Abstract
The mechanisms by which cannabinoid receptors CB1 and CB2 modulate immune function are not fully elucidated. Critical tools for the determination of the role of both receptors in the immune system are CB1/CB2 double null mice (CB1/CB2 null), and previous studies have shown that CB1/CB2 null mice exhibit exaggerated responses to various immunological stimuli. The objective of these studies was to determine the magnitude to which CB1/CB2 null mice responded to the respiratory allergen ovalbumin (OVA) as compared with wild-type C57BL/6 mice. The authors determined that in the absence of adjuvant, both wild-type and CB1/CB2 null mice mounted a marked response to intranasally instilled OVA as assessed by inflammatory cell infiltrate in the bronchoalveolar lavage fluid (BALF), eosinophilia, induction of mucous cell metaplasia, and IgE production. Many of the endpoints measured in response to OVA were similar in wild-type versus CB1/CB2 null mice, with exceptions being modest reductions in OVA-induced IgE and attenuation of BALF neutrophilia in CB1/CB2 null mice as compared with wild-type mice. These results suggest that T-cell responses are not universally exaggerated in CB1/CB2 null mice.
Keywords: cannabinoid, CB1, CB2, allergic airway hyperresponsiveness, inflammation
Introduction
Critical roles for cannabinoid receptors CB1 and CB2 have been identified in several physiological processes, which can be characterized with CB1/CB2 single or double knockout mice. In the immune system, for instance, there are many examples of cannabinoid receptor–dependent modulation of immune function by cannabinoid ligands. Using latex-sensitized, thioglycolate-elicited peritoneal cells derived from either wild-type or CB2 null mice to induce interleukin (IL)-2 production from T cells, Δ9-THC suppressed IL-2 production only when the wild-type peritoneal cells were used as stimulators, indicating that cannabinoid-induced suppression of accessory cell immune function occurs in a CB2-dependent manner (Buckley et al. 2000). Consistent with this, Δ9-THC suppressed the in vivo antisheep red blood cell IgM antibody-forming cell (anti-SRBC IgM AFC) response, and the in vitro CD40 ligand stimulated AFC in wild-type but not CB1/CB2 null mice (Springs et al. 2008). Interestingly, in CB1/CB2 null mice, there were few differences described in the overall magnitude of immune responses to a variety of pharmacological (i.e., phorbol ester plus calcium ionophore or α-CD3/α-CD28 antibodies) and physiological stimuli (i.e., allogeneic cells, lipopolysaccharide; (Springs et al. 2008).
Although there are a number of examples in which CB1/CB2 null mice responded with similar magnitude to various immune stimuli as compared with wild-type mice (Springs et al. 2008), there are immune responses that are critically regulated by CB1 and/or CB2 in the absence of exogenous cannabinoid ligand engagement, suggesting important roles for endocannabinoids. For instance, the magnitude of the in vitro anti-sRBC IgM AFC was remarkably low in splenocytes derived from CB1/CB2 null mice as compared with wild-type mice (Kaplan, Springs, and Kaminski 2008; Springs et al. 2008). However, there is more evidence to demonstrate that the immune response is enhanced in CB1/CB2 null mice in vivo. The immune response to influenza was more robust in CB1/CB2 null mice as compared with wild-type mice, as evidenced by lower viral burden in the lungs, enhanced induction of tumor necrosis factor (TNF)-α in lungs, and increased leukocytes in bronchoalveolar lavage fluid (BALF; Buchweitz et al. 2008). Moreover, allergic contact dermatitis induced by nickel ear clips and the contact allergen 2,4-dinitrofluorobenzene (DNFB) was enhanced in CB1/CB2 null mice as compared with wild-type mice (Karsak et al. 2007).
With the demonstration that CB1/CB2 null mice exhibited enhanced allergic responses to contact allergens, the objective of the present investigation was to determine if targeted deletion of CB1 and/or CB2 would lead to enhanced pulmonary responses to sensitization and challenge with the respiratory allergen ovalbumin (OVA). OVA exposure by inhalation or airway instillation induces functional and structural airway changes consistent with allergic asthma, including airway hyperresponsiveness (AHR), development of epithelial mucous cell metaplasia (MCM) or hyperplasia, eosinophilic inflammatory influx, and increases in OVA-specific IgE levels in both blood and BALF (Ewart et al. 2000; Finkelman and Wills-Karp 2008). The manner in which mice are exposed to OVA varies widely, including ip injections, intranasal instillations, or aerosolization by nebulization, and it often includes an adjuvant for the initial sensitization. In this study, intranasal instillation of OVA in the absence of adjuvant was used, which has been previously characterized in A/J mice (Farraj et al. 2003) and eliminates the potential of artificially skewing the immune response, as has been shown for some adjuvants (Rajananthanan et al. 1999).
Materials and Methods
Mice
Female wild-type C57BL/6J (seven to eight weeks at arrival) were obtained from Jackson Laboratories (Bar Harbor, ME). CB1/CB2 null mice, which are on a C57BL/6 background, were a generous gift from Dr. Andreas Zimmer (University of Bonn, Germany) and were bred at Michigan State University (MSU). Female wild-type or CB1/CB2 null were housed at no more than five mice/cage in polycarbonate boxes and were given food (Teklad 8640 rodent chow; Harlan Teklad, Madison, WI) and water ad libitum. Rooms were maintained at 21°C to 24°C and 40% to 60% humidity with a twelve-hour light-dark cycle. All procedures were performed in accordance with guidelines set forth by the Institutional Animal Care and Use Committee at MSU.
Allergen Sensitization and Challenge
OVA was purchased from Sigma (St. Louis, MO), and the same lot number was used throughout the experiments. The OVA exposure protocol was modified from that described by Farraj et al (2003). Wild-type or CB1/CB2 null mice were anesthetized with 4% isoflurane plus 96% oxygen and received either 30 μL 1% OVA in saline or saline alone via intranasal instillation (15 μL per nostril). Mice were sensitized once per day for four days, followed by one boost at day 14 and one challenge at day 21. Mice were sacrificed at 6 or 96 hours post-challenge (Figure 1).
Figure 1.
