamine receptors in separate microcircuits cooperate to drive Hebbian synaptic plasticity across a significant temporal window under intact inhibition. This mechanism should be important in functioning of interconnected PFC microcircuits, in which D1Rs and D2Rs are not colocalized but their coactivation is necessary.
Keywords: spike timing-dependent plasticity, PKA, GABAergic inhibition, learning, reward
Dopamine released in its terminal fields, linked to motivational arousal, is hypothesized to drive new learning and to consolidate posttrial memory traces, perhaps by attaching motivational salience to otherwise neutral environmental stimuli (1). The prefrontal cortex (PFC) is a major forebrain dopamine target that mediates executive functions (2). Certain PFC functions, such as rule learning and strategy shifting, may depend on lasting synaptic modifications. In its empirical form, the dopamine hypothesis of learning and motivation posits that dopamine, via D1-class receptors (D1Rs; D1 and D5) and D2-class receptors (D2Rs; D2, D3, D4), serves as an instructing signal that enables and/or facilitates synaptic modifications to reinforce ongoing associative learning and mnemonic processes (3). This might be particularly true for the PFC, as this association cortex differs significantly in plasticity mechanisms versus other areas. For instance, sensory experience is crucial in remodeling synaptic connections in sensory cortices (4), but is less so in scalping PFC circuits, which, on the other hand, are readily modified by drugs and stress known to elicit dopamine release (5).
Spike timing-dependent plasticity of glutamatergic synapses is a Hebbian synaptic learning rule that may underlie associative learning and cognitive processes (6, 7). t-LTP is induced when presynaptic spiking precedes postsynaptic spiking within a narrow temporal window (8–11). However, the rules governing t-LTP threshold, magnitude, and timing window are not rigid and are influenced by neuromodulatory inputs (12–14), including dopamine (15–17). Further, excitatory circuits are often embedded in a powerful local inhibitory network, which tightly controls the excitability, timing of firing, and synaptic plasticity of pyramidal cells via feedback or feedforward mechanisms (18, 19). Indeed, induction of LTP in native circuits is highly susceptible to GABAergic inhibition (20–24), suggesting that an endogenous role of these inhibitory mechanisms is to constrain synaptic modifications.
Both excitatory and inhibitory circuits in the PFC are innervated by dopamine. Dopaminergic terminals contact distal spines of deep layer PFC pyramidal cells (25, 26), where both D1Rs and D2Rs are concentrated, providing the structural basis for dopamine modulation of individual excitatory synapses (3, 27). Dopamine receptors are also found in PFC interneurons (28–30). Through these receptors, dopamine has been shown to modulate interneuron excitability (31–33) and inhibitory transmission to pyramidal cells (30, 34–36) to impose a powerful control over PFC excitatory circuits.
It is unknown how dopamine enables synaptic modifications in native PFC excitatory circuits. Using whole cell recordings on mouse PFC slices, we show that t-LTP is absent in layer V pyramidal neurons under intact GABAergic inhibition unless brief phasic dopamine is supplied. Importantly, this dopamine enabling of t-LTP requires cooperation between D1Rs in excitatory circuits and D2Rs in inhibitory circuits, whereby D2Rs gate t-LTP induction by suppressing GABAergic inhibition and D1Rs control the timing window for t-LTP induction, respectively. Cooperatively, these receptors in separate circuits define a window of at least 30 ms for t-LTP under normal inhibitory constraint.
Results
Dopamine Enables t-LTP in a Native PFC Circuit.
We investigated t-LTP induction in PFC slices with the GABAergic transmission unblocked to mimic physiological conditions in native circuits. Excitatory postsynaptic potentials (EPSPs) were recorded from layer V pyramidal neurons (Fig. 1A) at the resting membrane potential (−67 ± 1.1 mV) while stimulating layer II/III (37). The resting level is close to the reversal potential of inhibitory postsynaptic currents (IPSCs; approximately −67 mV; Fig. S1A). At this level, EPSPs were mediated primarily by AMPA receptors (AMPARs), with little contamination by inhibitory postsynaptic potentials (IPSPs) evoked as a result of direct excitation of local interneurons (i.e., monosynaptic) or activation of feedforward inhibitory (i.e., multisynaptic) pathways (Fig. S1B).
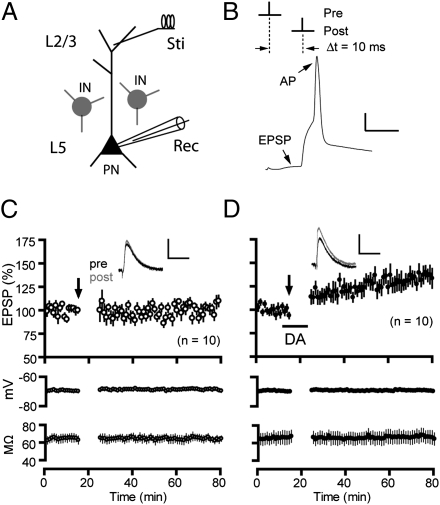
Fig. 1.
