Abstract
Psychological factors, including depression and social isolation, are important determinants of cardiovascular health. The current study uses a well-validated mouse model of cardiac arrest/cardiopulmonary resuscitation (CA/CPR) to examine the effect of social environment on several pathophysiological and behavioral responses to cerebral ischemia. Male experimental mice were either housed in pairs with an ovariectomized female or socially isolated for the duration of the experiment. Cardiac arrest increased the mRNA expression of the proinflammatory cytokines TNF-α, IL-1β, and IL-6, as well as the microglia marker MAC-1; expression of each of these factors, except IL-6, was further increased among socially isolated mice. Furthermore, socially isolated animals exposed to the CA/CPR procedure displayed significantly higher levels of neuronal cell death and microglia staining within the hippocampus at 7 d following surgery. Social isolation also exacerbated CA/CPR-induced depressive-like behavior and cardiac autonomic dysregulation. In the absence of ischemic damage, social environment had no significant effect on the expression of neuronal cell death, autonomic cardiac control, or behavior. Together, these data suggest that social factors influence the pathophysiological trajectory following cardiac arrest.
Keywords: ischemia, depression, sympathetic, parasympathetic
Cardiac arrest is a leading cause of mortality worldwide (1). Although survival rates following cardiac arrest and cardiopulmonary resuscitation (CA/CPR) remain staggeringly low, advancements in both patient care and public awareness of risk factors have allowed many individuals to survive incidents that would have previously proven fatal (2–4). Although such developments represent an important advancement, survivors of CA/CPR are predisposed to suffer from a myriad of chronic physiological and psychiatric conditions as a result of neuronal damage caused by widespread deprivation of blood flow to the brain, otherwise known as global cerebral ischemia (5). Indeed, neurological injury secondary to CA/CPR is thought to exacerbate health status through a broad spectrum of mechanisms including the induction of inflammation and attenuated central control of autonomic and neuroendocrine output (6, 7). Furthermore, cerebral ischemia increases the incidence of depression and perceived social isolation (8), both of which are inversely correlated with health status among patients with cardiovascular disease (9, 10).
Neurological injury following CA/CPR primarily results from a global cessation of cerebrovascular blood flow and subsequent reperfusion of blood (11). The pathophysiological responses to ischemia-reperfusion injury include various molecular and systems processes associated with neuronal dysfunction and death, including damage associated with excessive inflammation (7, 11). Proinflammatory cytokines increase dramatically within minutes of cerebral ischemia and result in the infiltration of circulating immune cells (e.g., monocytes) and activation of the resident immune cells of the brain [i.e., microglia (12)]. Subsequent neuronal cell death and inflammatory responses deprive higher level neural structures of the ability to control basic physiological responses, including autonomic and neuroendocrine output (13, 14). Indeed, evaluation of neurocardiac functioning serves as part of a risk stratification battery in patients with various cerebrovascular diseases (15, 16). Furthermore, alterations in autonomic outflow modulate peripheral inflammatory signaling, primarily through cholinergic α7-nicotinic (parasympathetic) and β-adrenergic (sympathetic) signaling mechanisms (17, 18). Indeed, the sympathetic nervous system is thought to fine tune many immune responses (17) and may mediate the rapid inflammatory response following cerebral ischemia (19). Additionally, vagal (parasympathetic) cholinergic transmission may serve to increase neural control over immunity through prevention of runaway inflammation (18), and cerebral ischemia-induced alterations in parasympathetic autonomic control may limit the ability of the nervous system to regulate immune function, a fact that is likely to play a causative role in the pathophysiological and immunomodulatory responses to cardiac arrest.
In addition to the pathophysiological responses mentioned above, global cerebral ischemia engenders dramatic increases in psychopathological conditions such as depression (8, 20). Importantly, the increased prevalence of depression following CA/CPR directly influences health outcome (21). Related to, but distinct from, levels of depression, perceived social isolation is a significant predictor of all-cause morbidity and mortality, on par with obesity and smoking (9). Similarly, socially isolated rodents display elevated depressive-like behavior following nerve injury (22), and social isolation exacerbates neuroinflammation and cell death following cerebral ischemia (23, 24).
The goal of the current study was to examine the influence of social interaction on autonomic, neuroimmune, and behavioral responses to CA/CPR. Specifically, peripheral and central cytokine response, autonomic function, cell death, and depressive-like behavior were compared in socially housed and socially isolated mice. As previously described (14, 23), CA/CPR was achieved through administration of potassium chloride to stop the heart for 8 min, and the mice were subsequently resuscitated with epinephrine and chest compressions. Autonomic output was assessed using a well-validated pharmacological methodology that can dissociate the individual contributions of sympathetic and parasympathetic autonomic control of the heart. At the 24- and 72-h collection time points, we determined the mRNA expression of proinflammatory cytokines (rtPCR) associated with ischemic damage. Tissue from another cohort of animals was collected at 7 d and processed for the quantification of cell death (Fluorojade) and microglia responses (MAC-1 immunhistochemistry). Additionally, we assessed circulating levels of corticosterone (RIA) and IL-6 (ELISA) following CA/CPR (at all tissue collection time points). Finally, we evaluated the influence of social housing conditions on depressive-like (forced swim test) and anxiety-like (central tendency) behaviors following CA/CPR. The general hypothesis was that social isolation will exacerbate ischemia-induced neuronal, inflammatory, autonomic, and behavioral responses.
Results
Housing Effects on CA/CPR Outcome: mRNA Expression at 24 and 72 h Following Surgery.
