Abstract
Objective
To examine factors causing inadequate cortisol responses to the 1 mcg ACTH stimulation test.
Design
Random test assignment (by age and gender) at 0800h or 1600h
Methods
We recruited 20 healthy adults to each of three age groups (<40 years, 40 – 55 years, and > 55 years; half female in each group). ACTH stimulation tests were performed in an outpatient clinic at the NIH Clinical Research Center. Plasma cortisol was measured just before, 30 and 60 minutes after administration of 1mcg ACTH (1-24). The ACTH concentration in diluted and administered solutions was measured.
Results
Twenty-five volunteers (19 at 1600h) had a subnormal cortisol response (peak cortisol 10.4-17.5 mcg/dl), using a criterion < 18 mcg/dl (497 nmol/L), for a specificity of 58% (CI 45 – 71%). Afternoon testing had a significant impact on failure rates (OR 6.98, CI 2.17 - 22.43), while gender and age did not. The stock solution contained 1 mcg ACTH, but after administration through tubing it contained only 0.5 to 0.8 mcg.
Conclusions
The high rate of abnormal results, especially in the afternoon, and loss of ACTH through tubing, suggest that morning testing and minimal tubing should be adopted to avoid an inappropriate diagnosis of adrenal insufficiency. Earlier time-points and standardized protocols would facilitate comparison of studies.
Introduction
Cosyntropin (ACTH 1-24, ACTH) stimulation is commonly used to diagnose adrenal insufficiency. Initially the test was performed with a 250 mcg dose 1. However, in response to concerns that patients with mild or recent secondary adrenal insufficiency may respond normally to this supraphysiologic dose, investigators developed a 1 mcg “low dose” test 2-4.
The population's potential risk of secondary adrenal insufficiency is significant, based on a report that 3% of adults are prescribed high dose corticosteroids 5. However, there is no consensus about which diagnostic test is optimal 6-9. Two meta-analyses have agreed that the 1 mcg test has better sensitivity, but only one found a worse specificity (79% vs. 94%) for central adrenal insufficiency 7, 10.
Since a falsely positive test may lead to unnecessary life-long glucocorticoid replacement therapy, with its attendant risks, it is important to enhance specificity. Few studies have examined the effects of time or method of administration of the agent or the influence of age or gender on the test responses. Therefore, to clarify the optimal protocol for the 1 mcg ACTH test, we evaluated factors associated with a falsely abnormal response in 60 healthy volunteers aged 22 to 71 years.
Methods
Volunteers and tests
We recruited healthy adults from three age groups (< 40 years, 40-55 years and > 55 years, evenly split by gender) using community flyers. Exclusion criteria included uncontrolled illnesses, abnormal cell blood count or electrolytes, pregnancy, lactation, recent use of imidazole or glucocorticoid medications or the presence of signs or symptoms of adrenal insufficiency (unintentional weight loss, nausea, fatigue or joint pain). Well-controlled chronic illnesses (e.g. hypertension) were allowed. Subjects received $100 (USD) upon study completion. The NICHD IRB approved the study (ClinicalTrials.gov identifier NCT00156767). Subjects provided written informed consent.
An outpatient visit at the NIH Clinical Center ensured eligibility based on medical history and measurement of blood count and chemistries and, in women, beta human chorionic gonadotropin.
Within each age group volunteers were evenly allocated to outpatient testing at 0800h or 1600h. At least 30 minutes after insertion of an intravenous (IV) line, 1 mcg ACTH 1-24 (Cortrosyn, Amphastar Pharmaceuticals, Rancho Cucamonga, CA) was given, followed by 10 ml saline. Serum cortisol was measured just before ACTH administration and 30 and 60 minutes later.
