Abstract
Accumulation of misfolded proteins in the endoplasmic reticulum (ER) triggers the unfolded protein response (UPR), an intracellular signaling pathway that adjusts the protein folding capacity of the ER according to need. If homeostasis in the ER protein folding environment cannot be reestablished, cells commit to apoptosis. The ER-resident transmembrane kinase-endoribonuclease inositol-requiring enzyme 1 (IRE1) is the best characterized UPR signal transduction molecule. In yeast, Ire1 oligomerizes upon activation in response to an accumulation of misfolded proteins in the ER. Here we show that the salient mechanistic features of IRE1 activation are conserved: mammalian IRE1 oligomerizes in the ER membrane and oligomerization correlates with the onset of IRE1 phosphorylation and RNase activity. Moreover, the kinase/RNase module of human IRE1 activates cooperatively in vitro, indicating that formation of oligomers larger than four IRE1 molecules takes place upon activation. High-order IRE1 oligomerization thus emerges as a conserved mechanism of IRE1 signaling. IRE1 signaling attenuates after prolonged ER stress. IRE1 then enters a refractive state even if ER stress remains unmitigated. Attenuation includes dissolution of IRE1 clusters, IRE1 dephosphorylation, and decline in endoribonuclease activity. Thus IRE1 activity is governed by a timer that may be important in switching the UPR from the initially cytoprotective phase to the apoptotic mode.
Keywords: receptor, oligomerization, kinase, RNase, fluorescent microscopy
Most secreted and transmembrane proteins are synthesized, modified, and folded in the lumen of the endoplasmic reticulum (ER). To ensure the fidelity of protein folding and maturation, cells turn on a network of signaling pathways, collectively termed the unfolded protein response (UPR), to adjust the protein-folding capacity of the ER according to need (1). Imbalances between protein load and folding capacity is monitored by three distinct UPR sensors: inositol-requiring enzyme 1 (IRE1) (2, 3), protein kinase RNA (PKR)-like ER kinase (PERK) (4), and activating transcription factor-6 (ATF6) (5). IRE1 is a conserved transmembrane protein with an ER–luminal domain that senses misfolded proteins in the ER, most likely by direct ligand-mediated recognition (6, 7). As a result, IRE1 activates its cytoplasmic kinase and endoribonuclease (RNase) domains, which initiate a nonconventional mRNA splicing reaction that results in the cleavage of the mRNA encoding the transcription factor XBP-1 (x-box binding protein 1) (8, 9), a conserved effector of the UPR that drives the transcription of a plethora of ER-stress responsive genes, including ER resident chaperones and protein modifying enzymes (10).
The structural analysis of the luminal stress-sensing domain of yeast Ire1 provided insights into Ire1 activation under ER stress. Upon UPR activation, the luminal domain of Ire1 undergoes oligomerization, which brings the cytosolic kinase domains into juxtaposition allowing their activation by transautophosphorylation and cofactor binding (11–13). Kinase activation in turn triggers conformational changes that activate the RNase domain of the enzyme. Recent structural work suggests that the cytosolic domain of yeast Ire1 can form high-order assemblies and that this process is instrumental to activating the endoribonuclease activity of Ire1 (13). Furthermore, in vivo studies in yeast demonstrate that high-order assembly of Ire1 is driven by its luminal domain, and that Ire1 clusters into distinct foci upon activation (14, 15). In contrast, previous studies of human IRE1 suggested that formation of IRE1 dimers is sufficient to fully activate the enzyme (16–19). It has remained unclear whether oligomerization of IRE1 is necessary for its activation in metazoans.
Here we show that the salient features of IRE1 activation, including its obligate oligomerization, are conserved, and describe additional regulation that evolved in metazoan cells.
Results
IRE1 Clustering in the ER Membrane of Human Cells.
To begin resolving the uncertainty and to determine whether IRE1 activation in mammalian cells involves high-order assembly, we engineered a GFP-tagged fluorescent human IRE1α fusion construct, IRE1–3-Flag-6-Histidine (3F6HGFP). The fluorescent tag was placed in the nonconserved linker between the transmembrane domain and the kinase domain (Fig. 1A). Yeast Ire1 tagged in the corresponding position was fully functional, whereas C- or N-terminally GFP tagged versions were not (14). As expected, IRE1-3F6HGFP restored splicing of Xbp-1 mRNA in response to ER-stress when expressed in Ire1α−/− mouse embryonic fibroblasts (MEFs) (Fig. 1B). Overexpression is known to activate WT IRE1 constitutively, which explains the constitutive Xbp-1 mRNA splicing observed in untreated MEFs.
