Abstract
We show that the oil sands industry releases the 13 elements considered priority pollutants (PPE) under the US Environmental Protection Agency's Clean Water Act, via air and water, to the Athabasca River and its watershed. In the 2008 snowpack, all PPE except selenium were greater near oil sands developments than at more remote sites. Bitumen upgraders and local oil sands development were sources of airborne emissions. Concentrations of mercury, nickel, and thallium in winter and all 13 PPE in summer were greater in tributaries with watersheds more disturbed by development than in less disturbed watersheds. In the Athabasca River during summer, concentrations of all PPE were greater near developed areas than upstream of development. At sites downstream of development and within the Athabasca Delta, concentrations of all PPE except beryllium and selenium remained greater than upstream of development. Concentrations of some PPE at one location in Lake Athabasca near Fort Chipewyan were also greater than concentration in the Athabasca River upstream of development. Canada's or Alberta's guidelines for the protection of aquatic life were exceeded for seven PPE—cadmium, copper, lead, mercury, nickel, silver, and zinc—in melted snow and/or water collected near or downstream of development.
Keywords: oil sands mining, oil sands processing, trace metals, airborne deposition, water contamination
Bitumen production in the Alberta oil sands increased from 482,000 to 1.3 million barrels/d between 1995 and 2008 (1, 2) and is projected to double by 2020 (3). By 2008, mining had disturbed 530 km2 of boreal landscape, with tailings ponds covering more than 130 km2 (4, 5). Development of the oil sands, including mining, processing, and tailings pond leakage, has raised concerns about pollution of the Athabasca River (AR) (5, 6). Downstream residents fear that increased cancer rates (7) may be related to pollution from the oil sands industry. Based in part on results from the Regional Aquatic Monitoring Program (RAMP), industry, gov-ernment and related agencies claim that human health and the environment are not at risk from oil sands development (8, 9) and that sources of elements and polycyclic aromatic compounds (PAC) in the AR and its tributaries are natural (10). However, the reliability of RAMP findings has been questioned repeatedly (11–13). Hence, accurate, independent assessments of the effects of the oil sands industry on concentrations of toxic elements in the AR and its tributaries are unavailable.
The north-flowing AR, its tributaries, the Athabasca Delta (AD), and Lake Athabasca (LA) (figure 1 in reference 13) were investigated to test the hypothesis that increased concentrations of elements in these waterbodies are entirely from natural sources. In February and June 2008, surface water was collected from 37 and 47 sites, respectively. In March, the accumulated winter snowpack was sampled at 31 sites. Sites on the AR were chosen upstream or downstream of oil sands mining and processing activity. Upstream sites and all sites near oil sands development are exposed directly to the McMurray Geologic Formation (McMF), where most oil sands occur (13). Using 2006 Landsat imagery, three sites along each of four tributaries affected by oil sands development were chosen. The first was located upstream of oil sands development and the McMF, the second was midstream within the McMF but upstream of mining, and the third was near stream mouths above the confluence with the AR, downstream of development and downstream of or within the McMF. Comparable sites were selected on two undeveloped reference tributaries. To increase statistical power, additional stream mouth sites, with and without upstream development, were sampled in June. Here we present results for the 13 elements on the US Environmental Protection Agency (EPA) list of priority pollutants (PPE): Sb, As, Be, Cd, Cr, Cu, Pb, Hg, Ni, Se, Ag, Tl, and Zn.
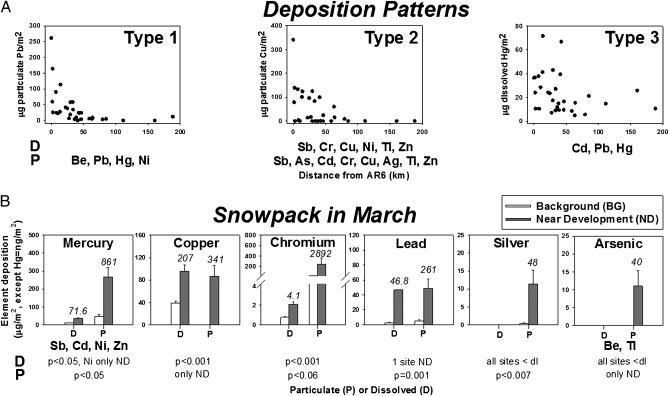
Fig. 1.
Snow deposition patterns (A) and deposition of PPE in snowpack collected in March (B). D, dissolved. P, particulate. (A) Type 1, exponential decline from upgrading facilities; Type 2, exponential decline from AR6 and local sources; Type 3, local sources only. (B) Dissolved and particulate deposition expressed in μg/m2 (except Hg, which is expressed in ng/m2). Data are presented as mean ± SE BG sites (white bars) and ND sites (gray bars). The numbers above the gray bars represent the maximum value near development. The distribution of elements listed below each panel is similar to that in the panel above, and statistics refer to all elements with similar distributions. dl, detection limit.
Based on 2008 Landsat imagery, it was determined that some midstream and stream mouth sites that were undeveloped in 2006 had been developed by the oil sands industry by 2008. To assess the effects of development on the AR and its tributaries, digital disturbance data [change analysis of forest ecozones within Alberta (1991–2001) and Canada access (roads, mines, forest fragments, and reservoirs buffered by 500 m), and the extent of oil sands development in 2008 (14)] were merged using geographic information system (GIS) software to create an index of relative overall land disturbance. Based on the percentage of disturbed area, each watershed was classified as either less disturbed (<25%) or more disturbed (>25%).
