Abstract
New results have brought to light the importance of the regulation of glucagon by β-cells in the development of diabetes. In this perspective, we examine the normal paracrinology of α- and β-cells in nondiabetic pancreatic islets. We propose a Sherringtonian model of coordinated reciprocal secretory responses of these juxtaposed cells that secrete glucagon and insulin, hormones with opposing actions on the liver. As insulin is a powerful inhibitor of glucagon, we propose that within-islet inhibition of α-cells by β-cells creates an insulin-to-glucagon ratio that maintains glycemic stability even in extremes of glucose influx or efflux. By contrast, in type 1 diabetes mellitus, α-cells lack constant action of high insulin levels from juxtaposed β-cells. Replacement with exogenous insulin does not approach paracrine levels of secreted insulin except with high doses that “overinsulinize” the peripheral insulin targets, thereby promoting glycemic volatility. Based on the stable normoglycemia of mice with type 1 diabetes during suppression of glucagon with leptin, we conclude that, in the absence of paracrine regulation of α-cells, tonic inhibition of α-cells improves the dysregulated glucose homeostasis. These results have considerable medical implications, as they suggest new approaches to normalize the extreme volatility of glycemia in diabetic patients.
Keywords: glucagon, glycemic control, insulin, leptin, type 1 diabetes
Traditional endocrinology focuses largely on actions of hormones on remote targets physically separated from the secreting organ. Scant attention is directed toward paracrine actions of hormones on target cells located within the secreting organ. In this perspective, we propose that certain paracrine interactions within the pancreatic islets play crucial roles in a vital homeostatic system and that disruption or dysfunction of these interactions can seriously compromise health.
Sherringtonian Paracrinology of α- and β-Cells
In his so-called Second Law, the British neurophysiologist Sir Charles Scott Sherrington proposed that reciprocal innervation of opposing muscles is required for coordinated muscular action. Thus, when the biceps contracts, the triceps must relax—and vice versa. Here we propose a Sherringtonian law of reciprocal secretion of the opposing hormones, insulin and glucagon, in tight coordination provided by the physical proximity of α- and β-cells. As insulin is a powerful glucagon suppressor (1–4), an increase in insulin will suppress glucagon secretion, and a decrease will increase it. We suggest that coordination of these two hormones, which diametrically oppose each other's action on hepatic fuel metabolism (5), provides a level of glycemic stability that could not otherwise exist.
The ability of insulin and glucagon to maintain blood glucose levels within a narrow range during extremes of caloric stress provides a critical survival asset (5). They make it possible to switch almost instantly from insulin-mediated stockpiling of surplus calories in time of nutrient abundance to glucagon-mediated retrieval of these calories in time of acute or chronic need, as in fight, flight, and famine situations. Insulin, the anabolic hormone, promotes the storage of unused calories as glycogen in liver and skeletal muscle and as triacylglycerol in adipose tissue. Glucagon, the catabolic hormone, regulates caloric retrieval and redistribution by activating glycogenolysis, ketogenesis, and gluconeogenesis (6, 7). The relative concentrations of the two hormones help determine whether the liver functions as an organ of fuel storage or fuel production.
α–β-Cell Relationships in Rodents
Although the topography of islet cells differs in rodents and humans, in both species, β-cells form a core from which insulin can easily reach α-cells and manage their secretory activity before exiting the islet. In rodents, the β-cell core makes up approximately 70% of the total endocrine population of the microorgans (8). The glucagon cells are arrayed in the periphery of the islet, so that only a minority of β-cells are juxtaposed to α-cells. However, the intraislet microcirculation appears to provide an insulin pathway from β-cells to α-cells (9). Arteriolar blood enters the core of the islet, where β-cells enrich it with the highest insulin concentration in the body as it flows to the mantle of α-cells. The physiologic importance of this intraislet vascular connection between β- and α-cells is demonstrated by the fact that a neutralizing antiinsulin serum perfused into the artery of an isolated rat pancreas causes glucagon levels in the pancreatic venous effluent to soar precipitously, whereas nonimmune serum has no effect (10). We propose that, in rodents, this microcirculatory connection between β-cells and α-cells accounts for the remarkably coordinated responses of α- and β-cells to minor changes in blood glucose.
