Abstract
The conversion of naive T cells into Treg can be achieved in vivo by delivery of antigen under subimmunogenic conditions. Here we have examined several drugs for their ability to enhance the conversion process in vivo and have found that the rapamycin analog everolimus potently enhances Treg conversion by interfering with T-cell costimulation, reducing cell division and thereby activation of DNA methyltransferase 1 as well as by reducing T-cell activation through the ATP-gated P2×7 receptor controlling Ca2+ influx. The resulting Tregs exhibit increased stability of Foxp3 expression even when generated in TGFβ-containing media in vitro. Thus the mammalian target of rapamycin (mTOR) inhibitor everolimus in addition to inhibiting immune responses enhances Treg conversion by several distinct pathways. The converted Tregs can be further expanded by injection of IL-2/IL-2ab complexes. These complexes also increase the number of CD25+Foxp3− cells that, however, do not represent cytokine secreting effector cells but anergic cells, some of which can secrete IL-10 and can themselves be considered regulatory T cells as well. The combined use of everolimus and IL-2/IL-2ab complexes in vivo makes it feasible to achieve highly effective antigen-driven conversion of naive T cells into Treg and their expansion in vivo and thereby the described protocols constitute important tools to achieve immunological tolerance by Treg vaccination.
Keywords: antigen-specific Treg conversion, Foxp3, IL-2/IL-2ab complexes, mammalian target of rapamycin, prospective tolerance
At present it is unclear how much the conversion of naive T cells into Treg in peripheral lymphoid tissue contributes to the control of immunity by Treg. On the other hand, it seems reasonable to exploit this conversion process to control unwanted immune responses. In the past our lab has described several strategies to induce such conversion by exogenous antigens with the aim to study whether Treg conversion can be used to induce tolerance to antigens, including transplantation antigens, prospectively (1–3). We were successful in using this approach to induce prospective tolerance in female mice to a variety of male tissues, including skin and hematopoetic grafts (4).
With regard to mechanisms it became clear that Treg conversion in vivo is best achieved by subimmunogenic delivery of strong agonists under conditions that avoid functional activation of antigen presenting cells (5–9). Also, in accordance with more recent data (10), in this setting, best conversion is seen in T cells that undergo only limited proliferation, whereas higher doses of the strong agonist result in more extensive proliferation and diminished conversion (5). Thus these studies that addressed Treg conversion by monitoring the generation of Foxp3-expressing Treg in vivo confirmed anecdotal evidence of generating “dominant” tolerance by subimmunogenic antigen delivery (10).
Comparison of such converted Treg with Treg generated by T-cell stimulation in vitro in the presence of relatively high doses of transforming growth factor beta TGFβ (12) showed that in vitro-generated Treg exhibited marked differences: the in vitro conversion process was much faster and resulted in Treg with unstable Foxp3 expression that correlated with lack of demethylation of the Foxp3 locus, whereas the in vivo method resulted in complete demethylation and stable Foxp3 expression (13). In fact in some aspects the in vivo protocol was more akin to a different in vitro conversion method where T-cell activation was limited for an initial 18-h culture period in the absence of exogenously added TGFβ and activation of the PI3 kinase pathway was limited (14).
Because stability of Foxp3 expression appeared mandatory for the induction of prospective tolerance, we thought to make the in vivo conversion more efficient with drugs that would either enhance the conversion process or expand already converted cells because previous analyses had shown that converted cells could be easily expanded in vivo even under conditions when antigens were delivered in an immunogenic context (2, 3, 5). The results show that in vivo Treg conversion can be markedly enhanced by drugs that interfere with signals generated through the phosphatidyl inositol 3 kinase (PI3K) pathway and that converted Treg can be expanded by drugs that induce strong proliferation. With regard to expansion of Treg by IL-2/IL-2 ab complexes, we focused our attention on the properties of CD25+Foxp3− cells that could represent activated, cytokine-producing effector cells. The results, however, show that these cells resemble regulatory T cells in that they are anergic and secrete either no or only suppressive cytokines.
Results and Discussion
Enhancing Treg Conversion in Vivo.
