Abstract
Pollen tubes are highly polarized plant cells specialized in delivering sperm for fertilization. Pollen tube growth is rapid, occurs exclusively at the tip, and can reach distances thousands of times the diameter of the pollen grain without cell division, thus representing an excellent model system for studying asymmetric cell growth. In flowering plants, pollen tube growth is dependent on the actin cytoskeleton, which supports an efficient vesicle trafficking system to deliver membrane and cell-wall materials to the tube tip. A highly dynamic subapical actin structure and an apical vesicular zone are known to be critical for the tip-growth process. How this apical organization is maintained, how the subapical actin structure is assembled, and direct evidence for its functional coupling with tip growth remain to be established. Here, we show that a tip-located, cell membrane-anchored actin-nucleating protein, the Arabidopsis formin homology5 (FH5), stimulates actin assembly from the subapical membrane, provides actin filaments for vesicular trafficking to the apical dome, and mediates assembly of the subapical actin structure. Moreover, FH5-expressing pollen tubes provided a unique opportunity to demonstrate that assembly of the subapical actin structure is concomitant with the acquisition of rapid tip growth, providing further support for their functional coupling. Together, our results show that FH5 plays a pivotal role in establishing the subapical actin and apical vesicular organization critical for tip-focused growth in pollen tubes.
Keywords: actin dynamics, actin polymerization, actin-nucleating proteins, polarized cell growth, vesicle trafficking
Polarized cell growth underlies many fundamental processes, ranging from budding and mating in yeast, hyphal extension in filamentous fungi, axon outgrowth in animals, and pollen tube growth in plants. Pollen tubes elongate within the female reproductive organ; growth is strictly at the apex and responds to growth-regulating factors and directional cues from the female (1). Pollen tubes from many species are capable of growing in a defined medium, maintaining rapidity, polarity, and the ability to respond to guidance molecules, providing convenient experimental systems that produced much of our understanding of the intracellular processes and extracellular factors that control this apical growth process (2, 3). In flowering plants, pollen tube growth is supported by an elaborate and dynamic actin cytoskeleton and membrane trafficking system (Fig. 1A). Long axially aligned actin bundles in the shank of the tube transport organelles and vesicles to the subapical cytoplasm along the cortical region and recycle them back to the rear of the tube in the core region, giving rise to a “reverse-fountain” cytoplasmic streaming pattern. Densely packed vesicles occupy a dynamic, “inverted cone”-shaped region, also referred to as the “clear zone,” in the apical cytoplasm, where large organelles, such as Golgi bodies, rarely enter. The subapical region is marked by a collection of shorter and thinner actin cables that gather into a morphologically dynamic structure variously referred to as an “actin band,” “ring,” “funnel,” “basket,” “mesh,” or “fringe” (2–4); the term “subapical actin structure” is used here to reflect this morphological range. Perturbing actin dynamics—that is, the cycling of actin monomers on and off the ends of actin filaments (F-actin)—results in rapid dismantling of the subapical actin structure, disruption of the reverse fountain cytoplasmic streaming pattern, and of tip-focused growth, consistent with a close functional relationship between the apical cellular organization and tip growth (5–9).
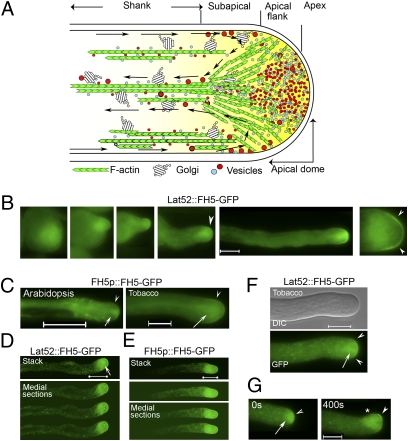
Fig. 1.
FH5-GFP is localized to the pollen-tube tip. (A) A schematic representation of angiosperm pollen-tube actin cytoskeleton and the apical inverted cone region (or “clear zone”) of vesicles. Arrows trace the “reverse-fountain” cytoplasmic streaming pattern. (B–F) Localization of formin homology-5 (FH5) promoter (FH5p) and the pollen-specific Lat52 promoter-expressed FH5-GFP to the apical and subapical membrane of Arabidopsis and tobacco elongating pollen tubes. (B) Emerging to elongating (Left to Right) Arabidopsis tubes. (D and E) Whole-tube projection of Arabidopsis tubes (Upper) by confocal imaging; three medial sections are shown (with increased γ to reveal signal from the shank, where no cell membrane-associated GFP-signal was detected). (F) Typical FH5-GFP localization pattern of prominent association with the apical and subapical, tip-focused collection of vesicles, and vesicular congregates that streamed along the cytoplasm (Movie S1). (G) A reorienting tobacco tube. Arrowheads highlight the apical flank and subapical regions; arrows indicate inverted cone region; asterisk in G indicates where the tip was at 0 s. See Fig. S2 for additional details and FH5p:GUS expression in transformed Arabidopsis pollen. Arabidopsis tubes were from stably transformed pollen. Tobacco tubes were transiently transformed by either 2 μg of Lat52::FH5-GFP or 10 μg of FH5p::FH5-GFP; elongating pollen tubes showed comparable FH5-GFP signals. (Scale bars, 10 μm.)