In addition to watershed disturbance, the process of upgrading bitumen to synthetic crude oil involves coking, coke combustion, and production of wastes and fly ash that contain PPE (15–17). Environment Canada's National Pollutant Release Inventory shows that upgrading is a substantial and increasing source of PPE to air.
Some PPE are of particular concern in the lower AR. There is a fish consumption advisory for Hg in walleye (Sander vitreus) (18), and the toxicity of PAC discharged by oil sands development (13) can be increased by coexposure to As (19, 20). Concern also exists over Sb, As, Cd, Cr, Cu, Pb, Ni, and Se concentrations in water and/or sediment from the AR (12).
Results
Deposition of PPE in Snow.
Four deposition patterns were identified for particulate (Dataset S1) and dissolved (Dataset S2) PPE in snow. PPE with deposition masses that decreased exponentially with distance from upgrading facilities near site AR6, similar to particulates and PAC (13), were classified as type 1 (Fig. 1A). These PPE include Pb, Hg, Ni, and Be associated with particulates. PPE with deposition patterns in which masses deposited decreased exponentially with distance from upgrading facilities (like type 1) but also increased locally near oil sands development because of land clearing, mining, road dust, or other emissions were classed as type 2 (Fig. 1A). PPE in this class include particulate Sb, As, Cd, Cr, Cu, Ag, Tl, and Zn and dissolved Sb, Cr, Cu, Ni, Tl, and Zn. Deposition of some PPE was from local sources only and was classified as type 3 (Fig. 1A). Type 3 PPE included dissolved Cd, Pb, and Hg. PPE that were not detected either in particulate (Se) or in dissolved form (As, Be, Se, Ag) were classified as type 4.
Sites were designated as background (BG) or near development (ND), depending on their location and deposition, for each PPE. For PPE with type 1 deposition, all sites within 50 km of the upgraders at AR6 were considered ND, and all sites more than 50 km away were considered BG (Datasets S1 and S2). However, Be exhibited type 1 deposition only within 2 km of AR6, and the designations of ND or BG were adjusted accordingly. For PPE with type 2 or 3 deposition patterns, the magnitude of deposition near oil sands development varied among sites and PPE. These differences reflect the wide variety of possible sources and the chemical-physical properties of each element. Hence, each particulate and dissolved PPE was graphed in order of descending deposition, and for each PPE the difference between ND and BG was defined as the point between a marked decrease in deposition from sites ND and consistent deposition at BG sites (SI Text). Occasionally, concentrations of PPE greater than BG were observed at sites distant from oil sands development, such as the northernmost AR, AD, or LA sites. Based on distance, these greater concentrations probably were from local sources unrelated to oil sands mining and processing. Thus, these sites were designated BG.
Particulate-bound PPE deposition in snow.
Upgrading facilities were identified as a source of particulate PPE. The mean deposition of type 1 and 2 PPE at sites ND was up to 30-fold greater than BG (two-sample t test; P < 0.05; Fig. 1B and Fig. S1A), and maximum deposition was as much as 120-fold greater than BG. Some PPE, such as As, Be, Cu, and Tl, were detected only at sites ND (Fig. 1B and Dataset S1), with maximum concentrations at site AR6. Although mean Cr deposition was 14-fold greater than BG at sites ND, these concentrations were not quite significantly different from BG (two-sample t test; P = 0.06) (Fig. 1B, Fig. S1A, and Dataset S1).
Type 1 particulate PPE deposition in snow was correlated with deposition of particulate PAC (13) (r2 > 0.8, except for Hg, r2 = 0.5; all P < 0.002). Estimated total depositions of Pb, Hg, and Ni over 4 mo at sites within a 50-km radius of site AR6 were 162, 1.1, and 583 kg, respectively (SI Text and Dataset S1).
Local inputs of PPE with type 2 deposition caused by oil sands development were discernible as far as 85 km from site AR6 (Fig. 1A, Fig. S1A, and Dataset S1), with mean and maximum loadings as much as 28- and 169-fold greater than at BG sites, respectively (Fig. 1B).
Dissolved PPE deposition in snow.
Deposition of dissolved PPE in snow generally was less than that of particulate PPE (Fig. 1 and Datasets S1 and S2). Dissolved PPE with a type 2 deposition pattern, including Ni, Sb, Cr, Cu, Tl, and Zn, were as much as 5-fold greater than BG at ND sites (Fig. 1B, Fig. S1B, and Dataset S2), with maxima at sites other than AR6. Deposition of dissolved Cd, Pb and Hg, with a type 3 pattern, were as much as 18-fold greater than BG at ND sites (Fig. 1B, Fig. S1B, and Dataset S2). Sites with the greatest concentrations of Sb, Cd, Cr, Cu, Pb, Tl, and Zn were not always the same as sites with the greatest deposition (Dataset S2), because snow depth and density were greater at some ND sites.