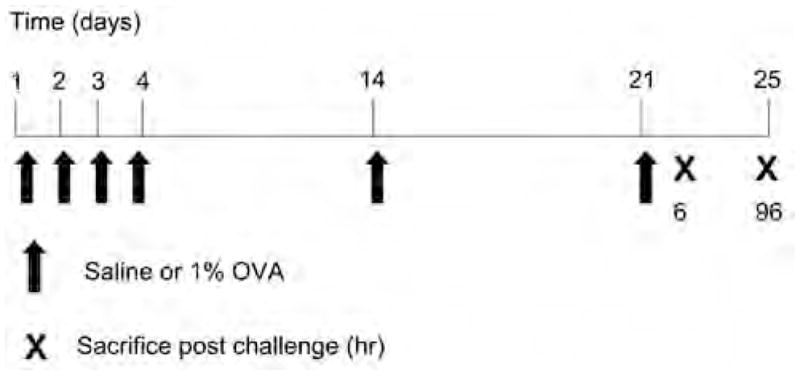
Experimental design. Mice received 1% ovalbumin by intranasal instillation for four days, boosted on day 14 and challenged on day 21. Mice were sacrificed at 6 and 96 hours postchallenge.
Necropsy, Tissue Collection, and Preparation
Mice were anesthetized with 0.1 mL 12% pentobarbital and euthanized via exsanguination of the abdominal aorta. Blood was collected into plasma separator tubes on ice (BD Medical, Franklin Lakes, NJ) and centrifuged at 3500 rpm (2536 × g) for 15 minutes. The trachea was cannulated and the heart/thymus/lung removed en bloc. The lungs were lavaged with 2 × 0.9 mL volumes of saline, and BALF cells were enumerated using a hemacytometer. BALF was then placed on a microscope slide using a Shandon 3 Cytospin (Thermo Fisher Scientific, Waltham, MA) at 600 rpm (70 × g) for 10 minutes. Following BALF collection, the four right lung lobes were ligated, removed, and placed in RNA Later (Qiagen, Valencia, CA) for subsequent RNA extraction and analysis. The left lung lobe was perfused intratracheally with 10% normal buffered formalin (NBF) at a constant pressure (30 cm H2O). After at least 1 hour, the left lung was ligated and stored in 10% NBF for subsequent histopathologic examination. The spleen was removed, and one-third of it was placed in RNA Later (Qiagen) for subsequent RNA extraction and analysis.
Determination of BALF Cellularity
Slides were stained using the Diff-Quick Stain Set (Dade Behring, Newark, DE). A minimum of 150 total cells was enumerated, and the percentage of monocytes, lymphocytes, eosinophils, or neutrophils was determined. Cells/mL for each cell type was calculated using the total cell hemacytometer count.
Preparation of Lung Sections for Histopathological Examination
After fixation, the left lobe was microdissected along the main axial airway, and two transverse tissue blocks were excised at the level of the fifth (proximal) and eleventh (distal) airway generation (G5 and G11, respectively) for further processing, as has been previously reported (Farraj et al. 2003). Tissue blocks were embedded in paraffin, and the anterior surface of each block was cut at a thickness of 5 μm. Lung sections were stained with hematoxylin and eosin for routine light microscopic examination or Alcian Blue (pH 2.5)/Periodic Acid Schiff sequence (AB/PAS) for detection of stored intraepithelial mucosubstances in the conducting airways. Other unstained and hydrated paraffin sections from the same airway locations were immuohistochemically stained for major basic protein (MBP) within eosinophils, using a polyclonal rabbit antibody directed against murine MBP (generously provide by Dr. Jamie Lee, Mayo Clinic, Scottsdale, AZ) and as previously described in detail (Farraj et al. 2003). Those cells that stained positively and exhibited distinct eosinophilic morphology were enumerated. The length of the lamina propria was calculated using morphometric techniques as previously described (Wagner et al. 2007).
Quantification of Stored Intraepithelial Mucosubstances in Pulmonary Airways
The amount of stored mucosubstances in the surface epithelium lining the proximal and distal axial airways in the lung, airway generations 5 and 11, respectively, were estimated by measuring the volume densities of AB/PAS-stained mucosubstances using computerized image analysis and standard morphometric techniques previously reported (Farraj et al. 2003). The area of AB/PAS-stained mucosubstances was calculated by circumscribing the perimeter of the stained material using the Scion Image program (Scion Corporation, Frederick, MD). The length of the basal lamina underlying the surface epithelium was calculated from the contour length of the digitized image of the basal lamina. The volume of stored mucosubstances (volume density [Vs]) per unit of surface area was estimated using the method described in detail by Harkema et al. (1987).
Quantitation of Airway Eosinophils
The severity of the influx of eosinophils was estimated by dividing the number of eosinophils in the subepithelial interstitium of the axial airway wall (airway generation 5 and 11, respectively) by the length of the corresponding basal lamina. Eosinophils were identified by their morphologic features (i.e., slightly larger than neutrophils with bilobed nuclei) and by the positive immunohistochemical staining for MBP in the large cytoplasmic granules (dark pink-red granules).
RNA Extraction and Analysis
Right lung lobes or spleen that had been stored in RNA Later were weighed and placed in RLT buffer (Qiagen) containing 1% 2-mercaptoethanol (600 μL buffer for every 30 mg tissue). The lungs were then homogenized using a Pro 250 tissue homogenizer (Pro Scientific, Oxford, CT), centrifuged at 12,000 × g for 4 minutes and stored at −80°C. RNA extraction was performed using the RNeasy RNA Isolation Kit from Qiagen according to the manufacturer’s instructions. All samples were treated with DNase using the RNase-free DNase Set (Qiagen) during the total RNA isolation. Real-time polymerase chain reaction (PCR) was performed using a 7900 HT Thermocycler (Applied Biosystems, Foster City, CA). All Taqman primers and pairs were purchased from Applied Biosystems using FAM-MGB probes. Assay IDs were as follows: IL-2, Mm00434256_m1; IL-4, Mm00445259_m1; interferon-γ, Mm00801778_m1; IL-12p40, Mm01288992_m1; IL-5 and IL-13 were from predeveloped assay reagents (catalog numbers 4329591E and 4333916F, respectively). Quantification of fold change was calculated using the ΔΔCt method (Livak and Schmittgen 2001) with 18S (VIC-MGB probe) as the control. Additional calculations for PCR can be found in the Statistical Analysis section.