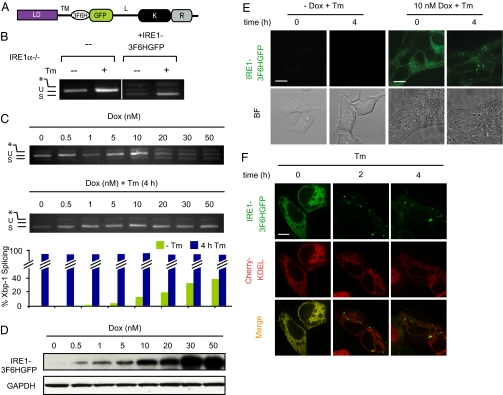
Fig. 1.
IRE1 Clustering in the ER membrane of human cells. (A) Schematic of IRE1-3F6HGFP imaging construct. Luminal domain (“LD”), transmembrane domain (“TM”), linker domain (“L”), kinase domain (“K”), and endoribonuclease domain (“R”) of human IRE1 and inserted tag consisting of three FLAG epitopes followed by six histidines and a GFP (“3F6H”, “GFP”) are indicated. (B) IRE1-3F6HGFP construct rescues Xbp-1 mRNA splicing in Ire1α−/− MEFs. Xbp-1 mRNA splicing was determined by RT-PCR. Products resulting from unspliced (“u”) an spliced (“s”) Xbp-1 mRNA are indicated. Asterisk identifies a hybrid amplicon resulting from spliced and unspliced Xbp-1 mRNA. (C) Doxycycline (“Dox”) titration in T-REx293 cells ± 5 μg/mL tunicamycin (Tm). Dox was added to the cells for 24 h and withdrawn before Tm treatment. Xbp-1 mRNA splicing was determined by RT-PCR as in B. (D) IRE1-3F6HGFP protein levels were determined by Western blotting using anti-IRE1 antibodies. Blotting for the metabolic enzyme GAPDH was used as a loading control. (E) Localization and clustering of IRE1-3F6HGFP in stably transfected T-REx293 cells. Fluorescent and brightfield images of cells grown ± 10 nM Dox before and after a 4-h treatment with 5 μg/mL Tm. (Scale bar, 10 μm.) (F) Stable T-REx293 cells bearing IRE1-3F6HGFP allele were transiently transfected with Cherry-KDEL construct to covisualize the ER.
We next established a stable T-REx293 cell line, in which the IRE1-3F6HGFP expression construct was integrated at a unique frt site in the genome, and driven by a tetracycyline-inducible CMV promoter (20, 21), allowing us to control the expression level of IRE1. Increasing concentrations of doxycycline (Dox) led to constitutive activation of Xbp-1 mRNA splicing in the absence of ER stress (Fig. 1C, Upper, 20–50 nM Dox), similar to what was observed in MEFs. Based on these findings, IRE1-3F6HGFP expressed as a transgene in T-REx293 cells is therefore functional. Titration of Dox allowed us to establish conditions under which constitutive IRE1-3F6HGFP activation in the absence of ER-stress inducer tunicamycin (Tm), an inhibitor of N-linked glycosylation, was minimized (Fig. 1C, Upper; compare lower Dox concentrations 5 and 10 nM Dox with 30 and 50 nM Dox). No IRE1-3F6HGFP fusion protein was detected by Western blotting using anti-total IRE1 antibody or by fluorescent microscopy in the absence of Dox (Fig. 1 D and E), indicating that the promoter is tightly controlled. Endogenous IRE1 protein was not detected by this antibody, likely due to low abundance of IRE1 in T-REx293 cells.
Addition of Tm robustly induced Xbp-1 mRNA splicing at all Dox concentrations (Fig. 1C, Middle and Lower). The splicing observed in the absence of Dox is due to endogenous IRE1 activity, whereas the splicing at higher Dox concentrations reflects the combined activities of endogenous IRE1 and exogenous IRE1-3F6HGFP. IRE1-3F6HGFP expressed at 10 nM Dox is clearly detectable by fluorescence microscopy and is found to be diffusely distributed in the ER membrane (Fig. 1E, Upper), as indicated by its colocalization with the ER marker protein Cherry-KDEL (22) (Fig. 1F, “Tm, 0 h”).
