Abstract
Charges are inherently incompatible with hydrophobic environments. Presumably for this reason, ionizable residues are usually excluded from the hydrophobic interior of proteins and are found instead at the surface, where they can interact with bulk water. Paradoxically, ionizable groups buried in the hydrophobic interior of proteins play essential roles, especially in biological energy transduction. To examine the unusual properties of internal ionizable groups we measured the pKa of glutamic acid residues at 25 internal positions in a stable form of staphylococcal nuclease. Two of 25 Glu residues titrated with normal pKa near 4.5; the other 23 titrated with elevated pKa values ranging from 5.2–9.4, with an average value of 7.7. Trp fluorescence and far-UV circular dichroism were used to monitor the effects of internal charges on conformation. These data demonstrate that although charges buried in proteins are indeed destabilizing, charged side chains can be buried readily in the hydrophobic core of stable proteins without the need for specialized structural adaptations to stabilize them, and without inducing any major conformational reorganization. The apparent dielectric effect experienced by the internal charges is considerably higher than the low dielectric constants of hydrophobic matter used to represent the protein interior in electrostatic continuum models of proteins. The high thermodynamic stability required for proteins to withstand the presence of buried charges suggests a pathway for the evolution of enzymes, and it underscores the need to mind thermodynamic stability in any strategy for engineering novel or altered enzymatic active sites in proteins.
Keywords: dielectric effect, electrostatics, hydration, pKa, bioenergetics
The transfer of an ion from water into a less polar and polarizable environment, such as the hydrophobic interior of a protein, is energetically unfavorable. Internal charges usually destabilize the folded states of proteins, which is primarily why charged groups are largely excluded from the hydrophobic interior and found instead at the protein-water interface, where they can interact with bulk water (1). Paradoxically, internal ionizable groups in proteins are essential for biological energy transduction. These type of ionizable groups are found in the active sites of enzymes (2), and are necessary for e- transfer and H+ transport in proteins such as ATPase (3) and cytochrome c oxidase (4), for ion homeostasis (5, 6), and for light-activated processes in proteins such as bacteriorhodopsin (7, 8). The structural adaptations necessary for proteins to tolerate internal ionizable groups, and the factors that stabilize internal charges, are poorly understood. For this reason, our understanding of fundamental aspects of function and evolution of proteins is still limited, as is our ability to manipulate and design novel enzymes.
To examine systematically the capacity of globular proteins to tolerate the presence of buried charges, we measured the pKa of 25 internal glutamic acid residues (Glu) that were introduced with mutagenesis into internal hydrophobic positions in staphylococcal nuclease (SNase). Because substitution of internal hydrophobic positions with Glu is usually destabilizing, the experiments were performed with a highly stable form of SNase known as Δ+PHS, which has a stability of 11.8 kcal/mol at 298 K (9). We know from existing crystal structures of some variants of SNase with internal Glu, Asp, and Lys at positions 66 (9–12), 9 (13), and 38 (14, 15) that ionizable side chains engineered by substitution of internal hydrophobic amino acids with ionizable ones are, indeed, internal. We have also shown previously that at pH 7 the Glu-substituted variants of Δ+PHS nuclease are thermodynamically stable and that their conformation is comparable to that of the background protein (16). The goal of the present set of experiments was to measure the pKa values of the internal ionizable groups and to examine the effects of internal charges on the protein’s conformation. To this end we measured the thermodynamic stability of the Glu-containing variants over a wide range of pH values and used Trp fluorescence and far-UV CD spectroscopy to monitor structural consequences of ionization of internal Glu residues.
Results and Discussion
Thermodynamic Stability.
