Summary
Objective
The objective of this study is to characterize mouse temporomandibular joint (TMJ) following partial discectomy, since there is no documentation of whether or not partial discectomy can induce early-onset osteoarthritis (OA) in mouse TMJ.
Methods
Partial discs of TMJ in mice were removed by microsurgery. Histology was performed to characterize articular cartilages from the TMJ of mice. The morphology of the articular cartilages was evaluated using a modified Mankin scoring system. Immunohistostaining was carried out to examine the expression of discoidin domain receptor 2 (Ddr2), a type II collagen receptor, matrix metalloproteinase 13 (Mmp-13), and Mmp-derived type II collagen fragments in the articular cartilage of condyles from the mouse TMJ.
Results
Articular cartilage degeneration was seen in the mouse TMJ post discectomy, including increased proteoglycan staining in the extracellular matrix at 4 weeks, the appearance of chondrocyte clusters at 8 weeks, reduced proteoglycan staining and fibrillation at 12 weeks and the loss of articular cartilage at 16 weeks. Increased immunostaining for Ddr2, Mmp-13, and Mmp-derived type II collagen fragments was detected.
Conclusion
Results indicate that partial discectomy induces early-onset OA in mouse TMJ and that increased expression of Mmp-13, likely due to the elevated expression of Ddr2, may be one of the factors responsible for the early-onset OA in mouse TMJ.
Keywords: temporomandibular joint, articular cartilage, discectomy, osteoarthritis
Introduction
The fibrous disc in temporomandibular joint (TMJ) serves as a cushion during joint movements, evenly distributing pressure-loads on the articular surfaces of the condyle and the fossa of temporal bone (1,2). Data from studies with rats and rabbits indicate that a partial or total removal of the disc in TMJ results in early-onset osteoarthritis (OA) (3–5). However, to our knowledge, there are no reports on discectomy of mouse TMJ. Clinical studies suggest that injury to the disc might be the most frequent cause of TMJ OA (6–8). Therefore, an understanding of the molecular basis of articular cartilage degeneration in TMJ may provide novel insights into the pathogenesis of TMJ OA.
Investigation of the mechanisms of OA with the complicated etiology is a formidable challenge. However, the task is made simpler by the fact that there is a typical pattern of OA progression regardless of the nature of the initiating events (9). The earliest indication of articular cartilage degeneration is the over-production of proteoglycans and other extracellular matrix molecules, and the appearance of chondrocyte clusters. Then, gradual loss of proteoglycans occurs in the surface of articular cartilage, along with cleavage of type II collagen fibrils. Cracks gradually develop along the surface, producing a histological image of fibrillation (10–12). This pattern of the degeneration indicates that there may be a common chain of molecular events underlying the degeneration. Many studies suggest that articular cartilage degeneration is mediated by numerous biochemical factors (13–16). Matrix metalloproteinase (Mmp)-13 is one of these factors and is of particular importance because of its ability to cleave triple-helical type II collagen (17,18). Mmp-13 is expressed at a very low level in normal articular cartilage and at a high level in degenerative ones (19–21). This is consistent with the observation that constitutive expression of Mmp-13 results in OA-like changes in mouse knee joints (22). Results from our recent studies indicated that the activity and expression of Mmp-13 were increased in mouse OA knee joints. We also found that the increased expression of Mmp-13 might result from the elevated expression of a cell membrane type II collagen receptor, discoidin domain receptor 2 (Ddr2) (23). Furthermore, we found that increased expression of Mmp-13 was associated with a high-level of Ddr2 expression in human OA cartilages. Thus we hypothesize that increased expression of Ddr2 may be a common event in articular cartilage degeneration.
To investigate if the expression of Ddr2 is increased in degenerative TMJ induced by a non-genetic factor, we performed microsurgery to destabilize mouse TMJ by removing a portion of the intraarticular disc. We then examined the articular cartilage of surgically destabilized joints for evidence of articular cartilage degeneration, changes in Ddr2 and Mmp-13, and alterations in the amount of Mmp-derived type II collagen fragments.