Dopamine enables t-LTP in the PFC under intact GABAergic inhibition. (A) Schematic of stimulation and recording configuration. IN, interneuron; PN, pyramidal neuron; Sti, stimulation; Rec, recording. (B) t-LTP induction protocol (Δt = 10 ms). A representative EPSP-AP response during a paired stimulus is shown. (Scale bar: 20 mV, 10 ms.) (C) Lack of lasting changes in EPSP amplitude following t-LTP induction in normal extracellular bath. (D) t-LTP induced in the presence of dopamine (100 μM) applied to the bath during EPSP-AP pairings. Lower panels in C and D show stability of membrane potentials and series resistance during experiments. Insets are averages of five EPSPs recorded 5 min before and 30 min after t-LTP induction (arrows). (Scale bars for EPSPs: 2 mV, 25 ms.) Values in parentheses indicate numbers of cells examined except as noted otherwise.
To induce t-LTP, presynaptic stimulation (resulting in an EPSP) was paired with a single postsynaptic action potential (AP) for 10 min at 0.1 Hz, with the onset of EPSP proceeding the AP (i.e., prepost) by a timing interval (Δt) of 10 ms (Fig. 1B). This protocol did not produce lasting changes in EPSP amplitude (102.6 ± 3.8% of baseline; Fig. 1C), indicating the absence of t-LTP under physiological conditions of intact inhibition. However, when dopamine (100 μM) was applied (approximately 12 min) during EPSP-AP pairings, significant (135.3 ± 7.6%; P < 0.01 vs. no dopamine; Fig. 1D) and long-lasting (>60 min) enhancement of EPSPs was observed. A similar result was also obtained for dopamine at 20 μM (137.8 ± 4.8%; Fig. S2). Presynaptic (95.2 ± 2.0%; Fig. S3A) or postsynaptic (101.2 ± 2.0%; Fig. S3B) stimulation alone failed to induce significant changes in EPSPs, suggesting that this t-LTP depends on near-coincident prepost spiking, a characteristic of Hebbian synaptic plasticity. The dopamine-enabled t-LTP was not caused by a delayed potentiation of EPSPs by dopamine itself because bath-applied dopamine in the absence of EPSP-AP pairings produced a reversible depression of EPSPs, mediated by D1Rs (Fig. S4). In addition, dopamine had little effect on the intrinsic excitability of these neurons (Fig. S5). Importantly, the same prepost pairing protocol did not alter the efficacy of GABAergic inputs to pyramidal neurons (Fig. S1 C–E), regardless of dopamine, ruling out the possibility that any residual inhibitory components undetectable under our experimental conditions might contribute to the dopamine enabling of t-LTP. Taken together, dopamine is necessary to gate t-LTP in a PFC synapse when GABAergic inhibition is intact.
We then investigated the induction and expression mechanisms underlying the dopamine-enabled t-LTP (Δt = 10 ms). Loading postsynaptic cells with the Ca2+ chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA; 15 mM) blocked t-LTP induction (100.6 ± 0.9%; Fig. S6A). Application of APV, an NMDA receptor (NMDAR) antagonist (50 μM), to the bath not only blocked LTP, but also unmasked a long-term depression (74.4 ± 7.6%; Fig. S6B). Thus, the induction of this t-LTP requires an increase of postsynaptic Ca2+ concentrations and is likely mediated by NMDARs. We also analyzed paired-pulse facilitation (PPF), a measure of presynaptic release probability (38) before and after t-LTP induction. There was no significant difference in PPF (interpulse interval: 50 ms) before and after t-LTP induction (Fig. S6 C and D), suggesting no change in release probability associated with t-LTP expression. Therefore, expression of the dopamine-enabled t-LTP may also involve a postsynaptic mechanism.
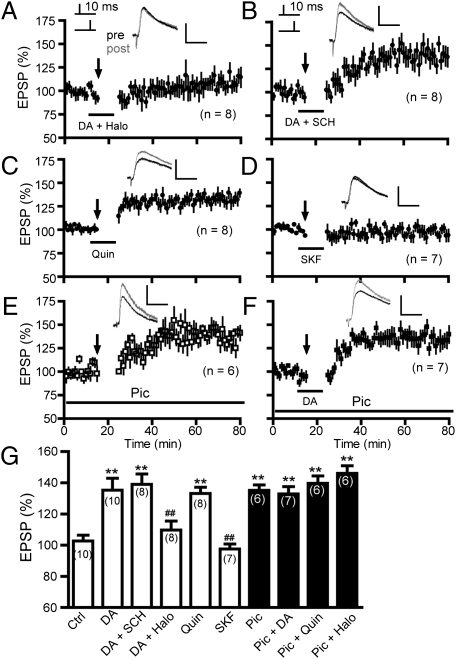
We determined the dopamine receptor class mediating the dopamine-enabled t-LTP induction (Δt = 10 ms). Blockade of D2Rs by haloperidol (2 μM) during dopamine application completely abolished this t-LTP (109.6 ± 5.8%; Fig. 2 A and G). In contrast, selective blockade of D1Rs by SCH23390 (10 μM) was without effect (138.9 ± 6.7%; Fig. 2 B and G). In addition, substituting dopamine with the D2R agonist quinpirole (10 μM; 133.1 ± 4.0%; Fig. 2 C and G), but not the D1R agonist SKF81297 (2 μM; 97.6 ± 3.2%), was sufficient to induce t-LTP (Fig. 2 D and G). These results indicate that dopamine gates PFC t-LTP at an Δt of 10 ms via D2Rs under intact GABAergic transmission.
