Abstract
Objective
Humanin, an endogenous anti-apoptotic peptide, has previously been shown to protect against Alzheimer’s disease and a variety of cellular insults. We evaluated the effects of a potent analog of humanin, HNG, in an in vivo murine model of myocardial ischemia and reperfusion (MI-R).
Methods
Male C57BL6/J mice (8–10 week old) were subjected to 45 min of left coronary artery occlusion followed by 24 hr reperfusion. HNG or vehicle was administered intra-peritoneally one hour prior or at the time of reperfusion. The extent of myocardial infarction per area-at-risk was evaluated at 24 hrs using Evans Blue dye and 2,3,5 triphenyltetrazolium chloride (TTC) staining. Left ventricular (LV) function was evaluated at one week post ischemia using high-resolution, 2- D echocardiography (VisualSonics Vevo 770). Myocardial cell signaling pathways and apoptotic markers were assessed at various time points (0–24 hrs) following reperfusion. Cardiomyocyte survival and apoptosis in response to HNG were assessed in vitro.
Results
HNG reduced infarct size relative to the area-at-risk in a dose dependent fashion, with a maximal reduction at the dose of 2 mg/kg. HNG therapy enhanced LV ejection fraction and preserved post-ischemic LV dimensions (end-diastolic and end-systolic), resulting in improved cardiac function. Treatment with HNG significantly increased the expression of pAMPK and p-eNOS in the heart and attenuated Bax and Bcl-2 levels following MI-R. HNG improved cardiomyocyte survival and decreased apoptosis in response to daunorubicin in vitro.
Conclusions
These data show that HNG provides cardioprotection in a mouse model of MI-R potentially through activation of AMPK-eNOS mediated signaling and regulation of apoptotic factors. HNG may represent a novel agent for the treatment of acute myocardial infarction.
Keywords: Humanin, myocardium, ischemia-reperfusion
Introduction
Heart disease is a major cause for morbidity and mortality and is the leading cause of death in the United States. In 2009, heart disease was projected to cost more than $304.6 billion, including health care services, medications, and lost productivity (1). Among heart diseases, Coronary heart disease is the principal type accounting for about 1 of every 5 deaths in the United States (2). Therefore, identifying novel therapeutic agents that improve survival in coronary vascular disease is of paramount significance considering the tremendous health care burden associated with this disease.
We have recently studied the role of a novel peptide called Humanin (HN) on its role in improving insulin action (3). HN is a 24 aminoacid polypeptide that was initially characterized for its protective effects against various Alzheimer’s disease associated insults (4; 5). HN has been shown to enhance cell survival in prion induced apoptosis (6), as well as in serum deprived conditions in lymphocytes (7). The cytoprotective effects are broad, extending to both neuronal and non-neuronal cells (8–10) and the effects appear to be mediated both via an extracellular receptor such as CNTFR-α/gp130/WSX-1 complex (11) as well as intracellular mechanisms (12). HN binds pro-apoptotic Bax, a member of the Bcl-2 family, inhibits its mitochondrial localization, and attenuates Bax-mediated apoptosis activation (12). The Bcl-2 family consists of both pro-apoptotic factors such as Bid and Bim, and anti-apoptotic proteins including Bcl-2. The ability of the Bcl-2 family to regulate cellular survival has been shown to be determined in part by a balance between its pro-apoptotic and anti-apoptotic members. HN has also been shown to bind and inactivate other pro-apoptotic members of the Bcl2 family including Bid and Bim (13) (14). In addition to direct protein-protein interactions, HN has been shown to activate STAT-3 (15), Jnk (16) and tyrosine kinases (15), all of which have been implicated in its actions.
Since its initial discovery, several cDNAs sharing similar sequence homology to HN have been identified in plants, nematodes, and rodents demonstrating that HN is evolutionarily conserved (12). HN is transcribed from an open reading frame within the mitochondrial 16S ribosomal RNA (17). Endogenous HN is both an intracellular and secreted protein and has been detected in skeletal muscle, brain, liver, testis and colon at specific stages of development (3; 17). In addition, HN is present in cerebral spinal fluid (CSF), seminal fluid and plasma (3).