Genomic DNA Extraction and Analysis
Genomic DNA was isolated from mouse tails using the Wizard Genomic DNA Purification Kit according to the manufacturer’s protocol (Promega, Madison, WI). A total of 10 ng of isolated DNA was assayed in a total volume of 20 μL in a real-time PCR reaction using standard amplification procedures (50°C for 2 minutes, 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute) with a 7900 HT Thermocycler (Applied Biosystems). The presence of CB1 was determined using CNR1 stock primers from Applied Biosystems. The presence of CB2 was determined using primers designed by Applied Biosystems according to known information on the CB2 deletion (Buckley et al. 2000): CB2 forward 5′-CCTGATAGGCTGGAAGAAGTATCTAC-3′, CB2 reverse 5′-ACATCAGCCTCTGTTTCTGTAACC-3′.
Enzyme-Linked Immunosorbent Assay
OVA-specific IgG1 and IgE antibody levels were determined as previously described using a modified enzyme-linked immunosorbent assay (ELISA) with OVA as coating antigen (Birmingham et al. 2003). Previous optimization of coating antigen concentration showed that high levels of IgG did not interfere with IgE determination and that IgG depletion using protein G was not necessary with this method. Blood was collected after the day 14 boost and at necropsy. Blood was separated from plasma and subsequently used in ELISA (1:10 dilution for IgE and 1:1,000 dilution for IgG1).
Statistical Analysis
The mean ± standard error was determined for each treatment group. Differences between means were determined with a two-way analysis of variance. When significant differences were detected, treatment groups were compared using Bonferroni’s test. For PCR data, Grubb’s outlier test was performed for each treatment group using Delta Ct (Ct target gene−Ct 18S). In addition, fold-change values were transformed using ln (fold change +1) prior to statistical analysis. Statistical analyses were performed using GraphPad Prism version 4.0a for Macintosh OSX, GraphPad software (San Diego, CA).
Results
Verification of Genotype
Real-time PCR was performed on genomic DNA isolated from the tails of each mouse to verify the genotype. The average Ct value for CB1 and CB2 in the wild-type mice was 28.44 ± 1.52 and 28.25 ± 0.74, respectively. All Ct values for both genes in the CB1/CB2 null mice were below the level of quantifiable product and were reported as undetermined.
Effect of OVA Sensitization and Challenge on Inflammatory Cell Infiltration in BALF
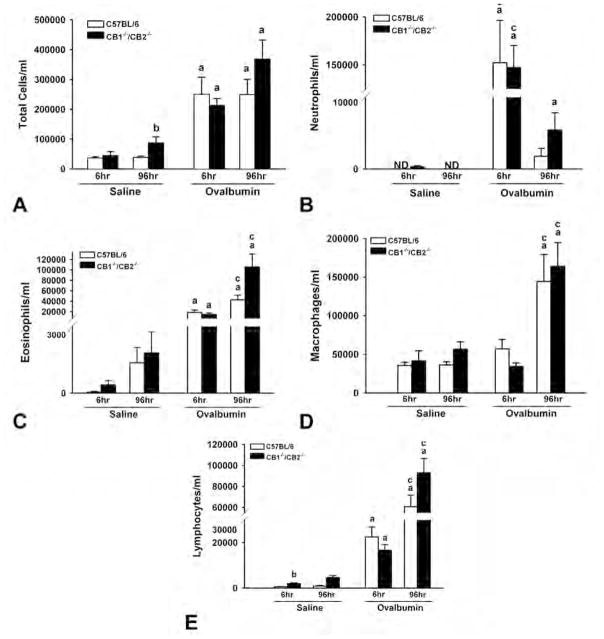
Although it has been reported that C57BL/6 mice are relatively resistant to OVA-induced AHR as compared with other TH2-biased mouse strains (Ewart et al. 2000), intranasal sensitization and challenge with OVA induced marked inflammatory cell infiltration in BALF in both wild-type and CB1/CB2 null mice (Figure 2A). Total inflammatory cells were increased at 6 and 96 hours following OVA challenge, with neutrophils being the predominant cell type observed in the BALF at 6 hours (Figure 2B). By 96 hours, the cellular composition in the BALF was comprised mainly of monocytes, lymphocytes, and eosinophils (Figures 2C–E). Overall, the inflammatory cell infiltrate in BALF was strikingly similar between wild-type and CB1/CB2 null mice. One exception was a small difference in neutrophils at 96 hours post–OVA challenge, suggesting the possibility that the kinetics of neutrophil infiltration or resolution are different in CB1/CB2 null mice.
Figure 2.
Ovalbumin (OVA)-induced inflammatory cells in the bronchoalveolar lavage fluid. Lungs from saline- or OVA-treated wild-type and CB1/CB2 null mice were washed twice with saline, and differential staining of cell types was performed. Cells were enumerated using a 40× objective. (A) Total. (B) Neutrophils. (C) Eosinophils. (D) Macrophages. (E) Lymphocytes. (a) p < .05 versus respective saline. (b) p < .05 treatment-matched genotype difference. (c) p < .05 treatment-matched time difference.
Pulmonary Histopathology
No histopathology was found in the lungs of saline-instilled wild-type or CB1/CB2 null mice. In contrast, 6 hours after OVA challenge, the principal morphologic alteration in the lungs of both wild-type and CB1/CB2 null mice was a moderate-marked mixed inflammatory influx centered around conducting airways and pulmonary vessels. The inflammatory response was most severe in the more proximal lung section near the hilus of the lung lobe (G5 tissue section). This mixed inflammatory cell response consisted primarily of neutrophils intermixed with large and small lymphocytes. Eosinophils were also present but in lesser numbers. Inflammatory infiltrates were most conspicuous in the interstitial spaces surrounding (1) the conducting airways (axial, preterminal, and terminal bronchioles) and (2) interstitial tissues surrounding pulmonary veins in the alveolar parenchyma and pulmonary arteries adjacent to conducting airways. There was also prominent pavemaking of inflammatory cells along the endothelial surfaces of these vessels. Some extension of the inflammatory cells in the alveolar septa and airspaces immediately surrounding the large and small conducting airways was also present. No obvious morphologic differences in the character or severity of the OVA-induced inflammatory response between wild-type and CB1/CB2 null mice were histologically evident at this early postchallenge time point. In addition, no changes in airway epithelium were associated with this early inflammatory response to OVA challenge.