Preparation of ACTH
In the U.S., ACTH is available only in vials containing 250 mcg of sterile lyophilized powder. A one mcg dose was prepared just before administration as follows: 2.5 ml of sterile 0.9% saline was injected into the vial, yielding a 100 mcg/ml solution; 0.1 ml was injected into a vial containing 9.9 ml of 0.9% saline, yielding a 1 mcg/ml solution for administration.
IV tubing
The routine IV tubing used for endocrine testing in our clinic included a 2.5 cm catheter with attached polyurethane tubing and hub (ProtectIV Plus, Medex, Inc., Carlsbad, Ca) connected to 20.3 cm of tubing with an injection port (Baxter Healthcare Corp., Deerfield, IL).
Assays
The NIH Department of Laboratory Medicine measured cortisol using a chemiluminescent competitive immunoassay (Siemens, Los Angeles, CA). The inter-assay and intra-assay coefficients of variation are < 11.1% and 7.4%, respectively. The functional detectable value is 28 nmol/l. CBG was measured by radioimmunoassay (Quest Diagnostics Nichols Institute). The inter-assay and intra-assay coefficients of variation are less than 22.4% and less than 5.8%, respectively. The least detectable value is 0.1 mg/l (2 nmol/l).
In vitro study
To investigate a higher than anticipated failure rate (see Results), we evaluated if ACTH was diluted correctly, absorbed by the IV tubing or degraded by delayed administration. This was done by measuring ACTH levels in the following samples: (a) 1 mcg/ml stock solution prepared as described, (b) one ml aliquot of the stock solution and the saline flush after pushing through the IV tubing; samples (c) and (d) repeated these steps after leaving the stock solution syringe at room temperature for 60 minutes.
An alternative dilution technique was evaluated whereby 250 mcg of ACTH was added to 250 ml of 0.9% saline. Samples a – d were collected for each dilution method, flash frozen and stored at −80C. A single sample for the two dilutions at steps a – d was assayed in duplicate for ACTH (below) and the results were averaged.
ACTH radioimmunoassay
ACTH levels were measured by radioimmunoassay with reagents purchased from MP Biomedicals (Orangeburg, NY). The rabbit anti-porcine ACTH antibody has 100% cross-reactivity with ACTH (1-24) verified using synthetic, HPLC-purified ACTH 1-24 (Phoenix Pharmaceuticals, Belmont, CA). The tracer was synthetic human ACTH-125I. Samples were assayed in duplicate at dilutions of 1:5,000 and 1:10,000 using zero ACTH standard as diluent, to give an assayed ACTH result of 22-300 pg/ml (standard curve range: 11 to 1050 pg/ml). Intra- and inter-assay coefficients of variation are 4.1-6.8% and 3.9-10.7%, respectively.
Data analysis
Clinical characteristics were summarized using descriptive statistics
A published criterion for a normal cortisol response to ACTH (497 nmol/l [18 mcg/dl] or greater at 30 minutes) was chosen to minimize falsely abnormal results and was used to classify the results 11-13.
Results are presented as mean (SD). Logistic regression analysis determined the influence of time of test, gender and age on the “pass” or “fail” rate. Odds ratios (OR) and their 95% confidence intervals 14 were determined from logistic regression analyses, and specificity and Fisher's exact 95% CI were computed.
All statistical analyses were two-tailed, with significance defined as a p-value of ≤ 0.05. Data were analyzed using SAS system software, release 9.1 (SAS Institute, Inc., Cary, North Carolina).
Results
Volunteers
Characteristics of the subjects are shown in Table 1. Four women (13%) were taking oral contraceptives (n = 3) or estrogen-containing hormone replacement therapy (n = 1); 12 were postmenopausal. No other subjects were receiving medication known to affect cortisol or CBG levels. Some had well-controlled chronic illnesses, including hypertension (n = 4), hyperlipidemia (n = 6), diabetes (n = 2), depression/anxiety (n =7), migraine headache (n =2), sleep apnea (n = 1), GERD (n =3), seasonal allergies (n = 6), osteopenia (n = 3) and osteoarthritis (n = 1).