Formins are actin-nucleating proteins that accelerate the rate-limiting step of dimer and trimer formation from actin monomers to provide nuclei for rapid actin polymerization and play key roles in many polarized cellular and growth-related processes in animals and yeast (10, 11). Formins are multidomain proteins with a variable N-terminal domain, a proline-rich domain, formin homology-1 (FH1), and an activity domain, FH2 (Fig. S1A). FH2 is sufficient for nucleating actin and FH1 interacts with profilin and enhances FH2’s activity. Most formins studied to date are cytoplasmic proteins recruited to the cell membrane. A subset of plant formins (referred to as Group I) has evolved an N-terminal extension comprised of a transmembrane and an extracellular domain (Fig. S1A) (12). Studies on plant formins are beginning to reveal their functional roles in various polarized processes, ranging from cytokinesis to polarized cell growth (13–18).
In Arabidopsis, there are 10 Group I formins and 11 cytoplasmic formins (Group II). Miroarray analyses (19) indicate that as a group actin-nucleating proteins are expressed at very low levels in pollen compared with proteins that regulate actin dynamics (e.g., actin depolymerizing factors and profilins) or compared with signaling molecules that mediate pathways that regulate actin dynamics (e.g., Rho GTPases) (2, 20, 21), implying that nascent F-actin synthesis must be maintained at relatively low levels. Microarray data (19) and promoter activity assays (13) showed that multiple formins are expressed in pollen, with the Group I FH3 and FH5 being predominant, and two Group II formins among the more highly represented. FH3 is specific to pollen, whereas FH5 is broadly expressed but at even lower levels than in pollen. We showed previously that the Group I formin AFH1 stimulated actin assembly along the pollen-tube cell membrane and that deregulating actin nucleation activity disrupted the tip-growth process (13). RNAi knockdown of FH3 in Arabidopsis resulted in reduced abundance of the axially-aligned actin cables and inhibited in vitro pollen tube growth (17). In providing nascent F-actin, actin-nucleating proteins should conceivably contribute to the assembly of higher order actin structures. Whether any pollen formin impacts the formation and maintenance of the subapical actin structure remains unknown. Like several other Group I Arabidopsis formins (13–16, 22) and consistent with their predicted transmembrane and extracellular domain-containing structure (12), FH5-GFP has been localized to the cell plate, a membrane-rich structure synthesized from highly polarized secretory activity in dividing cells (14). Loss of FH5 function resulted in delayed endosperm cellularization, implying compromised cytokinesis (14), although vegetative phenotypes were normal and pollen-related phenotypes were not reported. Here we show FH5 (At5g54650) localizes to the apical dome (Fig. 1A) of elongating pollen tubes, stimulates actin assembly most prevalently around the subapical membrane, and plays a crucial role in controlling pollen-tube tip growth by facilitating assembly of the subapical actin structure and apical vesicular trafficking.
Results and Discussion
Arabidopsis FH5 Is Apical Membrane-Associated and Regulates Actin and Vesicular Organization in the Apical Cytoplasm.
Elongating Arabidopsis and tobacco pollen tubes expressing comparable levels of FH5-GFP expressed from its own promoter (FH5p) (Fig. S2D) or from the pollen-specific Lat52 promoter (23) showed similar apical localization (Fig. 1 B–F; Fig. S1B shows transgene constructs). FH5-GFP-labeled vesicles accumulated in the apical clear zone, where vesicular congregates rapidly cycled in and out in the typical reverse-fountain pattern (Movie S1). The apical localization pattern was established early during tube emergence (Fig. 1B) and was distinct from those of membrane proteins that are located throughout the pollen-tube surface (13, 24, 25) (Fig. S2). When growth was reorienting, FH5-GFP consistently relocalized to the new growth front (Fig. 1G). Together, these observations suggest a role for FH5 in organizing tip growth.
FH5 Regulates the Prominence of Subapical Actin Structure.
To decipher how FH5 mediates its impact on pollen tube growth, we took advantage of tobacco pollen tubes because their robust and easily monitored growth, cellular structural and dynamic properties in vitro, and relatively facile transient transformation system render them prominently used for functional dissection of pollen-tube components (e.g., refs. 3, 7, 8, 25, 27). Pollen tubes coexpressing the FH1and FH2 domains of FH5 and actin reporter confirmed that FH5 was active in stimulating supernumerary actin cables in the pollen-tube cytoplasm (Fig. S3A), establishing in vivo its activity to stimulate actin polymerization, as previously demonstrated by in vitro actin polymerization assays (14). Pollen tubes are known to be highly sensitive to increased actin polymerization (9, 13), consistent with actin nucleating proteins being maintained at very low levels (19). Transiently transformed pollen tubes showed dose-dependent growth responses to FH5 expressed from increasing amounts of input FH5 transgenes, ranging from moderate growth enhancement to inhibition, with the positive impact most noticeable between 4 and 8 h after transformation by 0.25 μg of the FH5 transgene (Fig. 2A), a growth period and transgene amount used for subsequent experiments where we analyzed pollen tubes that displayed robust tip growth. The most noticeable impact FH5 has on these transformed pollen tubes was a striking increase in the prominence of their subapical actin structure than in control tubes (Fig. 2 B and C). When pollen tubes were monitored individually during growth, the FH5 transformed tubes showed a moderate but significant increase in average growth rates and significantly increased subapical actin prominence relative to the controls (Fig. 2 E–H and Movies S2 and S3).