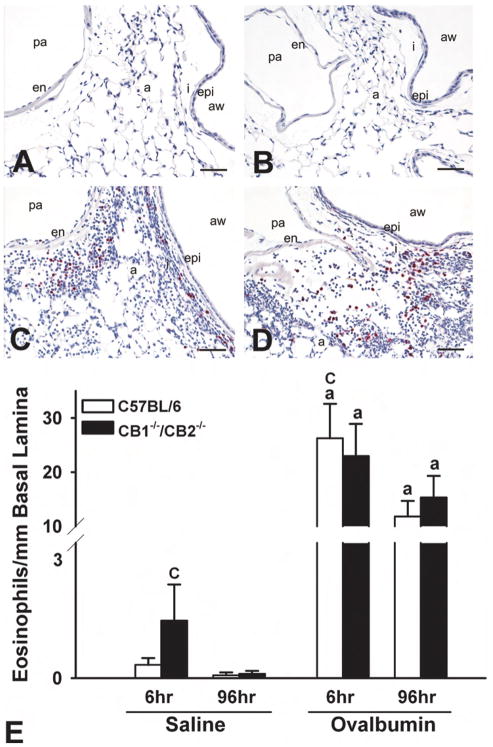
At 96 hours after OVA challenge, the periairway and perivascular distribution of the inflammatory cell influx was very similar to that observed in similar treated mice that were sacrificed at 6 hours postchallenge. The cellular makeup of inflammatory response, however, was different. At this later time point, the mixed inflammatory cell infiltrates were mainly composed of eosinophils and mononuclear cells (lymphocytes and plasma cells) and lesser numbers of neutrophils (Figure 3A–D). In addition, there was an associated MCM in the surface epithelium lining the large-diameter conductive airways (axial and preterminal bronchioles) that was most evident in the more proximal G5 lung lobe section compared with the more distal G11 section. Numerous AB/PAS-positive mucous cells, normally devoid in bronchiolar epithelium of mice, characterized this metaplastic change in the airway epithelium at 96 hours after OVA challenge (Figure 4A–D). This airway epithelial change was not present in saline-instilled control mice sacrificed at this similar time point after challenge. Like with the inflammatory changes induced by OVA, the character and severity of the OVA-induced MCM in airway epithelium was similar in wild-type and CB1/CB2 null mice.
Figure 3.
Ovalbumin (OVA)-induced eosinophilia in the basal lamina. (A–D) Photomicrographs of respiratory epithelium at G5 stained with major basic protein (MBP) at 96 hours postchallenge. (A) C57BL/6 sal. (B) CB1/CB2 null sal. (C) C57BL/6 OVA. (D) CB1/CB2 null OVA. (E) Enumeration of MBP+ eosinophils in the basal lamina at 6 and 96 hours postchallenge. (a) p < .05 versus respective saline. (b) p < .05 treatment-matched genotype difference. (c) p < .05 treatment-matched time difference. a = alveoli; aw = airway; en = endothelium; epi = epithelium; I = interstitium; pa = pulmonary artery.
Figure 4.
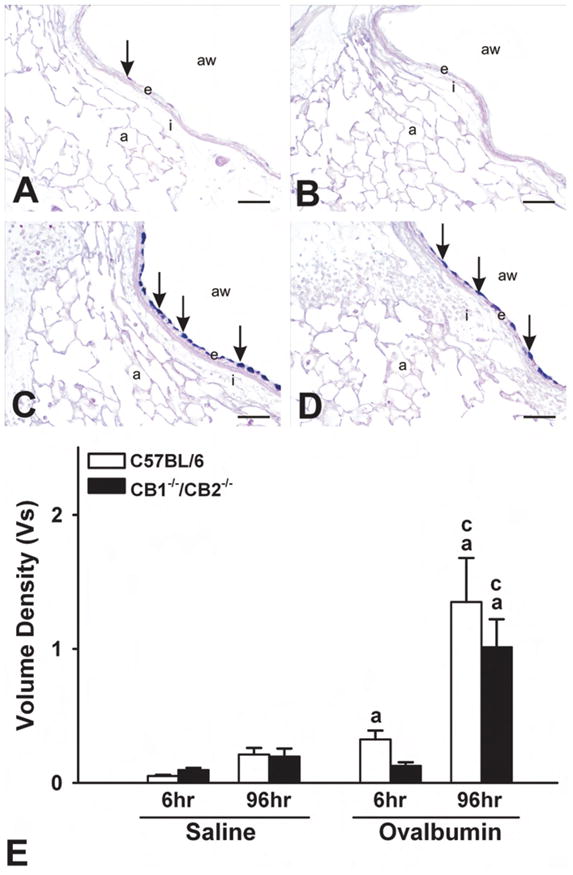
Ovalbumin (OVA)-induced mucous cell metaplasia. (A–D) Photomicrographs of respiratory epithelium at G5 stained with Alcian Blue (pH 2.5)/Periodic Acid Schiff sequence at 96 hours postchallenge. (A) C57BL/6 sal. (B) CB1/CB2 null sal. (C) C57BL/6 OVA. (D) CB1/CB2 null OVA. (E) Enumeration of AB-PAS+ epithelial cells at 6 and 96 hours postchallenge. (a) p < .05 versus respective saline. (b) p < .05 treatment-matched genotype difference; (c) p < .05 treatment-matched time difference. a = alveoli; aw = airway; e = endothelium; I = interstitium. Arrows indicate positive staining of stored intraepithelial mucosubstances.
Effect of OVA Sensitization and Challenge on Eosinophilic Infiltration in the Lamina Propria
Based on the identification of an inflammatory infiltrate in the lungs of wild-type and CB1/CB2 null mice following exposure to OVA, the number of eosinophils surrounding the proximal axial airway (G5) was quantified. Eosinophils were increased in the periairway interstitial tissue within 6 hours and persisted for 96 hours, regardless of genotype (Figure 3A–B). The small increase in the saline group was due to a modest response in a few mice.
Effect of OVA Sensitization and Challenge on MCM
One of the hallmarks of allergic AHR to OVA is mucous cell metaplasia in the airway epithelium (Blyth et al. 1996; Kuperman et al. 1998; Zuhdi Alimam et al. 2000). In response to intranasally instilled OVA, there was an increase in stored mucosubstances in the airway epithelium at G5 in both wild-type and CB1/CB2 null mice (Figure 4A–B). There were no significant differences in the amount of intraepithelial mucosubstances in the airways between genotypes.