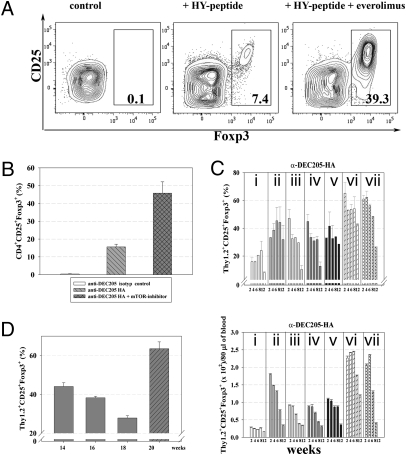
Because costimulation is counterproductive to Treg conversion in vivo (5) and because in vitro results showed that interference with the PI3K pathway, especially inhibition of the mammalian target of rapamycin (mTOR), could affect Treg conversion (14–16), we delivered antigen either by osmotic minipumps or DEC205 fusion antibodies in combination with or without the rapamycin analog everolimus, which inhibits mTOR. Everolimus was given for 14 d either together with the peptide when peptide was delivered by osmotic minipumps or after antigen was delivered at day 1 in the form of 40 ng of DEC205 fusion antibody. As a readout, two T-cell receptor (TCR) transgenic systems coupled with a Foxp3 reporter (17) were used; i.e., either female Marilyn mice (18) that express TCR transgenes encoding a receptor specific for HY peptide presented by I-Ab MHC complexes and also expressed a Foxp3 reporter knockin. Alternatively, we used naive T cells expressing an HA-specific TCR (19) and the Foxp3 reporter, which were transferred into congenic unmanipulated BALB/c mice before injection with 40 ng of DEC205 antibody fused with the HA peptide.
As shown in Fig. 1 i.p. application of everolimus (at a dose of 100 μg/mouse/d = 5 mg/kg) markedly increased the Treg-conversion rate such that a much higher proportion of T cells in both experimental systems expressed Foxp3. Representative data of Foxp3 expressing Tregs from spleens are shown (Fig. 1 A and B). Similar results of Foxp3+ expressing Tregs were obtained by analysis of mesenteric and peripheral lymph nodes. Everolimus resulted in a high proportion of Tregs even at later stages after application, whereas absolute numbers declined due to a general decline in transferred cells (Fig. 1C Lower). The combination of antigen plus everolimus injection followed by injection of IL-2/IL-2 antibody complexes clearly represented the most powerful means of increasing Treg numbers, but as earlier demonstrated (20), the expanded proportion and absolute number of Tregs were not stable over prolonged time periods. Thus in contrast to the conversion enhanced by everolimus, which resulted in a nearly stable high proportion of converted Tregs up to several weeks after conversion (Fig. 1C), the expansion of Treg is transient. The deficit in Tregs observed several weeks after expansion with IL-2/IL-2 antibody complexes could be ameliorated by renewed injection of these compounds (Fig. 1D).
Fig. 1.
Everolimus enhances antigen-specific Treg conversion in vivo. (A) Female HY–TCR transgenic Rag−/− Foxp3 reporter Marilyn mice were infused with HY peptide (10 μg/d) and everolimus (5 mg/kg i.p.) was given in parallel for 14 d. Numbers in dot plots indicate the percentages of CD4+CD25+Foxp3+ Tregs from spleen analyzed by FACS. (B) Naive CD4+CD25−Thy1.2+HA–TCR transgenic T cells were adoptively transferred into unmanipulated Thy1.1+ BALB/c mice before injection with 40 ng of anti-DEC205-HA or control antibody. Everolimus was given for 14 d. Representative results from spleen are shown. (C) Kinetic of Foxp3 expression following HA-specific Treg conversion at 2-wk intervals analyzed in blood. Shown are percentages of Thy1.2+CD4+CD25+Foxp3+ Tregs (Upper) and absolute numbers of Thy1.2+CD4+CD25+Foxp3+ Tregs (Lower) per 80 μL of blood. Bars are starting from week 2 after in vivo conversion until week 12. Mice were treated during the initial 14-d Treg conversion process as follows: i, no drugs; ii, + everolimus; iii, + IL-2/IL-2ab complexes (day 10–12); iv, + DNMT1-inhibitor doxorubicin; v, + everolimus + DNTM1-inhibitor doxorubicin; vi, + everolimus + IL-2/IL-2ab complexes; vii, + everolimus + doxorubicin + IL-2/IL-2ab complexes. (D) Second application of everolimus for 14 d + IL-2/IL-2ab complexes (for 3 d, shaded bar) starting at 18 wk after the initial Treg conversion process. Bars show averages (±SD of four mice per group), representative of at least two independent experiments.