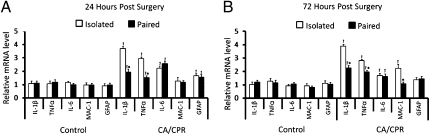
mRNA expression.
The sham and hypothermic CA/CPR groups were collapsed into one control group because they were comparable across all dependent variables (P > 0.05); the sham group serves as a nonischemic surgical control, although the hypothermic CA/CPR group serves as an ischemic control in which neuronal damage, neuroinflammation, and endocrine alterations fail to develop. Hypothermia has been shown to mitigate the pathophysiological effects of neuronal trauma (25, 26). The absence of any statistical differences between the sham and hypothermic groups rules out potential effects of potassium chloride, epinephrine, and chest compressions on the dependent measures in animals without brain damage. As expected, the CA/CPR procedure resulted in increased hippocam-pal expression of IL-1β (F1,50 = 49.96, P < 0.05), TNFα (F1,50 = 60.12, P < 0.05), GFAP (F1,50 = 13.57, P < 0.05), and IL-6 (F1,50 = 35.07, P < 0.05) at 24 h following surgery, relative to controls (Fig. 1A). A similar pattern was observed at 72 h postsurgery, with CA/CPR animals displaying increased hippocampal expression of IL-1β (F1,46 = 66.69, P < 0.05), TNFα (F1,46 = 97.50, P < 0.05), IL-6 (F1,46 = 10.31, P < 0.05), and MAC-1 (F1,46 = 45.32, P < 0.05) (Fig. 1B). Social housing conditions had a significant effect on gene expression among the CA/CPR group but not the control group, which led to a significant housing × surgery interaction at both the 24- (Fig. 1A) and 72-h (Fig. 1B) time points. The interaction reflects significantly higher mRNA expression of IL-1β (F1,48 = 13.58, P < 0.05) (Fig. 1A) and TNFα (F1,48 = 15.48, P < 0.05) (Fig. 1A) among socially isolated mice at 24 h following CA/CPR. Similarly, socially isolated CA/CPR mice displayed elevated hippocampal mRNA levels of IL-1β (F1,44 = 8.31, P < 0.05) (Fig. 1B), TNFα (F1,44 = 11.17, P < 0.05) (Fig. 1B), and MAC-1 (F1,44 = 13.13, P < 0.05) (Fig. 1B) at the 72-h time point, as compared with both the paired CA/CPR and control groups. Gene expression was quantified through real-time PCR (rtPCR). All animals were similar in weight before surgical manipulations (P > 0.05).
Fig. 1.
Social influences of hippocampal mRNA expression. Hippocampal mRNA levels of IL-1β, TNFα, IL-6, MAC-1, and GFAP at 24 (A) and 72 h (B) following surgery. As compared with pair-housed animals, socially isolated CA/CPR mice displayed significantly higher levels of IL-1β and TNFα at both 24 and 72 h postsurgery and significantly higher MAC-1 expression at 72 h following surgery. At 24 h, there also was no significant impact of housing on the expression of these genes among the control group. The control group was comprised of sham-operated mice and mice that underwent hypothermic CA/CPR and as a result did not sustain neuroinflammation or neuronal damage. The data are presented as mean ± SEM. *Significantly different from isolated CA/CPR animals. (P < 0.05). †Significantly different from controls (P < 0.05).
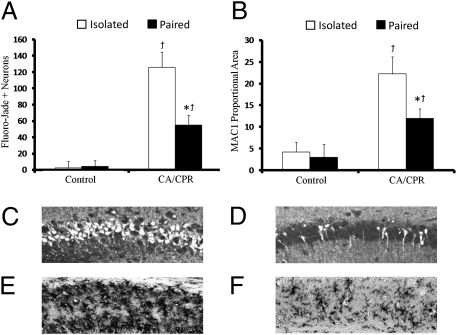
Cell death and MAC-1 response to CA/CPR.
CA/CPR mice displayed significantly higher numbers of dead or dying Fluorojade positive (FJ+) neurons within the hippocampus (F1,48 = 46.01, P < 0.05) (Fig. 2A) at 7 d postischemia. The number of FJ+ neurons was minimal in the control group (Fig. 2A). The extent of CA/CPR-induced cell death was further exacerbated by social isolation as revealed by a significant surgery × housing interaction (F1,46 = 8.19, P < 0.05; Tukey < 0.05) (Fig. 2A). Indeed, although socially paired mice show elevations in neuronal cell death following CA/CPR, socially isolated CA/CPR animals displayed more than twice the neuronal damage as socially housed CA/CPR mice (Fig. 2A). Histological analysis of post-CA/CPR MAC-1, a pattern recognition complement receptor protein expressed on macrophage and microglia cells, revealed similar increases in proportional area staining within the hippocampus of CA/CPR animals as compared with controls (F1,46 = 21.31, P < 0.05) (Fig. 2B). A housing × surgery interaction revealed that socially isolated CA/CPR animals had elevated levels of MAC-1 expression relative to pair-housed CA/CPR and control animals (F1,44 = 8.31, P < 0.05; Tukey < 0.05) (Fig. 2B).
Fig. 2.