Effect of OVA Sensitization and Challenge on Cytokine mRNA Expression
OVA-induced allergic AHR can also be characterized by induction of various inflammatory cytokines, including TH2-associated cytokines IL-4, -5, and -13. IL-4, -5, and -13 were all robustly induced in the lungs following OVA sensitization and challenge. Comparing CB1/CB2 null mice with wild-type mice, IL-4 and IL-13 were not differentially induced by OVA, and IL-5 induction was only modestly different between wild-type and CB1/CB2 null mice (Table 1). Since there was no clear trend of modulation in TH2-associated cytokines in response to OVA between genotypes, TH1-associated cytokines were also examined, and again, there was no clear trend of modulation in TH1-associated cytokines in response to OVA between genotypes. The lack of induction of IL-4 in the spleen of either genotype provides more evidence that intranasal instillation of OVA induced a local response (Table 1). Moreover, there was no induction of cell surface CD40 ligand on CD4+ T cells in response to OVA in splenocytes regardless of genotype (data not shown).
Table 1.
OVA-Induced Cytokine mRNA Levels
| T Subset | Cytokine Gene | C57BL/6 Saline | CB1−/−/CB2−/− Saline | C57BL/6 OVA | CB1−/−/CB2−/− OVA |
|---|---|---|---|---|---|
| TH2 | IL-4 | 1.10 ± 0.22 | 1.07 ± 0.21 | 22.29 ± 5.62a | 43.02 ± 12.62a |
| IL-5 | 1.04 ± 0.13 | 0.63 ± 0.06 | 9.85 ± 3.08a | 4.07 ± 1.90a,b | |
| IL-13 | 1.25 ± 0.35 | 0.47 ± 0.03 | 27.69 ± 8.45a | 10.83 ± 3.72a | |
| IL-4 (spleen) | 1.59 ± 0.58 | 0.43 ± 0.11 | 0.53 ± 0.12 | 1.06 ± 0.71 | |
| TH1 | IFN-γ | 1.03 ± 0.09 | 0.41 ± 0.07 | 11.18 ± 2.59a | 5.04 ± 0.79a,b |
| IL-12 p40 | 1.02 ± 0.09 | 0.39 ± 0.06b | 1.47 ± 0.32 | 1.14 ± 0.18a | |
| IL-2 | 1.02 ± 0.09 | 0.76 ± 0.14 | 5.64 ± 0.95a | 5.62 ± 0.68a |
Total RNA was isolated from four right lung lobes of saline- or ovalbumin (OVA)-treated wild-type or CB1/CB2 null mice. The values represent the mean fold induction of mRNA Levels ± standard error for at least five determinations per treatment group as compared with the C57BL6 saline group. IL = interleukin; IFN = interferon.
p < .05 versus respective saline.
p < .05 treatment-matched genotype difference.
Effect of OVA Sensitization and Challenge on Plasma IgG1 and IgE
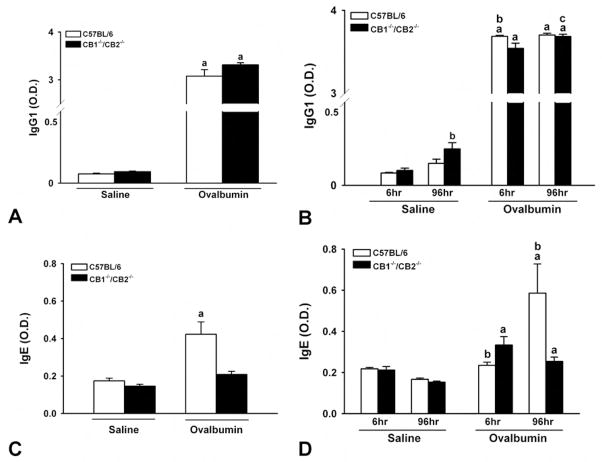
Induction of OVA-specific antibody production correlates with OVA-induced AHR. IgG1 and IgE plasma levels were evaluated during OVA exposure (at day 17, after boost 1, prior to challenge) and at necropsy (6 and 96 hours post–OVA challenge). Although OVA-specific IgG1 was induced significantly by day 17 and levels were maintained at 6 and 96 hours post–OVA challenge, there was no difference between wild-type and CB1/CB2 mice (Figure 5A, C). Higher dilutions of the plasma were also performed, which lowered the O.D. values but did not alter the outcome. However, OVA-specific IgE was induced only in wild-type mice at day 17 and to a greater degree in wild-type mice at 6 and 96 hours postchallenge. Plasma derived from CB1/CB2 null mice demonstrated a modest induction of OVA-specific IgE at 6 and 96 hours post-challenge (Figure 5B, D).
Figure 5.
Ovalbumin (OVA)-induced specific IgE and IgG1. Plasma was collected from saline- or OVA-treated wild-type or CB1/CB2 null mice on day 17 (A, C) or at necropsy (B, D). OVA-specific plasma IgE and IgG1 were enumerated by enzyme-linked immunosorbent assay. Plasma was diluted 1:1,000 for IgG1; further dilution to 1:10,000 did not alter the outcome. (a) p < .05 versus respective saline. (b) p < .05 treatment-matched genotype difference. (c) p < .05 treatment-matched time difference.
Discussion
The objective of this study was to address the role of CB1 and CB2 in a model of OVA-induced allergic AHR. In light of previous observations in which CB1/CB2 null mice exhibited enhanced responses to influenza virus or contact allergens (Buchweitz et al. 2008; Karsak et al. 2007), it was hypothesized that CB1/CB2 null mice would also exhibit exacerbated responses to OVA sensitization and challenge as compared with wild-type mice. Although many of the OVA-induced airway responses were similar in CB1/CB2 null mice and wild-type mice using an OVA sensitization and challenge model in the absence of adjuvant, there was a modest reduction in IgE in CB1/CB2 null mice as compared with wild-type mice.