In contrast, upon induction of ER stress with Tm, IRE1-3F6HGFP relocalized into discrete foci in the ER membrane (Fig. 1 E and F, “Tm, 2 h and 4 h”). These results suggest that, like in yeast, activation of mammalian IRE1 by ER stress leads to its oligomerization in the ER membrane.
Oligomerization of Kinase/RNase Domain of Human IRE1 in Vitro.
To show that IRE1 oligomerization not only correlates with its activation but also is an intrinsic mechanistic requirement for its full activity, we characterized the enzymatic activity of human IRE1 in vitro. To this end, we purified the cytosolic portion of human IRE1 as previously described (23). The expressed fragment contained the kinase/RNase domains extended by 43 amino acids at its N terminus (Fig. 2A, “IRE1(KR43)”). These extra 43 amino acids are part of the linker region that serves to tether IRE1’s kinase/RNase to the transmembrane domain. The extra 43-amino acid extension was included because in yeast, a similar extension of the kinase domain is required to activate Ire1 by oligomerization (13).
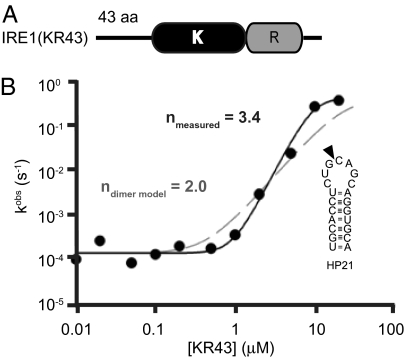
Fig. 2.
Oligomerization of the kinase/RNase domain of human IRE1 in vitro. (A) Schematic of the IRE1(KR43) construct expressed in insect SF9 cells. IRE1(KR43) contains a 43-aa portion of linker, kinase (“K”) and endoribonuclease (“R”) domains of human IRE1. (B) Cooperative activation profile for IRE1(KR43) obtained using 5′-32P-HP21. Solid curve indicates profile of IRE1(KR43) with n = 3.4; dashed curve indicates predicted activation profile with n = 2 for comparison purpose. Schematic representation of RNA substrate (HP21) used in the in vitro experiments. Arrowhead marks site of specific cleavage by human IRE1 (KR43).
We assayed IRE1(KR43) for its activity to site-specifically cleave a 5′-32P-labeled stem–loop oligoribonucleotide (“HP21”) derived from the Xbp-1 mRNA (Fig. 2B and Fig. S1A). The observed rate constant for RNA cleavage exhibited non–Michaelis-Menten dependence on the enzyme concentration and increased cooperatively with a Hill coefficient of n = 3.4 (Fig. 2B), which suggests that IRE1 forms a tetramer and/or larger species to be fully activated. The theoretical curve calculated for dimerization model (n = 2) clearly does not conform to the data observed (Fig. 2B, dashed line). Velocity-analytical ultracentrifugation experiments revealed the presence of high-order species of IRE1 detecting up to eight mers (Fig. S1B), in agreement with the observations from the kinetic studies. The lack of odd numbered oligomers indicates that, like yeast, human IRE1α oligomerizes by assembly of dimeric building blocks. High-order (n > 2) IRE1 oligomers were also observed upon gel electrophoresis after chemical crosslinking (data not shown). We conclude that the endoribonuclease activity of human IRE1 is activated by self-association of four or more IRE1 monomers into an oligomeric assembly, presumably similar to that observed with yeast Ire1 (13).
Disruption of IRE1’s ER–Luminal Dimerization Interface Blocks Clustering and RNase Activity of Human IRE1.
