Abstract
Background & Purpose: Tuberculosis is an infectious disease that affects the lungs and results in poor lung compliance secondary to diffuse fibrotic changes to lung tissue. Consequently, people with pulmonary tuberculosis experience impaired gas exchange resulting in a decline in functional capacity. The purpose of this study was to evaluate the physical functional capacity (VO2max) in a group of older (50 – 65 years) people with pulmonary tuberculosis and to compare them to an age-matched healthy group. A secondary purpose was to develop reference equations that could be used to predict 6 minute walk test (6MWT) distance in older, healthy people in India. Methods: Sixty healthy subjects (30 male and 30 female) and 60 subjects with a diagnosis of pulmonary tuberculosis (30 male and 30 female) participated in the study. All subjects underwent a 6MWT. Walk-work was calculated and used for evaluating functional capacity. Group comparison for functional capacity was done using 2-tailed t-tests. Pearson product correlation was used to examine for significant relationships and regression analysis was used to derive reference equations. Results: There was a significant difference between groups in regard to functional capacity and 6MWT distance (p < 0.001). Reference equations were developed that use age, height, and weight as predictors for 6MWT distance in the healthy group. Conclusion: The sequelae from pulmonary tuberculosis have considerable impact on functional capacity in older people in India.
Key Words: six minute walk test, tuberculosis, functional capacity
INTRODUCTION
In 2008, India ranked first globally in the total number of incident cases of tuberculosis (1.6-2.4 million cases) with an incidence rate of 168/100,000 cases per year.1 Tuberculosis often results in diffuse fibrotic changes to lung tissue as well as lung tissue consolidation leading to a reduction in overall lung compliance. Consequently, the functional status of persons following tuberculosis is diminished secondary to poor ventilation and gas exchange leading to progressive dyspnea, deconditioning, and an overall decline in functional status.
Functional walk tests are typically administered as a means of evaluating functional status, monitoring effectiveness of treatment, and establishing prognosis. The ability to ambulate for a distance is a quick and inexpensive measure of physical function and an important component of quality of life since it reflects the capacity for undertaking everyday activities.2 Functional walk tests either alone or in combination with other exercise tests have been validated in people with chronic cardiac and pulmonary disease,3,4 chronic obstructive pulmonary diseases and asthma,5 older adults with chronic heart failure,6 and in healthy, elderly adults.2
The six minute walk test (6MWT) is an example of a functional walk test that is practical and simple and only requires the ability to walk. The distance that a patient can walk on a flat surface in 6 minutes may be used as a generic one-time measure of functional status or as an outcome measure from a rehabilitation program. The outcome of the 6MWT can also be expressed in terms of work (body weight × meters walked), which considers the energy required to move the body mass a given distance. 7 The 6MWT is a well-established, valid, and reliable measure of aerobic capacity in older people with cardiac, pulmonary, and peripheral vascular disorders.8–11 The 6MWT is strongly correlated with performance-based measures of functional limitations such as chair stand time and gait velocity in healthy older adults.12 Furthermore, 6MWT performance is strongly associated with established functional measures such as leg muscle strength and power in healthy older adults.13 Currently, there is little research describing the differences in the functional limitation in patients with pulmonary sequelae as a result of tuberculosis as compared to those who are healthy. An understanding of the functional limitations in these patients as compared to age-matched healthy people may be useful in developing appropriate rehabilitation programs in these patients as well as quantifying the level of disability.
The 6MWT would be useful for characterizing the functional limitations in people who have pulmonary sequelae from tuberculosis; however, reports on exercise testing and physical functional capacity evaluation in people with chronic respiratory failure as a consequence of tuberculosis are very rare. This is quite surprising considering that a high number of people in India have pulmonary sequelae from tuberculosis. To correctly interpret the results of the 6MWT, the result from a given patient must be compared to the appropriate reference values for that specific population. Since there are many predictors for 6MWT distance (6MWD), it is also necessary to develop reference equations to better interpret the results for comparison between the healthy and tuberculosis sequelae populations. Therefore, the purpose of this study was two-fold. First, we evaluated the physical functional capacity of older people with tuberculosis sequelae and compared the findings to an age-matched group of people. The second purpose of this study was to derive reference equations that could be used to predict 6MWD in healthy older people in India.