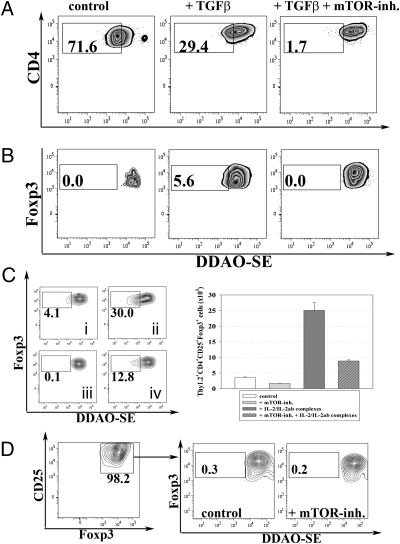
In further in vivo as well as in vitro experiments, we analyzed possible pathways by which everolimus enhanced Treg conversion to establish whether combinations of different drugs would be useful. When DDAO-SE–labeled T cells were cultured, everolimus reduced rather than enhanced T-cell proliferation, whereas published data claim that rapamycin enhances Treg proliferation (21). Our data are in line with earlier experiments indicating that Treg conversion was best achieved with antigenically stimulated T cells that exhibited little proliferation (5). To address this question further in vivo, DDAO-SE–labeled Tregs were adoptively transferred into congenic unmanipulated BALB/c hosts and treated with everolimus daily for a period of 5 d. Reanalysis of transferred Tregs from such mice exhibited no signs of Treg expansion (Fig. 2C). Similarily, in vitro experiments conducted with ex vivo Treg in the presence of everolimus showed that everolimus interfered with T-cell proliferation of converted Treg (Fig. 2D).
Fig. 2.
Everolimus inhibits T-cell proliferation in vitro and in vivo. Naive CD4+ T cells from Foxp3 GFP reporter mice were DDAO-SE labeled and subjected to TGFβ-dependent in vitro conversion assays (anti-CD3 and anti-CD28 at 5 μg/mL each). At day 3, Foxp3 expression and DDAO-SE dilution of all T cells as shown in A as well as of converted Treg, shown in B were analyzed by FACS. (C) CD4+CD25+Thy1.2+Foxp3+ Tregs were sorted to high purity, labeled with DDAO-SE, and adoptively transferred into normal congenic Thy1.1+ BALB/c mice. Animals were left either: untreated (i); injected with IL-2/IL-2ab complexes (ii), everolimus (iii), 3 mg/kg i.p., or the combination of everolimus and IL-2/IL-2ab complexes (iv) daily for 5 d. Shown are DDAO-SE dilution profiles of transferred Tregs (Left) as well as bars representing absolute numbers of Thy1.2+CD4+CD25+Foxp3+ Tregs (Right, ±SD of four mice per group). (D) Ex vivo purified CD4+CD25+Foxp3+ Tregs from Foxp3 GFP reporter mice were DDAO-SE labeled, cultured in vitro using anti-CD3 and anti-CD28 at 5 μg/mL each with or without everolimus (10 nM). Proliferation of Tregs was assessed by DDAO-SE dilution. Data are representative of at least three independent experiments.
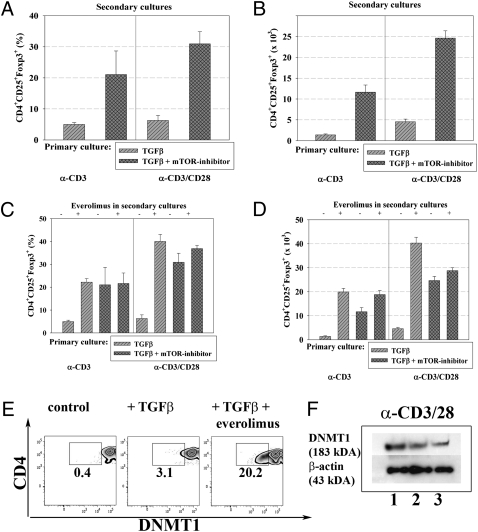
In the absence of data supporting the notion that everolimus can augment Treg proliferation, we addressed other mechanisms that could account for the increased number of Tregs in vivo in the presence of this drug: Fig. 3 shows that when everolimus is added to cultures in which T cells undergo TGFβ-dependent in vitro conversion, everolimus increased Foxp3 stability, which becomes evident when the converted Treg cells are subsequently cultured in the absence of TGFβ (secondary cultures). The stabilizing effect of everolimus on Foxp3 expression could also be observed, when the drug was added to restimulation cultures of Tregs that had been converted previously in the presence of TGFβ only (Fig. 3 C and D).
Fig. 3.