The current OVA dosing paradigm is based on a model previously established in our laboratory using intranasal adjuvant-free OVA to induce AHR in A/J mice (Farraj et al. 2003). As compared with A/J mice, C57BL/6 mice, which are the background strain for the CB1/CB2 null mice, exhibited a lesser but measurable immune reaction to OVA challenge (Brewer, Kisselgof, and Martin 1999; Ewart et al. 2000; Tomkinson et al. 2001; Whitehead et al. 2003). This is likely due, in part, to the TH2 bias that has been observed with some strains, such as A/J. Indeed, we also observed lower immune responses for mucus production and airway cell infiltration in C57BL/6 wild-type and CB1/CB2 null mice as compared with our previously established model in A/J mice (Farraj et al. 2003). Although the airway-associated immune responses are more modest in magnitude in C57BL/6 mice as compared with other strains, our results do suggest that this OVA dosing paradigm induces a measurable immune response in a low-responding strain. The results further suggest that the use of 1% OVA is an appropriate dose for adjuvant-free intranasal instillation of OVA in C57BL/6 mice.
The use of intranasal instillation as the sole route of aeroallergen exposure closely mimics the natural route of exposure of agents known to induce asthma-like symptoms. The use of adjuvants in combination with ip administration of the allergen, a more commonly used paradigm of exposure, altered the type of immune response typically elicited by an aeroantigen as well as induced a basal systemic response (Tomkinson et al. 2001). In the present study, the immune response to OVA remained localized within the airways as no observable markers of immune reactivity were observed in the spleen, including induction of IL-4 mRNA and CD40 ligand cell surface expression. Although increased levels of IgE were detected in the plasma of OVA-challenged CB1/CB2 null and wild-type mice, the presence of IgE in the plasma was not unexpected since the lung is a highly perfused organ.
IgE production is one of several hallmarks of an allergic airway response to OVA, which also includes MCM and airway eosinophilia (Ewart et al. 2000). When comparing CB1/CB2 null and wild-type mice, there was no difference in OVA-induced MCM or the influx of airway eosinophils, indicating that neither CB1 nor CB2 plays a critical role in these responses. However, plasma OVA-specific IgE was significantly induced to a greater degree in wild-type mice as compared with CB1/CB2 null mice both at day 17 postboost and at 96 hours post-challenge. The decreased IgE in CB1/CB2 null mice was consistent with previous results showing that the synthetic cannabinoid CP55940 induced IgE in IL-4 plus anti-CD40–stimulated murine B cells in a CB2-dependent manner (Agudelo et al 2008).
While the difference in OVA-induced IgE between wild-type and CB1/CB2 null mice did not correlate with OVA-induced TH1- or TH2-associated cytokine mRNA levels, it is notable that cytokine profiles were assessed in lung tissue early after OVA challenge. With the sole focus on lung tissue, it is not possible to discern if there is an overall TH1/TH2 imbalance in CB1/CB2 null mice, which is suggested by observations that cannabinoids are capable of modulating the TH1/TH2 balance (Newton, Klein, and Friedman 1998; Yuan et al. 2002).
An additional role for CB1 and/or CB2 was elucidated from examination of BALF cellularity. Differential cell counts in the BALF showed similar elevations of eosinophils, lymphocytes, and macrophages in CB1/CB2 null and wild-type mice in response to OVA challenge. However, there was an increased number of neutrophils in the BALF at 96 hours postchallenge in the CB1/CB2 null mice, suggesting either that there are more neutrophils in general in the CB1/CB2 null mice or that the resolution of the early neutrophilic infiltrate at 6 hours postchallenge in the wild-type mice did not occur to the same degree in the CB1/CB2 null mice. The exact mechanism for the increase is not known but is consistent with our previous study using influenza in which the CB1/CB2 null mice had modest increases in neutrophils in the BALF on day 7 postinfection (Buchweitz et al. 2008). Overall, it is noteworthy that although this was not an exhaustive kinetic study, the trend of early neutrophilia with subsequent recruitment of eosinophils was observed and is consistent with other reports of OVA-induced AHR, including those conducted in the presence of adjuvant (Gleich, Adolphson, and Leiferman 1993; Tomkinson et al. 2001).
With the exception of the enhanced neutrophilia and decreased IgE production in CB1/CB2 null mice, other measured endpoints in response to the OVA-induced AHR were remarkably similar in CB1/CB2 null as compared with wild-type mice. These results are in contrast with the enhanced immune responses to nickel ear clips and the contact sensitizer DNFB observed in CB1/CB2 null mice (Karsak et al. 2007). There are several differences between the DNFB contact sensitizer study and the present study. First, the OVA-induced immune response is predominantly a Type I hypersensitivity response involving the production of allergen-specific antibodies, whereas the nickel- and DNFB-induced immune responses are predominantly Type IV hypersensitivity reactions, which is a cell-mediated immune response. For instance, the sensitization and functional end line responses are different in the two responses: sensitization for DNFB involves Langerhans cells presenting antigen to TH1 cells (Gober and Gaspari 2008), whereas OVA involves airway dendritic cells presenting antigen to TH2 cells (Lambrecht, Hoogsteden, and Pauwels 2001). Although speculative, one hypothesis is that CB1 and/or CB2 is critical for Langerhans cell function but not airway dendritic cells. Alternatively, but consistent with a differential effect of CB1 and/or CB2 on different populations of antigen presenting cells (APC), there may be compensatory mechanisms allowing for the presentation of OVA-derived antigens but not for the skin-localized Langerhans cells in CB1/CB2 null mice. A critical role for CB2 has also been shown in an oxazolone model of contact dermatitis (Oka et al. 2006).
There is additional evidence for a putative role for CB1 and/or CB2 for APC function, particularly since macrophages and other APC express relatively high levels of CB1 and CB2 (Galiegue et al. 1995). For example, there was a similar magnitude in the response to sheep erythrocyte antigen between CB1/CB2 null and wild-type mice in vivo (Springs et al. 2008). However, in vitro studies with sheep erythrocyte antigen showed a reduction of B-cell antibody–forming cell response in splenocytes derived from CB1/CB2 null and wild-type mice (Springs et al. 2008). Data from the same study using the polyclonal B-cell activator CD40 ligand in vitro demonstrated a similar magnitude of response between genotypes, suggesting that APC, either B cells or macrophages, may be critically regulated by CB1 and/or CB2. Furthermore, several other studies have demonstrated a critical role for CB1 and/or CB2 in APC function (Buckley et al. 2000; Lu et al. 2006; Wacnik et al. 2008).