α–β-Cell Relationships in Human Islets
The architecture of normal islets in man differs from that of rodents in two ways. First, in humans, the β-cells form a lower percentage of the endocrine population than in rodent islets, but as in rodent islets, they occupy a core position surrounded by mantles of α-cells in pseudolobular subdivisions, with blood vessels at their periphery (11, 12). Second, in human islets the α- and β-cells are mixed together, with more than 70% of β-cells in contact with non–β-cells (13) (Fig. 1A). If anything, the morphologic difference in the human islets compared with rodents would tend to increase the possibility of paracrine interactions.
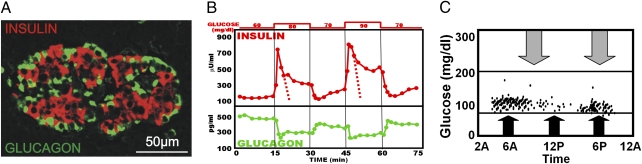
Fig. 1.
(A) Confocal microscopy of a normal human islet shows the extensive juxtaposition of insulin and glucagon-containing cells. Note that clusters of insulin-containing cells (red) are surrounded by glucagon-containing cells (green), which seem to line the blood vessels. (Photomicrograph provided by L.O.) (B) Demonstration of the reciprocal responses of insulin and glucagon to 20 mg/dL changes in the concentration of glucose perfusing the isolated pancreas of rats. Note the dramatic but short-lived spike of insulin secretion followed by a second, more prolonged, period of hyperinsulinemia. The dotted lines are intended to mark the full contour of the first phase, which is concealed by the second phase of insulin release. Note that the reciprocal change in glucagon occurs almost simultaneously with changes in insulin. (C) The range of plasma glucose levels in normal individuals. Even extremes of caloric stress do not raise or lower glycemia above this narrow range. Intensive exercise is marked by the gray arrows; oversized meal ingestion is marked by the black arrows.
In normal humans and rodents, β-cells respond to glucose with an immediate short-lived spike of insulin release, followed early in its downward course by a prolonged second phase (Fig. 1B). Based on calculations from published patterns of insulin release from perfused rat pancreas (14), we estimate that first phase represents only approximately 7% of the total insulin released in response to a stimulatory challenge. This seems insufficient by itself to exert a significant glycemic effect. The second phase must therefore provide most of the insulin for systemic metabolic regulation. Could the function of the brief but dramatic initial insulin spike be a paracrine signal that suppresses glucagon whenever glucose level increases to minimize postprandial hyperglycemia? Normally the insulin spike is always accompanied by a reciprocal decrease in glucagon, consistent with the possibility that it exerts a paracrine suppressive effect on α-cells. Conversely, when insulin levels decrease in response to a decrease in glucose, normally a reciprocal increase in glucagon occurs (Fig. 1B). We propose that it is these reciprocal changes in insulin and glucagon that provide the remarkable defense against glycemic perturbations that is characteristic of normal humans (Fig. 1C). Although autonomic innervation must play an important role in the coordination of reciprocal secretion, as depicted schematically in Fig. 2, normal regulation of α-cells requires the juxtaposed presence of normally functioning β-cells.
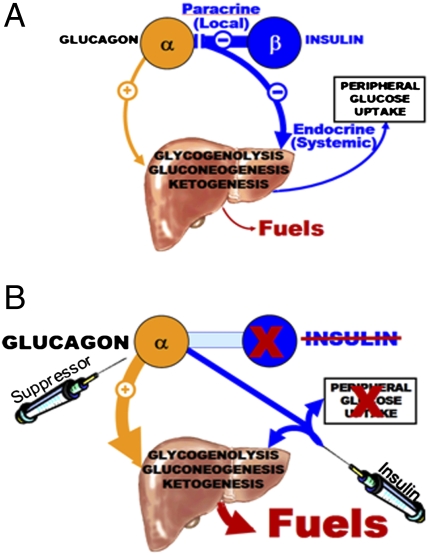
Fig. 2.