Table 1.
Characteristics of Healthy Volunteers
| Age Group (years) | |||
|---|---|---|---|
| < 40 | 40-55 | > 55 | |
| Number | 20 | 20 | 20 |
| Female / male | 10/10 | 10/10 | 10/10 |
| Mean age (SD) | 27.5 (4.1) | 46.7 (4.7) | 62.1 (4.9) |
| Age range | 22-38 | 40-53 | 56-71 |
| Race/Ethnicity (n) | |||
| White (43) | 14 | 13 | 16 |
| Black (11) | 3 | 6 | 2 |
| Asian (6) | 3 | 1 | 2 |
| Hispanic (3) | 1 | 2 | 0 |
All subjects completed the test without adverse events.
Cortisol responses
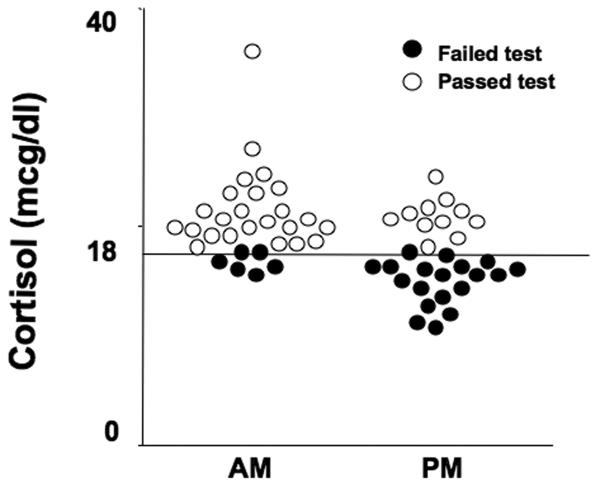
Surprisingly, in this group of healthy volunteers without signs or symptoms of adrenal insufficiency, 25/60 (42%, CI 29% - 55%) had cortisol responses less than 497 nmol/l (range 287 – 483 nmol/l; 10.4-17.5 mcg/dl) (Figure 1).
Figure 1.
Cortisol responses at 30 minutes after 1 mcg Cortrosyn administration between 0800-0900h (AM) and 1600-1700h (PM). Open circles denote subjects who passed the test, and closed circles show those who failed the test, based on a criterion of response of at least 497 (18 mcg/dl).
The time of testing influenced the failure rate: 19/30 (63%, CI 44% - 80%) volunteers tested at 1600h, and 6/30 (20%, CI 8% - 39%) tested at 0800h had abnormal responses (Figure 1). Afternoon testing had a significant impact on failure rates (OR 6.98, CI 2.17 - 22.43), while gender and age did not.
The absolute cortisol increment (30 minute minus 0 minute value) was not affected by the time of the test (AM: 10.6 ± 3.5 mcg/dl (292 ± 97 nmol/l) vs. PM: 10.2 ± 3.7 mcg/dl (281 ± 102 nmol/l, p = 0.74), but was marginally significant by pass/fail status (pass: 11.1 ± 4.0 mcg/dl (306 ± 110 nmol/l) vs. fail: 9.4 ± 2.5 mcg/dl (259 ± 69 nmol/l, p = 0.049; Figure 2). However, the absolute change in cortisol did not predict a pass or fail outcome for individual patients (Figure 2). Those who failed the test had lower baseline values but similar incremental results (Figure 2).
Figure 2.
Absolute change (delta TF) in total cortisol at 30 minutes from baseline total cortisol (TF) at 0 minutes of the 1 mcg ACTH stimulation test performed in the morning (AM) or afternoon (PM). The line divides subjects who passed or failed based on the criterion of a response of at least 497 nmol/L (18 mcg/dl).