Materials and methods
PARTIAL DISCECTOMY OF TMJ IN MICE
The experimental procedure was performed following approval from the Forsyth Institutional Animal Care. Eighty C57BL/6j mice at the age of 3 months (Jackson Laboratory, Maine) were used for the surgery. Each mouse was anesthetized with intra-peritoneal 70µg Ketamine-15µgXylazine/g bodyweight. An incision was made over the left TMJ and then through the subcutaneous and muscle layers. The lateral part of the disc was removed. All of the removed discs were saved to evaluate association, if any, of the cartilage damage with the size of the removed discs. For control (sham surgery), TMJ in mice underwent a similar preparation and surgical procedure but their discs were not cut. Buprenex (analgesic) was also provided subcutaneously at 15ng/g bodyweight twice per day for 3 days post-surgically. Mice were fed with powder food for a week immediately after the surgery and then with regular food for the remainder of the experiment. Body weights of all the mice were recorded prior to the surgery and 3 times per week after the surgery, in order to monitor whether the mice were losing weight as a result of the procedure.
HISTOLOGY
At 2, 4, 8, 12 and 16 weeks post surgery, 8 heads from the discectomy and 8 heads from the sham surgery were fixed in 4% paraformaldehyde for 6 hours at room temperature. The experimental procedure for histology has been described in our previous study (24). Serial sections were cut at thickness of 6 µm. It took about 100 sections to cover a mouse TMJ from anterior to posterior. Every fifteenth section (total six sections) was collected for evaluation of each TMJ. Mouse articular cartilages were evaluated based upon pericellular and background Safranine O staining, chondrocyte arrangement, and structural condition of the articular cartilage. The score for normal mouse articular cartilage is 0 and the maximum score for degenerative articular cartilage is 10, see table 1.
Table 1.
Modified Mankin scoring system used to evaluate articular cartilage in mice
| 1) Pericellular Safranine-O staining | ||
| a. | Normal | 0 |
| b. | Slightly enhanced | 1 |
| c. | Intensely enhanced | 2 |
| 2) Background Safranine-O staining | ||
| a. | Normal | 0 |
| b. | Slight increase or decrease | 1 |
| c. | Severe increase or decrease | 2 |
| d. | No staining | 3 |
| 3) Arrangement of chondrocytes | ||
| a. | Normal | 0 |
| b. | Appearance of clustering | 1 |
| c. | Hypocellularity | 2 |
| 4) Cartilage structure | ||
| a. | normal | 0 |
| b. | fibrillation in superficial layer | 1 |
| c. | fibrillation beyond superficial layer | 2 |
| d. | missing articular cartilages | 3 |
IMMUNOHISTOCHEMISTRY
Polyclonal antibodies against DDR2 (Santa Cruz Biotechnology, CA, USA), Mmp-13 (Chemicon, Temecula, CA, USA), and type II collagen fragments (C1-2C, IBEX Tech. Inc. Montreal, QC, Canada) were used for immunostaining. Isotype-matched normal IgGs (Vector Laboratory, CA) were used for negative control staining. Every 15th section from 4 randomly selected TMJ in each group (2, 4 and 8 weeks post surgery) was collected for the staining. Sections were incubated with polyclonal antibodies (1:200), and then incubated with biotinylated secondary antibody. Color detection was performed with peroxidase substrate after incubation with a mixture of avidin and biotinylated HRP.
STATISTICAL ANALYSIS
Data from the modified Mankin scoring system was nonparametric (ordinal). Thus, the Mann-Whitney test was used to examine differences between discectomy and sham surgery.
Results
DISCECTOMY
There were no significant losses or gains in the body weights of the experimental and control mice. The sizes of the removed discs from mouse TMJ varied from a half to two thirds of the disc. Results from the histological analysis indicated that variations in the size of the removed disc were not correlated with the severity of the articular cartilage degeneration. Instead, the severity of the cartilage damage was associated with the time course following the surgery.