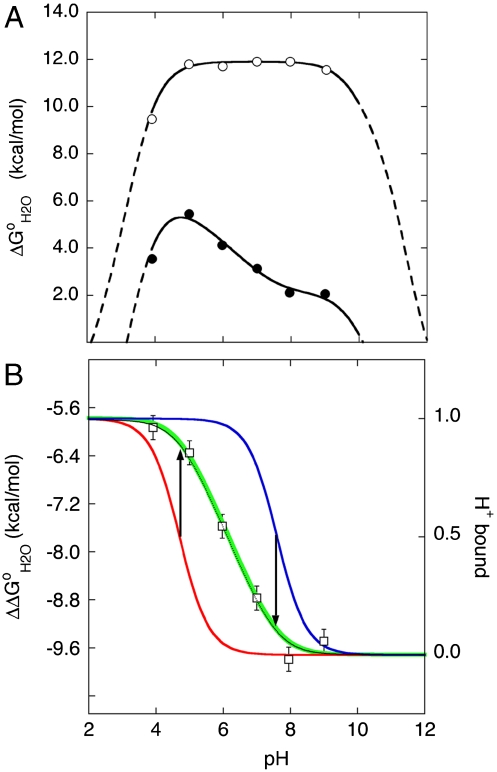
The apparent pKa values of the 25 internal Glu residues were determined by analysis of the pH-dependence of thermodynamic stability. Specifically, its the difference in thermodynamic stability (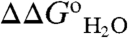 ) between the reference Δ+PHS protein and the Glu-substituted variants (Fig. 1) that contains information about the pKa values of the internal groups (10, 12, 14, 15, 17) (the
) between the reference Δ+PHS protein and the Glu-substituted variants (Fig. 1) that contains information about the pKa values of the internal groups (10, 12, 14, 15, 17) (the 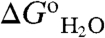 of each of the 25 variant proteins at many pH values is provided in Table S1, with fits used to extract pKa values). This method for measuring pKa values was useful because the pKa of the internal Glu residues tended to be highly perturbed. The principle behind the experiments used to measure pKa values is illustrated in Fig. 1. The red and blue curves in Fig. 1B (with reference to right axis) correspond to H+ titration of a representative internal Glu in the unfolded (pKa = 4.5) and native forms (pKa = 7.6) of a protein, respectively. These curves were simulated using the pKa values obtained by analysis of the
of each of the 25 variant proteins at many pH values is provided in Table S1, with fits used to extract pKa values). This method for measuring pKa values was useful because the pKa of the internal Glu residues tended to be highly perturbed. The principle behind the experiments used to measure pKa values is illustrated in Fig. 1. The red and blue curves in Fig. 1B (with reference to right axis) correspond to H+ titration of a representative internal Glu in the unfolded (pKa = 4.5) and native forms (pKa = 7.6) of a protein, respectively. These curves were simulated using the pKa values obtained by analysis of the 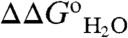 vs. pH data shown in Fig. 1B. The midpoints of the red and blue H+ titration curves represent the pKa; they also correspond to the regions with changing curvature in the
vs. pH data shown in Fig. 1B. The midpoints of the red and blue H+ titration curves represent the pKa; they also correspond to the regions with changing curvature in the 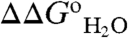 vs. pH data (black line in Fig. 1B). The area between these red and blue H+ titration curves, shown by the green curve in Fig. 1B (with reference to the left axis), corresponds exactly to the
vs. pH data (black line in Fig. 1B). The area between these red and blue H+ titration curves, shown by the green curve in Fig. 1B (with reference to the left axis), corresponds exactly to the 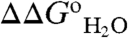 curve measured experimentally with chemical denaturation (black curve and square symbols in Fig. 1B).
curve measured experimentally with chemical denaturation (black curve and square symbols in Fig. 1B).
Fig. 1.
Measurement of pKa values of internal ionizable groups by analysis of pH dependence of thermodynamic stability (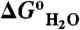 ). (A) Stability of the reference protein (circle) and of a variant with Leu-25 substituted with Glu (solid circle). All
). (A) Stability of the reference protein (circle) and of a variant with Leu-25 substituted with Glu (solid circle). All 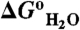 were measured with GdnHCl denaturation monitored by Trp fluorescence as described previously (34). The lines are meant to guide the eye (dashed line identifies the pH interval in which measurements of
were measured with GdnHCl denaturation monitored by Trp fluorescence as described previously (34). The lines are meant to guide the eye (dashed line identifies the pH interval in which measurements of 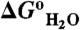 are not accessible owing to acid or base unfolding). (B) Difference between the two curves in (A) (square) (with reference to the left axis). The thin solid black curve represents the fit of equation 3 from Karp et al. (9) to obtain the apparent pKa of 7.6 for Glu-25. The vertical arrows describe graphically the relationship between the pKa values in the native (
are not accessible owing to acid or base unfolding). (B) Difference between the two curves in (A) (square) (with reference to the left axis). The thin solid black curve represents the fit of equation 3 from Karp et al. (9) to obtain the apparent pKa of 7.6 for Glu-25. The vertical arrows describe graphically the relationship between the pKa values in the native (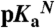 ) and unfolded (
) and unfolded (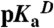 ) states, and regions with change in slope in the
) states, and regions with change in slope in the 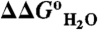 vs. pH curve. The H+ titration curves for Glu-25 with pKa values of 4.7 in the unfolded state (red) and 7.6 in the folded state (blue) are shown (with reference to the right axis). The green curve describes the pH dependence of the area between these two curves (with reference to left axis).
vs. pH curve. The H+ titration curves for Glu-25 with pKa values of 4.7 in the unfolded state (red) and 7.6 in the folded state (blue) are shown (with reference to the right axis). The green curve describes the pH dependence of the area between these two curves (with reference to left axis).