Social influences of cell damage and MAC-1 levels. (A) CA/CPR animals displayed increased cell damage within the hippocampus at 7 d following surgery. Chronic social isolation exacerbated the effect of CA/CPR on neuronal cell death. (B) CA/CPR animals displayed increased levels of MAC-1 within the hippocampus at 7 d following surgery. Representative photomicrographs of neuronal cell death (Fluorojade C+ neurons) within the CA1 region of the hippocampus in socially isolated (C) and socially paired (D) animals 7 d following CA/CPR. Representative photomicrographs of microglial responses (MAC-1/CD11b+ cells) within the CA1 region of the hippocampus in socially isolated (E) and socially paired (F) animals 7 d following CA/CPR. The effects of CA/CPR on Fluorojade C+ neurons and MAC-1 proportional area were significantly greater in socially isolated than pair-housed animals. The control group was comprised of sham-operated mice and mice that underwent hypothermic CA/CPR and as a result did not sustain neuroinflammation or neuronal damage. Data are presented as mean ± SEM. *Significantly different from isolated CA/CPR animals. (P < 0.05). †Significantly different from controls (P < 0.05).
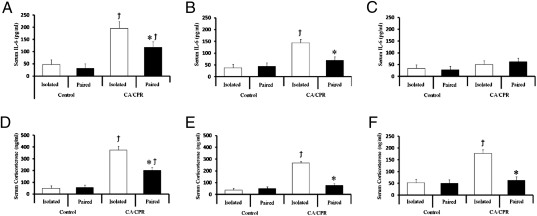
IL-6 and corticosterone.
CA/CPR resulted in significantly elevated serum IL-6 and corticosterone levels at 24 (all F1,46 < 8.01, P < 0.05; Fig. 3) and 72 h (all F1,46 < 4.99, P < 0.05; Fig. 3) following surgery, relative to controls. Socially isolated CA/CPR animals displayed increased circulating levels of IL-6 and corticosterone at 24 (all F1,44 < 6.22, P < 0.05; Tukey < 0.05; Fig. 3) and 72 h (all F1,44 = 6.02, P < 0.05; Tukey < 0.05; Fig. 3) following surgery. By 7 d following CA/CPR, socially isolated and pair-housed animals displayed comparable circulating IL-6 levels to that of controls (P > 0.05; Fig. 3). However, at 7 d following surgery, circulating corticosterone levels were elevated in socially isolated, but not pair-housed CA/CPR animals, as compared with controls (F1,44 = 4.96, P < 0.05; Tukey < 0.05; Fig. 3).
Fig. 3.
Social influences on serum IL-6 and corticosterone levels. CA/CPR animals displayed elevated levels of circulating IL-6 at (A) 24 and (B) 72 h postsurgery with socially isolated animals displaying significantly higher levels than their paired counterparts. (C) IL-6 levels returned to control levels in the CA/CPR groups by 7 d following surgery. (D) Both the socially isolated and paired CA/CPR groups displayed elevated serum corticosterone concentrations at 24 h relative to controls. Corticosterone concentrations had returned to control levels by 72 h postsurgery among the paired CA/CPR group (E and F), but remained significantly elevated at both the 72-h (E) and 7-d (F) time points among the socially isolated CA/CPR group. The control group was comprised of sham-operated mice and mice that underwent hypothermic CA/CPR and as a result did not sustain neuroinflammation or neuronal damage. Data are presented as mean ± SEM. *, significantly different from isolated CA/CPR animals. (P < 0.05). †Significantly different from controls (P < 0.05).
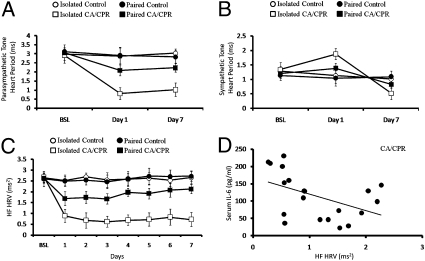
Autonomic cardiac control.
To determine the influence of social housing and CA/CPR on autonomic nervous system functioning, animals were implanted with telemetric devices and autonomic cardiac control was determined via a well-validated pharmacological manipulation protocol (27, 28). A 2 (surgery) × 3 (time) ANOVA of the dual pharmacological blockade data revealed that CA/CPR resulted in dramatic alterations within both the parasympathetic (PNS; F6,93 = 6.69, P < 0.05) (Fig. 4A) and sympathetic (SNS; F6,93 = 4.09, P < 0.05) (Fig. 4B) branches of the autonomic nervous system. Social housing proved to be an important moderator of the effects of CA/CPR on both PNS (F6,93 = 7.15, P < 0.05) (Fig. 4A) and SNS cardiac control (F6,93 = 4.01, P < 0.05) (Fig. 4B) as revealed by significant housing × surgery × time interactions. Specifically, among CA/CPR mice, social isolation increased sympathetic and decreased parasympathetic cardiac control at 24 h following surgery. Similarly, the effects of CA/CPR on autonomic tone were most pronounced in the socially isolated group at 7 d following surgery, with SNS values dropping below baseline (Fig. 4) and PNS cardiac control measurements maintaining subbaseline values. With regard to the pharmacological blockade study, the only significant alteration in autonomic tone of socially housed CA/CPR animals was a diminished level, relative to baseline, of PNS control at 72 h following surgery (F2,34 = 3.82, P < 0.05; Tukey < 0.05) (Fig. 4A). Parasympathetic cardiac control returned to baseline levels by 7 d postsurgery in the socially housed CA/CPR animals (F2,34 = 0.92, P > 0.05) (Fig. 4B). Housing conditions did not influence autonomic tone among control animals (P > 0.05). Autonomic cardiac control was quantified through pharmacological blockade of the sympathetic and parasympathetic branches using atropine and atenolol.
Fig. 4.