Studies using CB1/CB2 null mice are critical to understand the role of CB1 and CB2 in physiological processes. While one disadvantage of this model is the need to further characterize the role of the individual receptors when differences are detected, advantages of this model over those conducted with cannabinoid receptor antagonists in vivo are that cannabinoid receptor antagonists can exhibit inverse agonism, partial agonism, or off-target (i.e., non–CB1-, non–CB2-mediated) effects (Batkai et al. 2004; Krylatov et al. 2005; Landsman et al. 1997; Landsman et al. 1998; Portier et al. 1999; Rao and Kaminski 2006; Rhee and Kim 2002; Shire et al. 1999). It is also important to emphasize that these studies were not exhaustive kinetic analyses, nor did they address all endpoints, such as innate responses to OVA, AHR, or evaluation of T-cell responses in tracheobronchial lymph nodes. It is conceivable that additional differences in OVA-induced immune responses could be detected with alternative endpoints or using cells derived from various microenvironments. Moreover, these studies were conducted in the absence of exogenous ligands, which could highlight genotype differences. For example, in response to influenza, Δ9-THC suppressed influenza-induced inflammation in wild-type mice but greatly enhanced influenza-induced inflammation in CB1/CB2 null mice (Buchweitz et al. 2008). In summary, there were a few differences in the response to OVA in wild-type versus CB1/CB2 null mice, including decreased IgE production and an attenuation of neutrophilic resolution from the BALF at later time points in the CB1/CB2 null mice, suggesting that not all T-cell responses are not universally exaggerated in CB1/CB2 null mice.
Acknowledgments
This work was supported in part by the National Institutes of Health, National Institute on Drug Abuse (DA 07908), and the authors acknowledge funding from the MSU Respiratory Research Initiative (to B.L.F.K., N.E.K., and J.R.H.). The authors would like to thank Mrs. Lori Bramble, Mr. Robert Crawford, Ms. Lauren Topper, and Mr. Ryan Lewandowski for excellent technical assistance and Mrs. Kimberly Hambleton for assistance with submission of the manuscript.
Abbreviations
- AB/PAS
Alcian Blue (pH 2.5)/Periodic Acid Schiff sequence
- AHR
airway hyperresponsiveness
- APC
antigen presenting cells
- BALF
bronchoalveolar lavage fluid
- DNFB
2,4-dinitrofluorobenzene
- ELISA
enzyme-linked immunosorbent assay
- IFN
interferon
- IL
interleukin
- MBP
major basic protein
- MCM
mucous cell metaplasia
- MSU
Michigan State University
- NBF
normal buffered formalin
- OVA
ovalbumin
- PCR
polymerase chain reaction
- TNF
tumor necrosis factor
Footnotes
For reprints and permissions queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav.
References
- Agudelo M, Newton C, Widen R, Sherwood T, Nong L, Friedman H, Klein TW. Cannabinoid receptor 2 (CB2) mediates immunoglobulin class switching from IgM to IgE in cultures of murine-purified B lymphocytes. J Neuroimmune Pharmacol. 2008;3:35–42. doi: 10.1007/s11481-007-9088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batkai S, Pacher P, Jarai Z, Wagner JA, Kunos G. Cannabinoid antagonist SR-141716 inhibits endotoxic hypotension by a cardiac mechanism not involving CB1 or CB2 receptors. Am J Physiol Heart Circ Physiol. 2004;287:H595–600. doi: 10.1152/ajpheart.00184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham N, Payankaulam S, Thanesvorakul S, Stefura B, HayGlass K, Gangur V. An ELISA-based method for measurement of food-specific IgE antibody in mouse serum: an alternative to the passive cutaneous anaphylaxis assay. J Immunol Methods. 2003;275:89–98. doi: 10.1016/s0022-1759(03)00008-5. [DOI] [PubMed] [Google Scholar]
- Blyth DI, Pedrick MS, Savage TJ, Hessel EM, Fattah D. Lung inflammation and epithelial changes in a murine model of atopic asthma. Am J Respir Cell Mol Biol. 1996;14:425–38. doi: 10.1165/ajrcmb.14.5.8624247. [DOI] [PubMed] [Google Scholar]
- Brewer JP, Kisselgof AB, Martin TR. Genetic variability in pulmonary physiological, cellular, and antibody responses to antigen in mice. Am J Respir Crit Care Med. 1999;160:1150–56. doi: 10.1164/ajrccm.160.4.9806034. [DOI] [PubMed] [Google Scholar]
- Buchweitz JP, Karmaus PW, Williams KJ, Harkema JR, Kaminski NE. Targeted deletion of cannabinoid receptors CB1 and CB2 produced enhanced inflammatory responses to influenza A/PR/8/34 in the absence and presence of Delta9-tetrahydrocannabinol. J Leukoc Biol. 2008;83:785–96. doi: 10.1189/jlb.0907618. [DOI] [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, Glass M, Zimmer A. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2000;396:141–49. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- Ewart SL, Kuperman D, Schadt E, Tankersley C, Grupe A, Shubitowski DM, Peltz G, Wills-Karp M. Quantitative trait loci controlling allergen-induced airway hyperresponsiveness in inbred mice. Am J Respir Cell Mol Biol. 2000;23:537–45. doi: 10.1165/ajrcmb.23.4.4199. [DOI] [PubMed] [Google Scholar]
- Farraj AK, Harkema JR, Jan TR, Kaminski NE. Immune responses in the lung and local lymph node of A/J mice to intranasal sensitization and challenge with adjuvant-free ovalbumin. Toxicol Pathol. 2003;31:432–47. doi: 10.1080/01926230390213766. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Wills-Karp M. Usefulness and optimization of mouse models of allergic airway disease. J Allergy Clin Immunol. 2008;121:603–6. doi: 10.1016/j.jaci.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gleich GJ, Adolphson CR, Leiferman KM. The biology of the eosinophilic leukocyte. Annu Rev Med. 1993;44:85–101. doi: 10.1146/annurev.me.44.020193.000505. [DOI] [PubMed] [Google Scholar]
- Gober MD, Gaspari AA. Allergic contact dermatitis. Curr Dir Autoimmun. 2008;10:1–26. doi: 10.1159/000131410. [DOI] [PubMed] [Google Scholar]