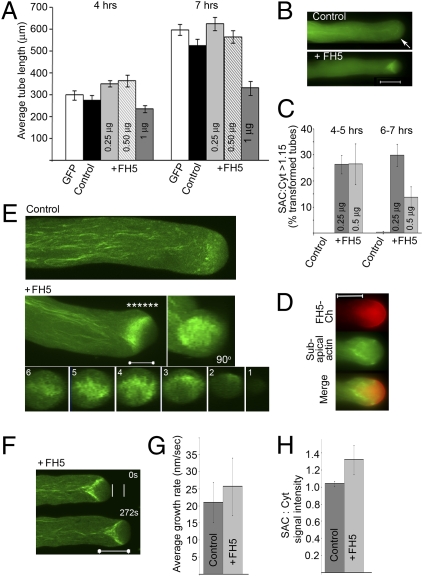
Fig. 2.
FH5 regulates the prominence of the subapical actin structure. Data are from transiently transformed tobacco pollen tubes. GFP-ADF was used as the actin reporter as it most efficiently reveals the subapical actin structure and least adversely affects tip growth among several plant actin reporters (3, 8, 36). Pollen tubes were either transformed by the actin reporter alone (control) or cotransformed by Lat52::FH5 (+FH5). (A) Growth response to FH5 transgene dosage (indicated in data bars). Data are the mean ± SE of the average tube lengths from triplicate samples (with 20–30 tubes per replicate) in one representative experiment. Difference between the +FH5 and control samples were significant (at 4 h, P = 5.8 × 10−5, 4.7 × 10−3, and 10−3 for the +0.25, +0.5, and +1 μg FH5 samples, respectively; at 7 h, P = 1.1 × 10−3, 2.9 × 10−3 and 3.2 × 10−4, respectively). Pollen tube-width comparison, which negatively correlates with growth quality (7, 17, 25), showed 0.25 μg FH5-transformed pollen tubes being slightly but significantly narrower than the controls (Fig. S3B), consistent with enhance growth quality. (B) Wide-field images showing the relatively subtle subapical actin structure in a control (arrow) and the substantially augmented structure in a +FH5 tube. The structures were elongating at 35 and 41.3 nm/s, respectively, over a 10-min period. The subapical actin structure signal intensity (SAC):distal cytoplasm signal intensity (Cyt) ratio was used as a measure for the prominence of the subapical actin structure. The SAC:Cyt ratio was 1.1 and 1.63 for the control and +FH5 tube, respectively. Fig. S3C shows details on SAC and Cyt analyses. (C) Percentage of tubes showing a prominent subapical actin structure (SAC:Cyt >1.15). Data shows average ± SD (n = 73–145 tubes analyzed for each sample). Differences from controls were significant (P = 10−4 and 2 × 10−3 at 4 h, 1.4 × 10−4, and 2.4 × 10−3 by 7 h for the +0.25 and 0.5 μg samples, respectively). The reduced percentage observed for the 7-h +0.5 μg FH5 sample reflected that many of the transformed tubes were already growth-inhibited and their subapical actin structure obliterated. Analyses in A to C established that 0.25 μg of Lat52::FH5 was optimum in obtaining the largest number of rapidly elongating transformed pollen tubes over the best growth period for observations (4–8 h after transformation) (3, 8, 36). (D) Wide-field coimaging of a FH5-Ch and actin reporter cotransformed pollen tube showing the spatial relationship between the subapical actin structure and the FH5-Ch–occupied apical dome membrane. (E) Whole-tube projections from confocal z-stack images of a control and a +FH5 tube. The frontal view (90°) and orthogonal sections of the +FH5 tube (1–6 taken at *, Right to Left) revealed a basket-shaped subapical actin structure anchored at the apical flank cortex, projecting centripetally to span the central cytoplasm at its base. (F) Medial sections of a +FH5 tube taken from a confocal time series (Movie S2). White lines show distance grown by a control tube for a comparable period (Movie S3), showing more rapid growth in the +FH5 tube (26.8 nm/s vs. 18.9 nm/s in the control). (G) Growth-rate comparison. Growth rates were averaged from control (n = 31) and +FH5 (n = 33) tubes individually monitored for durations of between 3 min and 1 h (with intermittent image acquisition) in several independent comparative experiments. Difference was significant (P = ∼5 × 10−3) and resulted from a higher percentage of FH5-transformed pollen tubes elongating at higher growth rates than controls (Fig. S3D). (H) SAC:Cyt intensity ratio comparison shows significantly more prominent subapical actin structures in FH5 transformed tube compared with controls (P = ∼4.7 × 10−7). Data were average ± SDs from the same samples analyzed in G. (Scale bars: 5 μm in E, 10 μm in all others.)
The FH5-augmented prominence of the subapical actin structure allowed its morphology during growth to be revealed with unprecedented clarity, showing actin cables projecting centripetally and distally from the apical flank cortex and merging to form a mesh-like structure spanning the central cytoplasm (Fig. 2E). This prominence also permitted rapid imaging of its morphological dynamics, allowing its structural reconfiguration and dynamic spatial relationship with the extending tip, circumferential to the FH5-occupied apical dome (Fig. 3D), to be documented in the millisecond time frame (Movie S4). Actin cables could be observed moving out of the base of the subapical structure to join the retrograde actin streams in the central cytoplasm (Movie S5). Taken together, these observations suggest an important contribution from apical actin assembly not only to the subapical actin organization, but also providing precursors to at least some of the long actin bundles in the core cytoplasm, thus contributing to the overall actin cytoskeleton in growing pollen tubes.