Everolimus increases Foxp3 stability. Naive CD4+ T cells from Foxp3 GFP reporter mice were stimulated with either anti-CD3 or anti-CD3/28 antibodies and subjected to TGFβ-dependent in vitro conversion assays ± everolimus (10 nm). Foxp3+ Tregs from these primary cultures were sorted to high purity and restimulated in the absence of exogenous TGFβ. Foxp3 expression in these secondary Treg cultures was then reassessed on day 4 of restimulation by FACS. Bars show percentages of CD4+CD25+Foxp3+Tregs at the end of the secondary culture (A) as well as absolute numbers (B). Data (averages ± SEM) are representative of at least four independent experiments. (C) Everolimus directly added to restimulation cultures of Tregs reduces their loss of Fopx3 expression. CD4+CD25+Foxp3+ Tregs were sorted to high purity from TGFβ-dependent in vitro conversion assays ± everolimus (primary culture) as described above and restimulated in the absence of TGFβ but ± everolimus (secondary cultures). Foxp3 expression from these secondary Treg cultures was then reassessed on day 4 of restimulation. Bars show percentages of CD4+CD25+Foxp3+Tregs at the end of the secondary culture (C) as well as absolute numbers (D). Data are averages ± SEM and representative of three independent experiments. (E) Naive CD4+T cells from Foxp3 GFP reporter mice were subjected to TGFβ-dependent in vitro conversion assays (anti-CD3 and anti-CD28 at 5 μg/mL each) ± everolimus as described above. DNMT1 expression was assessed at the end of the conversion process by intracellular staining (E) or by Western blot analysis as shown in F. (F) Cell lysates from cultures as described in E were separated by SDS/PAGE gel with the following conditions: (1) untreated, (2) + TGFβ (0.6 ng/mL), and (3) TGFβ (0.6 ng/mL) + everolimus (10 nM). Representative data of four independent experiments are shown.
These data could be a result of inhibition of cell cycling by everolimus (Fig. 2) because cell cycling increases the expression of certain methylating enzymes such as DNA methyltransferase 1 (DNMT1), which could interfere with stable Foxp3 expression (13, 22, 23). Complete demethylation of CpG motifs at the foxp3 locus was shown to correlate with a stable human (24, 25) and mouse Treg phenotype (13). Pharmacologic inhibition of the DNMT1 activity and knocking down or ablating DNMT1 gene markedly increased the efficacy of induction and stability of Foxp3 expression (13, 22, 23). Moreover, the absence of DNMT1 in vivo resulted in Foxp3 expression by antigenically stimulated T cells rather than in their immune activation (22). In our experiments, addition of everolimus to TGFβ-dependent in vitro conversion assays resulted in a significant reduction of DNMT1 expression (Fig. 3 E and F). This reduction may explain why the use of DNMT1 inhibitors in addition to everolimus did not significantly increase Treg conversion, even though in the absence of everolimus they do so (Fig. 1C). These results suggest that everolimus works, at least in part, by interfering with DNMT1 expression, which results in increased Treg conversion. The mechanistic interpretation for these findings remains correlative, because demethylating enzymes like DNMT1 act genomwide (13, 22, 23) and there is only a correlation of Foxp3 demethylation and expression.
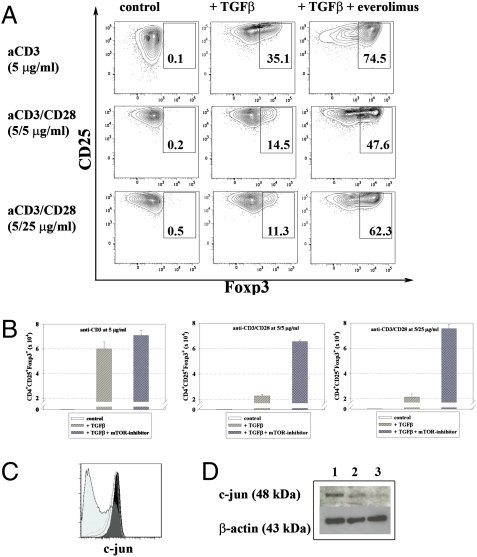
To address the impact of mTOR inhibition on costimulation during TGFβ-dependent in vitro conversion, we titrated CD28 antibodies. Everolimus could significantly enhance TGFβ-dependent conversion when cells were cultured with CD3 and CD28 antibodies at a 1:1 ratio. The magnitude of the Treg conversion-enhancing effect by inhibitors of the PI3K/Akt/mTOR pathway became even more obvious under conditions of strong costimulation (ratio 1:5), supporting the concept that Foxp3 induction can be increased by enhanced costimulatory signals in the presence of PI3K/Akt/mTOR inhibition (14), whereas CD28 signals have been shown to interfere with Treg conversion in the presence of TGFβ only. Synergism between TGFβ and mTOR inhibitors has been demonstrated (14, 26); however, blockade of the TGFβ signaling pathway did not interfere with Foxp3 induction enhanced by PI3K/mTOR inhibition, suggesting that different mechanisms are relevant for the observed increase of Treg conversion in the presence of TGFβ or PI3K/mTOR inhibitors. Everolimus interferes with the generation of AP-1 because addition of everolimus to CD4+ T cells subjected to TGFβ-dependent conversion assays in the presence of strong costimulation (anti-CD3 and anti-CD28 at 5 μg/mL each) resulted in a clear reduction of c-jun expression (Fig. 4). It remains an open question whether AP-1 regulates Foxp3 expression.