METHODS
Participants
Sixty (30 male and 30 female) patients with tuberculosis sequelae between 50-65 years of age were selected from the inpatient department of the Government Hospital of Chest Diseases in Puducherry-6. Sixty (30 male and 30 female) healthy age-matched people from the normal population were chosen as a comparison group.
All of the patients with tuberculosis were stable and all were taking medications to manage their tuberculosis. Patients with other pulmonary diseases were excluded from the group with tuberculosis sequelae. Exclusion criteria specific to the comparison group included excluding those who chewed tobacco, smoked, or drank alcohol. Persons with coronary artery disease, myocardial infarction, cardiac surgeries, abdominal surgeries within the previous 6 months, history of fracture within 6 months especially at the spine and hip, arthritis, back pain, acute illness or injury on the day of the functional assessment, renal disease, peripheral vascular disease, and lower limb weakness and deformities were excluded from both groups.
Experimental Design and Protocol
An observational case control study and a purposive sampling design were used. All test procedures, the purpose of the study and possible risks associated with participating in the study were explained in detail to all subjects who met the inclusion and exclusion criteria of the study. Informed consent was obtained from them in their vernacular language. The study was approved by the Pondicherry University ethical board.
The materials used to administer the 6MWT and the preparation of subjects was done using American Thoracic Society (ATS) guidelines.14 Test-retest reliability for measurements obtained with this test are 0.94 – 0.96 as obtained via intraclass correlation coefficients.4,11 Construct validity of the test in patients with pulmonary disease are 0.63 – 0.79 when correlating 6MWD and peak oxygen consumption.15 Sensitivity and specificity of the 6MWT for prediction of death in patients awaiting lung transplants is 0.80 and 0.27, respectively.16
General Assessment
Using a stadiometer, each subject's standing height and weight was measured with a balance beam scale that is calibrated monthly. Demographic details, present and past medical history, and surgical details, if any, were obtained on each subject. Their corresponding medical charts were also reviewed before conducting the test.
Cardiovascular Assessment
Prior to administration of the 6MWT, systolic blood pressure was recorded as well as Borg scores for dyspnea and leg fatigue and resting heart rate. History of stroke, hypertension, cardiac disease, or any past cardiac or pulmonary surgery was noted for excluding participants for the current study.
Pulmonary Assessment
Pulmonary data regarding medications taken for tuberculosis sequelae and limitation of activity secondary to disease was collected. History of lung cancer or any other serious pulmonary disease, lung surgery was also noted for excluding participants for the current study.
6MWT
A 30 m hospital corridor marked by colored tape at each end was used. Subjects were instructed to walk from end to end at their self-selected pace, while attempting to cover as much distance as possible in the 6 minutes. The time and distance covered were recorded. The Borg scales for dyspnea and leg fatigue, as well as heart rate were recorded before and immediately after completion of the walk test.
From the collected data, the distance covered in meters was converted to walk work (kg-m) by multiplying body weight in kilograms by distance covered. Walk work was then converted into energy expenditure by the following equations17:
Energy expenditure (Kcal) = walk work (Kg m) ÷ 426.8 (or) walk work × 2.342 × 10−3
Kcal/min = Energy expenditure (Kcal) ÷ 6
Finally, kcal/min was converted to oxygen consumption by the following equations15:
O2 (l/min) = (Kcal/min) × 5, since 1 litre = 5kcal
VO2 MAX (ml/kg/min) = [O2(l/min) × 1000]÷ body weight (kg)
Using these VO2max values, subjects were classified into functional levels (FL). The FL are as follows: FL-4 ≤ 3.5 ml/kg/min; FL-3 between 3.6 and 10.5 ml/kg/min; FL-2 between 10.6 and 21 ml/kg/min; FL-1 ≥ 21 ml/kg/min.18
Statistical Analysis
All data analysis was performed using statistical software SPSS 16.0 version. Descriptive statistics were calculated and expressed as mean ± standard deviation (SD). The data was checked for normality using the Kolmogorov-Smirnov Z test for individual variables of both groups. Pearson product correlation was used to assess for relationships among appropriate variables.
To discern statistically significant differences between groups, a two tailed independent sample ‘t’ test was used. The level of significance was set at 0.05 with a 95% confidence interval. Multiple linear regression analysis was calculated using the ordinary least square method. In these analyses, 6MWD was a dependent variable and age, height, weight, and BMI were independent variables used to explain the variance in 6MWD.