Social influences on cardiac autonomic control. (A) Pharmacological evaluation of parasympathetic cardiac control revealed that the CA/CPR procedure resulted in decreased parasympathetic cardiac control at 24 h and 7 d following surgery as compared with the control group. This effect was primarily the result of significantly diminished parasympathetic cardiac control in socially isolated CA/CPR animals. (B) Pharmacological evaluation of sympathetic cardiac control. CA/CPR animals exposed to chronic social isolation displayed significantly increased levels of sympathetic cardiac control (decreased heart period) at 24 h following surgery as compared with pair-housed CA/CPR animals and hypothermic controls. However, socially isolated animals displayed diminished sympathetic cardiac control by 7 d following CA/CPR as compared with pair-housed counterparts and hypothermic controls, illustrating an important time-dependent effect of social housing conditions on autonomic outcome following CA/CPR. (C) Confirming the data presented above, parasympathetic tone, as measured through HF HRV, was diminished following CA/CPR. Although HF HRV levels returned to baseline levels by day 7 in pair-housed CA/CPR animals, HF HRV tone remained significantly lower than baseline presurgical recordings. (D) At 72 h following surgery, HF HRV values were significantly correlated with circulating IL-6 levels. The control group was comprised of sham-operated mice and mice that underwent hypothermic CA/CPR and as a result did not sustain neuroinflammation or neuronal damage. Data are presented as mean ± SEM.
Analysis of high-frequency heart rate variability (HF HRV), a measure of parasympathetic cardiac control, corroborated the data provided above; HF HRV was diminished following CA/CPR (F2,31 = 18.94. P < 0.05; Tukey < 0.05) (Fig. 4C). The combination of CA/CPR and social isolation significantly reduced HF HRV relative to socially paired CA/CPR mice and controls as evidenced by a significant housing × surgery × time interaction (F14,217 = 7.39, P < 0.05; Tukey P > 0.05) (Fig. 4C). Interestingly, circulating IL-6 levels were significantly correlated with PNS activity at 72 h following CA/CPR, regardless of housing conditions (r2 = −0.33, P < 0.05) (Fig. 4D), with decreased HF HRV associated with increased circulating IL-6 levels (Fig. 4D). Housing conditions did not alter HF HRV among control animals (P > 0.05) (Fig. 4C).
Behavior.
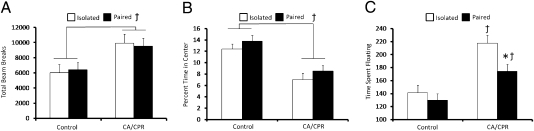
Mice exposed to CA/CPR displayed increased general locomotor activity at 5 d postischemia, regardless of housing conditions, relative to controls (F1,48 = 5.02, P < 0.05) (Fig. 5A). Furthermore, both CA/CPR groups spent less time exploring the center of the open field chamber, interpreted as increased anxiety-like behavior, relative to control mice (F1,48 = 5.21, P < 0.05) (Fig. 5B). Housing conditions did not influence locomotor and anxiety-like behavior in control animals (F1, 48 = 0.32, P < 0.05) (Fig. 5).
Fig. 5.
Social influences on behavioral outcome. (A) The CA/CPR procedure resulted in significantly increased locomotor activity independent of housing conditions. (B) CA/CPR also led to increased levels of anxiety-like behavior regardless of housing conditions, as measured by decreased central tendency in the open field. (C) CA/CPR engendered increased levels of floating in the forced swim task, which is interpreted as increased depressive-like behavior. Social isolation exacerbated this effect with isolated CA/CPR mice displaying increased depressive-like behavior as compared with pair-housed CA/CPR mice and controls. The control group was comprised of sham-operated mice and mice that underwent hypothermic CA/CPR and as a result did not sustain neuroinflammation or neuronal damage. Data are presented as mean ± SEM. *Significantly different from isolated CA/CPR animals. (P < 0.05). †Significantly different from controls (P < 0.05).
A 2 (housing) × 2 (surgery) ANOVA revealed an overall effect of surgery on levels of depressive-like behavior (F1,46 = 9.91, P < 0.05; Tukey P > 0.05) (Fig. 5C); CA/CPR animals spent more time floating in the forced swim test (FST). A significant housing × surgery interaction revealed that housing conditions significantly influenced depressive-like behavior among CA/CPR mice; socially isolated CA/CPR mice spent more time floating in the FST as compared with paired CA/CPR mice 5 d following CA/CPR (F1,46 = 7.21, P < 0.05) (Fig. 5C). Housing conditions did not influence depressive-like behavior among control animals (F1,46 = 0.19, P > 0.05) (Fig. 5C).
Discussion
This study establishes that social environment strongly affects the physiological and behavioral responses to global cerebral ischemia. Replicating and extending previous findings (23, 24), socially isolated mice experienced elevations in ischemia-induced central proinflammatory cytokine expression (Fig. 1 A and B) and nearly double the amount of cell death (Fig. 2A) and microglial expression (Fig. 2B) as pair-housed CA/CPR animals. Furthermore, housing conditions proved to be an important determinant of the serum IL-6 response to cerebral ischemia; socially isolated CA/CPR mice displayed significantly higher concentrations of serum IL-6 as compared with pair-housed CA/CPR mice and controls at 24 and 72 h following surgery (Fig. 3). Furthermore, circulating IL-6 levels were negatively correlated with levels of parasympathetic activity at 72 h following CA/CPR surgery (HF HRV) (Fig. 4D). Furthermore, socially isolated mice displayed more severe dysregulation of autonomic tone after CA/CPR as compared with socially housed animals (Fig. 4). Additionally, socially isolated mice showed increased depressive-like behavior at 5 d following CA/CPR (Fig. 5C). Thus, social interaction moderates the effects of global cerebral ischemia on the behavioral, neuronal, inflammatory, and autonomic outcomes. Physical contact is likely the critical neuroprotective component of social interaction in the current study (29); the absence of group differences in presurgical body mass, autonomic functioning, and postsurgical inflammatory measures among the paired and socially isolated control groups suggest that a metabolic syndrome had not developed among the socially isolated mice by the time of CA/CPR induction. Also, whether CA/CPR outcome is exacerbated by social isolation or mitigated by social interaction cannot be conclusively determined in this study. However, because mice, like humans, typically live in social groups, it is likely that the response exhibited by the paired mice more closely approximates the baseline response to CA/CPR and that it is exacerbated by social isolation.