Fig. 4.
Everolimus blocks costimulation and thereby increases TGFβ-dependent in vitro conversion. (A) Naive CD4+ T cells form Foxp3 GFP reporter mice were cultured with anti-CD3 or anti-CD3 + anti-CD28 at a ratio of 1:1 or 1:5 in the presence of TGFβ (0.6 ng/mL) ± (10 nm). Numbers in dot plots indicate percentages of CD4+CD25+Foxp3+ Tregs. Absolute numbers of CD4+CD25+Foxp3+ Tregs are shown in B. (C) c-jun expression from conversion cultures (anti-CD3 and anti-CD28 at 5 μg/mL each) was assessed by intracellular staining: black histogram (filled), untreated control; gray lined histogram (unfilled), TGFβ (0.3 ng/mL) (solid line) and TGFβ (0.6 ng/mL) (dotted line); light gray histogram (shaded), TGFβ (0.6 ng/mL) + everolimus (10 nM). (D) Cell lysates from the cultures as in C were prepared from the following conditions (1) control; (2) TGFβ at 0.6 ng/mL; and (3) TGFβ (0.6 ng/mL) + everolimus (10 nM), subjected to SDS/PAGE and immunoblotting using c-jun as well as β-actin-specific antibodies. Representative data out of at least five independent experiments are shown.
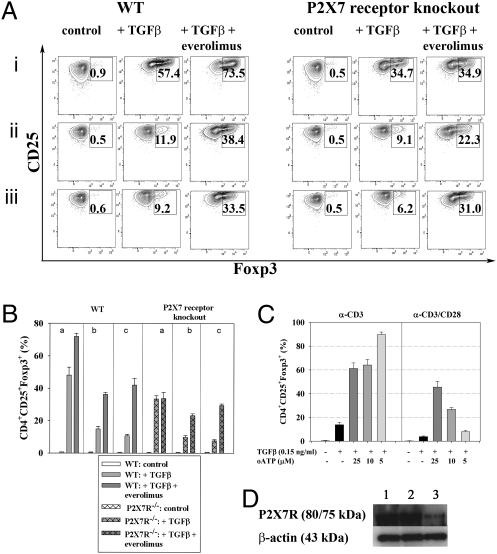
Another possible pathway downstream of mTOR may operate through the P2X7 receptor (27, 28). P2X7 receptors are membrane cation channels gated by extracellular ATP, playing a key role in calcium influx and downstream signaling events associated with the activation and proliferation of T cells. ATP causes the opening to nonselective cation pores (28). Oxidized ATP (29) functions as a potent and specific inhibitor of the P2X7 receptor and enhances TGFβ-dependent Treg conversion (Fig. 5).
Fig. 5.
Everolimus-mediated dampening of T-cell activation involves the P2X7 receptor. (A) Naive CD4+ T cells from WT or P2X7 receptor knockout animals were subjected to TGFβ-dependent (0.3 ng/mL) in vitro conversion assays ± everolimus (10 nm) in the presence of anti-CD3 only (i) as well as with anti-CD3 and anti-CD28 at a ratio of 1:1 (ii), or 1:5 (iii). Numbers in dot plots indicate percentages of CD4+CD25+Foxp3+ Tregs at the end of the conversion process. (B) Bars show combined results of percentages of CD4+CD25+Foxp3+ Tregs using the conditions described under A. Data are representative of four independent experiments. (C) The P2X7 receptor inhibitor oxidized ATP (oATP) increases TGFβ-dependent in vitro conversion. Naive CD4+ T cells from Foxp3 GFP reporter mice were subjected to TGFβ-dependent in vitro conversion assays ± oATP. Bars represent percentages (±SD) of CD4+CD25+Foxp3+ cells. Data are representative of four independent experiments. (D) Naive T cells from WT animals were subjected to TGFβ-dependent conversion assays (anti-CD3 and anti-CD28 at 5 μg/mL each) in the presence or absence of everolimus: (1) control, (2) TGFβ at 0.6 ng/mL, (3) TGFβ (0.6 ng/mL) + everolimus (10 nM) and subjected to SDS/PAGE and immunoblotting using P2X7 receptor as well as β-actin–specific antibodies. Representative data out of at least four independent experiments are shown.