Fig. 1 illustrates how the pKa values of the internal ionizable groups can be obtained directly by analysis of 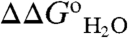 vs. pH data. pKa values measured this way are apparent pKa values because the pKa is assumed to be pH independent and because the analysis assumes that a single ionizable group with a highly perturbed pKa determines the
vs. pH data. pKa values measured this way are apparent pKa values because the pKa is assumed to be pH independent and because the analysis assumes that a single ionizable group with a highly perturbed pKa determines the 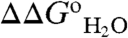 vs. pH curve. The validity of this last assumption was tested previously in one variant (9, 10, 14, 15) and by measurement of the pKa values of all surface ionizable groups in Δ+PHS nuclease (18, 19).
vs. pH curve. The validity of this last assumption was tested previously in one variant (9, 10, 14, 15) and by measurement of the pKa values of all surface ionizable groups in Δ+PHS nuclease (18, 19).
pKa Values of Internal Glu Residues.
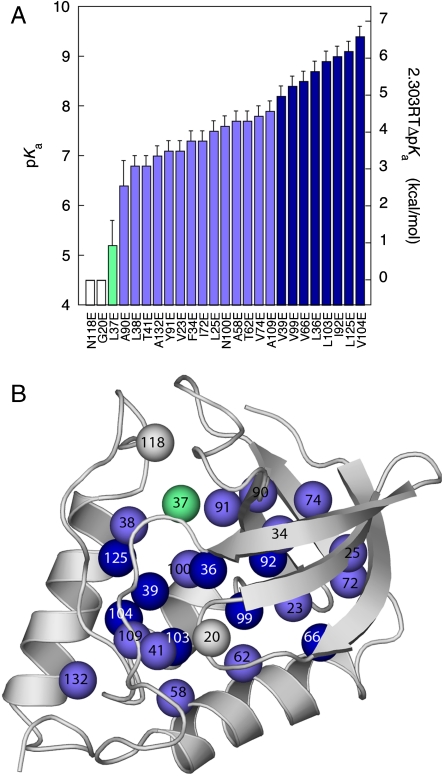
The apparent pKa values of Glu residues at 23 of the 25 internal positions (Fig. 2A and Table 1) are much higher than the normal pKa of 4.5 for Glu in water (19, 20). The upward shifts in pKa are consistent with the ionizable moieties of the Glu residues being internal, at least partially removed from bulk water, and from the influence of the large number of basic residues on the surface of the protein. The direction of the shifts demonstrates that for buried Glu residues the neutral state of the carboxylic groups is preferred over the charged one. The shifts suggest that the dehydration experienced by the buried carboxylic side chains is not compensated completely by favorable interactions between the carboxylate moiety and internal polar groups or surface charges, by polarization of their local microenvironments, or by any other factor that could influence the pKa of an internal group.
Fig. 2.
pKa values of Glu residues in 25 internal positions in staphylococcal nuclease. (A) Apparent pKa values. Color coding is only meant to guide the eye: white identifies groups with no measurable shift in pKa relative to the normal pKa of Glu in water; green was used for groups with pKa between 4.5–6.5; light blue for groups with pKa between 6.5–8.0; dark blue for groups with pKa higher than 8.0. The right axis describes the 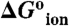 values from Table 1. (B) Location of 25 internal positions coded with color according to the pKa for Glu in that position as listed in (A).
values from Table 1. (B) Location of 25 internal positions coded with color according to the pKa for Glu in that position as listed in (A).
Table 1.
Apparent pKa values and energetic cost for creating charge at 25 internal positions in SNase
| Position | pKa* |
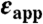 † †
|
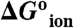 (kcal/mol)‡ (kcal/mol)‡
|
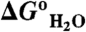 (kcal/mol)§ (kcal/mol)§
|
| V104E | 9.4 | 9.2 | 6.7 | 4.2 |
| L125E | 9.1 | 9.7 | 6.3 | 2.5 |
| I92E | 9.0 | 9.9 | 6.1 | 1.4 |
| L103E | 8.9 | 10.1 | 6.0 | 3.4 |
| L36E | 8.7 | 10.5 | 5.7 | 3.2 |
| V66E | 8.5 | 11.0 | 5.4 | 1.8 |
| V99E | 8.4 | 11.2 | 5.3 | 3.2 |
| V39E | 8.2 | 11.7 | 5.0 | 5.3 |
| A109E | 7.9 | 12.6 | 4.6 | 4.2 |
| V74E | 7.8 | 12.9 | 4.5 | 2.7 |
| A58E | 7.7 | 13.3 | 4.4 | 5.0 |
| T62E | 7.7 | 13.3 | 4.4 | 5.6 |
| N100E | 7.6 | 13.6 | 4.2 | 7.4 |
| L25E | 7.5 | 14.0 | 4.1 | 3.1 |
| F34E | 7.3 | 14.8 | 3.8 | 4.4 |
| I72E | 7.3 | 14.8 | 3.8 | 4.6 |
| V23E | 7.1 | 15.7 | 3.5 | 4.9 |
| Y91E | 7.1 | 15.7 | 3.5 | 3.7 |
| A132E | 7.0 | 16.2 | 3.4 | 3.7 |
| L38E | 6.8 | 17.3 | 3.1 | 7.3 |
| T41E | 6.8 | 17.3 | 3.1 | 8.2 |
| A90E | 6.4 | 20.1 | 2.6 | 4.0 |
| L37E | 5.2 | 38.1 | 1.0 | 9.1 |
| G20E | 4.5 | - | - | 8.2 |
| N118E | 4.5 | - | - | 9.9 |
*Apparent pKa values. Estimated error was 0.2 for all but Glu-37 and Glu-90, which have an estimated error of 0.5.