- Harkema JR, Plopper CG, Hyde DM, St George JA. Regional differences in quantities of histochemically detectable mucosubstances in nasal, paranasal, and nasopharyngeal epithelium of the bonnet monkey. J Histochem Cytochem. 1987;35:279–86. doi: 10.1177/35.3.2434556. [DOI] [PubMed] [Google Scholar]
- Kaplan BL, Springs AE, Kaminski NE. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT) Biochem Pharmacol. 2008;76:726–37. doi: 10.1016/j.bcp.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsak M, Gaffal E, Date R, Wang-Eckhardt L, Rehnelt J, Petrosino S, Starowicz K, Steuder R, Schlicker E, Cravatt B, Mechoulam R, Buettner R, Werner S, Di Marzo V, Tuting T, Zimmer A. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science. 2007;316:1494–97. doi: 10.1126/science.1142265. [DOI] [PubMed] [Google Scholar]
- Krylatov AV, Maslov LN, Lasukova OV, Pertwee RG. Cannabinoid receptor antagonists SR141716 and SR144528 exhibit properties of partial agonists in experiments on isolated perfused rat heart. Bull Exp Biol Med. 2005;139:558–61. doi: 10.1007/s10517-005-0344-9. [DOI] [PubMed] [Google Scholar]
- Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. 1998;187:939–48. doi: 10.1084/jem.187.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht BN, Hoogsteden HC, Pauwels RA. Dendritic cells as regulators of the immune response to inhaled allergen: recent findings in animal models of asthma. Int Arch Allergy Immunol. 2001;124:432–46. doi: 10.1159/000053778. [DOI] [PubMed] [Google Scholar]
- Landsman RS, Burkey TH, Consroe P, Roeske WR, Yamamura HI. SR141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol. 1997;334:R1–2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- Landsman RS, Makriyannis A, Deng H, Consroe P, Roeske WR, Yamamura HI. AM630 is an inverse agonist at the human cannabinoid CB1 receptor. Life Sci. 1998;62:PL109–13. doi: 10.1016/s0024-3205(97)01187-9. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu T, Newton C, Perkins I, Friedman H, Klein TW. Role of cannabinoid receptors in Delta-9-tetrahydrocannabinol suppression of IL-12p40 in mouse bone marrow-derived dendritic cells infected with Legionella pneumophila. Eur J Pharmacol. 2006;532:170–77. doi: 10.1016/j.ejphar.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Newton C, Klein T, Friedman H. The role of macrophages in THC-induced alteration of the cytokine network. Adv Exp Med Biol. 1998;437:207–14. doi: 10.1007/978-1-4615-5347-2_23. [DOI] [PubMed] [Google Scholar]
- Oka S, Wakui J, Ikeda S, Yanagimoto S, Kishimoto S, Gokoh M, Nasui M, Sugiura T. Involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in oxazolone-induced contact dermatitis in mice. J Immunol. 2006;177:8796–805. doi: 10.4049/jimmunol.177.12.8796. [DOI] [PubMed] [Google Scholar]
- Portier M, Rinaldi-Carmona M, Pecceu F, Combes T, Poinot-Chazel C, Calandra B, Barth F, le Fur G, Casellas P. SR 144528, an antagonist for the peripheral cannabinoid receptor that behaves as an inverse agonist. J Pharmacol Exp Ther. 1999;288:582–89. [PubMed] [Google Scholar]
- Rajananthanan P, Attard GS, Sheikh NA, Morrow WJ. Evaluation of novel aggregate structures as adjuvants: composition, toxicity studies and humoral responses. Vaccine. 1999;17:715–30. doi: 10.1016/s0264-410x(98)00256-4. [DOI] [PubMed] [Google Scholar]
- Rao GK, Kaminski NE. Cannabinoid-mediated elevation of intracellular calcium: a structure-activity relationship. J Pharmacol Exp Ther. 2006;317:820–9. doi: 10.1124/jpet.105.100503. [DOI] [PubMed] [Google Scholar]
- Rhee MH, Kim SK. SR144528 as inverse agonist of CB2 cannabinoid receptor. J Vet Sci. 2002;3:179–84. [PubMed] [Google Scholar]
- Shire D, Calandra B, Bouaboula M, Barth F, Rinaldi-Carmona M, Casellas P, Ferrara P. Cannabinoid receptor interactions with the antagonists SR 141716A and SR 144528. Life Sci. 1999;65:627–35. doi: 10.1016/s0024-3205(99)00285-4. [DOI] [PubMed] [Google Scholar]
- Springs AE, Karmaus PW, Crawford RB, Kaplan BL, Kaminski NE. Effects of targeted deletion of cannabinoid receptors CB1 and CB2 on immune competence and sensitivity to immune modulation by Delta9-tetrahydrocannabinol. J Leukoc Biol. 2008;84:1574–84. doi: 10.1189/jlb.0508282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkinson A, Cieslewicz G, Duez C, Larson KA, Lee JJ, Gelfand EW. Temporal association between airway hyperresponsiveness and airway eosinophilia in ovalbumin-sensitized mice. Am J Respir Crit Care Med. 2001;163:721–30. doi: 10.1164/ajrccm.163.3.2005010. [DOI] [PubMed] [Google Scholar]
- Wacnik PW, Luhr KM, Hill RH, Ljunggren HG, Kristensson K, Svensson M. Cannabinoids affect dendritic cell (DC) potassium channel function and modulate DC T cell stimulatory capacity. J Immunol. 2008;181:3057–66. doi: 10.4049/jimmunol.181.5.3057. [DOI] [PubMed] [Google Scholar]
- Wagner JG, Jiang Q, Harkema JR, Illek B, Patel DD, Ames BN, Peden DB. Ozone enhancement of lower airway allergic inflammation is prevented by gamma-tocopherol. Free Radic Biol Med. 2007;43:1176–88. doi: 10.1016/j.freeradbiomed.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead GS, Walker JK, Berman KG, Foster WM, Schwartz DA. Allergen-induced airway disease is mouse strain dependent. Am J Physiol Lung Cell Mol Physiol. 2003;285:L32–42. doi: 10.1152/ajplung.00390.2002. [DOI] [PubMed] [Google Scholar]
- Yuan M, Kiertscher SM, Cheng Q, Zoumalan R, Tashkin DP, Roth MD. Delta 9-tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J Neuroimmunol. 2002;133:124–31. doi: 10.1016/s0165-5728(02)00370-3. [DOI] [PubMed] [Google Scholar]
- Zuhdi Alimam M, Piazza FM, Selby DM, Letwin N, Huang L, Rose MC. Muc-5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. Am J Respir Cell Mol Biol. 2000;22:253–60. doi: 10.1165/ajrcmb.22.3.3768. [DOI] [PubMed] [Google Scholar]