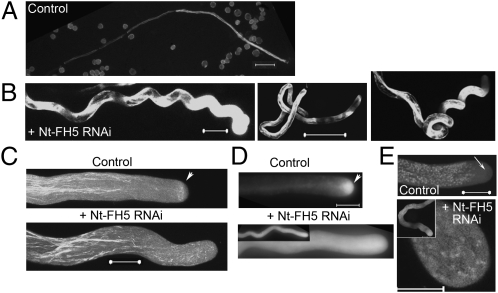
Fig. 3.
RNAi suppression of Nt-FH5 diminished the subapical actin structure and compromised tip-focused vesicular organization and growth. Data are from tobacco pollen tubes transiently transformed by marker GFP with or without RNAi Nt-FH5 (control). (A and B) Pollen tubes expressing GFP (control) (A) or coexpressing GFP and NtFH5 RNAi (B) at ∼10 h of in vitro growth when RNAi-induced phenotype was prevalent. An average of 80.3 ± 14% of Nt-FH5 RNAi transformed and 2.47 ± 0.75% of control pollen tubes developed severe meandering (p ∼3 × 10−4): n = 3 independent experiments, each with 40 to 55 control and >50 Nt-FH5 RNAi tubes each. (C–E) Actin cytoskeleton (C), apical vesicular accumulation pattern (D), and Golgi localization pattern (E) in control and Nt-FH5 RNAi transformed pollen tubes at ∼6 h of growth when the meandering phenotype was just developing. GFP-ADF was used as actin reporter. GFP-Rab11 and GFP-Rab2 was used as vesicle and Golgi reporters, respectively (24, 26). Insets in D and E show a longer profile of the meandering tubes. Arrowheads indicate subapex. Arrow in E indicates apical clear zone. (Scale bars: 50 μm in A and B Center and 10 μm in all others.) Fig. S4 shows control and Nt-FH5 antisense transformed tubes.
Loss of Apical Formins Diminished the Subapical Actin Structure and Compromised Tip-Focused Growth.
When Lat52-driven RNAi or antisense transgenes against the Nicotiana tabacum homolog of FH5, Nt-FH5 (Fig. S1C) were expressed in transiently transformed tobacco pollen tubes, severely meandering growth routinely occurred in close to 80% of Nt-FH5 RNAi-transformed pollen tubes by 8 to 10 h after germination, but control tubes rarely showed such drastic twists and turns (Fig. 3 A and B). The subapical actin structure and the apical vesicular accumulation in these Nt-FH5 RNAi and Nt-FH5 antisense transformed tobacco pollen tubes were also substantially diminished (Fig. 3 C and D and Fig. S4 C and D). Larger organelles, such as Golgi bodies, were no longer precluded from the clear zone but invaded the apical cytoplasm (Fig. 3E), reflecting perturbation of the normal vesicular trafficking pattern that maintained compartmentalization of vesicles and organelles. We cannot eliminate the possibility of off-target silencing because of a lack of sequence information for other tobacco pollen-expressed formins. However, the meandering phenotype was distinct from the FH3 RNAi-induced phenotype of shorter and wider Arabidopsis pollen tubes (17), but reminiscent of the winding property of pollen tubes whose tip-focused accumulation of vesicles was compromised (26). Taken together, our results suggest that the apically located FH5, with its ability to stimulate actin polymerization (14), is important for regulating the prominence of the subapical actin structure, maintaining the apical vesicular and subapical organelle segregation pattern and supporting tip-focused growth. These results further support the notion that these apical cellular organization and tip growth are critically linked.
FH5 Nucleates the Assembly of the Subapical Actin Structure.
The subapical actin structure has been observed only in its assembled form in elongating pollen tubes and its functional significance implied from its disassembly as tip growth was inhibited. The augmented subapical actin structure in FH5-transformed pollen tubes provided a unique opportunity to examine how the subapical actin structure was assembled and how its assembly relates to the tip-growth process.
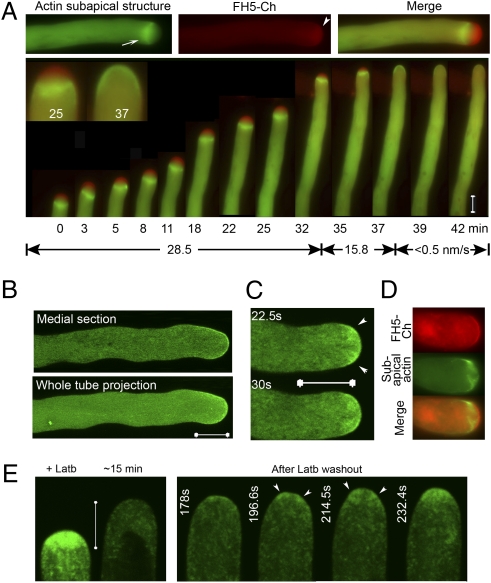
The actin dynamics inhibitor Latrunculin b (Latb) induces dissociation of the subapical actin structure and inhibits growth (5, 6). Latb-treated FH5-transformed pollen tubes readily returned to rapid tip-focused growth after removal of the inhibition and as growth recovered, a progressively more robust and morphologically dynamic subapical actin structure reformed (Fig. 4A). During early recovery, bursts of nascent actins were observed emanating centripetally and flanking the FH5-occupied region distal to the apical dome (e.g., 350-s and 402-s images in Fig. 4B). Within seconds, they merged to span the central cytoplasm (e.g., 357 s, 410 s). As rapid tip growth was reorganized, the bursts of subapical actin filaments became more distally located with respect to the tip, the subapical actin structure gained prominence and became dynamically maintained, most predominantly, about one-third to two-thirds of a pollen tube's diameter away from the extending apex (∼3–6 μm). Furthermore, during the early recovery phase, nascent actin cables were dislodged from the apical membrane and released into the apical cytoplasm (Fig. 4C), where they presumably could contribute to the assembling subapical actin structure, especially considering that increasing FH5 augmented the prominence of this structure (Fig. 2). Retrograde flows of actin from the newly reassembled subapical actin structure to the distal cytoplasm was evident in these recovering pollen tubes (Movie S8). Although occurring under the impact of increased apical actin assembly activity expressed from FH5, the de novo assembly of the subapical actin structure observed during tip-growth recovery nonetheless provided novel insight on how this structure may be assembled from subapical actin filaments, showed that its assembly was coupled to attaining rapid tip growth, and suggested a structural continuity between apical actin assembly, the subapical actin structure, and the overall actin cytoskeleton in growing pollen tubes.