Deposition of dissolved type 2 and 3 PPE (Fig. 1A, Fig. S1B, and Dataset S2) was greater than established BG concentrations as far as 85 km from AR6. This finding is consistent with the presence of localized sources of PPE in addition to upgraders (Fig. 1 A and B, Fig. S1B, and Dataset S2).
PPE in Tributaries.
In all six tributaries sampled, concentrations of PPE in water did not increase significantly from upstream sites outside the McMF to midstream and stream mouth sites within the McMF in either summer or winter (Dataset S3). Thus, PPE concentrations were unaffected by contact of river water with the McMF and were unrelated to the proportion of McMF within each watershed in summer or winter (r2McMF < 0.2; P > 0.2; df = 26). Differences among sites were not statistically significant, even in three tributaries with almost all development near stream mouths (two-way ANOVA; Dataset S3), and PPE concentrations sometimes were greater at sites upstream of the McMF than at midstream sites within the McMF. Within the McMF, concentrations of seven PPE increased up to 5-fold from midstream to stream mouth sites in summer, but not in winter, although differences were not significant (paired t test; Dataset S3). PPE concentrations during low flow under ice often were greater than in summer (Dataset S3).
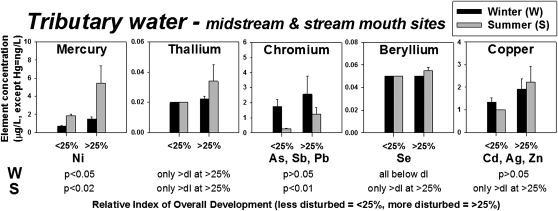
In contrast, concentrations of some PPE in tributary water increased significantly near oil sands development and were significantly correlated with overall land disturbance (e.g., Cd, Zn: r2disturbance = 0.2 and 0.3; P = 0.05 and P < 0.01, respectively; df = 18) and with the proportion of oil sands development within a watershed (e.g., Cd, Ni: r2oil sands = 0.4 and 0.3; P ≤ 0.01 and 0.02, respectively; df = 18). Concentrations of some PPE in winter, such as Hg, Ni, and Tl, were as much as 2-fold greater in watersheds with more development (two-way ANOVA and two-sample t test; P < 0.02; Fig. 2A, Fig. S1C, and Dataset S4) than in less developed watersheds. In summer, concentrations of PPE in watersheds exposed to >25% overall development were as much as 8-fold greater than in less developed watersheds (two-way ANOVA and two-sample t test; P < 0.02; Fig. 2, Fig. S1C, and Dataset S4).
Fig. 2.
Element concentrations (mean ± SE, expressed in μg/L, except for Hg, which is expressed in ng/L) in water from midstream and tributary mouth sites by relative index of overall disturbance by development: <25%, less disturbed; >25%, more disturbed. Black bars, winter (W); gray bars, summer (S). dl, detection limit; only >dl at >25%, above dl only at more disturbed sites. The distribution of elements listed below panels is similar to that in the panel above, and statistics refer to all elements with similar distributions.
At all midstream and stream mouth sites, Sb, Cd, Cr, Pb, Ni, Ag, and Zn concentrations were greater in winter (two-way ANOVA; P < 0.03), whereas Hg concentrations were greater in summer (P < 0.002). At more disturbed sites, concentrations of As, Be, Hg, and Se were greater in summer than in winter, but at less disturbed sites only Hg was more concentrated in summer. Similar patterns were observed when only stream mouth sites were considered (Dataset S4).
PPE in the Athabasca River, Athabasca Delta, and Lake Athabasca.
Neither summer nor winter concentrations of PPE in the AR at sites upstream from or near development were related significantly to the proportion of McMF within a watershed. Cd was an exception, with concentrations inversely related to McMF in winter (r2McMF = 0.4; P = 0.05; df = 8). In contrast, Zn concentrations in the AR during winter were related to the proportion of overall land disturbance within a watershed (r2disturbance = 0.7; P < 0.002; df = 8).
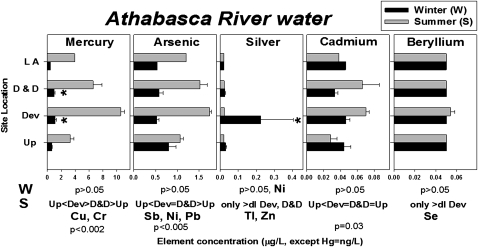
In winter, concentrations of Cr, Hg, Ni, and Ag in the AR under ice were up to 8-fold greater just downstream of tailings ponds, impoundments, or other oil sands development infrastructure than upstream. Hg remained slightly increased (1.5-fold) downstream and in the AD. However, none of the concentrations downstream was significantly greater than upstream (two-way and one-way ANOVA; P > 0.05; Fig. 3, Fig. S1 D, E, and F, and Dataset S5), probably because of low statistical power (β < 0.171). In summer, concentrations of Sb, As, Cr, Cu, Pb, Hg, and Ni, were up to 4-fold greater in the AR near oil sands development than upstream (one-way ANOVA, P < 0.01; multiple comparison, P < 0.05; Fig. 3, Fig. S1D, and Dataset S6). Concentrations of Be, Se, Ag, Tl and Zn were detectable near oil sands development but not upstream (Fig. 3, Fig. S1D, and Dataset S6).
Fig. 3.