Social Influences Cytokine Expression, Neuronal Damage, and Microglia Expression.
Among socially isolated animals, the CA/CPR procedure produced large increases in hippocampal mRNA levels of the proinflammatory cytokines IL-1β and TNF-α at 24 and 72 h postsurgery (Fig. 1 A and B) and increased mRNA expression of MAC-1 (Fig. 1B). The CA/CPR procedure also led to robust increases in hippocampal cell damage and increased microglial staining at 7 d post-CA/CPR (Fig. 2 A and B). Furthermore, histological analysis at 7 d following surgery revealed that socially isolated animals displayed increased neuronal damage and microglial expression within the hippocampus as compared with pair-housed and control animals (Fig. 2 A and B). These data provide further evidence of the powerful influence of social environment on neuroinflammation and neuronal damage after CA/CPR.
Previous reports have demonstrated the ability of social behavior to modulate immune function (23, 30, 31) and inflammatory responses to cerebral ischemia (23, 24, 29). Although the present study did not find significant housing differences in central IL-6, robust differences were detected for the proinflammatory cytokines IL-1β and TNFα. Furthermore, neuroinflammation following CA/CPR is thought to be a major contributor to neuronal cell death (11). Thus, the ability of social information to modulate the neuroinflammatory response to global cerebral ischemia is a likely mechanism for the reduced cell death among pair-housed CA/CPR animals.
Social Influences on Circulating IL-6 Levels Following Global Cerebral Ischemia.
CA/CPR produced significant increases in circulating IL-6 levels 24 h following surgery, regardless of housing conditions (Fig. 3). Socially isolated animals still had significantly elevated levels of serum IL-6 at 72 h (Fig. 3) relative to the control animals, whereas IL-6 concentrations had returned to baseline levels among pair-housed animals by this time point. By postsurgical day 7, IL-6 concentrations were similar for all groups. These data are consistent with previous work in humans (32) and experimental animals (24) demonstrating increased serum IL-6 levels following cerebral ischemia and the ability of social interaction to moderate this response.
Social Influences on Circulating Corticosterone Levels Following Global Cerebral Ischemia.
In the current study, mice that underwent CA/CPR had significantly greater postsurgery serum corticosterone concentrations compared with controls. At 24 h following CA/CPR, there was no effect of housing on serum corticosterone concentrations (Fig. 3); by 72 h, socially isolated mice had significantly greater serum corticosterone concentrations than pair-housed mice. This pattern was still evident 7 d following CA/CPR (Fig. 3). These data are consistent with a previous report describing the effects of social housing on the neuroendocrine response to global cerebral ischemia (23).
Social Influences on Neural Regulation of Cardiac Functioning Following CA/CPR.
CA/CPR resulted in dynamic alterations in sympathetic and parasympathetic cardiac regulation and, as above, social interaction proved to be an important factor in moderating this effect. Specifically, socially isolated animals displayed elevations in sympathetic autonomic outflow 24 h following normothermic CA/CPR (Fig. 4B). However, this trend was reversed by day 7, when sympathetic outflow values declined to well below baseline levels (Fig. 4B). The effects of CA/CPR on sympathetic outflow in pair-housed animals were minimal at all postsurgical time points (1, 3, and 7 d) (Fig. 4B). Comparable effects were detected in parasympathetic outflow as measured by pharmacological manipulation and HF HRV (Fig. 4 B and C). Specifically, among socially isolated CA/CPR mice, parasympathetic autonomic output was diminished at all postsurgery time points measured, as determined via pharmacological blockade (days 1 and 7 following surgery) (Fig. 4A). Similarly, HF HRV levels were dramatically reduced immediately following CA/CPR in socially isolated animals, and this effect was maintained through day 7 (Fig. 4C) as compared with pair-housed CA/CPR and control groups. Interestingly, HF HRV was negatively correlated with peripheral IL-6 levels following CA/CPR at 72 h, independent of housing conditions (Fig. 4D). In contrast to the CA/CPR groups, social housing did not alter autonomic cardiac regulation in control animals (Fig. 4 A and B). Thus CA/CPR produces profound dysregulation of overall autonomic control; a phenomenon greatly influenced by social interaction and previously shown to be associated with poorer health outcomes in humans (16, 33).
As mentioned above, quantification of autonomic function is widely used in clinical setting as a predictor of mortality in chronic heart disease patients (16). Furthermore, autonomic tone is often dysregulated in at-risk patients suffering from major depression (10). In the current study, HF HRV values were significantly correlated with circulating IL-6 levels at 72 h following surgery (Fig. 4D), thereby suggesting that decreased parasympathetic activity may partially mediate increases in cytokine levels following CA/CPR through the “cholinergic anti-inflammatory reflex” (18). Thus, global cerebral ischemia leads to dramatic alterations within both branches of the autonomic nervous system that may have important implications for the well-described immunomodulatory effects following cerebral ischemia (7). Furthermore, the ability of social interaction to mitigate the destructive effects of global cerebral ischemia on autonomic functioning provides an additional mechanism through which the social environment is able to influence health outcome following CA/CPR.