CBG results
Basal CBG levels were higher in women taking estrogen (50.0 ± 25.5 mg/dl, n=2), compared to women not taking estrogen (34.7 ± 13.0 mg/dl, n = 17), or men (26.3 ± 5.7, n=22) (p=0.003). As a result, total cortisol values were significantly greater in the 3 women taking oral contraceptives compared to the other women who were tested in the morning (20.9 ± 5.9 vs. 8.5 ± 3.2 mcg/dl, p=0.002). Basal CBG values were slightly higher in subjects who passed the ACTH stimulation test compared to those who failed, but this did not reach statistical significance (32.0 ±9.8 vs. 29.4 ±14.1 mg/dl, p = 0.0524).
In vitro ACTH levels
The stock solutions contained at least 1 mcg/ml ACTH (range 1.1-1.6 mcg/ml). Values were similar in samples kept at room temperature for 60 minutes (standard dilution method: 1.6 mcg/ml, alternative: 1.15 mcg/ml) or processed immediately (standard: 1.25 mcg/mL; alternative: 1.1 mcg/mL). Values in samples pushed through iv tubing were lower (immediately collected: standard 0.047mcg/ml, alternative 0.074 mcg/ml; kept at room temperature: standard 0.070 mcg/ml, alternative 0.082 mcg/ml). Thus, among samples pushed through the IV tubing, 21.6% to 58.6% of ACTH was not recovered, corresponding to administration of 0.5 to 0.8 mcg of ACTH.
Discussion
This study of healthy volunteers revealed a surprisingly high rate of abnormal responses to the 1 mcg ACTH test when a cortisol value less than 18 mcg/dl (497 nmol/l) was considered abnormal. This 58% specificity (CI 45 – 71%) is less than that reported by most others (mean 79%, CI 74 – 84%) 10. Further analysis attributed these abnormal results to afternoon testing and loss of cosyntropin in the intravenous tubing, suggesting a need for standardization of the testing protocol. Age and gender did not influence outcome.
In this study, afternoon testing was associated with a 7-fold increased likelihood of failing the 1 mcg test. However, time of day did not explain all abnormal responses, since 6/30 (20%) failed morning testing. The incremental (delta) cortisol response was similar at both times of day, but both the 0 and 30 minute values were lower in the afternoon.
Our findings of abnormal afternoon responses to 1 mcg ACTH differ from those of Park et al., who reported lower peak cortisol values in the afternoon than in the morning, but a normal response in all eight volunteers 12. The larger size of the current study may underlie these differences. Another study found no influence of afternoon testing in ten subjects 3. Perhaps as a result of this variability, there is no published consensus on the optimal time for testing. For example, one report suggests that the test may be performed at any time of the day 15.
The overall cortisol increment above baseline was similar at each time of day, but values for individual subjects did not predict the pass or fail outcome. As a result, although the incremental cortisol response is not influenced by the time of testing, these data do not support use of this value for diagnostic purposes.
What might explain the 20% failure rate with morning testing? The cortisol dose-response curve is extremely steep when ACTH is delivered as a subcutaneous dose of 2.5 to 10 ug 16. Thus, possible loss of ACTH during dilution or administration might sharply reduce the cortisol response. As reported earlier, we found that short term exposure to room temperature did not degrade ACTH 3. Although the lack of a commercial 1 mcg preparation might lead to dilution errors, in this study loss of ACTH due to adherence to plastic tubing appeared to be more significant. These findings corroborate the results of Murphy who showed losses up to 70% when ACTH was passed through a plastic 30 cm scalp vein set 17, but contrast with the report of Wood, who found that storage in a plastic tube did not decrease the delivered dose 3. The importance of minimizing or eliminating catheter length was emphasized in one recent review 18 but was not mentioned in others 7, 19. Relatively few papers explicitly cite catheter length; of those that do, both short 12 and long 14 catheters were used. We did not investigate the possibilities that the type of plastic and the length of tubing independently influence this effect. Taken together, these data suggest that the shortest possible length of tubing, or direct venous injection, should be used for ACTH administration.