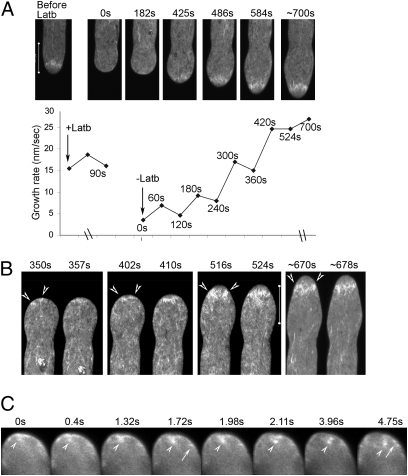
Fig. 4.
FH5 nucleates assembly of the subapical actin structure. Pollen tubes shown were transiently cotransformed by FH5 and actin reporter. (A and B) Subapical actin structure and growth recovery after Latb (12.5 nM) treatment. (A) Selected images from a confocal time series (Upper) and growth rates (Lower). (B) Subapical emergence of actin filaments (arrowheads), and their merging to form a structure spanning the central cytoplasm throughout the entire recovery period. More individual still frames and the entire time series are shown in Fig. S5 and Movie S6. (C) Selected images from a wide-field time series (after deconvolution) from a tube observed (∼5 min after Latb washout) when apical actin assembly was recovering from the apical membrane (Movie S7). Arrows, emanating actin filaments; arrowheads, dislodging actin fragments.
FH5-Nucleated Nascent Actin Filaments Support Apical Vesicular Trafficking.
The range of growth responses to transgene-expressed FH5 was most evident when individual transformed pollen tubes were observed over a period. These pollen tubes typically showed an initial period of rapid growth, followed by deceleration and arrest (Fig. 5A). Typically, FH5-transformed pollen tubes growing under similar experimental conditions were long and slender until growth was inhibited. This transition, although reflecting the effects of FH5 overexpression past a growth-enhancing level, provided a unique opportunity to derive additional insight about how apical actin assembly impacts the tip-growth process.
Fig. 5.
FH5-nucleated actin filaments support subapex-targeted vesicular trafficking. (A) Wide-field images monitoring growth of a tobacco pollen tube cotransformed by FH5-Ch and actin marker. (Upper) A representative image in individual channels and merged. Arrow, subapical actin structure; arrowhead, apical membrane and inverted cone region. The red signal was stretched equally in all merged images to illustrate the FH5-Ch labeled vesicular zone. Fig. S6 A and B show analysis details for the prominence of the subapical actin structure (SAC:Cyt) and of the apical vesicular zone (AV:Cyt) during transition from rapid to decelerated growth for this and other similarly transformed tubes. (B) A FH5-induced apical actin rim during the growth transition period. (C) A FH5-induced subapical vesicle targeting pattern (arrowheads) during the growth transition period. Two consecutive medial confocal sections are shown (7.5 s apart). (D) Subapex-focused actin filaments during a FH5-Ch induced growth transition. (E) Reaccumulation of vesicles during recovery from Latb-induced (+Latb) vesicle dispersal. Growth rate accelerated from < 7 nm/s to > 21 nm/s before and after 178 s. Arrowheads indicate subapical region. (Scale bars, 10 μm.)
The transition from FH5-regulated rapid to decelerated growth was consistently accompanied by the collapse of the subapical actin structure and formation of an apical actin rim [Fig. 5 A (37-min image) and B]. The FH5-induced apical actin rim coincided with its location and reflected its actin-nucleating activity around the apical dome membrane. Given that stable accumulation of readily detected levels of polymerized actin around the tube apex was almost never observed in rapidly growing pollen tubes (e.g., Figs. 2F and 4B and Movies S2, S3, and S4) (3, 13), the coincidence of apical rim formation with growth decline also suggested that to maintain rapid growth, apical actin assembly must be balanced by other endogenous actin regulatory activities that promote disassembly or remodeling (28).