(A) Representation of the Sherringtonian model of coordinated reciprocal paracrine hormone secretion in the regulation of homeostasis of fuel production and utilization. In this model, the β-cell controls basal α-cell secretion of glucagon via tonic paracrine inhibition. When a carbohydrate-containing meal stimulates insulin secretion, the paracrine insulin promptly suppresses the α-cells to reduce glucagon secretion and lower hepatic fuel production. Meanwhile, the endocrine insulin that enters the systemic circulation reduces hepatic fuel production by opposing glucagon action and also enhancing glucose uptake by skeletal muscle and adipocytes. (B) A representation of the paracrinopathy of T1DM and a novel strategy for its management. When the β-cells are absent and a paracrine source of insulin-mediated α-cell suppression is absent, unregulated hyperglucagonemia contributes to glycemic volatility. Peripheral injections of insulin cannot approach the high paracrine levels provided by local insulin secretion by juxtaposed β-cells without overinsulinizing the downstream targets of insulin, thus further destabilizing glycemia. By suppressing glucagon secretion with leptin, one can restore the tonic inhibition on α-cells while lowering peripheral insulin levels to a physiologic range and thus reduce risk of hypoglycemia.
Paracrinopathy of α-Cells in Type 1 Diabetes
In type 1 diabetes mellitus (T1DM), β-cells are destroyed and largely replaced by α-cells (Fig. 3A). These α-cells lack the tonic restraint normally provided by the high local concentrations of insulin from juxtaposed β-cells. As a consequence, inappropriate hyperglucagonemia ensues (1–4) (Fig. 3B). It has become increasingly certain that the lethal catabolic consequences of complete insulin deficiency do not occur if the hyperglucagonemia is suppressed by somatostatin (15–17) or by leptin (18, 19) or if its action is blocked by drugs (20) or by genetic disruption of glucagon receptors. Glucagon receptor-null mice remained completely normal for 2 to 3 mo despite complete insulin deficiency (18). This compelling evidence of the essentiality of hyperglucagonemia in mediating the lethal catabolic phenotype of insulin deficiency challenges one of the most deeply ingrained of all medical dogmas: that insulin lack alone is responsible for the phenotype of untreated T1DM and that it can be reversed or prevented only by insulin replacement. However, 40 y of evidence implicating hyperglucagonemia as the sine qua non of the T1DM phenotype now supports the concept that glucagon mediates the consequences of insulin deficiency.
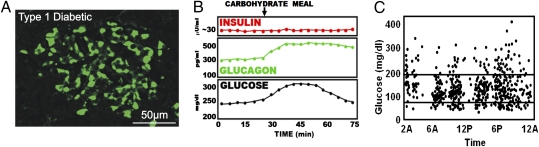
Fig. 3.
Morphological, paracrinological, and clinical features of pancreatic islets in T1DM. (A) Confocal microscopy of islet from a patient with T1DM shows replacement of insulin-containing cells with glucagon-containing cells (green) devoid of contact with β-cells. (Photomicrograph provided by L.O.) (B) In the absence of juxtaposed insulin-secreting cells, basal glucagon levels are increased and they increase further in a paradoxical response to hyperglycemia from a carbohydrate-containing meal, thereby worsening the basal hyperglycemia. (C) Even the subjects with optimally controlled T1DM exhibited severe glycemic volatility in a study by Derr et al. (31).