In T cells from P2X7 receptor knockout mice, the everolimus-mediated increase of TGFβ-dependent in vitro conversion is abolished under conditions of weak T-cell stimulation (anti-CD3 only), whereas under conditions of increased costimulation (anti-CD3:CD28 1:1 or 1:5) the everolimus-mediated increase is partly rescued, suggesting that the observed enhancement of Foxp3 expression by PI3K/mTOR inhibition in the presence of stronger costimulatory signals (14) is only partially dependent on P2X7 receptor signaling. Addition of everolimus to naive T cells from WT mice subjected to TGFβ-dependent in vitro conversion assays (anti-CD3 and anti-CD28 at 5 μg/mL each) resulted in a clear reduction of P2X7 receptor protein expression (Fig. 5D), consistent with the suggestion that everolimus works, at least in part, by controlling several distinct pathways of T-cell activation.
An enhancing effect of mTOR inhibition in vitro was also noted by Cobbold et al. (26) and interpreted to indicate that the PI3K/Akt/mTOR pathway acts as a nutrient sensor of essential amino acids required for proliferation. Our data establish that the mTOR inhibitor everolimus is a potent enhancer of Treg conversion in vivo. The in vivo results reported in this study are comparable with some more recent studies where limited TCR signaling in conjunction with mTOR inhibition resulted in Treg conversion in vitro (14) and less compatible with another in vitro study where Treg conversion was attempted in the presence of TGFβ (16). The latter procedure results in Tregs that do not stably express Foxp3, whereas limited TCR signaling in vivo results in stable Foxp3 expression in converted Tregs (14). Of note, addition of mTOR inhibitors to all of these conversion models resulted in Tregs that are more stable with regard to Foxp3 expression. mTOR inhibitors in the form of rapamycin, and its better-tolerated analog everolimus, not only stabilize Foxp3 expression but clearly increase the conversion rate of naive T cells stimulated by subimmunogenic antigen delivery in vivo. Other drugs like inhibitors of DNMT1 as 5-Aza-deoxycytidine or doxorubicin hydrochloride are much more toxic and offer less enhancement of Treg conversion in combination with everolimus. DNMT1 inhibitors are presumably ineffective because everolimus already sufficiently reduces DNMT1. Thus in our hands, everolimus is most effective on its own in promoting prospective tolerance by Treg vaccination. The drug also interferes with the activation of immune effector cells and thus appears well suited for tolerance induction through antigenic stimulation resulting in Treg conversion. Recent data suggest that the inhibition of the PI3K–Akt pathway by everolimus results in increased activity of Foxo proteins that up-regulate Foxp3 expression (reviewed in ref. 30).
Specific Expansion of Treg in Vivo.
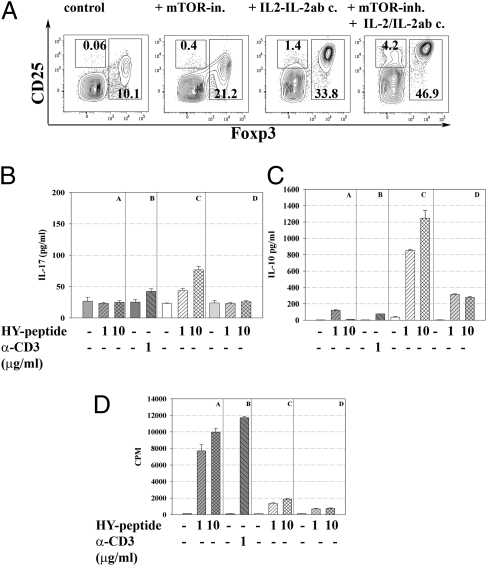
IL-2/IL-2ab complexes IL-2/IL-2ab complexes do not only expand naturally generated Treg in vivo as described (20) but can also be used to expand recently in vivo converted Tregs. It was noted, however, that the injection of IL-2/IL-2 complexes IL-2/IL-2ab complexes resulted not only in the expansion of Foxp3+ Tregs but also increased numbers of Foxp3−, CD25+ cells. Cells with this phenotype could be conceivably counterproductive to tolerance induction and given recent results on the activation of T cells by an agent (anti-CD28 superagonist antibodies) supposed to expand Tregs only (31) it appeared mandatory to analyze whether the injection of IL-2/IL-2 IL-2/IL-2ab complexes would result in the activation of other T cells generating cytokines that could be counterproductive to immunosuppression. Therefore CD25+Foxp3− cells were sorted (from various mice, i.e., injected with antigen alone (where very few such cells could be isolated) and mice injected with everolimus plus IL-2/IL-2 antibody complexes or IL-2/IL-2 anti complexes unify the term IL-2/IL-2ab complexes as seen above alone. In the latter case the increased number of such cells and their restimulation in vitro resulted in some (limited) IL-17 production and some increased IL-10 production (Fig. 6). When IL-2/IL-2 complexes IL-2/IL-2ab complexes were given after everolimus treatment the number of CD4+CD25+Foxp3− cells was also increased but neither IL-17 nor IL-10 production was observed after in vitro restimulation. Thus, everolimus is likewise useful to interfere with secretion of cytokines such as IL-17 when using this particular protocol to expand converted Treg. In terms of proliferation, the CD25+Foxp3− cells were anergic, which could suggest that they might be destined to become regulatory cells rather than immunological effector cells. Thus, at least the conversion protocol combining everolimus and IL-2/IL-2ab complexes does not increase activated effector cells simultaneously with enhancing Treg conversion and expansion (Fig. 6).