Distance walking for 6 minutes was calculated as a linear function of weight, height, BMI, and age. Accordingly, 6MWD was considered the dependent variable. Other study variables such as age, height, weight, and BMI measured with minimum error were independent variables. Linear relationship was established with all the independent variables. It was a forced regression as we have included all the study variables in the regression analysis. Even though the coefficient for age was not statistically significant, due to biological significance age was retained in the regression analyses.
RESULTS
Sixty males (30 healthy; 30 with TB) and 60 females (30 healthy; 30 with TB) completed the 6MWT. The VO2max values derived from the calculation of walk work was used as a measure of physical functional capacity and to functionally classify the subjects. All healthy subjects, both male and female had a VO2max > 21 ml/kg/min, while in the tuberculosis (TB) sequelae groups, only 60% of males and 36% of females had a VO2max > 21 ml/kg/min. Thirty-six percent of the male TB sequelae group had a VO2max between 10.6 and 21 ml/min/kg and 56.6% of females were in this same range of oxygen consumption. Only 3.3% of males with TB sequelae and 6.6% of females with TB sequelae had a VO2max between 3.6 and 10.5 ml/kg/min.
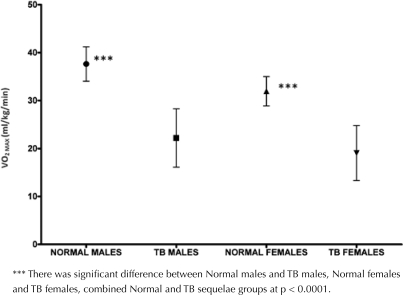
Descriptive statistics of each variable are shown in Table 1. The data was tested for normality using the Kolmogorov-Smirnov Z test under the null hypothesis that the sample data were normally distributed. The significance values for all study variables across all groups were greater than 0.05 indicating a normal distribution of the data (Table 2). There was a significantly lower mean 6MWD and VO2max in TB sequelae group than in older healthy group (p < 0.001) (Table 3). There was no significant difference between groups in regards to age and height, but participants with TB weighed significantly less than the healthy participants (Table 3).
Table 1.
Characteristics of Study Participants
| VARIABLE | NORMAL MALES (n = 30) | NORMAL FEMALES (n = 30) | TB - MALES (n = 30) | TB - FEMALES (n = 30) | ||||
|---|---|---|---|---|---|---|---|---|
| MEAN ± SD | RANGE | MEAN ± SD | RANGE | MEAN ± SD | RANGE | MEAN ± SD | RANGE | |
| ACE | 57.70 ± 5.36 | 15 | 56.47 ± 5.23 | 15 | 56.1 ± 5.1 | 15 | 56.3 ± 5.26 | 15 |
| HEIGHT | 156.67 ± 7.82 | 30 | 145.71 ± 8.49 | 36 | 159.63 ± 5.71 | 23 | 146.96 ± 6.38 | 21.9 |
| WEIGHT | 56.1 7 ± 10.76 | 41 | 57.53 ± 11.36 | 46 | 39.6 ± 5.73 | 23 | 36.9 ± 9.1 | 36 |
| BMI | 22.80 ± 3.48 | 14.3 | 26.80 ± 4.17 | 14.4 | 15.54 ± 2.11 | 8.1 | 16.69 ± 3.58 | 13.7 |
| 6MWD | 482 ± 45.89 | 180 | 408 ± 39.86 | 150 | 285 ± 79.18 | 285 | 245.5 ± 73.11 | 2 70 |
| VO2MAX | 37.74 ± 3.59 | 14.08 | 31.94 ± 3.05 | 11.75 | 22.19 ± 6.09 | 22.37 | 19.05 ± 5.73 | 21.14 |
Age in years, Height in centimetres, Weight in kilograms, 6MWD in meters, Vo2max in ml/kg/min.
Table 2.