Concomitant with the FH5-induced growth transition was also the dissipation of the tip-focused collection of vesicles. When FH5-Ch was coexpressed with actin reporter (Fig. 5A), the FH5-Ch-labeled apical vesicular zone abruptly dissipated as the subapical actin structure collapsed onto the apical membrane. In the 1 to 2 min before total dissipation of the apical vesicular collection, the most persistent vesicle accumulation remained focused at the apical flank (Fig. 5C), paralleling the subapex-focused actin filaments seen during the FH5-induced transition stage (Fig. 5D). Like most formins characterized to date, and as has been shown for its FH1FH2 domains during in vitro actin polymerization (10, 11, 14), FH5 should associate with the barbed (plus) end of elongating actin filaments that emanated along the apical membrane. Taken together, these observations imply that the added level of nascent actin filaments nucleated by the transgene-expressed FH5 supported the noticeably more persistent subapically-targeted vesicular transport. Complementing this conclusion was the observation that pollen tubes recovering from Latb-induced dispersal of the apical vesicles (26) also most rapidly reaccumulated subapically focused vesicles in a pattern closely mimicking that induced by FH5 (Fig. 5E), consistent with subapical actin assembly being most predominant under endogenous actin-nucleating conditions. The high density of vesicles in the apical dome of elongating pollen tubes has hampered deciphering vesicular trafficking pathways to and from the apical membrane. Our results provided direct support for the recently favored models of subapically targeted anterograde vesicular trafficking in elongating pollen tubes (29, 30).
A Subapical Actin Assembly Regulated Pollen Tube Growth Model.
Pollen tube growth relies on multiple cell surface and intracellular processes, ranging from lipid and Rho GTPase-mediated signaling on the cell surface to ionic conditions in the cytoplasm (2, 20, 21, 31, 32). These processes converge to regulate two interdependent cellular components: the actin cytoskeleton and membrane trafficking with actin cables supporting vesicle transport, which in turn delivers cell surface-located molecules critical for assembling and regulating the actin cytoskeleton. By focusing on understanding how the apical membrane-anchored FH5 impacts tip growth, our studies provided evidence for the interconnected structural and functional relationship between apical actin assembly, apical membrane-targeted vesicle trafficking, the subapical actin structure, and tip growth. We summarize these relationships in Fig. 6.
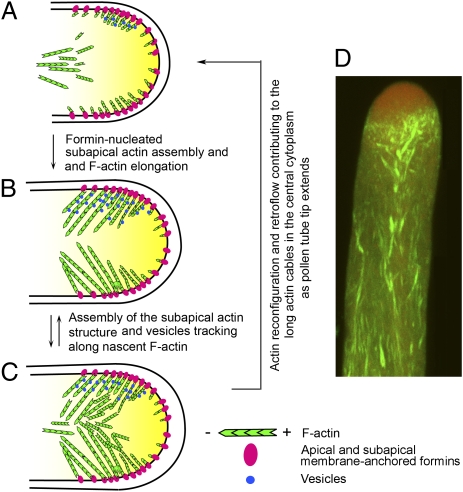
Fig. 6.
Formin-regulated apical actin assembly and the tip growth process. Elongating actin filaments are shown with their barbed end toward the membrane where formins are located. Short F-actins in the core cytoplasm where actin restructuring is expected are shown in random orientation. F-actins flowing out of the subapical structure are shown with their barbed end in the direction of the retroflow (37). Vesicles are shown only in the upper half of the tube. (A) Recently deposited apical dome membrane-associated formins most actively nucleates actin assembly from the subapical membrane as the previous subapical structure disassembles and actin cables flow rearward in the central cytoplasm. (B and C) Membrane-anchored actin filaments elongate centripetally to merge in the central cytoplasm; some are fragmented and released to the subapical cytoplasm providing nuclei for assembly into the subapical actin structure. These elongating nascent actin filaments, with their plus ends linked to membrane-anchored formins, support subapex-targeted vesicle trafficking. The morphological dynamics and variations observed for this subapical structure during growth reflect the rapid reconfiguration, disassembly and reassembly of this subapical collection of actin filaments. To maintain the spatial relationship with the extending tube tip, the process is reiterative, and as the actin cables from the assembled structure funnel into retro-streams of actin, a new round of subapical actin assembly ensues (C–A). (D) A confocal stack of an elongating, FH5-transformed pollen tube that were also coexpressing actin and vesicle markers, showing an apical vesicular zone (in red) closely subtended by the pronounced subapical actin structure trailed by retro-streams of actin bundles in the core cytoplasm (yellow).
During tip growth, actin assembly stimulated by apical dome membrane-anchored formins occurs most predominantly around the subapical membrane domain, providing actin tracks for membrane-targeted vesicular trafficking (Figs. 4–6 A and B). Perhaps in processes similar to those proposed for the dendritic-treadmilling model of actin assembly at the leading edge of growing cells (28), the elongating F-actin that emanates from the apical membrane could be severed and released into the subapical cytoplasm (Fig. 4C), either for disassembly or remodeling and incorporation into the subapical actin structure (Fig. 6 B and C). These processes would provide the modulating activities needed to prevent excessive apical membrane-associated actin polymerization, which inhibits growth (Fig. 5), and contribute to the construction (Fig. 4) and maintenance of the subapical actin structure (Figs. 2 and 3), which supports growth. The subapical actin structure also provides a pool of short actin cables for the retroflow of actin in the central cytoplasm (Movies S5 and S8), contributing to the overall actin cytoskeleton in elongating pollen tubes (Fig. 6 A and C). To maintain the dynamic spatial relationship between the subapical actin structure and the extending pollen-tube tip, these intracellular processes must occur reiteratively as the pollen-tube apex pushes forward (Fig. 6 A and C; and, for example, Fig. 4B and Fig. S5). Therefore, by providing subapically assembled F-actin to support vesicular trafficking and for construction and maintenance of the morphologically versatile subapical actin structure that closely subtends the apical vesicular zone during tip-focused growth (Fig. 6D), apical membrane-anchored formins play an important role in organizing the apical zone to support the dynamic pollen tube growth process.