Fig. 2.
Dopamine enabling of t-LTP induction is through depression of GABAergic transmission mediated by D2Rs. (A and B) Effects of the D2R antagonist haloperidol (Halo, A) and the D1R antagonist SCH23390 (SCH, B) on dopamine-enabled t-LTP. (C and D) The D2R agonist quinpirole (Quin, C), but not the D1R agonist SKF81297 (SKF, D) mimicked the effect of dopamine. (E) t-LTP was induced in the presence of picrotoxin (Pic) in the bath. (Scale bars: 2 mV, 25 ms.) (F) Gating of t-LTP by dopamine and picrotoxin was mutually occlusive. (G) t-LTP summary. **P < 0.01 vs. control (Ctrl) and ##P < 0.01 vs. dopamine (Student’s t test).
Suppressing GABAergic Inhibition Mimics D2R Activation in Gating t-LTP.
LTP induction at many excitatory synapses is sensitive to GABAergic inhibition (20–24). We found that PFC t-LTP (Δt = 10 ms) was induced (135.1 ± 3.5%) in the presence of the GABAA receptor (GABAAR) blocker picrotoxin (50 μM; Fig. 2 E and G), suggesting that GABAergic inhibition also constrains t-LTP in the PFC. The picrotoxin-enabled t-LTP was indistinguishable from that induced by dopamine (Fig. 2G); moreover, applying dopamine (132.9 ± 4.6%; Fig. 2F) or quinpirole (139.6 ± 4.8%; Fig. 2G) to the bath that already contained picrotoxin did not further increase the magnitude of t-LTP, compared with those enabled by dopamine or picrotoxin alone (Fig. 2G). Thus, suppressing GABAergic transmission occludes the effect of D2Rs. In addition, haloperidol no longer abolished t-LTP in the presence of picrotoxin (145.9 ± 4.9%; Fig. 2G), suggesting that GABA release to pyramidal neurons is downstream to D2R action. Together, our data indicate that dopamine enables t-LTP (Δt = 10 ms) by suppressing GABAergic inhibition, likely by activating presynaptic D2Rs in interneurons.
Dopamine Activates Presynaptic Interneuron D2Rs to Inhibit GABAergic Transmission.
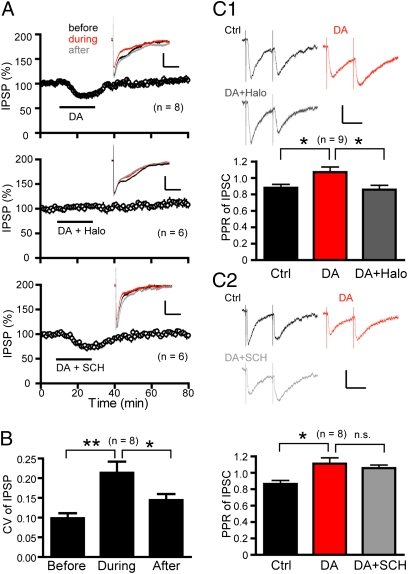
To directly investigate the dopamine suppression of GABAergic transmission to pyramidal neurons, we isolated monosynaptic IPSPs (Fig. 3A), mediated primarily by GABAARs, in the presence of NMDAR and AMPAR blockers. A brief application of dopamine elicited a significant and reversible decrease (75.3 ± 2.1% of baseline; P < 0.01) in IPSP amplitude. This depression was abolished by haloperidol, but not SCH23390, suggesting that similar to the D2R-depenndent gating of t-LTP, this dopamine suppression of IPSPs was also mediated by D2Rs (Fig. 3A). Further confirming this result, selective stimulation of D2Rs, but not D1Rs, reversibly decreased pharmacologically isolated IPSCs under voltage clamping at −65 mV (Fig. S7).
Fig. 3.
Dopamine inhibits GABAergic transmission via presynaptic D2Rs. (A) Dopamine reversibly depressed IPSPs via D2Rs. Vm = −55 mV. (Scale bars: 4 mV, 400 ms.) (Insets) IPSPs recorded before, during, and 30 min after drug applications, respectively. (B) CV of IPSPs before, during, and 30 min after drug applications. *P < 0.05 and **P < 0.01 (paired Student’s t tests). (C) PPR analysis: dopamine application increased the PPR of IPSCs, which was blocked by subsequent application of haloperidol (C1), but not SCH23390 (C2). Vc = −65 mV. (Scale bars: 25 pA, 20 ms.) *P < 0.05; n.s., not significant (paired Student’s t test).