Volatility Problem in T1DM
The single most serious therapeutic problem of open-loop insulin monotherapy of T1DM is glycemic volatility (Fig. 3C). It is in part the result of paradoxical behavior by α-cells during glycemic change. This behavior can be ascribed to the fact that the paracrine insulin level required to suppress α-cells is at least 50 times the levels required for glucoregulation of the peripheral targets This means that the hypersecreting α-cells cannot be suppressed by injected insulin without simultaneously “overinsulinizing” the liver and posthepatic tissues, as can be deduced from Fig. 2B.
It is not widely appreciated that, when glucose increases without a parallel insulin increase, hyperglycemia stimulates glucagon. This is crucially important in T1DM, in which insulin levels do not increase when glucose increases. The hyperglycemia will therefore paradoxically stimulate glucagon secretion (21, 22), as illustrated in Fig. 4. The mechanism of glucose stimulation of glucagon secretion is under investigation. And conversely, because in monotherapy with insulin, the levels of exogenous insulin do not decrease in parallel with a decrease in glucose, the increase in glucagon that would reverse the hypoglycemia cannot occur, as it does in nondiabetic subjects. It may, however, occur in T1DM patients receiving closed-loop insulin monotherapy (23, 24). In other words, the response of glucagon to glycemic change is regulated by glucose-induced change in insulin. In longstanding T1DM, the ablated paracrine defense against hypoglycemia ultimately is further compromised T1DM by autonomic neuropathy that impairs the adrenergically mediated component of the α-cell response to glucopenia (25).
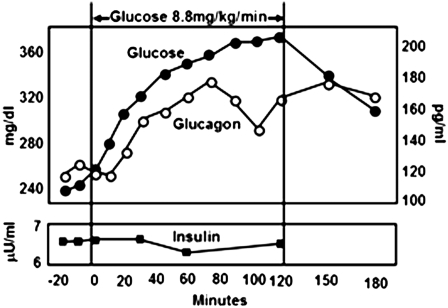
Fig. 4.
A demonstration of the “paradoxical” stimulation of glucagon secretion by hyperglycemia when unaccompanied by a concomitant increase in insulin. These experiments were performed in hypoinsulinemic alloxan-diabetic dogs, in which glucose was infused at a rate of 8.8 mg/kg/min to worsen the hyperglycemia. As blood glucose increased without an increase in insulin, glucagon levels increased paradoxically. Similar results have been obtained in cultured α-cells. These findings suggest that, in T1DM, the hyperglycemia induced by carbohydrate ingestion may be further exaggerated by inappropriate glucagon-stimulated hepatic glucose production [Figure modified from Braaten et al. (21)].
Glucagon Suppression in the Management of the Paracrinopathy of T1DM
Recent studies have demonstrated remarkable antidiabetic properties of leptin, a powerful suppressor of glucagon (18, 19, 26). Administration of recombinant leptin to insulin-deficient mice with uncontrolled T1DM reversed the entire catabolic syndrome as effectively as insulin. When leptin was added to low-dose insulin therapy at 10% of the optimal dose, the marked volatility of insulin monotherapy was eliminated (Fig. 5). This effect was attributed to tonic suppression of hyperglucagonemia, which eliminated the hyperglycemia, plus the fact that leptin permitted a approximate 90% lowering of insulin levels, which reduced the hypoglycemia. It is not yet known if leptin suppresses α-cells by direct action, via hypothalamic targets, or both.
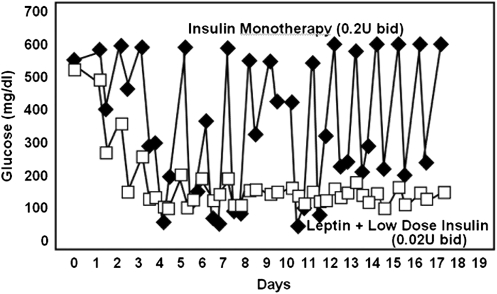
Fig. 5.