Element concentrations (mean ± SE, expressed in μg/L, except for Hg, which is expressed in ng/L) in water from the AR collected in winter (W, black bars) and summer (S, gray bars). Up, AR upstream (n = 3); Dev, AR downstream/near development (n = 7); D&D, AR downstream and Athabasca Delta (n = 6); LA, Lake Athabasca (n = 1); dl, detection limit and only >dl Dev, greater than dl only at sites near development. *Increases near development and downstream that were not statistically significant (power β < 0.171). The distribution of elements listed below panels is similar to that in the panel above, and statistics refer to all elements with similar distributions.
Downstream of the oil sands development, and extending as far as the AD, concentrations of many PPE remained significantly greater than upstream (Sb, As, Cr, Cu, Pb, Hg, Ni; one-way ANOVA, P < 0.02; multiple comparison, P < 0.05; Fig. 3, Fig. S1E, and Dataset S6). Ag, Tl, and Zn were detectable only near oil sands development and in the AD (Fig. 3, Fig. S1D, and Dataset S6). At LA, near the AR discharge, concentrations of eight PPE were as much as 2-fold greater than upstream of oil sands development (Sb, As, Cd, Cr, Cu, Pb, Hg, Ni; Fig. 3, Fig. S1F, and Dataset S6). Within the AR and AD, concentrations of Cr, Cu, Pb, Hg, Ni, and Tl were greater in summer than winter (two-way ANOVA; P < 0.02; Dataset S5), but concentrations of Zn were greater in winter (two-way ANOVA; P < 0.004; Dataset S5).
Discussion
Oil sands development releases significant masses of PPE to the AR and its watershed via air and water, confirming major transport pathways previously identified for PAC (13). Deposition patterns of PAC (13) and type 1 and 2 PPE were similar and were consistent with oil sands upgraders being an atmospheric source. In contrast to PAC, type 2 and 3 deposition patterns for PPE were consistent with local sources of airborne PPE. Local sources of PPE were identified as far as 85 km from AR6, indicating that PPE contamination is more widespread than PAC. In the AR and its tributaries, PPE were related to overall land disturbance, whereas PAC contamination was caused primarily by new development (13). Concentrations of PPE downstream of development on the AR, within the AD, and as far as one location on LA remained greater than upstream concentrations. Thus, during summer, PPE contamination was measurable further from the source than PAC.
Airborne Contaminants.
Within 50 km of upgrading facilities (AR6), 11,400 metric tons of airborne particulates were deposited in 2008 during ∼4 mo of snowfall (figure 2 of ref. 13). The majority of the particulates consisted of oil sands bitumen, as indicated by the large proportion of oil per unit particulate mass and similar distributions of PAC and PPE in oil sands and particulates (figure S2 a and b in ref. 13). Differential fractionation of PPE during upgrading and low solubility of some elements in water may be responsible for any differences in the distribution of PPE in oil sands and in particulates (Fig. S2 A and B). Coke and fly ash from upgraders contain significant amounts of Ni and elements other than PPE, and relative concentrations of elements in ashed coke and fly ash (16, 17) are similar to those in snow particulates. Particulate elements decline more rapidly with distance from AR6 than do dissolved elements. Thus, partitioning between phases likely occurred before emission rather than after melting of snow (Datasets S1 and S2).
Type 2 and 3 deposition patterns implicate local sources, such as land clearing, mining, road dust, and other emissions related to oil sands development, as substantial additional sources of PPE contamination (Datasets S1 and S2). Oil sands industries have emitted airborne PPE for at least 30 y. Increased deposition attributed to fly ash was found 25 km north and south and 10 km east and west of upgrading facilities during snow surveys in 1978 and 1981 (21, 22). Deposition declined from 1978 to 1981 after installation of precipitators (22). By 2008, the area contaminated by particulates was nearly 2-fold larger than in 1978–1981. Deposition of particulates was about 34,000 metric tons in 2008, five times greater than emission inventories and close to annual deposition rates before precipitators (13).
Although no PPE were measured in earlier studies, deposition of other elements (e.g., total K, Na, and Ca and dissolved Al) was significantly greater in 2008 than in 1978, before installation of precipitators (paired t test; P < 0.006) and was greater than in 1981, after installation (P < 0.04). In contrast, deposition of particulate Al, V, and Ti and dissolved V was significantly lower in 2008 than in 1978/1981 (P < 0.03; SI Text). Hence, the success of technologies in controlling different elements appears uneven, although differences among studies also may relate to site, sampling, and analytical differences.
Nonetheless, excessive deposition of elements has occurred for more than 30 y, and emissions of As, Pb, and Hg to the air by Suncor and Syncrude increased ∼3-fold between 2001 and 2008 (23). Our estimates of the annual particulate deposition of Pb, Hg, and Ni, integrated within a 50-km radius of AR6, were 36%, 96%, and 59% lower, respectively, than reported annual emissions. This difference probably indicates that some emissions are deposited outside the 50-km radius. Hg concentrations in fishes respond rapidly to changes in atmospheric deposition of Hg (24); these changes are of concern because Hg concentrations in fishes from the AR and AD are already high (18).
Riverborne Contaminants.