Affective Behavior Following Global Cerebral Ischemia.
Following CA/CPR, the normothermic mice displayed increased depressive-like behavior as measured by the forced swim test (Fig. 5C). Similarly, global ischemia increased both anxiety-like behavior and overall locomotor behavior (Fig. 5 A and B). Social isolation further exacerbated the effect of global cerebral ischemia on depressive-like behavior (Fig. 5) but had no effect on overall locomotor and anxiety-like behavior (Fig. 5 A and B).
As discussed above, clinical depression associated with neurological injury represents a significant source of suffering. Indeed, as many as 72% of cerebral ischemia patients are diagnosed with depression (34), and elevations in depressive scores are associated with mortality and functional deficits (35). Together, these data support the hypothesis that social isolation exacerbates the affective responses to cardiac arrest.
In sum, this study demonstrates that in conjunction with its effects on neuronal cell death and inflammatory response to ischemia, social interaction significantly moderates the effects of CA/CPR on both sympathetic and parasympathetic cardiac control across two time points. Mice exposed to 2 wk of social isolation before CA/CPR exhibited significantly greater neuroinflammation, serum corticosterone concentrations, serum IL-6 concentrations, and neuronal damage as compared with pair-housed mice that underwent CA/CPR. Social housing also proved to be an important moderator of the effects of CA/CPR on depressive-like behavior and autonomic regulation of the heart. Socially isolated CA/CPR mice demonstrated increased sympathetic tone immediately after ischemia, followed by a substantial loss in sympathetic control by day 7. Both HF HRV and pharmacological blockade of the autonomic nervous system revealed that CA/CPR induces significant loss in parasympathetic cardiac control, and this effect was more pronounced in socially isolated mice at all postsurgical time points. Together, these data provide further evidence of the powerful effect that social environment has on physiological processes and associated health outcomes.
Materials and Methods
Animals.
Adult male C57/BL6 mice (23–30 g, Charles River) were maintained on a 14:10 light/dark cycle in standard housing conditions within a temperature and humidity-controlled vivarium. Water and food were available ad libitum throughout the study. The study was conducted in accordance with National Institutes of Health guidelines for the care and use of animals and under protocols approved by the Ohio State University Institutional Animal Care and Use Committee. Animals were assigned to one of two housing conditions: 2 wk of social isolation or 2 wk of pair housing with an ovariectomized female. Animals then were randomly assigned to one of three conditions: surgical control (sham), an ischemic control that did not sustain neuronal damage (hypothermic CA/CPR), or the criterion groups (normothermic CA/CPR). Furthermore, animals were assigned to one of three tissue collection time points: 24 h, 72 h, or 7 d following surgery. Analysis revealed that hypothermic control animals did not statistically differ from sham surgery animals and, as such, hypothermic and sham groups were collapsed into a single control group for the purposes of statistical analysis and graphical representation as in previous studies (14).
Cardiac Arrest/CPR Procedure.
CPR was initiated via injection of epinephrine (16 μg epinephrine in 0.6 cc saline) and chest compressions (300/min). The sham animals underwent the same preparatory surgery as the CA/CPR mice but were not injected with potassium chloride or epinephrine and did not undergo chest compressions. A double lumen coil system was used to manipulate head temperature independently of body temperature during surgery. The periphery was maintained at 27 °C during CA/CPR or the sham surgery, which protects against peripheral organ damage in CA/CPR mice (14). The brains of CA/CPR experimental animals were maintained at 37 °C (normothermic) to induce neuronal damage, although the brains of the ischemic controls were maintained at 27 °C (hypothermic) to prevent neuronal damage. Hypothermia is neuroprotective during cerebral ischemia and is gaining acceptance clinically as a measure to improve neurological outcome after cardiac arrest in humans (25). Hypothermia is thought to reduce neuronal cell death through various mechanisms, including diminished mitochondrial dysfunction and excitotoxicity following neural injury (26). Hypothermic CA/CPR is an appropriate control for normothermic CA/CPR in the current study because the hypothermic animals are exposed to all aspects of the CA/CPR procedure but do not develop the neuronal damage, neuroinflammation, or endocrine changes that typically evolve after cerebral ischemia.
Determination of Autonomic Tone.
Heart rate variability.
At least 10 d before surgery, animals were implanted with telemetric recording devices (DSI). HF HRV, a well-validated measure of parasympathetic tone, was derived by spectral analysis of the interbeat interval series derived from the telemetric ECG implants by using commercial software (Mindware). Twenty-four hours before surgical manipulation, baseline recordings were taken over 90 sec and recorded each subsequent day following surgery. Analysis followed procedures recommended by the Society for Psychophysiological Research Committee on Heart Rate Variability and were adapted for use in rodents (36). High-frequency spectral power was integrated over the respiratory frequency band (1.5–5 Hz) based upon previous work in mice (37).
Blockade studies.
The contributions of the two branches of the autonomic nervous system to heart period (60,000/HR) were determined 1 d before surgery and 72 h and 7 d following surgery by using a specific muscarinic receptor antagonist (atropine methyl nitrate, 1 mg/kg i.p. to block parasympathetic signaling to the heart, Sigma–Aldrich), a β1 receptor antagonist (atenolol, 10 mg/kg i.p. to block sympathetic inputs to the heart, Sigma–Aldrich); both drugs in combination, and isotonic saline to determine the intrinsic heart period, were used as previously described (27, 28). Each mouse received each of the drug treatments i.p. in a counterbalanced order: (i) atropine injection alone, (ii) atenolol injection alone, (iii) atropine and atenolol given together, and (iv) isotonic saline injection. The four drug treatments were given at least 4 h apart in an attempt to limit drug interactions.