HISTOLOGY
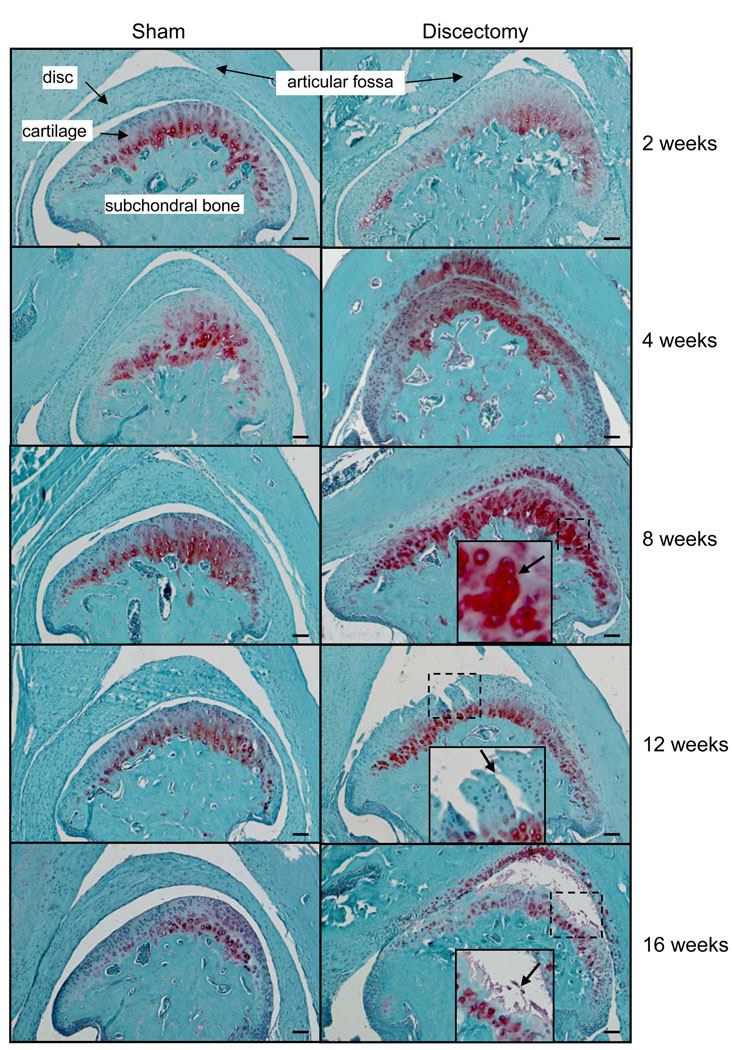
Results (figure 1) showed that the morphology of TMJ in sham surgery mice at different time points were similar to the appearance of TMJ of wild-type mice (10). There were no differences observed between the discectomy and sham mice 2 weeks post surgery. However, increased Safranine O staining for proteoglycans was seen throughout the entire articular cartilage of the condyle and in the articular cartilage of the fossa from mice 4 weeks post discectomy. At 8 weeks post discectomy, chondrocyte clusters were observed. At 12 weeks, fibrillation was seen in the discectomy TMJ. At 16 weeks post discectomy, the loss of articular cartilage was evident. No osteophytes and subchondral sclerosis were observed in discectomy mice.
Figure 1.
Histology of TMJ in mice post sham surgery and partial discectomy. There were no significant differences between the discectomy and sham mice at 2 weeks post surgery. However, at 4 weeks post discectomy, increased Safranine O staining for proteoglycans was seen throughout the entire articular cartilage of the condyle and in the articular cartilage of the fossa. At 8 weeks post discectomy, chondrocyte clusters appeared (see insert and arrow) and slight reduction of Safranine O staining in the fossa of the temporal bone and the superfacial layer of the condyles was observed. At 12 weeks, fibrillation was observed (see insert and arrow) in the discectomy mice. At 16 weeks, a dramatic overall reduction of Safranine O staining and the loss of articular cartilage (see insert and arrow) were also observed in the discectomy mice. No significant morphologic changes were observed in the subchondral plate and joint margins in the sham surgery or discectomy mice. (Bar=50 µm)
To evaluate the morphological condition of the articular cartilage of TMJ, 8 scores representing 8 animals from each group at each time point were obtained. Then each set of scores was used to calculate an average score for each group at each time point. A statistical comparison of the scores (table 2) obtained from the sham and discectomy mice, using the Mann-Whitney test, indicated a significant difference between the two groups at each time point; the P value was less than 0.001. Two-week post sham mice were used as a normal control (score=0). There were no significant differences between the morphologies of TMJ of discectomy and sham mice at two weeks post surgery. However, at 4 weeks, the morphologic changes observed in the discectomy mice were significantly different from those observed in the sham mice. As the length of time following the surgery increased, further degeneration of the articular cartilage was observed in the discectomy mice; indicating a close association between the post-surgery time course and the progression of articular cartilage degeneration. There were no significant changes observed among the sham surgery mice at the different time points.