Most previous studies used a 30-minute endpoint, based on data showing similar cortisol values at 20 and 30 minutes 20 or similar 30 minute values after 1 and 250 mcg ACTH 21 and a recent meta-analysis preferred this time-point 7. However, the 30-minute time point may not be optimal, as up to 20% of healthy volunteers fail the test at this time-point 6, 22-24. Other studies demonstrated that the peak cortisol response in healthy subjects occurs 20 to 30 minutes after low dose ACTH (1 mcg or 500 ng/1.73m2) 6, 12, and another recommends measurement of cortisol at both times 15. In another study no responses were abnormal when both 20 and 30-minute values were used. Notably, in patients with adrenal insufficiency, the times of peak total cortisol response tended to occur later than in the healthy volunteers, further supporting the use of earlier measurement intervals 12.
Differences among cortisol assays and different cut-off points might lead to either a “fail” or “pass”, and might underlie variability in published diagnostic accuracy. A recent meta-analysis found the 1 mcg test superior to the 250 mcg test only after converting plasma cortisol levels to serum values 7. Additionally, cortisol threshold criteria of have been proposed for various timepoints of the 1 mcg (500 to 600 nmol/L; 18 – 21.9 mcg/dL) and 250 ( 500 -725 nmol/L; 18 – 26.3 ug/dL) mcg tests 15, 20. It is not clear whether this variability results from differing assays, study populations or “gold standard” criteria for disease. Taken together, this variability suggests that it is important to validate diagnostic threshold criteria at each center.
As has been reported, higher CBG levels are associated with higher cortisol measurements, and oral estrogens can significantly increase CBG levels 25.Thus, estrogen administration is another confounding factor that might cause a falsely normal test response.
In conclusion, we identified two potential reasons for abnormal cortisol stimulation after 1 mcg ACTH in healthy volunteers: afternoon testing and long plastic tubing. Previous publications suggest that use of a single 30-minute endpoint also may lead to false results. Presumably these factors would influence falsely abnormal results in patients suspected of having adrenal insufficiency, especially those with long indwelling lines. We suggest that morning testing with 20 and 30-minute time-points, minimal IV tubing and standardized dilution methods be adopted routinely and that the potential influence of CBG be considered. If these requirements cannot be met, it may be prudent to consider alternative “gold standard” tests for the diagnosis, such as metyrapone or insulin stimulation in patients suspected of having recent or mild secondary adrenal insufficiency26 Alternatively, the standard 250 mcg test works well in the majority of patients, even those with ACTH deficiency 27.
Acknowledgements
We thank Nghi Huynh for his technical assistance and Bob Wesley for helpful statistical suggestions.
Funding: This work was supported in part by the intramural program of the National Institute of Child Health and Human Development, National Institutes of Health
Footnotes