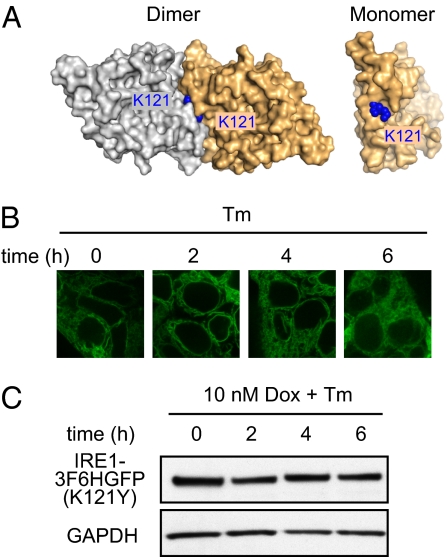
IRE1 activation occurs in response to ER stress. From the paradigms established in yeast, oligomerization of Ire1’s luminal domain in response to unfolded protein accumulation serves to laterally concentrate Ire1’s cytoplasmic domains, such that a threshold concentration is achieved to favor their oligomerization. Oligomerization in turn leads to trans-autophosphorylation and Ire1 RNase activation. In yeast, there are two luminal domain interfaces that govern Ire1 dimer and oligomer formation, and mutations to either of the two interfaces disrupt Ire1 foci formation and activation of the endoribonuclease (6, 14). Based on the crystal structure of the luminal domain of human IRE1 (19), we identified residue lysine (K) 121, which packs tightly within the domain's dimerization interface but is predicted to be solvent exposed in its monomeric form (Fig. 3A). We mutated human IRE1 K121 to a bulkier tyrosine (Y) residue to disrupt oligomerization by steric hindrance. Indeed, in Ire1α−/− MEFs reconstituted with mutant IRE1-3F6HGFP(K121Y) failed to splice Xbp-1 mRNA (Fig. S2A). To monitor foci formation, we next made a stable T-REx293 cell line expressing IRE1-3F6HGFP(K121Y) under the control of the Dox-inducible promoter. In contrast to IRE1-3F6HGFP, mutant IRE1-3F6HGFP(K121Y) failed to cluster into foci upon Tm treatment (Fig. 3B). The level of IRE1-3F6HGFP(K121Y) protein stayed constant over the time course of the experiment (Fig. 3C), and the splicing of Xbp-1 mRNA remained identical to that observed in cells that were not treated with Dox (Fig. S2B). In view of these results, we conclude that oligomerization of human IRE1 is required for its activation, which is controlled by its luminal domain.
Fig. 3.
Disruption of IRE1’s ER–luminal dimerization interface blocks clustering and RNase activity of human IRE1. (A) Crystal structure of human IRE1 luminal domain dimer and monomer (PDB ID code 2HZ6). Structure identifies K121 as tightly packed residue at dimerizing interface. (B) IRE1-3F6HGFP(K121Y) mutant construct localization with Dox (10 nM) and Tm (5 μg/mL) treatment for indicated hours. (C) Level of IRE1-3F6HGFP(K121Y) protein expressed is examined via immunoblotting against total 3F6HGFP-IRE1 under 10 nM Dox induction.
Sustained ER Stress Triggers Disassembly of IRE1 Clusters and IRE1 Dephosphorylation.
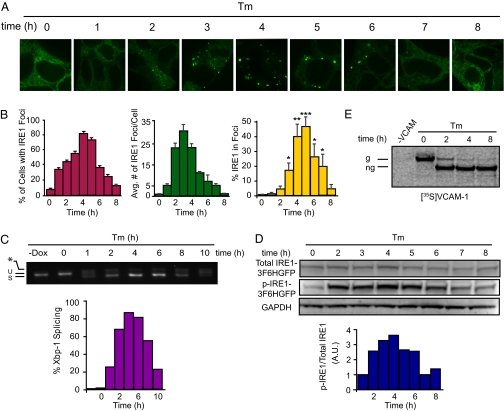
We previously reported that IRE1 signaling attenuates over time when HEK293 cells are exposed to persistent and unmitigated ER stress (24). Here we examined whether this IRE1 signaling attenuation is governed by the same timer as IRE1 cluster formation observed in this work. To this end, we followed IRE1 localization for 8 h after Tm treatment. IRE1-3F6HGFP clustered into many small foci after 2 h of Tm treatment (Fig. 4A and Movie S1), which, as stress persisted, converted into fewer, larger foci (Fig. 4B, middle graph). After 6 h of Tm treatment, IRE1 foci started to dissociate and vanished completely after 8 h. A concomitant reappearance of fluorescence staining took place in the ER network (Fig. 4 A and B), suggesting a diffused redistribution of IRE1-3F6HGFP over the ER membrane. The total IRE1-3F6HGFP fluorescence in foci followed the same temporal trend (Fig. 4B, right graph). Both the percentage of cells with IRE1 foci and total level of IRE1-3F6HGFP in foci correlated well with the Xbp-1 mRNA splicing (Fig. 4B, left graph, and 4C), establishing a clear connection between foci dynamics and our previous observations of dynamic IRE1 activity in HEK293 cells (24). During these time courses, new IRE1-3F6HGFP synthesis was prevented by removal of Dox before Tm addition. To further ascertain that the disappearance of foci at later time points resulted from redistribution of IRE1-3F6HGFP molecules rather than from protein degradation, we verified that IRE1-3F6HGFP protein level did not decline over the time course of the experiment (Fig. 4D, Upper).