Fig. 6.
Characterization of CD25+Foxp3− cells selected after treatment with IL-2/IL-2ab complexes during HY dependent in vivo conversion into Tregs. (A) HY–TCR transgenic Rag-deficient Foxp3 GFP reporter Marilyn mice were infused with HY peptide (10 μg/d) and left either untreated or treated with everolimus (3 mg/kg i.p.) for 14 d, with IL-2/IL-2ab complexes for 3 d at the end of the conversion process or with a combination of both drugs. Numbers in panels indicate percentages of CD4+CD25+Foxp3− as well as CD4+CD25+Foxp3+ T cells, respectively. CD4+CD25+Foxp3− cells from the respective treatment groups were sorted to high purity and restimulated in the presence of antigen (HY peptide) or anti-CD3 only. Supernatants of restimulation cultures were analyzed for IL-17 (B) and IL-10 production (C) after 48 h of culture by ELISA. (D) Analysis of [3H]thymidine incorporation was done after addition for the last 12 h of a 70-h culture period and followed by scintillation counting. For the bars shown in B–D the mice have been treated in the precedent HY-dependent in vivo conversion into Tregs as follows: A and B, untreated; C, + IL-2/IL-2ab complexes; and D, + everolimus + IL-2/IL-2ab complexes. Data are representative of three independent experiments.
Overall the use of everolimus represents an ideal Treg-vaccination approach, whereas the use of IL-2/IL-2ab complexes yields only a transient increase in Treg numbers. The latter method combined with everolimus to interfere with costimulation may in fact be more useful for treatment rather than prevention of disease.
Materials and Methods
Mice.
RAG2−/− Marilyn mice expressing a transgenic TCR for MHC class II restricted HY peptide were a generous gift from P. Matzinger (National Institutes of Health, Bethesda, MD), Foxp3–GFP-expressing Marilyn mice were obtained by crossing Marilyn mice with Foxp3–GFP reporter mice (kindly provided by A. Rudensky, Sloan Kettering Center, New York, ref. 17). P2X7 receptor knockout animals as well as C57BL/6J mice were from the Jackson Laboratory. Thy1.2 BALB/c Rag2−/− TCR–HA mice express a transgenic TCR specific for H-2I-Ed HA(107–119). Thy1.1 congenic BALB/c were used as hosts for adoptive transfer experiments. Animal care and all procedures were in accordance with the guidelines of the Animal Care and Use Committee of the Dana-Farber Cancer Institute. Antigen-specific in vivo conversion protocols into Tregs were done as recently described in more detail (32). Everolimus was from LC Laboratories, doxorubicin was from Sigma, and IL-2/IL-2ab complexes were used following a modification of the protocol initially described in refs. 20, 33.
Cell Sorting and Flow Cytometry.
mAbs specific for CD3ε (145-2C11), CD28 (37.51), Thy1.1 (OX-7), Thy1.2 (53-2.1), CD4 (RM4-5), CD25 (PC61), CD44 (IM7), CD62L (MEL-14), Foxp3 (FJK-16s), and IL-17 were from BD, eBioscience or Biolegend, and were used as biotin, FITC, PE, PerCP-Cy5.5, allophycocyanin (APC), or Pacific blue conjugates. Monoclonal antibody to TCR–HA (6.5) was purified and conjugated with Alexa fluor 647 following standard protocols. Fc-receptor blocking monoclonal antibody 2.4G2 was used as culture supernatant. Cell trace far red DDAO-SE reagent was from Molecular Probes (Invitrogen). Where indicated cells were labeled with DDAO-SE at 2 μM by incubation for 4 min at 37 °C in PBS plus 0.1% BSA. NT cells were sorted from 6- to 8-wk-old Foxp3 GFP reporter mice as CD4+CD25−CD44loCD62LhiFoxp3− from spleens and lymph nodes using a FACSAria (BD). Enumeration of cells and acquisition are performed by using FACSAria and FACSDiva software (BD). Single-cell data analyses are done by the use of the FlowJo software (Tree Star).