Based on previous findings (28), two equations were used to estimate autonomic control based on changes in HP in each of the blockade conditions. The averages of the two estimates were derived for each autonomic branch. The ECG was recorded beginning 5 min following injection and was averaged across a 90-sec span within each drug condition. Autonomic tone was taken as the change in HP from saline to the HP under blockade of the relevant branch (e.g., atropine for the parasympathetic branch), and the residual estimate was derived as the difference between the HP level after double autonomic blockade and after blockade of the other (nontarget branch, e.g., atenolol for the parasympathetic estimate). The former provides an estimate based on the change in HP with blockade of the target branch, and the latter provides a second estimate represented by the residual autonomic effects of the target branch after blockade of the other.
(i) cardiac vagal control estimate = [(HPsaline − HPatropine) + [(HPatenolol − HPatropine+atenolol)]/2
(ii) cardiac sympathetic control estimate = [(HPsaline − HPatenolol) [(HPatropine − HPatropine+atenolol)]/2
Tissue Collection, Processing, and Analysis.
Twenty-four– and 72–h survival.
Two separate cohorts of mice were used to quantify mRNA levels at 24 and 72 h after surgery. Mice were euthanized and then brains were removed using aseptic techniques. Hippocampi were dissected out, and total RNA was extracted using an RNeasy kit according to manufacturer's protocol (Qiagen); cDNA was created according to the manufacturer's protocol (Invitrogen). A TaqMan 18S Ribosomal RNA primer and probe set (Applied Biosystems) was used as the control gene for relative quantification. IL-1β, TNF-α, IL-6, GFAP, and MAC-1 inventoried primer probe kits were purchased from Applied Biosystems. Amplification was performed on an ABI 7000 Fast Sequencing Detection System.
Seven-day survival.
At 7 d postreperfusion, mice received an overdose with sodium pentobarbital and were perfused transcardially with ice cold 0.1 M PBS and 4% paraformaldehyde. Brains were removed and frozen on dry ice. A cryostat was used to cut 14-μm sections, and photographs of multiple hippocampal regions (CA1, CA2, CA3) were taken with a Nikon E800 microscope at 20×. Data from all regions were collapsed for statistical analysis. Images assessed using Image J software. The number of damaged neurons was identified via Fluorojade staining, and microglial levels were measured using an antibody directed against MAC-1/CD11b.
Forced swim task.
At 5 d following surgery, mice were placed into an opaque cylinder tank (24 cm in diameter, 53 cm high) filled to a depth of 30 cm with water maintained between 25–27 °C. Swimming behavior was recorded for 5 min and scored for time spent actively swimming versus floating. Quantification of float vs. swim time was performed with Observer software (Version 5; Exeter Software). An increase in floating is interpreted as an increase in depressive-like behavior (38).
Open field.
General activity and anxiety-like behavior were assessed during a 60-min session in an open field apparatus (40 × 40 × 37.5 cm) at day 5 following cardiac arrest using Flex Field photobeam activity (San Diego Instruments). Data were analyzed to determine general locomotor activity and the relative amount of activity occurring in the periphery versus the center of the apparatus (anxiety-like behavior).
Corticosterone.
Trunk blood samples were collected at the time of euthanasia (24 h, 72 h, and 7 d following surgery) and placed on ice. Corticosterone concentrations were determined by using an 125I corticosterone kit (MP Biomedical).
Data analysis.
The data are expressed as means ± SEM. Testing of statistical significance was performed using ANOVA. When a significant overall treatment effect was reported (P < 0.05), post hoc analyses were conducted using the Tukey test. Levels of significance were taken at P < 0.05. Autonomic tone responses for all experiments were analyzed using two-way repeated measures ANOVA assessing effects of time, housing conditions, and surgery. Histological, mRNA, corticosterone, and IL-6 data were analyzed using one-way ANOVAs.
Acknowledgments
This work was supported by grants from the American Heart Association (predoctoral fellowship to K.K.), The J. Parker and Kathryn Webb Dinius Fellowship (to G.J.N.), National Institute of Neurological Disorders and Stroke Behavioral Core Grant P30 NS045758, and Cardiac Arrest and Cardiopulmonary Resuscitation Grant R01 HL080249 (to A.C.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Rosamond W, et al. Heart disease and stroke statistics -2007 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Garza AG, et al. Improved patient survival using a modified resuscitation protocol for out-of-hospital cardiac arrest. Circulation. 2009;119:2597–2605. doi: 10.1161/CIRCULATIONAHA.108.815621. [DOI] [PubMed] [Google Scholar]
- 3.Drezner JA. Preparing for sudden cardiac arrest—the essential role of automated external defibrillators in athletic medicine: A critical review. Br J Sports Med. 2009;43:702–707. doi: 10.1136/bjsm.2008.054890. [DOI] [PubMed] [Google Scholar]
- 4.Tiainen M, et al. Arrhythmias and heart rate variability during and after therapeutic hypothermia for cardiac arrest. Crit Care Med. 2009;37:403–409. doi: 10.1097/CCM.0b013e31819572c4. [DOI] [PubMed] [Google Scholar]