We next investigated the mechanism underlying dopamine suppression of GABAergic transmission. Dopamine reduced the amplitude of both spontaneous IPSCs (sIPSCs) and miniature IPSCs (mIPSCs) (Fig. S8). This finding could reflect reductions in the presynaptic quantal content and/or the postsynaptic GABAAR responsiveness. However, currents elicited by perisomatic puffing of the GABAAR agonist muscimol (25 μM) were not affected by dopamine (Fig. S9A). Further, blockade of postsynaptic G proteins by including guanosine 5′-O-thiodiphosphate (GDPβS; 500 μM) in the patch electrode did not prevent dopamine suppression of IPSCs (Fig. S9B). Thus, postsynaptic GABAARs are unlikely to be modulated by dopamine. Dopamine also decreased the frequency of sIPSCs, but not mIPSCs, which may reflect decreased spontaneous firings in interneurons and/or reduced AP-evoked GABA release probability. The latter possibility appears most likely because dopamine predominately increases the intrinsic excitability of PFC interneurons (31–33, 35, 39). Together, these results suggest that dopamine inhibits presynaptic GABA release mechanisms—quantal content and release probability in particular—to suppress GABAergic transmission to pyramidal neurons.
The relatively small effect (approximately 92% of control) of dopamine on mIPSC amplitude argues that dopamine modulation of GABA release probability may represent the more dominant mechanism underlying dopamine suppression of GABAergic transmission. Indeed, dopamine significantly increased PPR of IPSCs (interpulse interval: 50 ms), a depression (i.e., paired-pulse depression) at this synapse (Fig. 3C), confirming that GABA release probability was decreased by dopamine. This effect was blocked by haloperidol, but not SCH23390, consistent with the role of D2Rs in mediating this presynaptic dopamine action. In these experiments, the GABABR antagonist SCH50911 (10 μM) was included in the bath to prevent activation of this presynaptic autoreceptor (40); thus, the dopamine inhibition of GABA release was unlikely mediated through GABAB autoreceptors. Finally, the coefficient of variation (CV), computed for IPSP amplitude in individual neurons (15, 36), was significantly increased following dopamine wash-in and returned to baseline level after dopamine wash-out (Fig. 3B), consistent with a decrease in GABA release probability. Together, our data indicate that dopamine suppresses GABAergic inhibition of pyramidal neurons primarily by depressing GABA release via D2Rs at presynaptic GABAergic terminals.
Postsynaptic D1Rs Extend t-LTP Induction Interval at Isolated Excitatory Synapses.
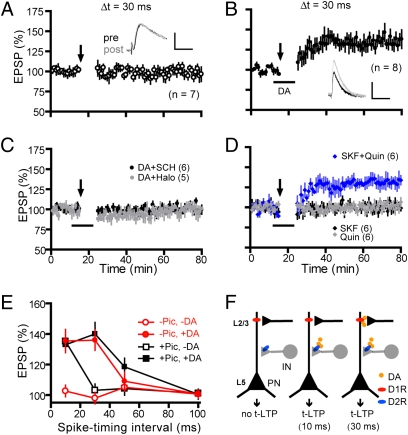
Results presented above demonstrate that D2Rs, but not D1Rs, gate t-LTP at a narrow timing interval (10 ms) under a physiological condition. However, a classical view holds that dopamine facilitates PFC LTP at glutamatergic synapses primarily via D1Rs (41, 42). To further investigate dopamine modulation of t-LTP, we examined the effects of dopamine on t-LTP in the presence of picrotoxin at an extended interval (Δt = 30 ms). t-LTP was absent at this interval (103.0 ± 4.2%; Fig. 4A). When dopamine was applied during EPSP-AP pairings, however, robust t-LTP was induced (140.0 ± 7.6%; Fig. 4B). This t-LTP was blocked by SCH23390 (102.1 ± 1.6%; Fig. 4C), but not haloperidol (138.0 ± 5.2%; Fig. 4D). Consistently, application of SKF81297 (132.4 ± 6.1%; Fig. 4E) allowed t-LTP at an Δt of 30 ms, which was indistinguishable from that enabled by dopamine, indicating that D1R activation is sufficient to mimic dopamine to enable t-LTP at this extended interval. Thus, dopamine broadens the PFC t-LTP timing window at pharmacologically isolated excitatory synapses, which is mediated by D1Rs.
Fig. 4.
Dopamine extends the t-LTP induction interval via D1R-coupled cAMP-PKA signaling in postsynaptic neurons of excitatory synapses. (A) Absence of t-LTP induction in normal bath. All experiments in this figure were done in a bath containing picrotoxin to remove GABAAR-mediated inhibition. t-LTP was induced by EPSP-AP pairings at an Δt of 30 ms. (B) t-LTP induced in the presence of dopamine during EPSP-AP pairings. (C and D) Dopamine enabled t-LTP was blocked by SCH23390 (C) but not haloperidol (D). (E) SKF81297 alone enabled t-LTP at an Δt of 30 ms. (F and G) SKF81297-enabled t-LTP was abolished by bath-applied RP-cAMPS (F) or PKI 14–22 (G). (Scale bars: 2 mV, 25 ms.) (H) Summary of t-LTP at an Δt of 30 ms. **P < 0.01 vs. control (Ctrl) and ##P < 0.01 vs. dopamine (Student’s t test).