Normality Test for Variables In Both Groups
| VARIABLE | KOLMOGOROV-SMIRNOV Z TEST (NORMALITY) | |||||
|---|---|---|---|---|---|---|
| NORMAL (n = 60) | TB SEQUELAE (n = 60) | |||||
| MEAN ± SD | Z-VALUE | SIGNIFICANE (2-tailed) | MEAN ± SD | Z-VALUE | SIGNIFICANCE (2-tailed) | |
| AGE (yrs) | 55.72 ± 5.28 | .939 | .342 | 56.06 ± 5.13 | 1.109 | .171 |
| HEIGHT (cm) | 151.19 ± 9.79 | .656 | .783 | 153.29 ± 8.76 | .585 | .883 |
| WEIGHT (kg) | 56.85 ± 10.99 | 1.033 | .236 | 38.25 ± 7.66 | .979 | .294 |
| BMI | 24.82 ± 4.31 | .690 | .727 | 16.11 ± 2.97 | .893 | .403 |
| 6MWD (m) | 445 ± 56.64 | 1.450 | .030 | 265.06 ± 78.13 | 1.201 | .112 |
| VO2MAX (ml/kg/min) | 34.79 ± 4.37 | 1.360 | .050 | 20.62 ± 6.07 | .980 | .293 |
Table 3.
Comparison of all Variables Between Normal and TB Sequelae Group
| VARIABLE | NORMAL vs. TB SEQUELAE | |||
|---|---|---|---|---|
| t VALUE | P VALUE | CONFIDENCE INTERVAL | ||
| LOWER LIMIT | UPPER LIMIT | |||
| AGE (yrs) | 0.928 | 0.355 | 1.542 | 2.222 |
| HEIGHT (cm) | 1.242 | 0.216 | 1.252 | 5.469 |
| WEIGHT (kg) | 10.752 | <0.0001 | 15.174 | 22.025 |
| BMI | 12.852 | <0.0001 | 7.349 | 10.026 |
| 6MWD (m) | 14.427 | <0.0001 | 155.077 | 204.422 |
| VO2MAX (ml/kg/min) | 14.675 | <0.0001 | 12.258 | 16.084 |
Pearson product correlations with 6MWD for height and body mass index (BMI) were significant in the normal group and for weight and BMI in the TB sequelae group (Table 4). Height, weight, and BMI were significant when entered into the regression model, whereas age was not. Significance level for the regression equation was tested using ANOVA in which the equation using age, height, and weight was highly significant (p < 0.001), while the equation using age and BMI was significant at the 0.05 level. The reference equations for 6MWD for the normal group are provided in Table 5.
Table 4.
Pearson Correlation Between 6MWD and Variables
| 6MWD | NORMAL (n = 60) | TB SEQUELAE (n = 60) | ||
|---|---|---|---|---|
| PEARSON CORRELATION | SIGNIFICANCE (2-tailed) | PEARSON CORRELATION | SIGNIFICANCE (2-tailed) | |
| AGE (yrs) | −.009 | .947 | .143 | .2 76 |
| HEIGHT (cm) | .526 (**) | .000 | .183 | .162 |
| WEIGHT (kg) | .059 | .653 | .368 (**) | .004 |
| BMI | −.311 (*) | .015 | .267 (*) | .039 |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
Table 5.
Regression Analysis for Six Minute Walk Distance
| REFERENCE EQUATIONS FOR SIX MINUTE WALK DISTANCE IN NORMAL OLDER CROUP: |
| 6MWD**= 14.708 − [(1.219) × (age)] + [(3.870) × (height)] − [(1.498) × (weight)]. |
| Alternate equation using BMI: |
| 6MWD* = 621.075 − [(1.1 35) × (age)] − [(4.486) × (BMI)]. |
| (Age in years, Height in centimetres, Weight in kilograms, BMI in kg/m2, Distance in meters) |
| Definition of abbreviations: BMI = body mass index; 6MWD = 6-min-ute walk distance. |
| REFERENCE EQUATION FOR 6MWD | R2 | R | p VALUE |
|---|---|---|---|
| Using age, height & weight | 0.336 | 0.580 | 0.000 |
| Using age & BMI | 0.107 | 0.327 | 0.038 |
P < 0.01
P < 0.05
A comparison of the calculated VO2max between the normal male and female groups with the TB sequelae male and female groups is shown in Figure 1.
Figure 1.
Comparison of VO2 MAX in all groups.