Comparison of blood glucose levels in nonobese T1DM mice treated with 0.2 U/d of insulin (■) or 0.02 U/d of insulin plus leptin injected twice daily (□). [Observed results from Wang M-Y et al. (18)]. Note that both the hyperglycemic spikes and the hypoglycemic dips are absent when leptin is injected.
Leptin therapy in diabetic mice was accompanied by three highly desirable side effects (18). The first was a remarkable reduction in the tissue content and the plasma concentrations of triacylglycerol and free fatty acid. Body fat was reduced well below the levels of insulin monotherapy, and lean body mass was increased. These changes would be expected to increase insulin sensitivity (27). The second desirable side effect was a down-regulation in the expression of cholesterologenic transcription factors SREBP-1a and SREB-2 and of 3-hydroxy-3-methyl glutaryl CoA reductase, the rate-limiting enzyme of cholesterol synthesis. Such changes might herald a reduction in the prevalence of coronary artery disease in T1DM patients, reported to exceed 90% after age 55 y (28, 29). A final beneficial side effect was a reduction in appetite that should greatly facilitate compliance with dietary restrictions that are a vital part of every diabetic regimen.
Paracrinopathy in Human Type 2 Diabetes
Type 2 diabetes (T2DM) is, by definition, a hyperglycemic disorder in which β-cells are present. Histologically, α- and β-cells in patients with T2DM do not appear to differ topographically from those of the nondiabetic pancreas (Fig. 6A). They are distributed in the same core-mantle arrangement and the frequency of contacts between α- and β-cells appears to be no less than in islets of nondiabetic humans (30). Significantly, the hyperglycemia of T2DM is unaccompanied by the glycemic lability of T1DM (Fig. 6C) (31). We attribute the stability of the hyperglycemia to restraint of glucagon secretion by β-cells juxtaposed to α-cells (Fig. 6A). Both the absolute hyperglucagonemia of T1DM and its extreme glycemic lability are prevented.
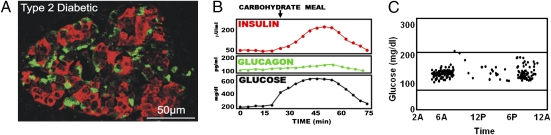
Fig. 6.
Morphological, paracrinological, and clinical features of pancreatic islets in T2DM. (A) Islet morphology in T2DM does not appear to differ grossly from that of normal islets. The juxtaposition of α- and β-cells appears to be approximately normal. (Photomicrograph provided by L.O.) (B) Despite the juxtaposition, α-cells are not suppressed by the hyperglycemia. This appears to be secondary to loss of the first-phase paracrine spike of insulin release immediately after the ingestion of a carbohydrate-containing meal. Without the spike, believed to be the cause of glucose-induced glucagon suppression, glucagon remains unsuppressed and elevated relative to the ambient glucose concentration. The failure of glucagon levels to decline during carbohydrate feeding exaggerates the postprandial hyperglycemia. (C) Despite the relative hyperglucagonemia and hyperglycemia, glycemic instability is not a problem in T2DM.
The α-cell dysfunction in T2DM would seem to result from failure of the juxtaposed β-cells to secrete the first phase of insulin (Fig. 6B) (32), or from insulin resistance of α-cells, or both. As mentioned, this insulin spike may be the key paracrine signal for prompt glucagon suppression by glucose. In its absence, glucagon is not suppressed and, as discussed, may even be paradoxically stimulated by the increasing glucose. In any case, T2DM is characterized by “relative” hyperglucagonemia, meaning that glucagon level is high relative to the ambient glucose level (33).