Fig. 4.
Sustained ER stress triggers disassembly of IRE1 clusters and IRE1 dephosphorylation. Stably transformed T-REx293 cells were grown in medium containing 10 nM Dox. At time 0, medium was replaced by medium containing no Dox but 5 μg/mL Tm to induce ER stress. (A) IRE1-3F6HGFP localization over 8-h time course. (B) Quantification of time course experiment. Percentage of cells with IRE1 foci, average number of IRE1 foci per cell, and percentage of IRE1 in foci were determined as described in Methods. Error bars represent SEM (*P < 0.05, **P < 0.005, ***P < 0.0005). Statistical significance of the difference between later time points and 0 h is indicated. (C) Xbp-1 mRNA splicing was determined by RT-PCR and quantified. (D) Total and phospho-IRE1-3F6HGFP protein levels were determined by immunoblotting. Histograms represent ratio of phosphoylated-IRE1-3F6HGFP and total IRE1-3F6HGFP. (E) T-REx293 cells were pulse-labeled for 1 h with [35S]methionine at the indicated times after beginning of the Tm treatment. Radiolabeled VCAM-1 (g, glycosylated; ng, nonglycosylated) was detected after immunoprecipitation and SDS gel electrophoresis.
We next asked whether we could detect molecular changes in IRE1 that accompany its redistribution in the ER. To this end, we monitored the phosphorylation state of IRE1 by Western blotting using a phosphorylation specific antibody. IRE1 phosphorylation peaked at 4 h of Tm treatment and then declined after 6 h of Tm treatment (Fig. 4D, Middle), in synchrony with foci formation and Xbp-1 mRNA splicing.
The kinetics of the induction of BiP, a transcriptional target of XBP-1, paralleled the kinetics of IRE1-3F6HGFP foci formation (Fig. S3A). The level of BiP protein continued to increase even after IRE1 foci began to dissociate, presumably due to delayed attenuation of ATF6 branch (24), which also targets BiP transcriptionally (25–27).
To ascertain that ER stress remained unmitigated over the time course of the experiment, we transfected T-REx293 cells with vascular cell adhesion molecule-1 (VCAM-1), a transmembrane protein that is cotranslationally inserted into the ER membrane, where it becomes N-glycosylated (28). The steady-state pool of VCAM-1 was fully glycosylated at the 0 h time point and gradually became nonglycosylated during the 8-h time course of Tm treatment with no sign of recovery (Fig. S3B). Moreover, all newly synthesized VCAM-1 detected after pulse labeling was nonglycosylated at all later time points of the time course (Fig. 4E). Therefore, the observed attenuation of IRE1 signaling after prolonged activation occurred despite the fact that Tm-induced ER stress persisted.
Reactivation of UPR After Reestablishment of ER Homeostasis.
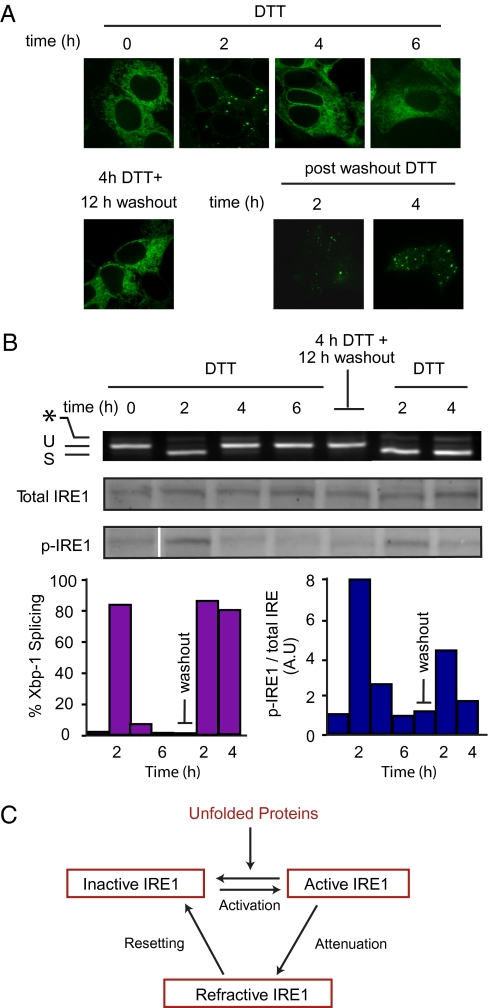
To determine whether IRE1 attenuation is restricted to the particular experimental conditions used to induce ER stress (impairment of N-glycosylation by Tm), we induced the UPR with DTT, which causes ER stress by interfering with disulfide bond formation. DTT treatment resulted in IRE1-3F6HGFP foci formation (Fig. 5A) concomitant with Xbp-1 mRNA splicing (Fig. 5B), which was followed by an attenuation phase in which IRE1-3F6HGFP redistributed in the ER membrane. The results mirror those observed with Tm treatments. Therefore, taken together, our work supports that IRE1 activity attenuation is a general phenomenon (24), although the timing of attenuation differs depending on the type or the strength of the stress.