Declaration of interest: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Wood JB, Frankland AW, James VH, Landon J. A RAPID TEST OF ADRENOCORTICAL FUNCTION. Lancet. 1965;191:243–245. doi: 10.1016/s0140-6736(65)91526-6. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosi B, Barbetta L, Re T, Passini E, Faglia G. The one microgram adrenocorticotropin test in the assessment of hypothalamic-pituitary-adrenal function. Eur J Endocrinol. 1998;139:575–579. doi: 10.1530/eje.0.1390575. [DOI] [PubMed] [Google Scholar]
- 3.Dickstein G, Shechner C, Nicholson WE, Rosner I, Shen-Orr Z, Adawi F, Lahav M. Adrenocorticotropin stimulation test: effects of basal cortisol level, time of day, and suggested new sensitive low dose test. J Clin Endocrinol Metab. 1991;72:773–778. doi: 10.1210/jcem-72-4-773. [DOI] [PubMed] [Google Scholar]
- 4.Tordjman K, Jaffe A, Grazas N, Apter C, Stern N. The role of the low dose (1 microgram) adrenocorticotropin test in the evaluation of patients with pituitary diseases. J Clin Endocrinol Metab. 1995;80:1301–1305. doi: 10.1210/jcem.80.4.7714104. [DOI] [PubMed] [Google Scholar]
- 5.van Staa TP, Leufkens HG, Abenhaim L, Begaud B, Zhang B, Cooper C. Use of oral corticosteroids in the United Kingdom. Qjm. 2000;93:105–111. doi: 10.1093/qjmed/93.2.105. [DOI] [PubMed] [Google Scholar]
- 6.Abdu TA, Elhadd TA, Neary R, Clayton RN. Comparison of the low dose short synacthen test (1 microg), the conventional dose short synacthen test (250 microg), and the insulin tolerance test for assessment of the hypothalamo-pituitary-adrenal axis in patients with pituitary disease. J Clin Endocrinol Metab. 1999;84:838–843. doi: 10.1210/jcem.84.3.5535. [DOI] [PubMed] [Google Scholar]
- 7.Kazlauskaite R, Evans AT, Villabona CV, Abdu TA, Ambrosi B, Atkinson AB, Choi CH, Clayton RN, Courtney CH, Gonc EN, Maghnie M, Rose SR, Soule SG, Tordjman K. Corticotropin tests for hypothalamic-pituitary- adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab. 2008;93:4245–4253. doi: 10.1210/jc.2008-0710. [DOI] [PubMed] [Google Scholar]
- 8.Tordjman K, Jaffe A, Greenman Y, Stern N. Comments on the comparison of low and high dose corticotropin stimulation tests in patients with pituitary disease. J Clin Endocrinol Metab. 1998;83:4532–4533. doi: 10.1210/jcem.83.12.5322-3. [DOI] [PubMed] [Google Scholar]
- 9.Thaler LM, Blevins LS., Jr. The low dose (1-microg) adrenocorticotropin stimulation test in the evaluation of patients with suspected central adrenal insufficiency. J Clin Endocrinol Metab. 1998;83:2726–2729. doi: 10.1210/jcem.83.8.5039. [DOI] [PubMed] [Google Scholar]
- 10.Nieman LK. Dynamic evaluation of adrenal hypofunction. J Endocrinol Invest. 2003;26:74–82. [PubMed] [Google Scholar]
- 11.Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350:1629–1638. doi: 10.1056/NEJMoa020266. [DOI] [PubMed] [Google Scholar]
- 12.Park YJ, Park KS, Kim JH, Shin CS, Kim SY, Lee HK. Reproducibility of the cortisol response to stimulation with the low dose (1 microg) of ACTH. Clin Endocrinol (Oxf) 1999;51:153–158. doi: 10.1046/j.1365-2265.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 13.Thaler LM. Comment on the low-dose corticotropin stimulation test is more sensitive than the high-dose test. J Clin Endocrinol Metab. 1998;83:4530–4531. doi: 10.1210/jc.83.12.4530-a. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Gonzalez JG, De la Garza-Hernandez NE, Mancillas-Adame LG, Montes-Villarreal J, Villarreal-Perez JZ. A high-sensitivity test in the assessment of adrenocortical insufficiency: 10 microg vs 250 microg cosyntropin dose assessment of adrenocortical insufficiency. J Endocrinol. 1998;159:275–280. doi: 10.1677/joe.0.1590275. [DOI] [PubMed] [Google Scholar]