Similarities between the relative concentrations of PPE in snow and river water link emissions of airborne elements to the AR and its tributaries (Fig. S2 C and D). PPE in the snow pack probably were released as a pulse during spring melt. In summer, PPE are deposited directly to waterbodies and the watershed. During snowmelt and rain events, elements are discharged to surface waters, but a proportion is retained in soil and vegetation. Particulate and dissolved Ni best represent the type 1 deposition pattern. In summer, when direct deposition of airborne contaminants to the river occurs, Ni concentrations are strongly correlated with concentrations of all other PPE in the AR and its tributaries (r2 > 0.8, except Ag = 0.5; P < 0.001). In winter, when airborne elements are deposited to snow on river ice, particulate deposition of all PPE except Se declines exponentially from AR6, although some also are affected by local sources. In river water under ice, concentrations of Ni were not correlated with other PPE, suggesting that concentrations under ice reflect inputs from erosion or effluent discharge, not atmospheric sources. Concentrations of three PPE and four other elements known to be increased in oil sands process water are much greater in the AR only near tailings ponds or oil sands development in winter. This finding suggests tailings pond leakage or discharge as sources of elements to the AR.
The pattern of increased PPE concentrations in snow and the river system does not support the claim that contamination of the AR and its tributaries is only from natural erosion of oil sands. Concentrations of PPE did not increase significantly as water flowed through the McMF from midstream to stream mouth sites, in winter or summer (Dataset S3), and element concentrations in bottom and suspended sediments of tributaries did not reflect greater exposure to natural oil sands (25). Previous records of upstream-to-downstream trends in waterborne Ni and Zn concentrations during high flow (26) probably reflect runoff of snowmelt and rain from disturbed areas or areas contaminated by atmospheric deposition.
Instead, our results indicate that the source of PPE was from oil sands development. In tributaries, overall land disturbance caused a major flux of PPE to water (Fig. 2 and Dataset S4). In summer, increased concentrations of many PPE were significantly related to development at midstream and tributary mouth sites (Fig. S1 and Dataset S4). At less disturbed tributary sites, concentrations of most PPE were greatest under winter ice (Dataset S4). However, at more disturbed tributary sites, concentrations of several PPE were greater in summer than winter (Dataset S4), indicating the impact of land disturbance. If the source were natural erosion of oil sands, concentrations at all sites would have been greater in summer than winter.
In the AR, PPE concentrations were greater downstream of oil sands development, particularly in summer, and many remained increased over upstream concentrations at downstream and AD sites (Fig. 3 and Dataset S6). This increase may be the result of natural increases in BG concentrations downstream, based on the river continuum concept (27). However, among all PPE, the pattern of increases over upstream concentrations in the AD and LA was similar to increases ND (Fig. S1), indicative of a persistent anthropogenic signal with oil sands development as the most likely source. Long-term trends (1989–2006) at an AR site downstream of oil sands development show that metal concentrations generally decreased, but with no significant trends after flow adjustment except for a decline in Pb (28). In contrast, from 1960–2007, stream flow and concentrations of three elements decreased, but turbidity, nutrients, As, and Al increased at Old Fort, just within the AD, probably because of anthropogenic disturbance (29). In sediments, metal concentrations increased downstream to the AD and LA (30), indicating deposition of suspended particulates in the meandering channels of the AD.
Increased airborne deposition of elements for ∼40 y probably has increased PPE and PAC (13) concentrations in surface soils, vegetation, snow, and runoff over a broad area of boreal forest. Although RAMP has sampled snow for hydrologic monitoring (31), pollutant concentrations were not reported, despite past recommendations for regional monitoring of contaminants in snow (21, 22). Given the lack of detailed long-term monitoring, it is difficult to tell how much upstream concentrations have increased over true BG as the result of long-term airborne deposition (13). Previously, concentrations of several elements in tributaries to the AR have exceeded guidelines for the protection of aquatic life during spring (26). These concentrations were thought to be natural and useful as baseline data to assess future emissions (26). However, reaches of these tributaries are within 50 km of AR6, where we show that deposition of many elements to snow is increased. Emissions from new and expanded upgrading facilities will further increase regional BG concentrations.
Concentrations of Cd, Cu, Pb, Hg, Ni, Ag, and Zn in melted snow and in tributary and AR water exceeded guidelines for the protection of aquatic life (SI Text) to the greatest extent at sites near development (Fig. S3). For example, seven PPE exceeded guidelines in snow at ND sites, whereas only Cd exceeded guidelines at some BG sites (Fig. S3). Cd in snow was 200- and 30-fold greater than the hardness-dependent and interim guidelines, respectively, of the Canadian Council of Ministers of the Environment (CCME) Water Quality Guidelines for the Protection of Aquatic Life, and Ag was 13-fold greater than CCME guidelines at one AR site in February (Fig. S3) (32). Other highly toxic metals (Cd, Cu, Pb, Hg, Ni, and Zn) exceeded guidelines by up to 5-fold (Fig. S3). Guidelines were exceeded more often in summer than in winter at AR sites.
Similarly, guidelines were exceeded during the spring freshet (26), when metals should be most toxic because water hardness decreases from >100 mg/L to <5 mg/L. For example, under these conditions, the CCME guideline of 2–4 μg Cu/L would be acutely lethal to minnow embryos (33), creating an annual risk of recruitment failure for 19 fish species that spawn in the AR and its tributaries in spring or early summer (34). Metal mixtures also can act synergistically (35), and some PPE potentiate PAC toxicity to aquatic organisms (19).