We next asked whether the D1R-dependent enabling of t-LTP at an Δt of 30 ms is mediated by the classical cAMP-PKA signaling. Coapplication of the cAMP inhibitor RP-cAMPS (50 μM) with SKF81297 during pairings prevented t-LTP (98.6 ± 2.5%; Fig. 4F). Protein kinase inhibitor (PKI) 14–22 (1 μM), an inhibitory peptide of PKA, also blocked the SKF81297-enabled t-LTP (101.4 ± 1.8%; Fig. 4G). Importantly, loading postsynaptic neurons with the membrane-impermeable form of PKI (PKI 6–22; 20 μM) completely abolished SKF81297-enabled t-LTP (96.4 ± 2.0%; Fig. 4H), suggesting that t-LTP at this expanded interval depends on postsynaptic cAMP-PKA activation. Our results suggest that dopamine acts at postsynaptic D1Rs and downstream cAMP-PKA signaling in pyramidal cells to enable t-LTP at an Δt of 30 ms, a normally ineffective timing interval, thus increasing the associability of pre- and postsynaptic stimuli.
D1Rs and D2Rs in Separate Circuits Cooperate to Broaden t-LTP Window.
The dual capabilities of dopamine to gate t-LTP by suppressing GABAergic inhibition via presynaptic D2Rs in interneurons and to increase the prepost coincidence associability via postsynaptic D1Rs in pyramidal neurons suggest that dopamine itself would be sufficient to drive t-LTP at extended timings even when GABAergic transmission is unblocked, and that this driving depends on both receptors in separate circuits. Indeed, under intact inhibition, no t-LTP (97.9 ± 3.7%) was induced at an Δt of 30 ms (Fig. 5A) unless dopamine was supplied (135.9 ± 6.6%; Fig. 5B). This t-LTP was blocked by either SCH23390 (100.3 ± 1.0%) or haloperidol (99.8 ± 4.9%; Fig. 5C), indicating that both receptors are necessary. Further, application of both SKF81297 and quinpirole (131.6 ± 5.7%), but not either alone (SKF81297, 101.6 ± 3.2%; quinpirole, 100.2 ± 1.8%; Fig. 5D), was required to induce this t-LTP. Thus, D1Rs and D2Rs cooperate to allow dopamine to enable t-LTP at the extended timing interval under intact inhibition.
Fig. 5.
D1R and D2R coactivation drives t-LTP induction at extended timing window under intact GABAergic inhibition. (A) Absence of t-LTP induction in normal bath. (B) t-LTP induced in the presence of dopamine during EPSP-AP pairings. (Scale bars: 2 mV, 25 ms.) (C) Dopamine enabled t-LTP at an Δt of 30 ms was blocked by either SCH23390 or haloperidol. (D) Both SKF81297 and quinpirole, but not either alone, were needed to enable t-LTP. Δt was 30 ms for experiments in A–D. (E) Summary of dopamine effects on t-LTP at various timing intervals. Some data presented are replotted for direct comparisons. (F) Working hypothesis: under native conditions, layer V PFC neurons are under powerful constraint by local GABAergic interneurons, as no t-LTP can be induced by our induction protocol (Left). At the 10-ms timing interval, activation of presynaptic D2Rs on local interneurons alone is sufficient to gate t-LTP, presumably by suppressing GABAergic inhibition (Center). At the 30-ms interval, coactivation of both D1Rs and D2Rs in different microcircuits is necessary to permit t-LTP, mediated by concurrent D2R suppression of GABAergic inhibition and postsynaptic D1R-activated cAMP-PKA signaling (Right).
Fig. 5E summarizes PFC t-LTP induction at Δt values of 10 ms, 30 ms, and beyond under various conditions. These data show that: (i) t-LTP is completely absent in standard extracellular bath; (ii) blocking GABAergic inhibition with picrotoxin allows a t-LTP induction window of at least 10 ms, consistent with the narrow t-LTP timing windows at most glutamatergic synapses (7); (iii) in the presence of picrotoxin, dopamine extends this window to at least 30 ms without affecting t-LTP induced at normally effective interval (e.g., 10 ms) or the maximum magnitude of t-LTP; and (iv) dopamine alone in the absence of picrotoxin is sufficient to operate this extended timing window. In conclusion, our data support a circuitry-based D1/D2 cooperation model in dopamine enabling of t-LTP, whereby D2R activation suppresses the GABAergic tone to gate the induction of t-LTP and D1R activation extends the timing window for t-LTP induction, respectively (Fig. 5F).
Discussion
We report here that dopamine enables the induction of timing-dependent synaptic modifications over a significant temporal window under physiological conditions of intact GABAergic transmission in the PFC. This dopamine enabling of t-LTP displays differential dependence on D1Rs and D2Rs: that induced at 10 ms requires only D2R activation but that induced at 30 ms requires D1R/D2R coactivation. Our study indicates that presynaptic D2Rs in interneurons play a permissive role and gate t-LTP, whereas postsynaptic D1Rs in pyramidal neurons regulate the associability of prepost spiking. Thus, dopamine receptors in separate circuits cooperate to drive associative synaptic plasticity in the PFC.
Presynaptic D2Rs in Inhibitory Circuits Gate PFC t-LTP.