Fig. 5.
Reactivation of UPR after restoration of ER homeostasis. (A) T-REx293 cells were treated with 10 nM Dox and DTT (1 mM) for indicated time. After 4 h of DTT treatment, DTT-containing medium was exchanged for regular medium for 12 h. Cells were then reexposed to ER stress by treatment with DTT (1 mM) ). (B) Xbp-1 mRNA splicing was determined by RT-PCR. Total and phosphorylated IRE1 were detected by Western blotting as in Figure 4. The normalized ratio of the signals from the two blots is displayed. (C) Three-state model for activation of mammalian IRE1.
If attenuation renders IRE1 insensitive to the protein folding status in the ER, we wondered whether removal of the inducing signal (as it would normally occur during homeostatic regulation when the UPR can correct the defect) would allow the signaling machinery to reset. To this end, we induced the UPR with DTT and followed IRE1-3F6HGFP foci formation and attenuation as described above. After 4 h, we washed out the DTT and allowed the cells to rest for 12 h. To verify that ER homeostasis was indeed restored after DTT removal, we examined the redox states of the newly synthesized pool of glycoprotein by 2D electrophoresis at 0 h and 2 h of DTT treatment and after 12-h washout (Fig. S4A). We observed no significant change in the redox state of the glycoprotein 12 h after DTT washout when compared with the 0-h untreated control. By contrast, the amount of glycoproteins isolated 2 h after DTT treatment was significantly reduced (Fig. S4B), which can be explained by the global translational inhibition by PERK activation.
We then added fresh DTT to reintroduce ER-stress. IRE1-3F6HGFP foci formation, IRE1 phosphorylation, and Xbp-1 mRNA splicing occurred in a manner comparable to the process observed in naive cells that were not subjected to a prior round of UPR induction (Fig. 5 A and B; compare panels before and after removal of DTT). Because Dox was removed during the DTT washout to prevent new synthesis of IRE1-3F6HGFP, these results demonstrate that cells can reset IRE1 signaling and that the molecular changes that mediate IRE1 attenuation are reversible.
Discussion
Transmembrane signaling receptors often concentrate in ultrastructural signaling centers, where dimerization or oligomerization of the signaling components serves to activate the downstream pathways. Oligomers of activated signaling receptors function as molecular platforms to which effectors are recruited, leading to their activation as their local concentration surpasses the threshold for activation (29). Based on our current and previous work (14, 15), oligomerization applies for IRE1 signaling from the ER, and the salient features of IRE1 activation appear conserved from yeast to human. Specifically, we have shown that activation of human IRE1 kinase/RNase module involves at least four IRE1 molecules, and we have visualized high-order oligomeric assembly of IRE1 in living cells.