Interestingly, single amino acid substitutions of HN can lead to significant alterations in its potency and biologic functions. HNG (HN in which the serine at position 14 is replaced by glycine), is a highly-potent analogue of HN. HNG has been shown to reverse the learning and memory impairment induced by scopolamine in mice (18) and has rescue activity against memory impairment caused by AD-related insults in vivo (19). HNG has also been shown to protect against neuronal cell death in an animal model of stroke (20).
Considering the role HN plays in promoting cell survival, we hypothesized that HNG may protect the heart against myocardial ischemia-reperfusion (MI-R) injury. We tested this hypothesis using a mouse model of MI-R and analyzed potential cytoprotective pathways and downstream signaling mechanisms.
Materials and Methods
Animal Preparation for in vivo Studies
Male, 8–10 week old C57BL/6J mice from the Jackson Laboratory (Bar Harbor, ME) were utilized in the present study. HNG peptide (18–20) was custom synthesized and >99% HPLC purified by GeneMed Inc (San Antonio, Texas). Synthetic HNG peptide was dissolved in saline and administered at different doses (0.04 mg/kg. 0.2 mg/kg, 0.4 mg/kg, 1 mg/kg or 2 mg/kg) as mentioned in the experiments while the control group received vehicle (saline). The route of administration was either intra-peritoneal (i.p., prior to ischemia) or intracardiac (at the time of reperfusion). The dose that produced the maximal response, 2 mg/kg was used in experimental groups that received HNG intra-cardiac at the time of reperfusion. The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine.
Myocardial Ischemia-Reperfusion Protocol (MI-R)
Surgical ligation of the left coronary artery (LCA) was performed as described previously (21–23). Briefly, mice were anesthetized with ketamine (50 mg/kg) and pentobarbital sodium (50 mg/kg), orally intubated, and connected to a rodent ventilator. A median sternotomy was performed; the LCA was visualized and ligated using a 7-0 silk suture mated to a BV-1 needle along with a short segment of PE-10 tubing. Mice were subjected to 45 min of LCA ischemia followed by varying periods of reperfusion. For determination of infarct size, a 24 hour reperfusion was performed. Left ventricular area at risk (AAR) and infarct size (Inf) were determined at 24 hours by methods previously described (21–23). For studies investigating signaling mediators, the time of reperfusion was based on the study groups with reperfusion periods ranging from 30 minutes to 24 hrs (i.e., 30 min, 4 hr, 12 hr, and 24 hr post-reperfusion). A 7-day reperfusion was performed in group of animals that had cardiac function assessed by echocardiogram.
Myocardial Area-at-Risk and Infarct Size Determination
Left ventricular area-at-risk (AAR) and infarct size (Inf) determination was performed using Evans Blue dye and 2, 3, 5-triphenyltetrazolium chloride (TTC; Sigma Chemical) staining method. All of the procedures for the AAR and Inf determination have been previously described (21–23). AAR and Inf determinations were performed in a blinded fashion.
Echocardiographic Assessment of Left Ventricular Structure and Function
Baseline echocardiography (ECHO) images were obtained 1 week before LCA ischemia to avoid any confounding effects of anesthesia as previously described using a Visualsonics VEVO 770 high-resolution ultrasound system (21). One week post MI-R, a repeat Echo was performed and images were analyzed in a blinded manner.
Signaling Studies and Protein Analysis
For acute signaling studies, mice were injected with either vehicle (sham) or HNG intra-peritoneally (i.p), and were sacrificed at 15 min, 30 min, 4 hr or 24 hr later. The animals were killed by decapitation without sedation; this method was specifically chosen to avoid the confounding effects of anesthesia on signaling mediators. Whole hearts were rapidly excised, rinsed thoroughly with saline, snap frozen in liquid nitrogen and stored at −80°C. For signaling studies following MI-R, mice were treated with either HNG or vehicle at the time of reperfusion and were sacrificed either 30 min, 4 hr, 12 hr or 24 hr post reperfusion. The ischemic portion of the heart comprising primarily the left ventricle was carefully dissected away from the non-ischemic area of the heart, frozen in liquid nitrogen and stored at −80°C.