TGFβ-Dependent in Vitro Conversion Assays.
The assays were performed as described in detail elsewhere (32). For analysis of cytokine production culture supernatants were harvested after 48 h and the concentration of IL-17 and IL-10 were analyzed by a mouse ELISA set (OptEIA, BD).
Immunoblotting.
For Western blot analyis, cells were sort purified and lysed after 48 h of culture using the Nuclear Protein extraction kit (Active Motif) according to the manufacturer's instructions. Lysates from 20,000 cells were separated on Nupage Novex 3–8% Tris-acetate gels for DNMT1 or on 4–12% Nupage Novex Bis-Tris gels for c-jun and P2X7 receptor. Blocking of unspecific binding was done using the ECL Advance blocking agent (GE Healthcare), followed by incubation with the primary and the appropriate horseradish peroxidase-conjugated secondary antibodies diluted in blocking agent. Immunoreactive proteins were detected by enhanced chemiluminescence (GE Healthcare). The specific antibodies for P2X7 receptor was from BD, for c-jun and DNMT1 from Cell Signaling Technology and from Abcam, respectively.
Acknowledgments
These studies were supported by National Institutes of Health Grant NIH-AI-53102 (to H.v.B.). C.D. was supported by a Leopoldina research fellowship (BMBF-LPD 9901/8-184) and by the LOEWE (Lipid Signaling Forschungszentrum Frankfurt) program of the Federal State of Hessen, Germany.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 2.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc Natl Acad Sci USA. 2003;100:8886–8891. doi: 10.1073/pnas.1533365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verginis P, McLaughlin KA, Wucherpfennig KW, von Boehmer H, Apostolou I. Induction of antigen-specific regulatory T cells in wild-type mice: Visualization and targets of suppression. Proc Natl Acad Sci USA. 2008;105:3479–3484. doi: 10.1073/pnas.0800149105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kretschmer K, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 6.Kretschmer K, Heng TS, von Boehmer H. De novo production of antigen-specific suppressor cells in vivo. Nat Protoc. 2006;1:653–661. doi: 10.1038/nprot.2006.105. [DOI] [PubMed] [Google Scholar]
- 7.Curotto de Lafaille MA, et al. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: More of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietrich FM, Weigle WO. Induction of tolerance to heterologous proteins and their catabolism in C57BL/6 mice. J Exp Med. 1963;117:621–631. doi: 10.1084/jem.117.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorstenson KM, Herzovi L, Khoruts A. A model of suppression of the antigen-specific CD4 T cell response by regulatory CD25+CD4 T cells in vivo. Int Immunol. 2005;17:335–342. doi: 10.1093/intimm/dxh213. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polansky JK, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 14.Sauer S, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delgoffe GM, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Lantz O, Grandjean I, Matzinger P, Di Santo JP. Gamma chain required for naïve CD4+ T cell survival but not for antigen proliferation. Nat Immunol. 2000;1:54–58. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- 19.Kirberg J, et al. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webster KE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: Induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 22.Josefowicz SZ, Wilson CB, Rudensky AY. Cutting edge: TCR stimulation is sufficient for induction of Foxp3 expression in the absence of DNA methyltransferase 1. J Immunol. 2009;182:6648–6652. doi: 10.4049/jimmunol.0803320. [DOI] [PubMed] [Google Scholar]
- 23.Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: A role for DNA methylation. J Exp Med. 2007;204:1543–1551. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baron U, et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol. 2007;37:2378–2389. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 25.Janson PC, et al. FOXP3 promoter demethylation reveals the committed Treg population in humans. PLoS ONE. 2008;3:e1612. doi: 10.1371/journal.pone.0001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cobbold SP, et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci USA. 2009;106:12055–12060. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 28.Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- 29.Murgia M, Hanau S, Pizzo P, Rippa M, Di Virgilio F. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem. 1993;268:8199–8203. [PubMed] [Google Scholar]
- 30.Hedrick SM. The cunning little vixen: Foxo and the cycle of life and death. Nat Immunol. 2009;10:1057–1063. doi: 10.1038/ni.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beyersdorf N, et al. Selective targeting of regulatory T cells with CD28 superagonists allows effective therapy of experimental autoimmune encephalomyelitis. J Exp Med. 2005;202:445–455. doi: 10.1084/jem.20051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daniel C, Ploegh H, von Boehmer H. Antigen-specific induction of regulatory T cells in vivo and in vitro. In: Kassiotis G, Liston A, editors. Regulatory T-Cells: Methods and Protocols (Methods in Molecular Biology) New York: Springer Science + Business Media; 2010. in press. [DOI] [PubMed] [Google Scholar]
- 33.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]