- 15.Beishuizen A, van Lijf JH, Lekkerkerker JF, Vermes I. The low dose (1 microg) ACTH stimulation test for assessment of the hypothalamo-pituitary-adrenal axis. Neth J Med. 2000;56:91–99. doi: 10.1016/s0300-2977(99)00111-4. [DOI] [PubMed] [Google Scholar]
- 16.Oelkers W, Boelke T, Bahr V. Dose-response relationships between plasma adrenocorticotropin (ACTH), cortisol, aldosterone, and 18-hydroxycorticosterone after injection of ACTH-(1-39) or human corticotropin-releasing hormone in man. J Clin Endocrinol Metab. 1988;66:181–186. doi: 10.1210/jcem-66-1-181. [DOI] [PubMed] [Google Scholar]
- 17.Murphy H, Livesey J, Espiner EA, Donald RA. The low dose ACTH test--a further word of caution. J Clin Endocrinol Metab. 1998;83:712–713. doi: 10.1210/jc.83.2.712-a. [DOI] [PubMed] [Google Scholar]
- 18.Dickstein G, Saiegh L. Low-dose and high-dose adrenocorticotropin testing: indications and shortcomings. Curr Opin Endocrinol Diabetes Obes. 2008;15:244–249. doi: 10.1097/MED.0b013e3282fdf16d. [DOI] [PubMed] [Google Scholar]
- 19.Reimondo G, Bovio S, Allasino B, Terzolo M, Angeli A. Secondary hypoadrenalism. Pituitary. 2008;11:147–154. doi: 10.1007/s11102-008-0108-4. [DOI] [PubMed] [Google Scholar]
- 20.Mayenknecht J, Diederich S, Bahr V, Plockinger U, Oelkers W. Comparison of low and high dose corticotropin stimulation tests in patients with pituitary disease. J Clin Endocrinol Metab. 1998;83:1558–1562. doi: 10.1210/jcem.83.5.4831. [DOI] [PubMed] [Google Scholar]
- 21.Nye EJ, Grice JE, Hockings GI, Strakosch CR, Crosbie GV, Walters MM, Jackson RV. Comparison of adrenocorticotropin (ACTH) stimulation tests and insulin hypoglycemia in normal humans: low dose, standard high dose, and 8-hour ACTH-(1-24) infusion tests. J Clin Endocrinol Metab. 1999;84:3648–3655. doi: 10.1210/jcem.84.10.6062. [DOI] [PubMed] [Google Scholar]
- 22.Choi CH, Tiu SC, Shek CC, Choi KL, Chan FK, Kong PS. Use of the low-dose corticotropin stimulation test for the diagnosis of secondary adrenocortical insufficiency. Hong Kong Med J. 2002;8:427–434. [PubMed] [Google Scholar]
- 23.Cohen O, Sidi Y, Barazin M, Karasik A. Low dose ACTH test--a word of caution. J Clin Endocrinol Metab. 1996;81:3129–3130. doi: 10.1210/jcem.81.8.8768887. [DOI] [PubMed] [Google Scholar]
- 24.Crowley S, Hindmarsh PC, Honour JW, Brook CG. Reproducibility of the cortisol response to stimulation with a low dose of ACTH(1-24): the effect of basal cortisol levels and comparison of low-dose with high-dose secretory dynamics. J Endocrinol. 1993;136:167–172. doi: 10.1677/joe.0.1360167. [DOI] [PubMed] [Google Scholar]
- 25.Klose M, Lange M, Rasmussen AK, Skakkebaek NE, Hilsted L, Haug E, Andersen M, Feldt-Rasmussen U. Factors influencing the adrenocorticotropin test: role of contemporary cortisol assays, body composition, and oral contraceptive agents. J Clin Endocrinol Metab. 2007;92:1326–1333. doi: 10.1210/jc.2006-1791. [DOI] [PubMed] [Google Scholar]
- 26.Grinspoon SK, Biller BM. Clinical review 62: Laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994;79:923–931. doi: 10.1210/jcem.79.4.7962298. [DOI] [PubMed] [Google Scholar]
- 27.Agha A, Tomlinson JW, Clark PM, Holder G, Stewart PM. The long-term predictive accuracy of the short synacthen (corticotropin) stimulation test for assessment of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 2006;91:43–47. doi: 10.1210/jc.2005-1131. [DOI] [PubMed] [Google Scholar]