†Apparent dielectric constant, calculated with equation 3 in Dwyer et al (10) using 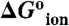 , rion = 2 Å and rprot = 12 Å.
, rion = 2 Å and rprot = 12 Å.
‡Calculated as 1.36 ∗ (pKa - pKa, mod), assuming a pKa, mod of 4.5. Estimated uncertainty, based on the uncertainty in apparent pKa is between 0.2 and 0.3 kcal/mol.
The pKa values of the 23 Glu residues with perturbed pKa ranged from 5.2–9.4, with an average value of 7.7. These are some of the highest pKa values ever measured for carboxylic groups in proteins. As large as the measured shifts in pKa were, they are much smaller than what would be calculated with standard continuum electrostatics methods using a protein dielectric constant of 2 or 4 (9, 10, 17). Indeed, when the differences between the measured pKa values and the normal pKa of 4.5 for Glu in water were analyzed with a simple Born model (equation 3 in Dwyer et al. (10)), the apparent dielectric constants (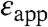 ) reported by the internal carboxylic groups ranged from 9–38 (Table 1). These apparent dielectric constants should not be confused with the dielectric constant of the protein. The apparent dielectric constants simply represent the values of the dielectric constant needed to reproduce an experimental pKa with a particular model (in this case, equation 3 in Dwyer et al (10), which is based on classical continuum electrostatic theory). The values of
) reported by the internal carboxylic groups ranged from 9–38 (Table 1). These apparent dielectric constants should not be confused with the dielectric constant of the protein. The apparent dielectric constants simply represent the values of the dielectric constant needed to reproduce an experimental pKa with a particular model (in this case, equation 3 in Dwyer et al (10), which is based on classical continuum electrostatic theory). The values of 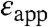 in Table 1 were calculated under the approximation that the shifts in pKa are governed solely by the dehydration of the buried charged groups and that the ionizable groups are buried infinitely far from bulk water. These
in Table 1 were calculated under the approximation that the shifts in pKa are governed solely by the dehydration of the buried charged groups and that the ionizable groups are buried infinitely far from bulk water. These 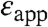 are approximate and model dependent; however, they are quite robust: the values of
are approximate and model dependent; however, they are quite robust: the values of 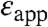 obtained with other continuum dielectric models are comparable to the values listed in Table 1. The significance of
obtained with other continuum dielectric models are comparable to the values listed in Table 1. The significance of 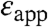 values is that they illustrate that the apparent polarizability reported by all internal Glu residues is high. Even the lowest values of
values is that they illustrate that the apparent polarizability reported by all internal Glu residues is high. Even the lowest values of 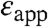 of 9.2, reported by Glu-104, already constitutes a high dielectric constant, comparable to that of a highly polarizable material. These results are consistent with earlier experimental studies with SNase that suggested that the protein interior can behave as a material with a high dielectric constant (9, 10, 17). Calculations based on MD simulations have also shown that the dielectric properties inside a protein can be comparable to the values of
of 9.2, reported by Glu-104, already constitutes a high dielectric constant, comparable to that of a highly polarizable material. These results are consistent with earlier experimental studies with SNase that suggested that the protein interior can behave as a material with a high dielectric constant (9, 10, 17). Calculations based on MD simulations have also shown that the dielectric properties inside a protein can be comparable to the values of 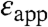 listed in Table 1 (21–23). More recently, Freed and coworkers have pointed out that high apparent dielectric constants inside proteins can also result when dielectric saturation effects for the reference state (i.e., a model Glu in water) are ignored in the calculations (24). A rigorous computational study of the dielectric effects that determine the ionization properties of the internal Glu residues in SNase is underway in our laboratory.
listed in Table 1 (21–23). More recently, Freed and coworkers have pointed out that high apparent dielectric constants inside proteins can also result when dielectric saturation effects for the reference state (i.e., a model Glu in water) are ignored in the calculations (24). A rigorous computational study of the dielectric effects that determine the ionization properties of the internal Glu residues in SNase is underway in our laboratory.
The structural and physical factors that govern the pKa values of internal groups are not well understood. The wide range of pKa values measured with the 25 internal Glu residues in SNase suggests that these factors differ significantly from location to location within the protein. No obvious correlation was observed between the magnitude of the shift in pKa and the location of the ionizable group (Fig. 2B), nor with various structural metrics, such as distance to polar or charged groups, depth of burial, etc. The pKa of Glu-118 and Glu-20 are normal or lower than normal, which was not surprising: Gly-20 is at a surface β-turn; therefore, Glu-20 is probably in bulk water. Glu-118 replaces Asn-118; therefore, in all likelihood its microenvironment is already adapted to accept polar groups. In contrast to these two cases, the pKa values of several of the other Glu residues are shifted by almost five pKa units; these are among the largest shifts in pKa values measured experimentally. The shifts in most pKa values were sufficiently large to render the majority of the Glu residues fully or at least partially neutral at pH 7. Most of the pKa values were in the range needed by naturally occurring internal carboxylic groups to facilitate H+ exchange reactions under physiological conditions.