DISCUSSION
The 12 minute walk test was introduced in 1968 as a guide to assess physical fitness19 and was later used in people with chronic obstructive pulmonary disease.20 It was subsequently determined that the time of the test could be decreased to 6 minutes without reducing the utility of the test.21 The 6MWT has been validated by high correlations of workload, heart rate, and oxygen saturation when compared to standard bicycle ergometry and treadmill exercise tests.22 A significant learning effect is observed when the test is performed on two successive days, with a mean 15% improvement in distance covered,21 but this effect was not important when determining cross-sectional correlations or when using the results as a baseline predictor of future events.
The 6MWD is intended to account for work (energy expenditure)24 that can be calculated as the force X distance traveled (6MW work). Consequently, it seems logical to include force (body weight) as well as walk distance, when assessing an individual's ability to ambulate. Carter et al7 used a receiver operating curve to demonstrate that the area under the curve was significantly greater for 6MW work in comparison to using only the 6MWD for the same individuals with improved sensitivity and specificity for the 6MW work calculation in comparison to the performance of the 6MWD. Therefore, the calculation and reporting of 6MW work appears to be a viable option for measuring functional capacity and provides the rationale for why we used the 6MW work calculation to determine VO2max in our subjects. Indeed, the BMI and the 6MWD of the subjects in the TB sequelae group was significantly lower than that of the normal subjects and our findings indicate that BMI is correlated to 6MWD. This implies that the body weight of a subject directly affects the work required to perform the walk.
The average 6MWD was 613 ± 93 m in a healthy, older population25 and was 659 ± 62 m in Caucasian healthy subjects.26 In contrast, the results of our study indicate that in the older, normal Indian population, the average 6MWD was lower. In comparison, the TB sequelae group had an average 6MWD that was even lower.
Although we did not directly measure oxygen saturation levels in our subjects, exercise hypoxia27 may have also played a role in the decreased 6MWD observed in the group with TB sequelae. Future studies should examine oxygen saturation as a possible contributor to the prediction of VO2max in this patient population.
The main limitation in this study was sample size, as a very large sample size is necessary for deriving highly predictive reference equations. In this study, we used walk work to calculate VO2max. Future studies should consider using rate pressure product, heart rate, and Borg's rating of perceived exertion to predict VO2max. Caution should be used when applying our reference equations to older people who have characteristics such as age, height, weight, BMI, and also inclusion and exclusion criterias that fall outside our cohort. A larger 6MWD may be expected from persons who have previously performed the 6MWT and when nonstandardized encouragement is provided.
CONCLUSION
There was a significant difference in physical functional capacity between a group of normal older people and those with TB sequelae. In the TB sequelae group, 51.6% had a VO2max below 21 ml/kg/min that resulted in a considerable impact of TB sequelae on cardiorespiratory endurance. The reference equations for 6MWD derived in this study computes the predicted 6MWD in an Indian population without TB. Future studies are recommended to examine other factors that may be included in the equation to predict VO2max.
ACKNOWLEDGEMENTS
This research work was supported by Dr. V. Balu, Dean, M.T.P.G & R.I.H.S; Prof. Supriya. K. Vinod, Principal, College of Physiotherapy & HOD Cardio-Respiratory Physiotherapy; Dr S. S. Prabu, Medical Superintendent, Government Hospital for Chest Diseases, Puducherry-6, granting permission for data collection; & Lecturer Mr. G. Alagumoorthi, College of Physiotherapy, M.T.P.G & R.I.H.S.