Immunoblotting was performed as described before (3; 24; 25). Briefly, frozen heart tissue was pulverized in a liquid-nitrogen cooled mortar and pestel and protein was extracted from powdered tissue using an ice-cold RIPA buffer and quantified using the BCA method. For Western blotting, 30 µg of total protein was electrophoresed on Bis-Tris 4–12% gradient gels (Biorad, Hercules, CA), and wet-transferred onto PVDF membranes(11). Bands were visualized by chemiluminescence (Super West Dura kit, Pierce Biotechnologies, Rockford, IL) with a 16-bit camera imaging system (Fuji LAS-3000, Fujifilm, Stamford, CT) until a saturated pixel was observed and densitometry performed using multi-gauge software. Antibodies to STAT-3, pSTAT-3Tyr705, AKT, pAKTSer473, eNOS, pENOSSer1177, AMPK, pAMPKThr172,MAPK, pMAPKThr202/Tyr204, Bcl2, GAPDH were all obtained from Cell Signaling (Danvers, MA) and anti-Bax antibody was obtained from Abcam (Cambridge, MA). Protein levels of endogenous mouse HN were measured using a rabbit polyclonal antibody to Rattin (RN; 1:500, GeneTex, Irvine, CA), which is the rat homolog to HN.
Cell specific expression of endogenous HN in heart
Cardiomyocytes were isolated from 10 wk old C57BL/6J mice using a modification of AFCS Procedure Protocol PP00000125 (26). Briefly, hearts were digested with modified Joklik’s media (11g/L Joklik’s medium, 34.5 mM NaHCO3, 0.49 mM MgSO4, and 10 mM 2,3-Butanedione Monoxime, pH 7.2) containing collagenase (Worthington Type II, Worthington Biochemical Corp., Lakewood, NJ), bovine serum albumin (1 mg/ml), and 12.5 µM CaCl2 for 10–15 min. Isolated cardiomyocytes were pelleted and then resuspended in myocyte culture medium (Medium 199 with Earle’s salts, L-glutamine, and 2.2 g/L NaHCO3 containing 0.1 mg/ml BSA, 100 U/ml Penicillin/Streptomycin, and 2 mM L-Glutamine). Cardiomyocytes were plated on laminin-coated 35 mm dishes at 35,000 cells per plate, incubated at 37°C, 5% CO2 for 1 hour, then non-adherent myocytes were aspirated, the plate rinsed with PBS, and snap frozen.
Mouse aortic smooth muscle cells were purchased from ATCC and Human umbilical vein endothelial cells were kindly provided by Dr. D. Casper at AECOM. The cells were cultured as per the recommended protocol and when confluent, were lysed, protein extracted and western blot analysis performed as described earlier for detection of endogenous HN.
Effect of HNG on cardiomyocyte survival and apoptosis in vitro
Rat heart myocardium cell line H9c2 (2-1) (cat# CRL-1446) was purchased from ATCC. Cells were cultured in DMEM containing antibiotics penicillin/streptomycin and 5% FBS as suggested by ATCC. They were incubated at 37°C in 5% CO2. Cell Viability was monitored using Resazurin Cell Viability Assay Kit (cat# 30025-1, Biotium Inc, Hayward, CA) in a 96 well plate as per the company’s protocol. In brief, to 5000 H9c2 cells per well, 100µl of 1µM daunorubicin (Sigma, St. Louis, MO) was added and incubated for 36h to induce apoptosis. To check the ability of HNG to protect against cell death, 1nM HNG was added 30min prior to the addition of daunorubicin.
CaspACE FITC-VAD-FMK (cat# G7461, Promega Corporation, was next used in an assay for apoptosis in H9c2 cells. This FITC conjugated cell permeable caspase inhibitor VAD-FMK binds to activated caspase serving as an in situ marker for apoptosis. H9c2 cells were grown on polylysine coated coverslips. They were treated with 100nM daunorubicin in presence or absence of 10nM HNG for 18 hr to induce apoptosis. HNG was added 30min prior to the addition of daunorubicin. 10µM CaspACE FITC-VAD-FMK in DMEM (without FBS) was added and cells were incubated for 20min in the incubator protected from light. Cells were washed to remove excess VAD-FMK and fixed in 4% formaldehyde for 15min, rinsed with PBS and stained with DAPI for 10 min. Coverslips were rinsed with PBS, mounted on slides using antifade mounting medium, observed using a Zeiss Axioscop Light microscope with attached camera, and images taken at 10X magnification for analysis (using Axiocam and Zeiss Axiovision software).