PPE concentrations in melted snow and in tributary and AR water did not exceed drinking water quality guidelines (Fig. S3). Nevertheless, increased deposition of elements considered priority pollutants under the US EPA Clean Water Act are of concern to human health. As indicated, a fish consumption advisory exists for Hg in walleye from the AR (18), and the AD wetlands provide an increased potential for Hg methylation (36). Links have been proposed between diseases prevalent in Fort Chipewyan and the carcinogenicity of PAC (6, 12). Effects of PAC can be potentiated by coexposure to As (20), which is above BG concentrations downstream of development as far as the AD. Also, high loadings of Cd to snow may present health risks because moose bioaccumulate Cd in liver and kidneys, reflecting regional distributions of Cd in vegetation (37). Monitoring PPE in vegetation and country foods where oil sands emissions fall on aboriginal treaty lands is essential.
Conclusions.
Contrary to claims made by industry and government in the popular press, the oil sands industry substantially increases loadings of toxic PPE to the AR and its tributaries via air and water pathways. This increase confirms the serious defects of RAMP (11–13), which has not detected such patterns in the AR watershed. Detailed long-term monitoring is essential to distinguish the sources of these contaminants and control their potential impacts on environmental and human health (13). A robust monitoring program to measure exposure and health of fish, wildlife, and humans should be implemented in the region affected by oil sands development (38, 39).
Methods
Study Design.
Sites were selected to distinguish contributions of oil sands development or natural sources to element loading. A detailed description of the study design, GIS analyses, and a study area map can be found in (13).
Field Sampling.
Snow was collected from 12 sites on the AR, AD, and LA and from 19 tributary sites in March 2008. To calculate areal deposition rates of elements, snow cores were collected as described in (13). An integrated sample of the snowpack was collected for Hg and other elements with a plastic shovel, acid-washed Teflon scraper, and an acid-washed Teflon scoop. Samples were placed into acid-washed 2-L Teflon jars (Hg) or acid-washed wide-mouthed high-density polyethylene bottles (other elements) and stored frozen until analysis.
In February and June unfiltered water samples were collected at all sites for analysis of Hg and 30 other elements using an ultraclean sampling protocol (40). Hg samples were acidified 500:1 with concentrated trace metal grade HCl. For other elements, 250-mL samples were acidified with 0.5 mL of optima grade nitric acid. Duplicates, a trip blank, and six field blanks were included in both winter and summer sampling campaigns. Oil sands sampling is described in (13).
Laboratory.
Snow was melted and filtered through 0.45-μm glass fiber filters. Filters were dried, and the mass of particulate was determined. Unfiltered (total) snow and river water and filtered (dissolved) snow samples were analyzed for Hg at the University of Alberta Low-Level Mercury Analytical Laboratory by cold vapor atomic fluorescence spectrometry (CVAFS). Samples were analyzed for other elements at the Queen's University Analytical Services Unit using inductively coupled plasma atomic emission spectroscopy with an ultrasonic nebulizer (ICP-AES) and at the Royal Military College Analytical Sciences Group using ICP-MS. Both laboratories are accredited by the Canadian Association for Laboratory Accreditation to International Organization for Standardization/International Electrotechnical Commission (ISO/IEC) standard 17025. Concentrations of elements in particulates were calculated as: particulate = total − dissolved.
Samples of oil sands were analyzed for elements at the Université du Québec á Rimouski Laboratoire de Chimie Marine et Spectrométrie de Masse, Institut des Sciences de la Mer de Rimouski, by ICP-MS.
Analytical and Statistical Methods.
To compare the relative importance of natural erosion and mining on element mobilization, element concentrations in water were regressed against the proportion of the watershed within the McMF, overall land disturbance, and land disturbed by oil sands mining in 2008. These comparisons were made for all tributaries combined and separately for the AR.
Details of analyses for Hg and other elements, quality assurance/quality control, treatment of samples below the detection limit (<dl), source identification of elements in snow, calculations of area-wide element deposition, designation of BG versus impacted for snow, percent above dl calculations, comparison with historical element-loading in snow, comparison with guidelines, and statistical methods are provided in SI Methods.
Supplementary Material
Acknowledgments
K. Timoney, R. Grandjambe, B. Fortin, R. Ladouceur, S. Laurent, T. Boag, P. Jordan, K. Vladicka-Davies, M. Davies, H. La, A. Rutter, D. Kelly, and R. Saint-Louis provided assistance with planning, field work, and/or laboratory analyses. Parks Canada and the Government of Alberta Sustainable Resource Development granted research permits. The Athabasca Chipewyan, Mikisew Cree, and Fort MacKay First Nations and Métis provided ecological knowledge and access to traditional territories. M. Hanneman and P. Lee supplied GIS data and defined watersheds. The Tides and the Walter and Duncan Gordon Foundations funded this work.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008754107/-/DCSupplemental.