The present study indicates that the induction of associative LTP in the PFC is normally under strong inhibitory control, as observed in other regions (20–24). Inhibitory inputs may deter LTP by limiting postsynaptic depolarization necessary for LTP induction via, e.g., quenching dendritic EPSPs (43), shunting back-propagating APs or dendritic Ca2+ spikes (44, 45), and/or derailing temporal summation of EPSPs during repetitive presynaptic activity (21, 46). These postsynaptic inhibitory mechanisms are expected to decrease activation of NMDARs and other determinants (e.g., Ca2+ channels) necessary for t-LTP during prepost spiking. Our data suggest that the inhibition is powerful but can be effectively relieved by dopamine.
The mechanism by which dopamine suppresses GABAergic transmission is likely presynaptic. This is supported by, following dopamine application, an increase in PPR of IPSCs, an increase in CV of IPSPs, a persistent IPSC suppression in the presence of postsynaptic GDPβS, and an unaltered muscimol-elicited postsynaptic GABAAR responsiveness. Interestingly, dopamine inhibits the frequency of sIPSCs, but not mIPSCs, and only slightly reduces the mIPSC amplitude. These observations, together with the largely excitatory role for dopamine in PFC interneuron excitability (31–33, 35, 39), support the idea that dopamine primarily affects AP-evoked GABA release mechanisms. Our data are in line with previous reports that dopamine inhibits GABAergic transmission to deep-layer PFC pyramidal neurons by directly modulating presynaptic GABA release (35, 36). These results, however, do not exclude the possibility that dopamine may regulate higher-order disinhibitory interneurons within the same feed forward pathway (21), which may indirectly contribute to dopamine suppression of GABAergic transmission to pyramidal neurons.
Our data suggest that D2Rs at interneuron terminals (28, 30, 47) mediate the dopamine suppression of evoked GABAergic transmission to layer V neurons, consistent with recent studies (30, 35). However, in a dual whole-cell recording study (36), Gao et al. showed that dopamine depressed inhibitory transmission between connected fast-spiking interneuron and pyramidal neuron pairs in layer V through D1Rs in presynaptic interneurons in the ferret PFC. A pyramidal neuron is likely bombarded by multiple interneurons with heterogeneous molecular, anatomic, synaptic, and functional properties. The variable results, although they do not exclude potential species-typical differences, suggest that certain interneurons (or subtypes) express D1Rs (29), and that the precise mode of dopamine modulation of GABAergic transmission to a target pyramidal cell may be interneuron subtype–dependent. The exact cell type(s) that mediate the D2R-dependent disinhibition in gating t-LTP remain to be determined.
The D2R-mediated enabling of PFC t-LTP is reminiscent of the dopaminergic gating of t-LTP (Δt = 10 ms) in the lateral amygdala, where dopamine directly reduces quantal content at inhibitory synapses onto projection neurons and indirectly enhances feed forward inhibition onto interneurons, both depending on presynaptic interneuron D2Rs (21). Norepinephrine also enables t-LTP (Δt = 4–6 ms) in the same pathway by decreasing local interneuron excitability (24). Thus, through varying mechanisms, neuromodulatory inputs appear to act as native disinhibitory systems to gate associative synaptic modifications in excitatory circuits. However, our study indicates that, although it can gate t-LTP induced by near-coincident prepost activity, disinhibition alone is incapable of driving all associative LTP, particularly those elicited by less correlated activity (Δt ≥ 30 ms). We have identified an additional D1-dependent postsynaptic mechanism that, in concert with the presynaptic disinhibition, can presumably drive associative synaptic modifications at all timings.
Postsynaptic D1Rs in Excitatory Circuits Regulate t-LTP Timing Window.
The classic facilitation of LTP by D1R signaling (41, 42, 48) often reflects changes in the magnitude of LTP, thought to result from modulation of postsynaptic AMPAR trafficking and phosphorylation, and/or presynaptic transmitter release (49). Our findings present an unconventional mode of facilitation whereby the induction timing window, but not the maximal level of t-LTP, is altered. As the width of t-LTP induction window depends on how close pre- and postsynaptic spiking are presented to the synapse, these findings suggest that D1Rs enhance the associability of prepost activity that leads to a gain in sensitivity for t-LTP induction. Similar mechanism has also been reported in dissociated hippocampal cultures (17) and slices (12), suggesting that it may represent a general, although underappreciated, mechanism by which dopamine and other neuromodulators (12) regulate the sensitivity and threshold of associative synaptic modifications. Our data further identify that D1Rs in postsynaptic pyramidal neurons, coupling to the cAMP-PKA signaling, mediate the dopamine enhancement of prepost associability in the PFC. This postsynaptic nature of dopamine modulation permits t-LTP timing window to be fine tuned at the single synapse level, providing a form of input specificity in dopamine modulation of synaptic plasticity.