Conformational Consequences of the Ionization of Internal Glu Residues.
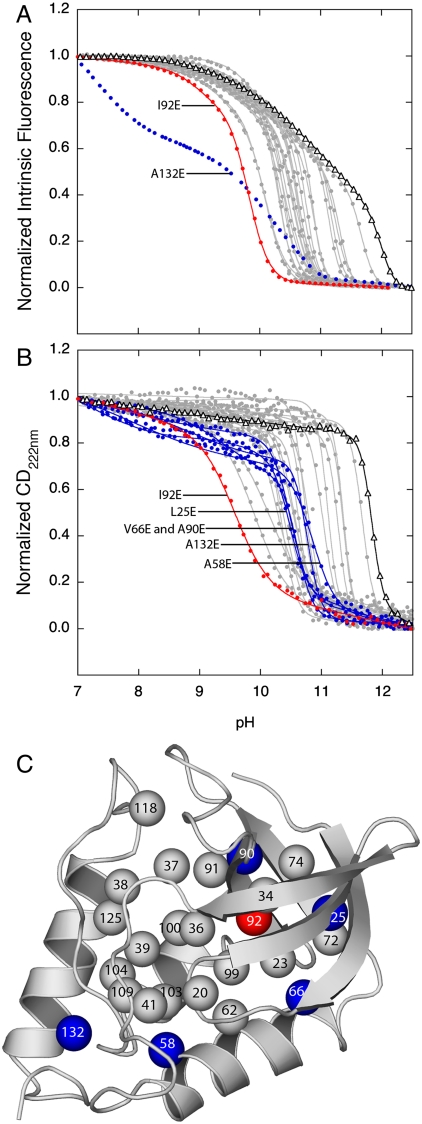
To examine effects of ionization of internal Glu residues on the conformation of the native state of the protein, we monitored H+ titrations with Trp fluorescence and far-UV CD at 222 nm over the range of pH where the internal Glu residues titrate (Fig. 3 A, B; thermodynamic parameters of base unfolding are listed in Table S2). The majority of the Glu-containing variant proteins were fully folded and native-like at pH values as high as 9.5, which corresponds to the highest pKa measured. The observation that the majority of the variants tolerated the ionization of the internal Glu residues without experiencing any detectable, global, conformational reorganization shows that charges can be tolerated in the hydrophobic interior of proteins, without the need for any specialized structural adaptations to stabilize the charge, even in a protein that did not evolve to use internal charges as part of its functional cycle. This inherent ability of proteins to withstand internal charges is consistent with the idea that the relatively hydrophobic and dehydrated interior of proteins behaves as a material with high dielectric constant. The physical and structural basis of this essential property of folded proteins is not understood and is currently under investigation in our laboratory. This property may involve the stabilization of internal charges through penetration of water into the hydrophobic core (9, 10, 13, 25), or through subtle structural rearrangement below the level of detection with optical spectroscopy (9, 14, 15, 26). Without this essential property proteins could not perform some of the most fundamental energy transduction processes essential for the living state.
Fig. 3.
Structural changes coupled to the ionization of internal Glu residues. (A) Base unfolding of variants with internal Glu monitored by Trp fluorescence. (B) Base unfolding of variants with internal Glu monitored by far-UV CD at 222 nm. The solid black line (triangle) identifies the base unfolding profile of the background protein used to engineer the Glu-containing variants. The gray lines (solid circle) represent fits of equation 1 or 2 from Karp et al (9) to obtain the midpoints of major and minor structural transitions, described in Table 1. Blue lines identify cases where predenaturational transitions suggest partial structural changes coupled to the ionization of the internal Glu. Red lines identify the case where the titration of the internal group coincides with the major unfolding transition. (C) Location of Glu residues that promote partial unfolding (blue), global unfolding (red), or no conformational reorganization coupled to ionization (gray).
Only one variant (I92E) out of the 25 that were studied was unfolded globally by the ionization of the internal Glu. The unfolding of the I92E variant is a consequence of both the high destabilization incurred by the substitution of Ile-92 with neutral Glu, and the large upward shift in its pKa*. If the pKa of Glu-92 could be measured in an even more stable form of SNase, it would, in all likelihood, be even higher than the measured value of 9.0. Five other variants (L25E, A58E, V66E, A90E, and A132E) showed a modest but clear predenaturational transition in the range of pH where the internal Glu residues became charged (Fig. 3 A, B). These pH-dependent changes in the spectroscopic signals are consistent with subtle and relatively minor conformational reorganization coupled to the ionization of the internal Glu (9, 26). Similar structural reorganization coupled to the ionization of internal groups has been reported in other proteins (27, 28). Some of the internal Glu residues in SNase that triggered local unfolding or reorganization are at the termini of elements of secondary structure, where fraying can occur (Fig. 3C). An extensive NMR spectroscopy study is underway in our laboratory to examine the extent to which high apparent dielectric constants reported by the internal Glu residues reflect local conformational reorganization.