The Future
Four decades of glucagon research have firmly established its hormonal status and physiologic roles in normal glucose homeostasis and its pathogenic roles in diabetes. Interest in the hormone has been revived by the recent demonstration that leptin, like insulin and somatostatin, is a powerful suppressor of glucagon that dramatically restores insulin-deficient mice to normal. A merging of the morphologic, physiologic, and clinical findings spanning these decades points to a vital but underappreciated paracrine action of insulin on juxtaposed α-cells that is essential for stable glucose homeostasis. T1DM is characterized by loss of both paracrine and endocrine actions of insulin. Conventional insulin treatment corrects the endocrine insulin deficiency but does not fully correct the paracrine insulin deficiency to which we attribute the glycemic volatility o T1DM. Although closed-loop insuin monotherapy may eliminate volatility as well as bitherapy with low-dose insulin plus leptin, it would not be expected to exert the antilipogenic and anticholesterologenic effects ascribed to bitherapy (18).The apparent ability to reduce glycemic volatility in T1DM rodents by tonic suppression of α-cells with leptin raises new hope for a vastly improved management strategy for human T1DM.
Acknowledgments
We thank Drs. Mike Brown, Daniel W. Foster, Philipp Scherer, and Pierre Cosson for helpful criticisms; S. Kay McCorkle for artwork; and Christie Fisher for editorial work. This work was supported by the Veterans Affairs North Texas Health Care System, Amylin Pharmaceuticals, and private donors (R.U.) and by grants from the Swiss National Science Foundation (to L.O.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Müller WA, Faloona GR, Unger RH. The effect of experimental insulin deficiency on glucagon secretion. J Clin Invest. 1971;50:1992–1999. doi: 10.1172/JCI106691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samols E, Bonner-Weir S, Weir GC. Intra-islet insulin-glucagon-somatostatin relationships. Clin Endocrinol Metab. 1986;15:33–58. doi: 10.1016/s0300-595x(86)80041-x. [DOI] [PubMed] [Google Scholar]
- 3.Unger RH, Aguilar-Parada E, Müller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970;49:837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unger RH, Orci L. Glucagon and the A cell: Physiology and pathophysiology (first two parts) N Engl J Med. 1981;304:1518–1524. doi: 10.1056/NEJM198106183042504. [DOI] [PubMed] [Google Scholar]
- 5.Exton JH, et al. The hormonal control of hepatic gluconeogenesis. Recent Prog Horm Res. 1970;26:411–461. doi: 10.1016/b978-0-12-571126-5.50014-5. [DOI] [PubMed] [Google Scholar]
- 6.Cherrington AD, Chiasson JL, Liljenquist JE, Lacy WW, Park CR. Control of hepatic glucose output by glucagon and insulin in the intact dog. Biochem Soc Symp. 1978;(43):31–45. [PubMed] [Google Scholar]
- 7.Keller U, et al. The roles of insulin, glucagon, and free fatty acids in the regulation of ketogenesis in dogs. Diabetes. 1977;26:1040–1051. doi: 10.2337/diab.26.11.1040. [DOI] [PubMed] [Google Scholar]
- 8.Baetens D, Malaisse-Lagae F, Perrelet A, Orci L. Endocrine pancreas: three-dimensional reconstruction shows two types of islets of langerhans. Science. 1979;206:1323–1325. doi: 10.1126/science.390711. [DOI] [PubMed] [Google Scholar]
- 9.Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes. 1982;31:883–889. doi: 10.2337/diab.31.10.883. [DOI] [PubMed] [Google Scholar]
- 10.Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest. 1984;74:2296–2299. doi: 10.1172/JCI111658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosco D, et al. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes. 2010;59:1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orci L. Macro- and micro-domains in the endocrine pancreas. Diabetes. 1982;31:538–565. doi: 10.2337/diab.31.6.538. [DOI] [PubMed] [Google Scholar]