This model is not intended to address all of the phenomena known to occur in tip growth. For example, although not essential to growth, pollen-tube growth is often oscillatory and correlates with multiple oscillatory intracellular processes and extracellular phenomena (31–33). How apical formin-nucleated actin polymerization, and the prominence and morphological dynamics of the subapical actin structure correlate with these oscillatory processes remain to be explored. Several fundamental aspects of FH5 also remain to be uncovered. For example, how its localization is restricted to the apical dome membrane and its activity regulated are to be established. Some animal and fungal formins are released from auto-inhibition via direct interaction with activated Rho GTPases (11). Although protein motifs similar to those involved in formin autoregulation have not been identified among plant formins (12), it is possible that signaling from the Rho GTPase-regulated molecular switch at the pollen-tube apex (3, 20, 21) and FH5 intersect functionally (e.g., through Rho-mediated regulation of actin and vesicular dynamics).
Materials and Methods
Plant and Pollen Growth and Transformation.
Arabidopsis was grown at 20 to 22 °C under 14-/10-h day/night cycles. Tobacco was grown in the greenhouse. Standard recombinant DNA and PCR-based strategies were used for chimeric gene construction; constructs used are shown in Fig. S1. Arabidopsis plant transformation followed ref. 34. In vitro tobacco and Arabidopsis pollen-tube growth followed previous methods (8, 35). Latb treatments used 12.5 nM; it was removed approximately 1 min after assembled actin or vesicles were stably dissipated from the apical zone. Transient tobacco pollen transformation was according to refs. 3 and 8). Unless otherwise stated, transgenes were expressed by the pollen-specific Lat52 promoter (23); 0.25 μg of Lat52:FH5 and 1.5 to 2 μg of Lat52:FH5-FP [GFP or mCherry (Ch)] were used per transformation. Fluorescent protein (GFP and Ch)-labeled actin and vesicle reporters, other FH5-related constructs, and the amounts used in transient transformation are described in Fig. S1. Mock vector DNA was used to equalize input DNA in transformation.
Pollen Tube Imaging and Analysis.
Wide-field microscopy was carried out on a Nikon Eclipse E800 microscope with a Spot CCD camera or Nikon Ti with a Coolsnap camera. Confocal imaging was carried out on a Zeiss Axiovert microscope and LSM 510 Meta Confocal System. Elongation rates were measured by monitoring individual pollen tubes for periods of between 5 min to 1 h (with intermittent imaging). Spot or NIS-Element software was used for length measurements; Image J was used for fluorescence intensity measurements; Student t-tests were used for statistics analysis. Images were assembled by Photoshop with no modification unless indicated. Unless otherwise stated, comparative pollen-tube length and morphology analyses were repeated at least in three independent experiments with comparable results. Data are presented as the mean ± SE from the averages from triplicate samples from one representative experiment.
Supplementary Material
Acknowledgments
We thank S. McCormick (Plant Gene Expression Center, Albany, CA) for the Lat52 promoter and R. Tsien (University of California, San Diego) for mCherry, S. McCormick for comments, M. Kieliszewski (Ohio University) for helpful suggestions on the manuscript, and O. Schmidt (University of Adelaide, Australia) for continual discussion, support, and comments on the manuscript. This work was supported by Grants MCB0618339 from the National Science Foundation and Cooperative State Research, Education, and Extension Service 2005-35304-16030 from the US Department of Agriculture.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008527107/-/DCSupplemental.
References
- 1.Cheung AY, Boavida LC, Aggarwal M, Wu HM, Feijó JA. The pollen tube journey in the pistil and imaging the in vivo process by two-photon microscopy. J Exp Bot. 2010;61:1907–1915. doi: 10.1093/jxb/erq062. [DOI] [PubMed] [Google Scholar]
- 2.Cheung AY, Wu HM. Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu Rev Plant Biol. 2008;59:547–572. doi: 10.1146/annurev.arplant.59.032607.092921. [DOI] [PubMed] [Google Scholar]
- 3.Cheung AY, et al. The dynamic pollen tube cytoskeleton: Live cell studies using actin-binding and microtubule-binding reporter proteins. Mol Plant. 2008;1:686–702. doi: 10.1093/mp/ssn026. [DOI] [PubMed] [Google Scholar]
- 4.Lovy-Wheeler A, Wilsen KL, Baskin TI, Hepler PK. Enhanced fixation reveals the apical cortical fringe of actin filaments as a consistent feature of the pollen tube. Planta. 2005;221:95–104. doi: 10.1007/s00425-004-1423-2. [DOI] [PubMed] [Google Scholar]
- 5.Gibbon BC, Kovar DR, Staiger CJ. Latrunculin B has different effects on pollen germination and tube growth. Plant Cell. 1999;11:2349–2363. doi: 10.1105/tpc.11.12.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vidali L, McKenna ST, Hepler PK. Actin polymerization is essential for pollen tube growth. Mol Biol Cell. 2001;12:2534–2545. doi: 10.1091/mbc.12.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu Y, Wu G, Yang Z. Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J Cell Biol. 2001;152:1019–1032. doi: 10.1083/jcb.152.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CY, Cheung AY, Wu HM. Actin-depolymerizing factor mediates Rac/Rop GTPase-regulated pollen tube growth. Plant Cell. 2003;15:237–249. doi: 10.1105/tpc.007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cárdenas L, Lovy-Wheeler A, Wilsen KL, Hepler PK. Actin polymerization promotes the reversal of streaming in the apex of pollen tubes. Cell Motil Cytoskeleton. 2005;61:112–127. doi: 10.1002/cm.20068. [DOI] [PubMed] [Google Scholar]