Table 2.
Modified Mankin scores of mouse TM joints
| Time point of post-surgery |
Sham | Discectomy | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of individual animals | No. of individual animals | |||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Aver. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Aver. | |
| 4 weeks | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0.38 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1.13* |
| 8 weeks | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0.38 | 3 | 4 | 3 | 3 | 4 | 3 | 3 | 3 | 3.24* |
| 12 weeks | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0.75 | 7 | 6 | 6 | 5 | 6 | 6 | 5 | 6 | 5.88* |
| 16 weeks | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0.63 | 7 | 6 | 8 | 6 | 6 | 7 | 6 | 6 | 6.50* |
p value < 0.001
IMMUNOHISTOCHEMISTRY
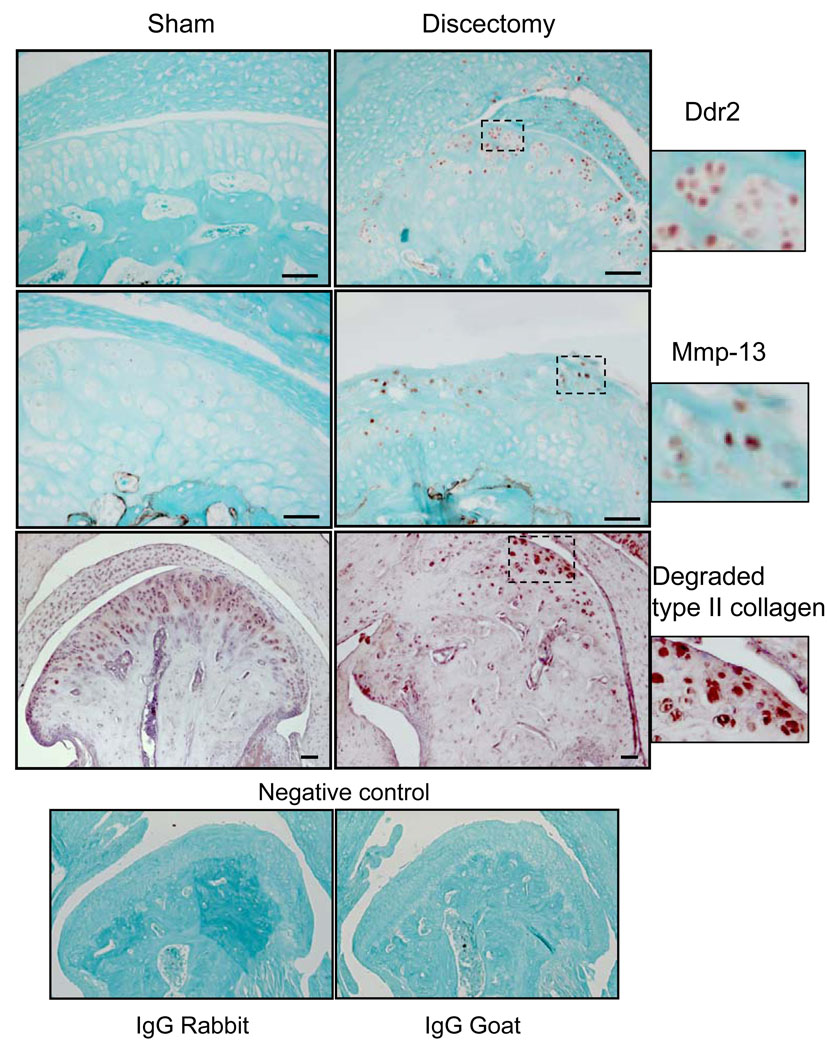
Results (figure 2) showed that the expression of Ddr2 and Mmp-13 was increased in the articular cartilage of discectomy TMJ. Mmp13 was seen in the majority of the sections selected at random from mice at 8 weeks post-discectomy. The number of positively stained cells for these two genes were consistent throughout the samples. Cells positively stained for Ddr2 and Mmp-13 were hardly detectable in the TMJ obtained from the sham mice at 8 weeks post-operation. At 2 and 4 weeks after the surgery, cells positively stained for Ddr2 and Mmp-13 were not detected in the TMJ of mice from either the sham or discectomy mice (data not shown). Results also indicated that there were increased amount of type II collagen fragments in the articular cartilage of TMJ in mice 8 weeks post discectomy. There were hardly any detectable type II collagen fragments in the articular cartilages of TMJ from mice at 2 and 4 weeks after the sham surgery or the discectomy (data not shown).