Free Energy of Formation of Charge Inside a Protein.
At pH 7 most of the internal carboxylic groups were fully or at least partially neutral. At this pH the destabilization of the native state by substitution of internal hydrophobic groups with Glu is not necessarily the result of introduction of charge into a hydrophobic environment. Instead, it reflects the substitution of the hydrophobic group with neutral Glu and the attendant shift in pKa. The actual cost of creating negative charge at the internal positions (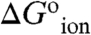 in Table 1) was calculated from the difference between the apparent pKa values in Table 1 and the normal pKa of 4.5 for Glu in water. The majority of the
in Table 1) was calculated from the difference between the apparent pKa values in Table 1 and the normal pKa of 4.5 for Glu in water. The majority of the 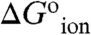 values range from 3.1–6.7 kcal/mol. These are large free energies, comparable to the net stability of many small globular proteins, but they are small compared to the cost of transfer of an ion from water into a strictly hydrophobic material with a dielectric constant in the range 2–4, calculated with a classical continuum electrostatic method (9, 10, 14, 15, 17). The relevance of continuum calculations of ion hydration remains to be established by comparison with fully microscopic simulations. Surprising results have been obtained for the hydration of ions at interfaces between regions with low and high dielectric constants, which cannot be explained with continuum electrostatics (29). A meaningful comparison of experimental
values range from 3.1–6.7 kcal/mol. These are large free energies, comparable to the net stability of many small globular proteins, but they are small compared to the cost of transfer of an ion from water into a strictly hydrophobic material with a dielectric constant in the range 2–4, calculated with a classical continuum electrostatic method (9, 10, 14, 15, 17). The relevance of continuum calculations of ion hydration remains to be established by comparison with fully microscopic simulations. Surprising results have been obtained for the hydration of ions at interfaces between regions with low and high dielectric constants, which cannot be explained with continuum electrostatics (29). A meaningful comparison of experimental 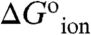 values with free energies of hydration is simply not yet possible. However, the experimental data, without any further analysis, are sufficient to suggest that the protein interior behaves as a material with high apparent polarizability, which explains why Δ+PHS nuclease was so resilient to the presence of internal charges.
values with free energies of hydration is simply not yet possible. However, the experimental data, without any further analysis, are sufficient to suggest that the protein interior behaves as a material with high apparent polarizability, which explains why Δ+PHS nuclease was so resilient to the presence of internal charges.
The detailed structural and physical origins of the high apparent polarizability reported by these internal Glu residues remains to be examined experimentally. The apparent polarizability could reflect contributions from all factors that stabilize the charged form of the internal Glu (i.e., interactions of the internal charge with surface charges, with dipoles, with the reaction field of bulk water, electronic polarization, conformational reorganization, water penetration, etc). Our data suggest that the dielectric constant of folded proteins is sufficiently low to prevent the unnecessary burial of ionizable groups, which would destabilize the folded state and perhaps promote aggregation, yet sufficiently high to allow the presence of ionizable groups and charges in internal hydrophobic environments when necessary for function. How this delicate balance is achieved remains to be explained.
Implications.
The pKa and 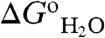 values in Table S2 and in Table S1 will be invaluable for benchmarking and critical assessment of computational methods for structure-based electrostatics calculations. The calculation of pKa values of internal ionizable groups and of electrostatic energies in the protein interior still represents a formidable challenge. These equilibrium thermodynamic parameters reflect a balance between large contributions (e.g., Coulomb effects and hydration effects) of opposite sign, each of which is difficult to calculate accurately. Our data will be useful to calibrate algorithms to improve their performance and their usefulness for examination of functional electrostatics in energy transduction processes of biological systems.
values in Table S2 and in Table S1 will be invaluable for benchmarking and critical assessment of computational methods for structure-based electrostatics calculations. The calculation of pKa values of internal ionizable groups and of electrostatic energies in the protein interior still represents a formidable challenge. These equilibrium thermodynamic parameters reflect a balance between large contributions (e.g., Coulomb effects and hydration effects) of opposite sign, each of which is difficult to calculate accurately. Our data will be useful to calibrate algorithms to improve their performance and their usefulness for examination of functional electrostatics in energy transduction processes of biological systems.