- 5.Cronberg T, Lilja G, Rundgren M, Friberg H, Widner H. Long-term neurological outcome after cardiac arrest and therapeutic hypothermia. Resuscitation. 2009;80:1119–1123. doi: 10.1016/j.resuscitation.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Adrie C, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 7.Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience. 2009;158:1098–1111. doi: 10.1016/j.neuroscience.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sunnerhagen KS, Johansson O, Herlitz J, Grimby G. Life after cardiac arrest; a retrospective study. Resuscitation. 1996;31:135–140. doi: 10.1016/0300-9572(95)00903-5. [DOI] [PubMed] [Google Scholar]
- 9.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 10.Frasure-Smith N, et al. Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation. 2009;120:134–140. doi: 10.1161/CIRCULATIONAHA.109.851675. [DOI] [PubMed] [Google Scholar]
- 11.Harukuni I, Bhardwaj A. Mechanisms of brain injury after global cerebral ischemia. Neurol Clin. 2006;24:1–21. doi: 10.1016/j.ncl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Qiu J, et al. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28:927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- 13.Micieli G, Cavallini A. The autonomic nervous system and ischemic stroke: A reciprocal interdependence. Clin Auton Res. 2008;18:308–317. doi: 10.1007/s10286-008-0495-7. [DOI] [PubMed] [Google Scholar]
- 14.Neigh GN, et al. Cardiac arrest and cardiopulmonary resuscitation dysregulates the hypothalamic-pituitary-adrenal axis. J Cereb Blood Flow Metab. 2009;29:1673–1682. doi: 10.1038/jcbfm.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balanescu S, Corlan AD, Dorobantu M, Gherasim L. Prognostic value of heart rate variability after acute myocardial infarction. Med Sci Monit. 2004;10:CR307–CR315. [PubMed] [Google Scholar]
- 16.Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Moynihan J, Kruszewska B, Madden K, Callahan T. Sympathetic nervous system regulation of immunity. J Neuroimmunol. 2004;147:87–90. doi: 10.1016/j.jneuroim.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Offner H, et al. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 20.Neigh GN, et al. Anxiety after cardiac arrest/cardiopulmonary resuscitation: Exacerbated by stress and prevented by minocycline. Stroke. 2009;40:3601–3607. doi: 10.1161/STROKEAHA.109.564146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Empana JP, et al. Clinical depression and risk of out-of-hospital cardiac arrest. Arch Intern Med. 2006;166:195–200. doi: 10.1001/archinte.166.2.195. [DOI] [PubMed] [Google Scholar]
- 22.Norman GJ, et al. Social interaction prevents the development of depressive-like behavior post nerve injury in mice: A potential role for oxytocin. Psychosom Med. 2010;72:519–526. doi: 10.1097/PSY.0b013e3181de8678. [DOI] [PubMed] [Google Scholar]
- 23.Weil ZM, et al. Social isolation potentiates cell death and inflammatory responses after global ischemia. Mol Psychiatry. 2008;13:913–915. doi: 10.1038/mp.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karelina K, et al. Social isolation alters neuroinflammatory response to stroke. Proc Natl Acad Sci USA. 2009;106:5895–5900. doi: 10.1073/pnas.0810737106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yenari M, Kitagawa K, Lyden P, Perez-Pinzon M. Metabolic downregulation: A key to successful neuroprotection? Stroke. 2008;39:2910–2917. doi: 10.1161/STROKEAHA.108.514471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corbett D, Thornhill J. Temperature modulation (hypothermic and hyperthermic conditions) and its influence on histological and behavioral outcomes following cerebral ischemia. Brain Pathol. 2000;10:145–152. doi: 10.1111/j.1750-3639.2000.tb00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berntson GG, Cacioppo JT, Quigley KS. Autonomic cardiac control. I. Estimation and validation from pharmacological blockades. Psychophysiology. 1994;31:572–585. doi: 10.1111/j.1469-8986.1994.tb02350.x. [DOI] [PubMed] [Google Scholar]
- 28.Weil ZM, Norman GJ, DeVries AC, Berntson GG, Nelson RJ. Photoperiod alters autonomic regulation of the heart. Proc Natl Acad Sci USA. 2009;106:4525–4530. doi: 10.1073/pnas.0810973106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karelina K, Norman GJ, Zhang N, DeVries AC. Social contact influences histological and behavioral outcomes following cerebral ischemia. Exp Neurol. 2009;220:276–282. doi: 10.1016/j.expneurol.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 30.Kiecolt-Glaser JK, et al. Psychosocial modifiers of immunocompetence in medical students. Psychosom Med. 1984;46:7–14. doi: 10.1097/00006842-198401000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Cole SW, et al. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waje-Andreassen U, et al. IL-6: An early marker for outcome in acute ischemic stroke. Acta Neurol Scand. 2005;111:360–365. doi: 10.1111/j.1600-0404.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- 33.Berntson GG, Norman GJ, Hawkley LC, Cacioppo JT. Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology. 2008;45:643–652. doi: 10.1111/j.1469-8986.2008.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pohjasvaara T, et al. Frequency and clinical determinants of poststroke depression. Stroke. 1998;29:2311–2317. doi: 10.1161/01.str.29.11.2311. [DOI] [PubMed] [Google Scholar]
- 35.Berg A, Palomäki H, Lehtihalmes M, Lönnqvist J, Kaste M. Poststroke depression: An 18-month follow-up. Stroke. 2003;34:138–143. doi: 10.1161/01.str.0000048149.84268.07. [DOI] [PubMed] [Google Scholar]
- 36.Berntson GG, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 37.Howden R, et al. The genetic contribution to heart rate and heart rate variability in quiescent mice. Am J Physiol Heart Circ Physiol. 2008;295:H59–H68. doi: 10.1152/ajpheart.00941.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porsolt RD. Animal models of depression: Utility for transgenic research. Rev Neurosci. 2000;11:53–58. doi: 10.1515/revneuro.2000.11.1.53. [DOI] [PubMed] [Google Scholar]