Figure 2.
Immunostaining of Ddr2, Mmp-13 and Mmp-derived type II collagen fragments in TMJ of sham and discectomy mice. Ddr2- and Mmp-13 positive cells (see brown color staining cells) were observed in the TMJ of the discectomy mice at 8 weeks following the surgery. However, Ddr2 and Mmp-13 positive cells were hardly detected in the TMJ of the sham-surgery mice at 8 weeks. No positively stained cells were detected in the sham and discectomy mice at 2 and 4 weeks (data not shown). More intense staining for Mmp-derived type II collagen fragments (see insert) was observed in the superficial layer of articular cartilage in the TMJ of mice 8 weeks post discectomy than was observed in the sham-surgery mice. Diffuse non-significant staining for Mmp-derived type II collagen fragments in the extracellular matrix appeared in both sham and discectomy mice at 8 weeks. Immunostaining for Mmp-derived type II collagen fragments was undetectable in the articular cartilage of TMJ from mice 4 weeks post sham and partial discectomy (data not shown). Stainings with isotype-matching normal IgG (rabbit IgG for Ddr2 and Mmp-derived type II collagen; goat IgG for Mmp-13) and without primary antibody (data not shown) were also performed as negative controls. No positive stainings were observed in the controls. Counter staining for Ddr2 and Mmp-13 is Fast Green and for degraded type II collagen is hematoxylin. (Bar=50 µm)
Discussion
To our knowledge, this is the first time to demonstrate that partial discectomy can induce early-onset OA in mouse TMJ. The data revealed a typical pattern of articular cartilage degeneration following partial discectomy. Although precise mechanisms that initiate early-onset OA in TMJ of discectomy mice are unknown, we believe that a partial removal of the disc can dramatically affects the distribution of the pressure and the capacity for load absorption on surfaces of the TMJ, resulting in the presence of excessive mechanical force on a small area of articular surfaces. This results in early-onset OA. In this study, we used a modified Mankin scoring system to characterize morphologic changes of TMJ. Others and we have used this system in previous studies (10, 25). We found that this modified score system reliably represented the morphological conditions of mouse joints at different developmental stages. We notice that there are other scoring systems for mouse or small animal joints that have been used in numerous studies since there is no a consensus in the grading system for mouse articular cartilage or small animals.
To understand mechanisms underlying articular cartilage degeneration in TMJ of discectomy mice, we examined expression patterns of Ddr2 and Mmp13. One question that remained was which molecules, if any, can stimulate chondrocytes to synthesize and release Mmp-13 in articular cartilage, prior to the significant degradation of the cartilage at the early stage of the degeneration. We found that the activation of Ddr2 resulted in increased expression of Mmp-13 in chondrocytes in vitro (23). We also observed that the levels of Ddr2 and Mmp-13 mRNA and proteins were elevated in the articular cartilage of human OA hip and knee joints. In addition, we found increased expression of Ddr2 and Mmp-13 in knee and TMJ of two genetic mouse OA models. These results suggest that Ddr2 may play an important role in the pathogenesis of OA. Ddr2 is a cell membrane tyrosine kinase receptor and preferentially bound to native type II collagen (26–28). There is little or no type II collagen molecules around chondrocytes in the pericellular region (29). This suggests that there is little or no contact between chondrocytes and type II collagen molecules in mature articular cartilage under normal conditions. Consequently, we speculate that the exposure of the collagen network to chondrocytes, under any circumstance, such as after depletion of proteoglycans, will permit interaction of type II collagen with chondrocytes, resulting in the activation of Ddr2. The activated Ddr2 induces the expression of Mmp-13 as well as expression of Ddr2 itself. In this study, we found that expression of Ddr2 and Mmp-13 was increased in the articular cartilage of the TMJ at 8 weeks post partial discectomy, when the degradation of proteoglycans was already evident. This is consistent with our hypothesis that regardless of the nature of OA initiation events (genetic or non-genetic factors), degradation of proteoglycans increases exposure of chondrocytes to type II collagen. This, in turn, elicits interaction of Ddr2 with type II collagen, resulting in elevated expression of the receptor itself and induction of Mmp-13 expression in chondrocytes. Therefore, inhibitors of Ddr2 or its downstream effectors may turn out to be useful for the treatment of OA.