In enzymes and in proteins involved in H+ transport, the pKa values of ionizable groups that donate or accept H+ are tuned (e.g., depression of pKa for basic groups and elevation for acidic ones) to facilitate H+ transfer between them (30). Our data show that the tuning of pKa values for functional purposes does not necessarily require the evolution of dipolar cages or other specialized structural microenvironments. Although different regions in the interior of SNase appear to respond differently to the presence of negative charge, the data in Fig. 2A show that at least in this highly stable form of SNase, simply by virtue of being internal, the pKa values of internal Glu residues are shifted into the range of pKa values used by naturally occurring carboxylic groups for H+ transport and other H+-activated biological processes. Studies are underway with internal basic residues to determine the extent to which the ionization energetics of internal basic groups are comparable to those of internal acidic ones.
Thus far the results obtained with internal Glu residues suggests that the evolution of function in proteins that depend on internal ionizable groups might have been governed more by the evolution of the thermodynamic stability required to tolerate internal ionizable groups (31, 32), than by the evolution of special dynamics or structural microenvironments with high polarity or whatever other properties are necessary to tune pKa values for functional purposes. Our results also suggest a strategy for de novo design of enzymes or for the engineering of enzymes with modified function, which exploits the relationship between thermodynamic stability and the capacity of proteins to tolerate the presence of internal charges and of internal ionizable groups with shifted pKa values. In de novo enzyme design the enzyme’s intrinsic thermodynamic stability is likely to be as important a factor as the design of the actual polar or charged functional groups required to achieve a specific chemistry.
Materials and Methods
Protein Engineering.
Glu-containing variants of the Δ+PHS variant of SNase were prepared with QuickChange site-directed mutagenesis on a pET24A+ vector as described previously (9, 16). Purification was performed as described previously (33).
Thermodynamic Stability Measurements.
Stability measurements were performed with guanidinium chloride titrations using an Aviv Automated Titration Fluorimeter 105, as described previously (34). Linkage analysis of pH dependence of stability to obtain pKa values was performed as described previously (9, 10, 12).
Optical Spectroscopy.
pH titrations monitored with CD at 222 nm or with Trp fluorescence were performed with an Aviv Automated Titration Fluorimeter model 105 and with an Aviv circular dichroism spectrometer model 215, respectively. The experiments were performed following protocols published previously (34).
Supplementary Material
Acknowledgments.
This work was supported by Grant GM-RO1-061597 (to B.G.M.E.)
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004213107/-/DCSupplemental.
*The thermodynamic stability of a protein with a Glu with an elevated pKa in the native state decreases by 1.36 kcal/mol for every unit shift in the pKa.
References
- 1.Perutz MF, Kendrew JC, Watson HC. Structure and function of haemoglobin: II. Some relations betwen polypeptide chain configuration and amio acid sequence. J Mol Biol. 1965;13:669–678. [Google Scholar]
- 2.Warshel A. Energetics of enzyme catalysis. Proc Nat'l Acad Sci USA. 1978;75:5250–5254. doi: 10.1073/pnas.75.11.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrahams JP, Leslie AGW, Lutter R, Walker JE. Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- 4.Iwata S, Ostermeier C, Ludwig B, Michel H. Structure at 2.9 A resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 2002;376:660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y, et al. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 6.Doyle DA, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 7.Luecke H, Richter HT, Lanyi JK. Proton transfer pathways in bacteriorhodopsin at 2.3 A resolution. Science. 1998;280:1934–1937. doi: 10.1126/science.280.5371.1934. [DOI] [PubMed] [Google Scholar]
- 8.Pebay-Peyroula E, Rummel G, Rosenbusch JP, Landau EM. X-ray structure of bacteriorhodoopsin at 2.5 A from microcrystals grown in lipidic cubic phases. Science. 1997;277:1676–1681. doi: 10.1126/science.277.5332.1676. [DOI] [PubMed] [Google Scholar]
- 9.Karp DA, et al. High apparent dielectric constant inside a protein reflects structural reorganization coupled to the ionization of an internal Asp. Biophys J. 2007;92:2041–2053. doi: 10.1529/biophysj.106.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dwyer J, et al. High apparent dielectric constants in the interior of a protein reflect water penetration. Biophys J. 2000;79:1610–1620. doi: 10.1016/S0006-3495(00)76411-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-Moreno EB, et al. Experimental measurement of the effective dielectric in the hydrophobic core of a protein. Biophys Chem. 1997;64:211–224. doi: 10.1016/s0301-4622(96)02238-7. [DOI] [PubMed] [Google Scholar]