What mechanism(s) might underlie the postsynaptic D1R broadening of t-LTP timing window? The window for t-LTP induction may depend on several processes (7): the kinetics of Mg2+ unblocking of glutamate-bound NMDARs (a classical coincidence detector) by back-propagating APs, the magnitude and dynamics of postsynaptic Ca2+ transients, or downstream Ca2+ signaling mechanisms. The D1R-mediated postsynaptic cAMP-PKA signaling could modulate NMDARs and/or dendritic ion conductances (27, 50), influencing coincidence detection, back-propagating APs (51), and/or Ca2+ dynamics. Activation of this cascade could also alter downstream kinase/phosphatase signaling important for LTP, e.g., CaMKII and protein phosphatase 1 (49, 52). Regardless of the mechanisms, our data indicate that postsynaptic D1Rs within individual synapses may serve as a coincidence modulator that can determine whether a synapse will undergo potentiation in response to certain activity patterns.
Circuit-Level D1/D2 Cooperation Driving Synaptic Plasticity.
Despite largely opposite intracellular signaling profiles mediated by D1Rs and D2Rs, behavioral and electrophysiological studies find cooperative effects of these receptors in dopamine target areas, including the PFC (53, 54). The D1/D2 synergism is best illustrated by dopamine modulation of neuronal firing rates in the basal ganglion and may be explained at the single-cell level by a G protein βγ subunit-dependent mechanism (55). However, these receptors are often expressed in different cells (56, 57) and a circuit-level cooperation between them is theoretically possible. Such cooperative mechanism may be important in interconnected microcircuits, where D1Rs and D2Rs are not colocalized but their coactivation is necessary, allowing these distributed receptors to drive separate cortical circuits simultaneously. Here, we provide experimental evidence that D1Rs in excitatory circuits and D2Rs in inhibitory circuits act cooperatively to drive a Hebbian synaptic plasticity in the PFC. This circuit mechanism may allow dopamine to gate associative synaptic modifications globally while regulating the timing window at individual synapses locally. This mechanism can play a crucial role in PFC-mediated cognitive functions that depend on activity-dependent synaptic plasticity.
Materials and Methods
Coronal PFC slices were prepared from young C57BL/6J mice as described (37). t-LTP was induced by 60 EPSP-AP pairs (0.1 Hz) with variable prepost timing intervals. LTP was quantified as the ratio of the average of 20 EPSPs between 50 and 60 min following LTP induction to the average of 20 EPSPs during a 10-min baseline recording. Data are expressed as mean ± SEM. Statistical comparisons were made with paired or unpaired Student’s t tests as appropriate. More details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Roger D. Spealman for comments. This work was supported by National Center for Research Resources Grant RR000168 (to the New England Primate Research Center), National Institutes of Health Grants DA021420 and NS057311, the National Alliance for Research on Schizophrenia and Depression, and the Williams F. Milton Fund (W.-D.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004108107/-/DCSupplemental.
References
- 1.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 2.Fuster JM. The Prefrontal Cortex. New York: Academic; 2008. pp. 333–385. [Google Scholar]
- 3.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 4.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 5.Kolb B, Cioe J. Organization and plasticity of the prefrontal cortex of the rat. In: Otani S, editor. Prefrontal Cortex: From Synaptic Plasticity to Cognition. Dordrecht, The Netherlands: Kluwer; 2004. pp. 1–32. [Google Scholar]
- 6.Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- 7.Caporale N, Dan Y. Spike timing-dependent plasticity: A Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- 8.Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- 9.Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- 10.Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sjöström PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–1164. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 12.Lin YW, Min MY, Chiu TH, Yang HW. Enhancement of associative long-term potentiation by activation of beta-adrenergic receptors at CA1 synapses in rat hippocampal slices. J Neurosci. 2003;23:4173–4181. doi: 10.1523/JNEUROSCI.23-10-04173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couey JJ, et al. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Seol GH, et al. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pawlak V, Kerr JN. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J Neurosci. 2008;28:2435–2446. doi: 10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang JC, Lau PM, Bi GQ. Gain in sensitivity and loss in temporal contrast of STDP by dopaminergic modulation at hippocampal synapses. Proc Natl Acad Sci USA. 2009;106:13028–13033. doi: 10.1073/pnas.0900546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonas P, Bischofberger J, Fricker D, Miles R. Interneuron diversity series: Fast in, fast out—temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci. 2004;27:30–40. doi: 10.1016/j.tins.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 20.Wigström H, Gustafsson B. Facilitated induction of hippocampal long-lasting potentiation during blockade of inhibition. Nature. 1983;301:603–604. doi: 10.1038/301603a0. [DOI] [PubMed] [Google Scholar]