Acknowledgments
This work was supported by Renée and Milton Glass Family Fellowship for TMJD Research (to Xu) and by NIH grants R01-AR-051989 (to Xu and Li), and R01-AR-36819 (to Olsen).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof The manuscript will undergo copyediting, typesetting, and review of the resulting proof process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Nickel JC, McLachlan KR. In vitro measurement of the stress-distribution properties of the pig temporomandibular joint disc. Arch Oral Biol. 1994;39:439–448. doi: 10.1016/0003-9969(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 2.Beek M, Aarnts MP, Koolstra JH, Feilzer AJ, van Eijden TM. Dynamic properties of the human temporomandibular joint disc. J Dent Res. 2001;80:876–880. doi: 10.1177/00220345010800030601. [DOI] [PubMed] [Google Scholar]
- 3.Lekkas C, Honee GL, van den Hooff A. Effects of experimental defects of the articular disc of the temporomandibular joint in rats. J Oral Rehabil. 1988;15:141–148. doi: 10.1111/j.1365-2842.1988.tb00763.x. [DOI] [PubMed] [Google Scholar]
- 4.Miyaki K, Murakami K, Segami N, Iizuka T. Histological and immunohistochemical studies on the articular cartilage after experimental discectomy of the temporomandibular joint in rabbits. J Oral Rehabil. 1994;21:299–310. doi: 10.1111/j.1365-2842.1994.tb01145.x. [DOI] [PubMed] [Google Scholar]
- 5.Sato S, Goto S, Motegi K. Changes of the collagen fibril arrangement of the rabbit temporomandibular joint following discectomy. J Craniomaxillofac Surg. 2002;30:178–183. doi: 10.1054/jcms.2002.0301. [DOI] [PubMed] [Google Scholar]
- 6.Wilkes CH. Internal derangement of the temporomandibular joint: pathologic variations. Arch Otolaryn-Head Neck Surg. 1989;115:469–477. doi: 10.1001/archotol.1989.01860280067019. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97(3):761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg MB. Posttraumatic temporomandibular disorders. J Orofac Pain. 1999;13:291–294. [PubMed] [Google Scholar]
- 9.Hamerman D. The biology of osteoarthritis. The New Engl J Med. 1989;320:1322–1330. doi: 10.1056/NEJM198905183202006. [DOI] [PubMed] [Google Scholar]
- 10.Xu L, Flahiff CM, Waldman BA, Wu D, Olsen BR, Setton LA, et al. Osteoarthritis-like changes and decreased mechanical function of articular cartilage in the joints of mice with the chondrodysplasia gene (cho) Arthritis Rheum. 2003;48:2509–2518. doi: 10.1002/art.11233. [DOI] [PubMed] [Google Scholar]
- 11.Wadhwa S, Embree MC, Kilts T, Young MF, Ameye LG. Accelerated osteoarthritis in the temporomandibular joint of biglycan/fibromodulin double-deficient mice. Osteoarth Cart. 2005;13(9):817–827. doi: 10.1016/j.joca.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Hu K, Xu L, Cao L, Flahiff CM, Setton LA, Youn I, et al. Pathogenesis of Osteoarthritis-like Changes in Joints of Type IX Collagen-Deficient Mice. Arthritis Rheum. 2007;9:2891–2900. doi: 10.1002/art.22040. [DOI] [PubMed] [Google Scholar]
- 13.Pelletier JP, Faure MP, DiBattista JA, Wilhelmm S, Visco D, Martel-Pelletier J. Coordinate synthesis of stromelysin, interleukin-1, and oncogene proteins in experimental osteoarthritis. An immunohistochemical study. Am J Pathol. 1993;142(1):95–105. [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol. 1998;10(5):602–608. doi: 10.1016/s0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- 15.Arner EC, Pratta MA, Trzaskos JM, Decicco CP, Tortorella MD. Generation and characterization of aggrecanase. A soluble, cartilage-derived aggrecan-degrading activity. J Biol Chem. 1999 May;274(10):6594–6660. doi: 10.1074/jbc.274.10.6594. [DOI] [PubMed] [Google Scholar]