- 12.Stites WE, Gittis AG, Lattman EE, Shortle D. In a staphylococcal nuclease mutant the side chain of a lysine replacing valine 66 is fully buried in the hydrophobic core. J Mol Biol. 1991;221:7–14. doi: 10.1016/0022-2836(91)80195-z. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen DM, Reynald RL, Gittis AG, Lattman EE. X-ray and thermodynamic studies of staphylococcal nuclease variants I92E and I92K: insights into polarity of the protein interior. J Mol Biol. 2004;341:565–574. doi: 10.1016/j.jmb.2004.05.066. [DOI] [PubMed] [Google Scholar]
- 14.Harms MJ, et al. The pKa values of acidic and basic residues buried at the same internal location in a protein are governed by different factors. J Mol Biol. 2009;389:34–47. doi: 10.1016/j.jmb.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harms MJ, et al. A buried lysine that titrates with a normal pKa: role of conformational flexibility at the protein water interface as a determinant of pKa values. Protein Sci. 2008;17:833–845. doi: 10.1110/ps.073397708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isom DG, et al. High tolerance for ionizable residues in the hydrophobic interior of proteins. Proc Natl Acad Sci USA. 2008;105:17784–17788. doi: 10.1073/pnas.0805113105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitch CA, et al. Experimental pKa values of buried residues: analysis with continuum methods and role of water penetration. Biophys J. 2002;82:3289–3304. doi: 10.1016/s0006-3495(02)75670-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castañeda CA, et al. Molecular determinants of the pKa values of Asp and Glu residues in staphylococcal nuclease. Proteins. 2009;77:570–588. doi: 10.1002/prot.22470. [DOI] [PubMed] [Google Scholar]
- 19.Lee KK, Fitch CA, Lecomte JTJ, García-Moreno EB. Electrostatic effects in highly charged proteins: salt sensitivity of pKa values of histidines in staphylococcal nuclease. Biochemistry. 2002;41:5656–5667. doi: 10.1021/bi0119417. [DOI] [PubMed] [Google Scholar]
- 20.Matthew JB, et al. pH-dependent properties in proteins. CRC Crit Rev Biochem. 1985;18:91–197. doi: 10.3109/10409238509085133. [DOI] [PubMed] [Google Scholar]
- 21.Simonson T, Brooks CL., III Charge screening and the dielectric constant of proteins: insights from molecular dynamics. J Am Chem Soc. 1996;118:8452–8458. [Google Scholar]
- 22.Simonson T, Perahia D. Internal and interfacial dielectric properties of cytochrome c from molecular dynamics in aqueous solution. Proc Nat'l Acad Sci USA. 1995;92:1082–1086. doi: 10.1073/pnas.92.4.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith PE, Brunne RM, Mark AE, van Gunsteren WF. Dielectric properties of trypsin inhibitor and lysozyme calculated from molecular dynamics simulations. J Phys Chem-US. 1993;97:2009–2014. [Google Scholar]
- 24.Gong H, Hocky G, Freed KF. Influence of nonlinear electrostatics on transfer energies between liquid phases: charge burial is far less expensive than Born Model. Proc Natl Acad Sci USA. 2008;105:11146–11151. doi: 10.1073/pnas.0804506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlessman JL, et al. Crystallographic study of hydration of an internal cavity in engineered proteins with buried polar or ionizable groups. Biophys J. 2008;94:3208–3216. doi: 10.1529/biophysj.107.122473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karp DA, Stahley MR, García-Moreno EB. Conformational consequences of ionization of Lys, Asp, and Glu buried at position 66 in staphylococcal nuclease. Biochemistry. 2010;49:4138–4146. doi: 10.1021/bi902114m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dao-pin S, et al. Structural and thermodynamic consequences of burying a charged residue within the hydrophobic core of T4 lysozyme. Biochemistry. 1991;30:11521–11529. doi: 10.1021/bi00113a006. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Z, Sosnick TR. Protein vivisection reveals elusive intermediates in folding. J Mol Biol. 2010;397:777–788. doi: 10.1016/j.jmb.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iuchi SHC, Paesani F, Voth GA. The hydrated excess proton at water-hydrophobic interface. J Phys Chem B. 2009;113:4017–4030. doi: 10.1021/jp805304j. [DOI] [PubMed] [Google Scholar]
- 30.Gunner M, Alexov E. A pragmatic approach to structure based calculation of coupled proton and electron transfer in proteins. Biochimica et Biophysica Acta. 2000;1458:63–87. doi: 10.1016/s0005-2728(00)00060-8. [DOI] [PubMed] [Google Scholar]
- 31.Bloom JD, Labthavikul ST, Otey CR, Arnold FH. Protein stability promotes evolvability. Proc Natl Acad Sci USA. 2006;103:5869–5874. doi: 10.1073/pnas.0510098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokuriki N, Stricher F, Serrano L, Tawfik D. How protein stability and new functions trade off. PLoS Computational Biol. 2008;4:1–7. doi: 10.1371/journal.pcbi.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shortle D. Guanidine hydrochloride denaturation studies of mutant forms of staphylococcal nuclease. J Cell Biochem. 1986;30:281–289. doi: 10.1002/jcb.240300402. [DOI] [PubMed] [Google Scholar]
- 34.Whitten ST, García-Moreno EB. pH dependence of stability of staphylococcal nuclease: evidence of substantial electrostatic interactions in the denatured state. Biochemistry. 2000;39:14292–14304. doi: 10.1021/bi001015c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.