References
- 1.Energy Resources Conservation Board (ERCB) (2005) Alberta's reserves 2004 and supply/demand outlook 2005-2014. ST98-2005, Power Point file of graphs and data from 2005 report. [Accessed September 15, 2009]. http://www.ercb.ca/portal/server.pt/gateway/PTARGS_0_0_308_265_0_43/http%3B/ercbContent/publishedcontent/publish/ercb_home/publications_catalogue/publications_available/serial_publications/st98.aspx.
- 2.Energy Resources Conservation Board (ERCB) (2009) Alberta's energy reserves 2008 and supply/demand outlook 2009-2018. ST98-2009, Power Point file of graphs and data from 2009 report. [Accessed September 15, 2009]. http://www.ercb.ca/portal/server.pt/gateway/PTARGS_0_0_308_265_0_43/http%3B/ercbContent/publishedcontent/publish/ercb_home/publications_catalogue/publications_available/serial_publications/st98.aspx.
- 3.Canadian Association of Petroleum Producers (CAPP) Crude Oil Forecast, Markets and Pipeline Expansions. Calgary, AB, Canada: Canadian Association of Petroleum Producers; 2009. [Google Scholar]
- 4.Government of Alberta . Environmental Management of Alberta's Oil Sands. Edmonton, AB, Canada: Oil Sands Management Division; 2008. [Google Scholar]
- 5.Price M. 11 Million Litres a Day: The Tar Sands’ Leaking Legacy. Toronto: Environmental Defense; 2008. [Google Scholar]
- 6.Timoney KP, Lee P. Does the Alberta tar sands industry pollute? The scientific evidence. Open Conservation Biology Journal. 2009;3:65–81. [Google Scholar]
- 7.Chen Y. Cancer Incidence in Fort Chipewyan, Alberta 1995-2006. Edmonton, AB, Canada: Alberta Cancer Board, Division of Population Health and Information Surveillance, Alberta Health Services; 2009. [Google Scholar]
- 8.Alberta Oil Sands Community Exposure and Health Effects Assessment Program (HEAP) (2000) Summary report Health Surveillance, Alberta Health and Wellness, Government of Alberta, Edmonton, AB, Canada. [Accessed October 28, 2009]. http://www.health.alberta.ca/newsroom/pub-environmental-health.html.
- 9.Wood Buffalo Environmental Association Human Exposure Monitoring Program (HEMP) (2007) Methods report and 2005 monitoring year results Wood Buffalo Environmental Monitoring Association, Fort McMurray, AB, Canada. [Accessed October 28, 2009]. http://www.health.alberta.ca/newsroom/pub-environmental-health.html.
- 10.Regional Aquatics Monitoring Program (RAMP) Joint Community Update 2008 Reporting our Environmental Activities to the Community. Fort McMurray, AB, Canada: RAMP, WBEA, and CEMA; 2008. Wood Buffalo Environmental Association (WBEA), (Cumulative Environmental Management Association) (CEMA) [Google Scholar]
- 11.Ayles GB, Dubé M, Rosenberg D. Oil Sands Regional Aquatic Monitoring Program (RAMP) Scientific Peer Review of the Five Year Report (1997-2001) Winnipeg, MB: Fisheries and Oceans Canada; 2004. [Google Scholar]
- 12.Timoney K. A Study of Water and Sediment Quality as Related to Public Health Issues, Fort Chipewyan, Alberta. Fort Chipewyan, AB: Nunee Health Board Society; 2007. [Google Scholar]
- 13.Kelly EN, et al. Oil sands development contributes polycyclic aromatic compounds to the Athabasca River and its tributaries. Proc Natl Acad Sci USA. 2009;106:22346–22351. doi: 10.1073/pnas.0912050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Global Forest Watch Canada Recent anthropogenic changes in Alberta's forest ecozones, Canada_Access_Combined, and Athabasca bituminous sands industrial footprint, 2008: Mapped from Landsat TM Multi-spectral scanner – path 42/Row 20, 19/03/2008 [digital data] 2009 Available upon request from info@globalforestwatch.ca. [Google Scholar]
- 15.Allen EW. Process water treatment in Canada's oil sands industry: I. Target pollutants and treatment objectives. Journal of Environmental Engineering and Science. 2008;7:123–138. [Google Scholar]
- 16.Jack TR, Sullivan EA, Zajic JE. Leaching of vanadium and other metals from Athabasca Oil Sands coke and coke ash. Fuel. 1979;58:589–594. [Google Scholar]
- 17.Jang H, Etsell TH. Mineralogy and phase transition of oil sands coke ash. Fuel. 2006;85:1526–1534. [Google Scholar]
- 18.Government of Alberta 2009 Alberta Guide to Sportfishing Regulations. 2009. [Accessed December 5, 2009]. http://www.albertaregulations.ca/fishingregs/other-information.html#mercurycontamination.