- 21.Bissière S, Humeau Y, Lüthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- 22.Meredith RM, Floyer-Lea AM, Paulsen O. Maturation of long-term potentiation induction rules in rodent hippocampus: Role of GABAergic inhibition. J Neurosci. 2003;23:11142–11146. doi: 10.1523/JNEUROSCI.23-35-11142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tully K, Li Y, Tsvetkov E, Bolshakov VY. Norepinephrine enables the induction of associative long-term potentiation at thalamo-amygdala synapses. Proc Natl Acad Sci USA. 2007;104:14146–14150. doi: 10.1073/pnas.0704621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci USA. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: Synaptic targets and relation to dopamine terminals. J Comp Neurol. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Mrzljak L, et al. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- 29.Muly EC, 3rd, Szigeti K, Goldman-Rakic PS. D1 receptor in interneurons of macaque prefrontal cortex: Distribution and subcellular localization. J Neurosci. 1998;18:10553–10565. doi: 10.1523/JNEUROSCI.18-24-10553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiu CQ, Puente N, Grandes P, Castillo PE. Dopaminergic modulation of endocannabinoid-mediated plasticity at GABAergic synapses in the prefrontal cortex. J Neurosci. 2010;30:7236–7248. doi: 10.1523/JNEUROSCI.0736-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou FM, Hablitz JJ. Dopamine modulation of membrane and synaptic properties of interneurons in rat cerebral cortex. J Neurophysiol. 1999;81:967–976. doi: 10.1152/jn.1999.81.3.967. [DOI] [PubMed] [Google Scholar]
- 32.Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- 33.Gao WJ, Goldman-Rakic PS. Selective modulation of excitatory and inhibitory microcircuits by dopamine. Proc Natl Acad Sci USA. 2003;100:2836–2841. doi: 10.1073/pnas.262796399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Islas C, Hablitz JJ. Dopamine inhibition of evoked IPSCs in rat prefrontal cortex. J Neurophysiol. 2001;86:2911–2918. doi: 10.1152/jn.2001.86.6.2911. [DOI] [PubMed] [Google Scholar]
- 35.Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao WJ, Wang Y, Goldman-Rakic PS. Dopamine modulation of perisomatic and peridendritic inhibition in prefrontal cortex. J Neurosci. 2003;23:1622–1630. doi: 10.1523/JNEUROSCI.23-05-01622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu TX, et al. Hyperdopaminergic tone erodes prefrontal long-term potential via a D2 receptor-operated protein phosphatase gate. J Neurosci. 2009;29:14086–14099. doi: 10.1523/JNEUROSCI.0974-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 39.Tseng KY, et al. Excitatory response of prefrontal cortical fast-spiking interneurons to ventral tegmental area stimulation in vivo. Synapse. 2006;59:412–417. doi: 10.1002/syn.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang ZJ. GABAB receptor isoforms caught in action at the scene. Neuron. 2006;50:521–524. doi: 10.1016/j.neuron.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci. 2000;20:RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang YY, Simpson E, Kellendonk C, Kandel ER. Genetic evidence for the bidirectional modulation of synaptic plasticity in the prefrontal cortex by D1 receptors. Proc Natl Acad Sci USA. 2004;101:3236–3241. doi: 10.1073/pnas.0308280101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llinas R, Nicholson C. Electrophysiological properties of dendrites and somata in alligator Purkinje cells. J Neurophysiol. 1971;34:532–551. doi: 10.1152/jn.1971.34.4.532. [DOI] [PubMed] [Google Scholar]
- 44.Miles R, Tóth K, Gulyás AI, Hájos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 45.Tsubokawa H, Ross WN. IPSPs modulate spike backpropagation and associated [Ca2+]i changes in the dendrites of hippocampal CA1 pyramidal neurons. J Neurophysiol. 1996;76:2896–2906. doi: 10.1152/jn.1996.76.5.2896. [DOI] [PubMed] [Google Scholar]
- 46.Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- 47.Negyessy L, Goldman-Rakic PS. Subcellular localization of the dopamine D2 receptor and coexistence with the calcium-binding protein neuronal calcium sensor-1 in the primate prefrontal cortex. J Comp Neurol. 2005;488:464–475. doi: 10.1002/cne.20601. [DOI] [PubMed] [Google Scholar]
- 48.Otani S, Daniel H, Roisin MP, Crepel F. Dopaminergic modulation of long-term synaptic plasticity in rat prefrontal neurons. Cereb Cortex. 2003;13:1251–1256. doi: 10.1093/cercor/bhg092. [DOI] [PubMed] [Google Scholar]
- 49.Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: The DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 51.Johnston D, Hoffman DA, Colbert CM, Magee JC. Regulation of back-propagating action potentials in hippocampal neurons. Curr Opin Neurobiol. 1999;9:288–292. doi: 10.1016/s0959-4388(99)80042-7. [DOI] [PubMed] [Google Scholar]
- 52.Lisman JE, Zhabotinsky AM. A model of synaptic memory: A CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron. 2001;31:191–201. doi: 10.1016/s0896-6273(01)00364-6. [DOI] [PubMed] [Google Scholar]
- 53.Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: Beyond working memory. Psychopharmacology (Berl) 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- 54.Matsuda Y, Marzo A, Otani S. The presence of background dopamine signal converts long-term synaptic depression to potentiation in rat prefrontal cortex. J Neurosci. 2006;26:4803–4810. doi: 10.1523/JNEUROSCI.5312-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hopf FW, Cascini MG, Gordon AS, Diamond I, Bonci A. Cooperative activation of dopamine D1 and D2 receptors increases spike firing of nucleus accumbens neurons via G-protein betagamma subunits. J Neurosci. 2003;23:5079–5087. doi: 10.1523/JNEUROSCI.23-12-05079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerfen CR. The neostriatal mosaic: Multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 57.Vincent SL, Khan Y, Benes FM. Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J Neurosci. 1993;13:2551–2564. doi: 10.1523/JNEUROSCI.13-06-02551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.