Statistical Analysis
All data are expressed as means ± SE. Comparisons were made by either Student’s paired two-tailed t tests or ANOVA when appropriate. When a significant main effect was observed, planned contrasts were performed with Tukey’s adjustment. Probability values of p ≤ 0.05 were considered statistically significant. All statistical analyses were performed using SPSS v16 (SPSS Inc, Chicago, IL).
Results
HN is expressed in Heart
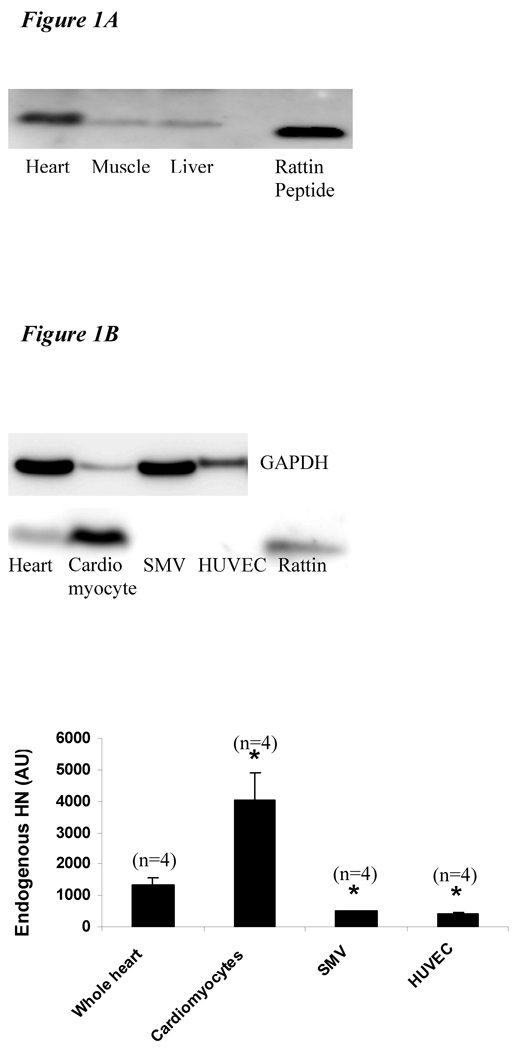
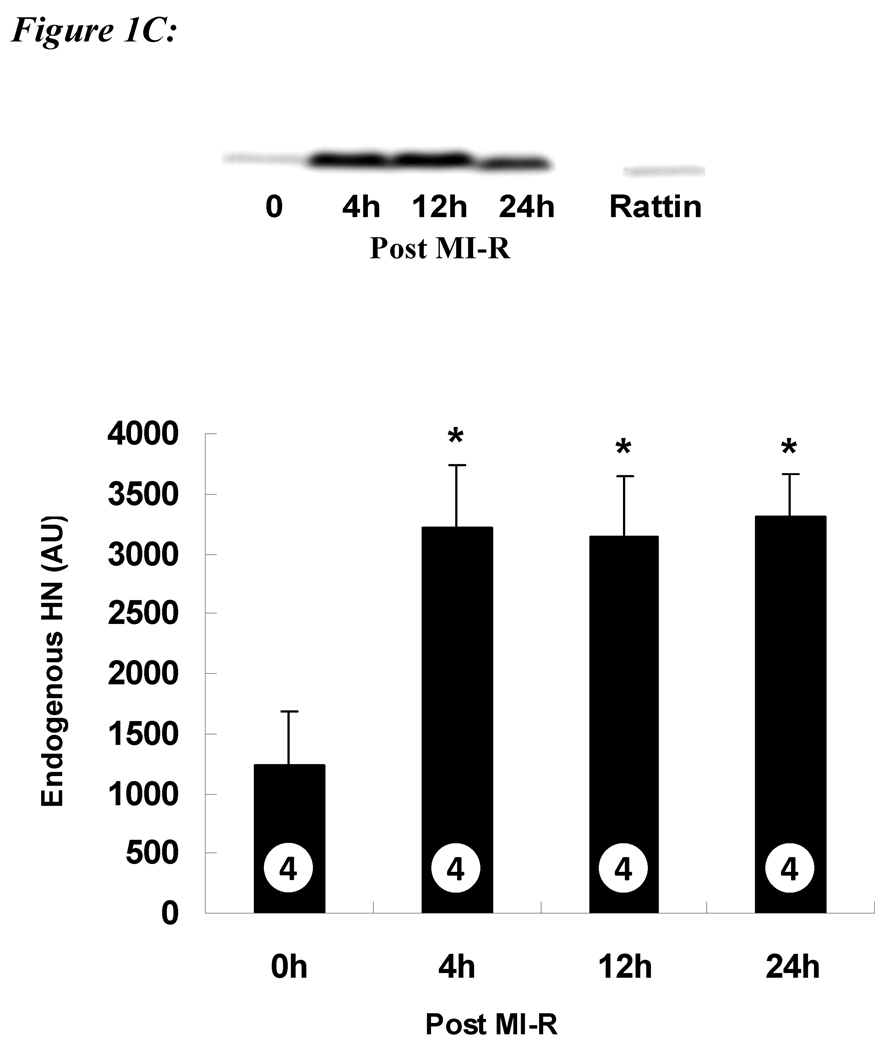
To confirm the presence of HN, we analyzed the expression level of HN protein in the heart. HN is highly expressed in the heart as demonstrated by Western blot (Figure 1A). Analysis of endogenous HN protein in different cell types shows that HN is predominantly present in the cardiomyocytes (Figure 1B) with minimal expression in SMV and endothelial cells.
Figure 1.
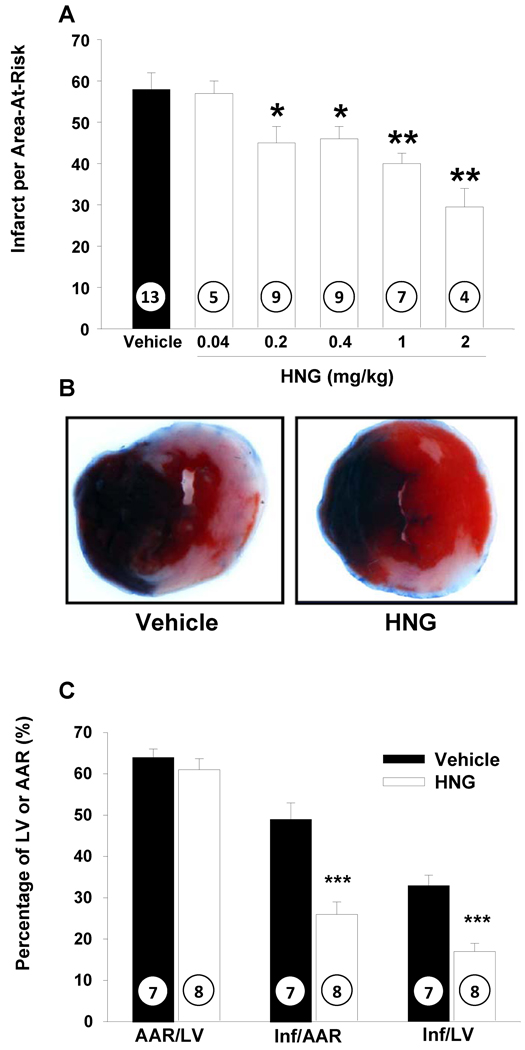
A) Protein level of HN in different tissues in mice: A total of 30 µg of protein from mouse heart (lane 1), skeletal muscle (lane 2), liver (lane 3) and 3 ng of synthetic RN (rodent homolog of HN) peptide (lane 5) were loaded. Higher amounts of HN protein were detected in heart compared to muscle and liver. B) Protein level of HN in different cell types in heart: 30 µg of mouse heart (lane 1), cardiomyocyte (lane 2), smooth muscle vascular cell (lane 3), human umbilical vein endothelial cells (lane 4) and 3 ng of synthetic RN peptide (lane 5) were loaded. HN levels were highest in the cardiomyocytes (*p< 0.001 vs. other cell lines). C) Protein levels of HN post MI-R: Protein extracts from left ventricle at basal (lane 1), 4 hr (lane 2), 12 hr (lane 3) and 24 hr (lane 4) post MI-R were loaded. The levels of HN increase post MI-R and persist up to 24 hrs post injury (*p < 0.01 vs. baseline).