- 10.Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–477. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- 11.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 12.Grunt M, Zárský V, Cvrcková F. Roots of angiosperm formins: The evolutionary history of plant FH2 domain-containing proteins. BMC Evol Biol. 2008;8:115. doi: 10.1186/1471-2148-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung AY, Wu HM. Overexpression of an Arabidopsis formin stimulates supernumerary actin cable formation from pollen tube cell membrane. Plant Cell. 2004;16:257–269. doi: 10.1105/tpc.016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingouff M, et al. Plant formin AtFH5 is an evolutionarily conserved actin nucleator involved in cytokinesis. Nat Cell Biol. 2005;7:374–380. doi: 10.1038/ncb1238. [DOI] [PubMed] [Google Scholar]
- 15.Yi K, et al. Cloning and functional characterization of a formin-like protein (AtFH8) from Arabidopsis. Plant Physiol. 2005;138:1071–1082. doi: 10.1104/pp.104.055665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deeks MJ, et al. Arabidopsis group Ie formins localize to specific cell membrane domains, interact with actin-binding proteins and cause defects in cell expansion upon aberrant expression. New Phytol. 2005;168:529–540. doi: 10.1111/j.1469-8137.2005.01582.x. [DOI] [PubMed] [Google Scholar]
- 17.Ye J, et al. Arabidopsis formin3 directs the formation of actin cables and polarized growth in pollen tubes. Plant Cell. 2009;21:3868–3884. doi: 10.1105/tpc.109.068700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidali L, et al. Rapid formin-mediated actin-filament elongation is essential for polarized plant cell growth. Proc Natl Acad Sci USA. 2009;106:13341–13346. doi: 10.1073/pnas.0901170106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 2004;136:2621–2632. doi: 10.1104/pp.104.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YJ, Yang Z. Tip growth: Signaling in the apical dome. Curr Opin Plant Biol. 2008;11:662–671. doi: 10.1016/j.pbi.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kost B. Spatial control of Rho (Rac-Rop) signaling in tip-growing plant cells. Trends Cell Biol. 2008;18:119–127. doi: 10.1016/j.tcb.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Banno H, Chua NH. Characterization of the Arabidopsis formin-like protein AFH1 and its interacting protein. Plant Cell Physiol. 2000;41:617–626. doi: 10.1093/pcp/41.5.617. [DOI] [PubMed] [Google Scholar]
- 23.Twell D, Klein TM, Fromm ME, McCormick S. Transient expression of chimeric genes delivered into pollen by microprojectile bombardment. Plant Physiol. 1989;91:1270–1274. doi: 10.1104/pp.91.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung AY, et al. Rab2 GTPase regulates vesicle trafficking between the endoplasmic reticulum and the Golgi bodies and is important to pollen tube growth. Plant Cell. 2002;14:945–962. doi: 10.1105/tpc.000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, McCormick S. A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2007;104:18830–18835. doi: 10.1073/pnas.0705874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Graaf BH, et al. Rab11 GTPase-regulated membrane trafficking is crucial for tip-focused pollen tube growth in tobacco. Plant Cell. 2005;17:2564–2579. doi: 10.1105/tpc.105.033183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klahre U, Kost B. Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. Plant Cell. 2006;18:3033–3046. doi: 10.1105/tpc.106.045336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 29.Bove J, et al. Magnitude and direction of vesicle dynamics in growing pollen tubes using spatiotemporal image correlation spectroscopy and fluorescence recovery after photobleaching. Plant Physiol. 2008;147:1646–1658. doi: 10.1104/pp.108.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zonia L, Munnik T. Vesicle trafficking dynamics and visualization of zones of exocytosis and endocytosis in tobacco pollen tubes. J Exp Bot. 2008;59:861–873. doi: 10.1093/jxb/ern007. [DOI] [PubMed] [Google Scholar]
- 31.Holdaway-Clarke T, Hepler P. Control of pollen tube growth: Role of ion gradients and fluxes. New Phytol. 2003;159:539–563. doi: 10.1046/j.1469-8137.2003.00847.x. [DOI] [PubMed] [Google Scholar]
- 32.Michard E, Alves F, Feijó JA. The role of ion fluxes in polarized cell growth and morphogenesis: The pollen tube as an experimental paradigm. Int J Dev Biol. 2009;53:1609–1622. doi: 10.1387/ijdb.072296em. [DOI] [PubMed] [Google Scholar]
- 33.McKenna ST, et al. Exocytosis precedes and predicts the increase in growth in oscillating pollen tubes. Plant Cell. 2009;21:3026–3040. doi: 10.1105/tpc.109.069260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 35.Fan LM, Wang YF, Wang H, Wu WH. In vitro Arabidopsis pollen germination and characterization of the inward potassium currents in Arabidopsis pollen grain protoplasts. J Exp Bot. 2001;52:1603–1614. [PubMed] [Google Scholar]
- 36.Wilsen K, et al. Imaging the actin cytoskeleton in growing pollen tubes. Sex Plant Reprod. 2006;19:51–62. [Google Scholar]
- 37.Lenartowska M, Michalska A. Actin filament organization and polarity in pollen tubes revealed by myosin II subfragment 1 decoration. Planta. 2008;228:891–896. doi: 10.1007/s00425-008-0802-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.