- 13.Cabrera O, et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerich JE. Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes. 2002;51(suppl 1):S117–S121. doi: 10.2337/diabetes.51.2007.s117. [DOI] [PubMed] [Google Scholar]
- 15.Dobbs R, et al. Glucagon: Role in the hyperglycemia of diabetes mellitus. Science. 1975;187:544–547. doi: 10.1126/science.1089999. [DOI] [PubMed] [Google Scholar]
- 16.Gerich JE, et al. Prevention of human diabetic ketoacidosis by somatostatin. Evidence for an essential role of glucagon. N Engl J Med. 1975;292:985–989. doi: 10.1056/NEJM197505082921901. [DOI] [PubMed] [Google Scholar]
- 17.Raskin P, Unger RH. Hyperglucagonemia and its suppression. Importance in the metabolic control of diabetes. N Engl J Med. 1978;299:433–436. doi: 10.1056/NEJM197808312990901. [DOI] [PubMed] [Google Scholar]
- 18.Wang MY, et al. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci USA. 2010;107:4813–4819. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci USA. 2008;105:14070–14075. doi: 10.1073/pnas.0806993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivera N, et al. A novel glucagon receptor antagonist, NNC 25-0926, blunts hepatic glucose production in the conscious dog. J Pharmacol Exp Ther. 2007;321:743–752. doi: 10.1124/jpet.106.115717. [DOI] [PubMed] [Google Scholar]
- 21.Braaten JT, Faloona GR, Unger RH. The effect of insulin on the alpha-cell response to hyperglycemia in long-standing alloxan diabetes. J Clin Invest. 1974;53:1017–1021. doi: 10.1172/JCI107638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Marchand SJ, Piston DW. Glucose suppression of glucagon secretion: metabolic and calcium responses from alpha-cells in intact mouse pancreatic islets. J Biol Chem. 2010;285:14389–14398. doi: 10.1074/jbc.M109.069195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hovorka R, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010;375:743–751. doi: 10.1016/S0140-6736(09)61998-X. [DOI] [PubMed] [Google Scholar]
- 24.Wilinska ME, et al. Overnight closed-loop insulin delivery with model predictive control: Assessment of hypoglycemia and hyperglycemia risk using simulation studies. J Diabetes Sci Tech. 2009;3:1109–1120. doi: 10.1177/193229680900300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tominaga M, et al. Morphologic and functional changes in sympathetic nerve relationships with pancreatic alpha-cells after destruction of beta-cells in rats. Diabetes. 1987;36:365–373. doi: 10.2337/diab.36.3.365. [DOI] [PubMed] [Google Scholar]
- 26.Hedbacker K, et al. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 2010;11:11–22. doi: 10.1016/j.cmet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Shulman GI. Unraveling the cellular mechanism of insulin resistance in humans: new insights from magnetic resonance spectroscopy. Physiology (Bethesda) 2004;19:183–190. doi: 10.1152/physiol.00007.2004. [DOI] [PubMed] [Google Scholar]
- 28.Larsen J, et al. Silent coronary atheromatosis in type 1 diabetic patients and its relation to long-term glycemic control. Diabetes. 2002;51:2637–2641. doi: 10.2337/diabetes.51.8.2637. [DOI] [PubMed] [Google Scholar]
- 29.Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care. 2006;29:2528–2538. doi: 10.2337/dc06-1161. [DOI] [PubMed] [Google Scholar]
- 30.Bonner-Weir S, O'Brien TD. Islets in type 2 diabetes: In honor of Dr. Robert C. Turner. Diabetes. 2008;57:2899–2904. doi: 10.2337/db07-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derr R, Garrett E, Stacy GA, Saudek CD. Is HbA(1c) affected by glycemic instability? Diabetes Care. 2003;26:2728–2733. doi: 10.2337/diacare.26.10.2728. [DOI] [PubMed] [Google Scholar]
- 32.Ward WK, Beard JC, Halter JB, Porte D., Jr Pathophysiology of insulin secretion in diabetes mellitus. Adv Exp Med Biol. 1985;189:137–158. doi: 10.1007/978-1-4757-1850-8_9. [DOI] [PubMed] [Google Scholar]
- 33.Müller WA, Faloona GR, Aguilar-Parada E, Unger RH. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970;283:109–115. doi: 10.1056/NEJM197007162830301. [DOI] [PubMed] [Google Scholar]