In agreement with previous work in mammalian cells (24), we found that IRE1 signaling is governed by a timer: even if the inducing stress has not been mitigated, IRE1 signaling shuts off as oligomers disassemble concomitantly with IRE1 dephosphorylation. In contrast, if ER stress is resolved, IRE1 resets so that it can become reactivated. Thus similar to other signaling receptors and ion channels, IRE1 can exist in three states (Fig. 5C): (i) an inactive state that can be activated by ER stress, (ii) an active, oligomeric state, which initiates Xbp-1 mRNA splicing, and (iii) a refractive state in which IRE1 enters after prolonged activation and in which it no longer responds to unresolved ER stress. The inactive and refractive states are both not recognized by the anti–phospho-IRE1 antibody, suggesting that dephosphorylation plays a role in entering the refractive state; yet the molecular features that distinguish the inactive and refractive states, if any, remain unknown. More subtle differences in the phosphorylation pattern or association with other yet-to-be-identified regulators may render IRE1 refractive to activation, perhaps akin to the phosphorylation and arrestin binding to G-protein–coupled receptors. Mounting evidence suggests that IRE1 phosphorylation is subject to complex regulation. A recent study revealed a role for the adaptor protein RACK1 in modulating the dynamic activation of IRE1α signaling in pancreatic β cells in response to physiological stimulation by glucose. RACK1 may regulate IRE1 phosphorylation by forming a ternary assembly of IRE1-RACK1-PP2A phosphatase (30), possibly resetting IRE1 foci. If modulation of IRE1’s cytosolic domain drives its disassembly, it remains puzzling how the luminal domain of IRE1 would dissociate in response to prolonged ER stress, when unfolded proteins remain in the ER. A mechanism would need to exist by which this signaling event is actively shut off. One possible explanation would allow the refractive state to be established from the ER lumen, perhaps by producing or activating a putative “IRE1 oligomerization antagonist” in the ER lumen. Such a role might be played by the excessive concentrations of BiP or other chaperones that accumulate upon prolonged ER stress. The observed change in IRE1’s phosphorylation status would then be a consequence rather than the cause of IRE1 becoming refractive to ER stress. The results presented here demonstrate that prolonged ER stress instructs IRE1 to deactivate reversibly rather than causing its degradation or inflicting other irreversible damage.
We previously proposed that attenuation of IRE1 activity upon persistent ER stress serves as the tipping point in the life–death decision such that the UPR drives cells into apoptosis if ER homeostasis cannot be restored (21, 24, 31). In previous work, IRE1 signaling was experimentally prolonged by addition of activating drugs under persistent ER stress induction regimes (24, 31), which resulted in an increased cell survival. Attenuation of IRE1 activity in the face of persistent ER stress thus may provide a timer that limits the duration of the cytoprotective phase of the UPR, which is likely to be driven by the transcriptional regulatory network overseen by XBP-1. It is interesting to note that both apoptotic and antiapoptotic regulators, such as BAX/BAK and BAX Inhibitor-1 associate with IRE1 directly (32, 33), and it is possible that their regulated association or release from IRE1 in distinct activation states may directly affects the cell's apoptotic predisposition. Analysis of the timing of recruitment of other components to IRE1 will be a crucial next step to unravel the mechanism of UPR-linked cell death.
The foci formed by active IRE1 on the ER membrane are highly dynamic, continually changing in size and intracellular localization over the time course of UPR activation. We have recently shown in yeast by fluorescent energy transfer that IRE1 molecules come into close physical contact in these structures (7). The cooperative RNase activation shown here in vitro and early crosslinking studies in yeast (11) further corroborate the notion that active IRE1 molecules are tightly packed in foci. Such packing can provide a concentrated, specialized molecular microenvironment, which could attract low affinity binders with high avidity. Thus the dynamic foci assembly may be an additional control principle in modulating the UPR and perhaps other signaling pathways. The ability to visualize IRE1 signaling centers thus signifies an important advance to study their composition and dynamics, as well as the functional consequences that arise from these features. The results reported here therefore hold great promise to inform our understanding of general design principles of signaling pathways that deploy protein oligomerization as a mechanistic principle.
Methods
Cell Culture.
T-REx293 and Ire1α−/− MEFs cells were maintained at 37 °C, 5% CO2 in DMEM media supplemented with FBS, glutamine, and antibiotics (Invitrogen). Transient and stable transfections were performed using the Lipofectamaine 2000 (Invitrogen) and FuGene6 reagent (Roche). Cherry-KDEL construct was derived from pShooter plasmid pCMV-Myc-GFP-KDEL (Invitrogen). IRE1-3F6HGFP stable cell lines were made with pcDNA5-IRE1-3F6HGFP-frt.
Live Cell Imaging.
T-REx293 cells were split 2 d before imaging onto glass-bottom microwell dishes (MatTek) at 5 × 104 cells/dish. Imagining media consists of HBSS (GIBCO), 2% FBS, and 5 mM HEPEs at a pH of 7. Cells were visualized on a Yokogawa CSU-11 spinning disk confocal on a Nikon TE2000 microscope. Images were analyzed using a customized MatLab script to determine the fraction of IRE1-3F6HGFP in foci. The annotated MatLab script is available in the SI Text.
Details of methods are given in SI Methods.