- 16.Lark MW, Bayne EK, Flanagan J, Harper CF, Hoerrner LA, Hutchinson NI, et al. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J Clin Invest. 1997;100(1):93–106. doi: 10.1172/JCI119526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell RJ. Etiology of temporomandibular disorders. Curr Opin Dent. 1991;4:471–475. [PubMed] [Google Scholar]
- 18.Freije JM, Diez-Itza I, Balbin M, Sanchez LM, Blasco R, Tolivia J, et al. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem. 1994;269(24):16766–16773. [PubMed] [Google Scholar]
- 19.Shlopov BV, Lie WR, Mainardi CL, Cole AA, Chubinskaya S, Hasty KA. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum. 1997;40(11):2065–2074. doi: 10.1002/art.1780401120. [DOI] [PubMed] [Google Scholar]
- 20.Poole AR. Imbalances of anabolism and catabolism of cartilage matrix components in osteoarthritis. In: Kuettner KE, Godberg VM, Rosemont, editors. Ostoearthritic Disorders. Illinois: American Association of Orthopaedic Surgeons; 1995. pp. 247–260. [Google Scholar]
- 21.Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46(10):2648–2657. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- 22.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107(1):35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu L, Peng H, Wu D, Hu K, Goldring MB, Olsen BR, et al. Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J Biol Chem. 2005;280:548–555. doi: 10.1074/jbc.M411036200. [DOI] [PubMed] [Google Scholar]
- 24.Lam NP, Li Y, Waldman AB, Brussiau J, Lee PL, Olsen BR, et al. Age-dependent increase of discoidin domain receptor 2 and matrix metalloproteinase 13 expression in temporomandibular joint cartilage of type IX and type XI collagen-deficient mice. Arch Oral Biol. 2007;52:579–584. doi: 10.1016/j.archoralbio.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bomsta BD, Bridgewater LC, Seegmiller RE. Premature osteoarthritis in the Disproportionate micromelia (Dmm) mouse. Osteoarth Cart. 2006;14:477–485. doi: 10.1016/j.joca.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Leitinger B, Steplewski A, Fertala A. The D2 period of collagen II contains a specific binding site for the human discoidin domain receptor, DDR2. J Mol Biol. 2004;344:993–1003. doi: 10.1016/j.jmb.2004.09.089. [DOI] [PubMed] [Google Scholar]
- 27.Konitsiotis AD, Raynal N, Bihan D, Hohenester E, Farndale RW, Leitinger B. Characterization of high affinity binding motifs for the discoidin domain receptor DDR2 in collagen. J Biol Chem. 2008;283(11):6861–6868. doi: 10.1074/jbc.M709290200. [DOI] [PubMed] [Google Scholar]
- 28.Ichikawa O, Osawa M, Nishida N, Goshima N, Nomura N, Shimada I. Structural basis of the collagen-binding mode of discoidin domain receptor 2. EMBO J. 2007;(18):4168–4176. doi: 10.1038/sj.emboj.7601833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunziker EB, Michel M, Studer D. Ultrastructure of adult human articular cartilage matrix after cryotechnical processing. Microsc Res Tech. 1997;37(4):271–284. doi: 10.1002/(SICI)1097-0029(19970515)37:4<271::AID-JEMT3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]