- 19.Fleeger JW, Gust KA, Marlborough SJ, Tita G. Mixtures of metals and polynuclear aromatic hydrocarbons elicit complex, nonadditive toxicological interactions in meiobenthic copepods. Environ Toxicol Chem. 2007;26:1677–1685. doi: 10.1897/06-397r.1. [DOI] [PubMed] [Google Scholar]
- 20.Fischer JM, et al. Co-mutagenic activity of arsenic and benzo[a]pyrene in mouse skin. Mutat Res. 2005;588:35–46. doi: 10.1016/j.mrgentox.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Barrie LA, Kovalick J. A Wintertime Investigation of the Deposition of Pollutants Around an Isolated Power Plant in Northern Alberta. 1980. p. 115. Prepared for the Alberta Oil Sands Environmental Research Program by Atmospheric Environment Service. AOSERP Report 90. [Google Scholar]
- 22.Murray WA. The 1981 Snowpack Survey in the AOSERP Study Area. 1981. p. 115. Prepared for the Alberta Oil Sands Environmental Research Program by Promet Environmental Group. AOSERP Report 125. [Google Scholar]
- 23.National Pollutant Release Inventory (NPRI) (2010) [Accessed May 13, 2010]. www.ec.gc.ca/inrp-npri/
- 24.Harris RC, et al. Whole-ecosystem study shows rapid fish-mercury response to changes in mercury deposition. Proc Natl Acad Sci USA. 2007;104:16586–16591. doi: 10.1073/pnas.0704186104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conly FM, Crosley RW, Headley JV, Quagraine EK. Assessment of metals in bed and suspended sediments in tributaries of the Lower Athabasca River. Journal of environmental science and health. Part A, Toxic/hazardous substances and environmental engineering. 2007;42:1021–1028. doi: 10.1080/10934520701418433. [DOI] [PubMed] [Google Scholar]
- 26.Headley JV, Crosley B, Conly FM, Quagraine EK. The characterization and distribution of inorganic chemicals in tributary waters of the lower Athabasca River, oil sands region, Canada. Journal of environmental science and health. Part A, Toxic/hazardous substances and environmental engineering. 2005;40:1–27. doi: 10.1081/ese-200033418. [DOI] [PubMed] [Google Scholar]
- 27.Vannote RL, Minshall GW, Cummings KW, Sedell JR, Cushing CE. The River Continuum Concept. Canadian Journal of Fisheries and Aquatic Sciences. 1980;37:130–137. [Google Scholar]
- 28.Glozier NE, Donald DB, Crosley RW, Halliwell D. Wood Buffalo National Park Water Quality: Status and Trends from 1989-2006 in Three Major Rivers; Athabasca, Peace and Slave. Saskatoon, SK: Prairie and Northern Office, Water Quality Monitoring and Surveillance Division, Water Science and Technology Directorate, Environment Canada; 2009. [Google Scholar]
- 29.Hebben T. Analysis of Water Quality Conditions and Trends for the Long-Term River Network: Athabasca River, 1960-2007. Edmonton, AB: Alberta Environment, Water Policy Branch, Environmental Assurance; 2009. [Google Scholar]
- 30.Allan R, Jackson TA. Heavy Metals in Bottom Sediments of the Mainstem Athabasca River System in the AOSERP Study Area. 1978. Prepared for the Alberta Oil Sands Environ Res Program by Inland Waters Directorate, Fisheries and Environment Canada. AOSERP Report 34 (Alberta Oil Sands Environmental Research Program) [Google Scholar]
- 31.Regional Aquatics Monitoring Program (RAMP) 2008 Final Technical Report. Hatfield Consultants, Kilgour and Associates Ltd., Klohn Crippe Berger Ltd. and Western Resource Solutions; 2009. [Google Scholar]
- 32.Canadian Council of Ministers of the Environment Canadian water quality guidelines for the protection of aquatic life. 2007. [Accessed November 4, 2009]. Summary Table. Update 7.1. December 2007 http://www.ccme.ca/publications/ceqg_rcqe.html?category_id=124.
- 33.Welsh PG, et al. Estimating acute copper toxicity to larval fathead minnows in soft water from measurements of dissolved organic carbon, calcium and pH. Canadian Journal of Fisheries and Aquatic Sciences. 1996;53:1263–1271. [Google Scholar]
- 34.Scott WB, Crossman EJ. Freshwater Fishes of Canada. Oakville, ON: Galt House; 1998. p. 996. [Google Scholar]
- 35.Wah Chu K, Chow KL. Synergistic toxicity of multiple heavy metals is revealed by a biological assay using a nematode and its transgenic derivative. Aquat Toxicol. 2002;61:53–64. doi: 10.1016/s0166-445x(02)00017-6. [DOI] [PubMed] [Google Scholar]
- 36.St. Louis VL, et al. Importance of wetlands as sources of methyl mercury to boreal forest ecosystems. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51:1065–1076. [Google Scholar]
- 37.Glooschenko V, et al. Cadmium levels in Ontario moose and deer in relation to soil sensitivity to acid precipitation. Sci Total Environ. 1988;71:173–186. doi: 10.1016/0048-9697(88)90165-9. [DOI] [PubMed] [Google Scholar]
- 38.NRBS (Northern River Basins Study) Northern River Basins Study Report to the Ministers. Alberta, and Northwest Territories: Government of Canada; 1996. [Google Scholar]
- 39.Alberta Health . Northern River Basins Human Health Monitoring Program Report. Edmonton, AB: Health Surveillance, Government of Alberta; 1999. [Google Scholar]
- 40.St. Louis VL, et al. Production and loss of methylmercury and loss of total mercury from boreal forest catchments containing different types of wetlands. Environ Sci Technol. 1996;30:2719–2729. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.