Supplementary Material
Acknowledgments
We thank Marc Shuman and Peter Walter laboratory members for their critical review of the manuscript and for stimulating discussions, and J. H. Lin, C. H. Deng, and F. Sanchez for their valuable suggestions and technical support. We also thank K. Thorn and staff at the Nikon Imaging Center for their invaluable help with imaging. This worked is supported by a National Science Foundation predoctoral fellowship (to H.L. and S.L.B.) and a Jane Coffin Childs fellowship (to A.V.K.). P.W. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010580107/-/DCSupplemental.
References
- 1.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 2.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 3.Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2??related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74:743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- 4.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 5.Haze K, et al. Identification of the G13 (cAMP-response-element-binding protein-related protein) gene product related to activating transcription factor 6 as a transcriptional activator of the mammalian unfolded protein response. Biochem J. 2001;355:19–28. doi: 10.1042/0264-6021:3550019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Credle JJ, Finer-Moore JS, Papa FR, Stroud RM, Walter P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2005;102:18773–18784. doi: 10.1073/pnas.0509487102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pincus D, et al. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010;8:e1000415. doi: 10.1371/journal.pbio.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calfon M, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 10.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shamu CE, Walter P. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 1996;15:3028–3039. [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss A, Schlessinger J. Switching signals on or off by receptor dimerization. Cell. 1998;94:277–280. doi: 10.1016/s0092-8674(00)81469-5. [DOI] [PubMed] [Google Scholar]
- 13.Korennykh AV, et al. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457:687–693. doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aragón T, et al. Messenger RNA targeting to endoplasmic reticulum stress signalling sites. Nature. 2009;457:736–740. doi: 10.1038/nature07641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimata Y, et al. Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins. J Cell Biol. 2007;179:75–86. doi: 10.1083/jcb.200704166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu CY, Schröder M, Kaufman RJ. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J Biol Chem. 2000;275:24881–24885. doi: 10.1074/jbc.M004454200. [DOI] [PubMed] [Google Scholar]
- 17.Liu CY, Wong HN, Schauerte JA, Kaufman RJ. The protein kinase/endoribonuclease IRE1alpha that signals the unfolded protein response has a luminal N-terminal ligand-independent dimerization domain. J Biol Chem. 2002;277:18346–18356. doi: 10.1074/jbc.M112454200. [DOI] [PubMed] [Google Scholar]
- 18.Liu CY, Xu Z, Kaufman RJ. Structure and intermolecular interactions of the luminal dimerization domain of human IRE1alpha. J Biol Chem. 2003;278:17680–17687. doi: 10.1074/jbc.M300418200. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, et al. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc Natl Acad Sci USA. 2006;103:14343–14348. doi: 10.1073/pnas.0606480103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen HR, Panning B. XIST RNA exhibits nuclear retention and exhibits reduced association with the export factor TAP/NXF1. Chromosoma. 2007;116:373–383. doi: 10.1007/s00412-007-0100-1. [DOI] [PubMed] [Google Scholar]
- 21.Han D, et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 23.Niwa M, Sidrauski C, Kaufman RJ, Walter P. A role for presenilin-1 in nuclear accumulation of Ire1 fragments and induction of the mammalian unfolded protein response. Cell. 1999;99:691–702. doi: 10.1016/s0092-8674(00)81667-0. [DOI] [PubMed] [Google Scholar]
- 24.Lin JH, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K. Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem. 2004;136:343–350. doi: 10.1093/jb/mvh122. [DOI] [PubMed] [Google Scholar]
- 26.Acosta-Alvear D, et al. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell. 2007;27:53–66. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida H, et al. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000;20:6755–6767. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garrison JL, Kunkel EJ, Hegde RS, Taunton J. A substrate-specific inhibitor of protein translocation into the endoplasmic reticulum. Nature. 2005;436:285–289. doi: 10.1038/nature03821. [DOI] [PubMed] [Google Scholar]
- 29.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu Y, et al. A crucial role for RACK1 in the regulation of glucose-stimulated IRE1alpha activation in pancreatic beta cells. Sci Signal. 2010;3:ra7. doi: 10.1126/scisignal.2000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han D, et al. A kinase inhibitor activates the IRE1alpha RNase to confer cytoprotection against ER stress. Biochem Biophys Res Commun. 2008;365:777–783. doi: 10.1016/j.bbrc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 32.Hetz C, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. [Google Scholar]
- 33.Lisbona F, et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. 2009;33:679–691. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.