REFERENCES
- 1.World Health Organization Tuberculosis – TB in South-East Asia-Epidemiology. http://www.who.int/tb/publications/global_report/2009/update/tbu_9.pdf Accessed January 31, 2010. [Google Scholar]
- 2.Enright PL, Sherril DL. Reference equations for the sixminute walk in health adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 3.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 4.Guyatt GH, Thompson PJ, Berman LB, et al. How should we measure function in patients with chronic heart and lung disease? J Chronic Dis. 1985;38:517–524. doi: 10.1016/0021-9681(85)90035-9. [DOI] [PubMed] [Google Scholar]
- 5.Mak VH, Bugler JR, Roberts CM, Spiro SG. Effect of arterial oxygen desaturation on six minute walk distance, perceived effort, and perceived breathlessness in patients with airflow limitation. Thorax. 1993;48:33–38. doi: 10.1136/thx.48.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riley M, McParland J, Stanford CF, Nicholls DP. Oxygen consumption during corridor walk testing in chronic cardiac failure. Eur Heart J. 1992;13:789–793. doi: 10.1093/oxfordjournals.eurheartj.a060258. [DOI] [PubMed] [Google Scholar]
- 7.Carter R, David B, Holiday DB, et al. 6-Minute walk work for assessment of functional in patients with COPD. Chest. 2003;123:1408–1415. doi: 10.1378/chest.123.5.1408. [DOI] [PubMed] [Google Scholar]
- 8.Simonsick EM, Gardner AW, Poehlman ET. Assessment of physical function and exercise tolerance in older adults: reproducibility and comparability of five measures. Aging (Milano) 2000;12:274–280. doi: 10.1007/BF03339847. [DOI] [PubMed] [Google Scholar]
- 9.Bittner V, Weiner DH, Yusuf S, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA. 1993;270:1702–1707. [PubMed] [Google Scholar]
- 10.Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardio respiratory domain. Chest. 2001;119:256–270. doi: 10.1378/chest.119.1.256. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc. 1998;46:706–711. doi: 10.1111/j.1532-5415.1998.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 12.Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80:837–841. doi: 10.1016/s0003-9993(99)90236-8. [DOI] [PubMed] [Google Scholar]
- 13.Bean JF, Kiely DK, Leveille SG, et al. The 6-minute walk test in mobility-limited elders: what is being measured? J Gerontol A Biol Sci Med Sci. 2002;57:M751–M756. doi: 10.1093/gerona/57.11.m751. [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society Guidelines for the six minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 15.Cahalin L, Pappagianopoulos P, Prevost S, Wain J, Ginns L. The relationship of the 6-min walk test to maximal oxygen consumption in transplant candidates with end-stage lung disease. Chest. 1995;108:452–459. doi: 10.1378/chest.108.2.452. [DOI] [PubMed] [Google Scholar]
- 16.Kadikar A, Maurer J, Kesten S. The six-minute walk test: a guide to assessment for lung transplantation. J Heart Lung Transplant. 1997;16:313–319. [PubMed] [Google Scholar]
- 17.William DM, Frank IK, Victor LK. Exercise Physiology: Energy, Nutrition, and Human Performance, 6th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007. p. 203. 1062, 1068. [Google Scholar]
- 18.Fortuin NJ, Weiss JL. Exercise stress testing. Circulation. 1977;S56(5):699–712. doi: 10.1161/01.cir.56.5.699. [DOI] [PubMed] [Google Scholar]
- 19.Cooper KH. A means of assessing maximal oxygen intake. Correlation between field and treadmill testing. JAMA. 1968;203:201–204. [PubMed] [Google Scholar]
- 20.McGavin CR, Artvinli M, Naoe H, McHardy CJ. Dyspnea, disability, and distance walked: comparison of estimates of exercise performance in respiratory disease. BMJ. 1978;2:241–243. doi: 10.1136/bmj.2.6132.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butland RJA, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12-minute walk tests in respiratory disease. BMJ. 1982;284:1607–1608. doi: 10.1136/bmj.284.6329.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langenfeld H, Schneider B, Grimm W, et al. The six minute walk test: an adequate exercise test for pacemaker patients? PACE. 1990;13:1761–1765. doi: 10.1111/j.1540-8159.1990.tb06886.x. [DOI] [PubMed] [Google Scholar]
- 23.Leach RM, Davidson AC, Chinn S, Twort CH, Cameron IR, Bateman NT. Portable liquid oxygen and exercise ability in severe respiratory disability. Thorax. 1992;47:781–778. doi: 10.1136/thx.47.10.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuang ML, Lin IF, Wasserman K. The body weight-walking distance product as related to lung function, anaerobic threshold and peak VO2 in COPD patients. Respir Med. 2001;95:618–626. doi: 10.1053/rmed.2001.1115. [DOI] [PubMed] [Google Scholar]
- 25.Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999;14:270–274. doi: 10.1034/j.1399-3003.1999.14b06.x. [DOI] [PubMed] [Google Scholar]
- 26.Camarri B, Eastwood P, Cecins N, Thompson PJ, Jenkins S. Six minute walk distance in healthy subjects aged 55–75 years. Respir Med. 2006;100:658–665. doi: 10.1016/j.rmed.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Kawashiro T. Evaluation of respiratory failure due to sequelae of tuberculosis. Kekkaku. 2005;80(6):491–497. [PubMed] [Google Scholar]