Endogenous HN increases following MI-R
To assess changes in endogenous HN in the heart following MI-R, we analyzed the expression level of HN protein in the left ventricle at different time points post MI-R. Endogenous HN levels increase following MI-R and persist as long as 24 hr (Figure 1C).
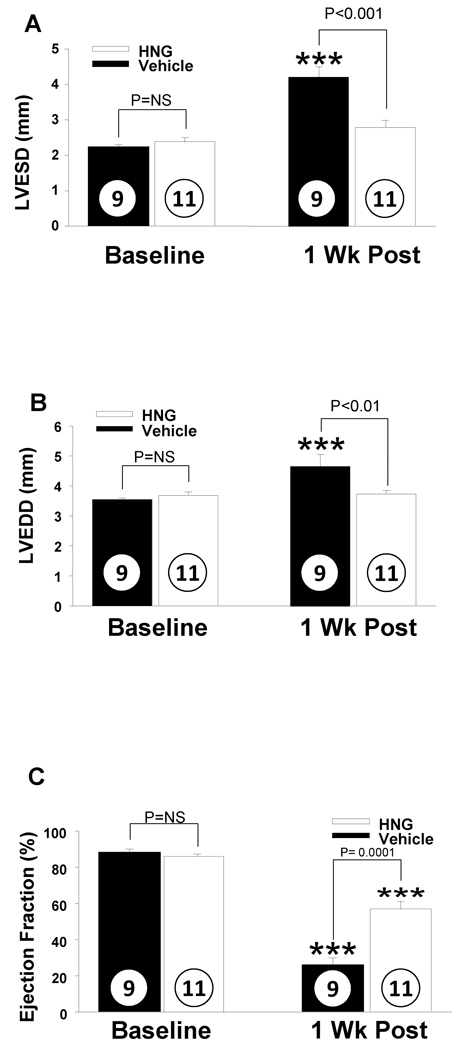
HNG Decreases Infarct Size in a Dose Dependent Fashion
Eight-week old male C57BL6/J mice were subjected to 45 min of left coronary artery ligation followed by 24 hr reperfusion, at which time the extent of myocardial infarction was evaluated. HNG or vehicle was administered (i.p.) 1 hr prior to ischemia. The total area at risk (AAR) was ~60% of the left ventricle, similar in both vehicle and HNG treated animals (p = NS, Figure 2B). The left ventricular infarct size in mice receiving vehicle was 58 ± 4% of AAR. There was a decrease in infarct size relative to the AAR in a dose dependent manner in HNG treated animals. Specifically, the size of infarct was significantly decreased to 44 ± 4% with 5 µg of HNG (0.2 mg/kg), 39 ± 2 % with 25 µg of HNG (1 mg/kg) and a maximal reduction to 28 ± 4 % with 50 µg of HNG (2 mg/kg; p < 0.01, Figure 2A). Further increase in dose to 100 µg (4 mg/kg) did not confer additional protection than that observed with 50 µg HNG.
Figure 2.
HNG decreases myocardial infarct size in mice following MI-R: A) myocardial infarct size in mice receiving doses of HNG ranging from 0.2 to 2 mg/kg. HNG significantly decreased myocardial infarct size compared with vehicle in a dose dependent manner. B) Representative mid-ventricular photomicrographs of mouse hearts are shown after 45 min of myocardial ischemia and 24 hr of reperfusion. Areas of the myocardium that appear blue represent the areas of myocardium not at risk for infarction. In contrast, the areas of myocardium that stain red (i.e., TTC positive) represent viable myocardium that was at risk for infarction. Myocardium that appears pale (i.e., TTC negative) indicates areas of myocardium at risk that are necrotic (i.e., infarcted). C) HNG administered at the time of reperfusion decreased infarct size significantly compared to AAR. Values are means ± SE. Numbers inside bars indicate the number of animals investigated in each group. **p <0.01; ***p <0.001 vs. vehicle.
Clinically, patients receive medical treatment for acute myocardial infarction after the onset of symptoms. Therefore to evaluate for therapeutic potential, we administered HNG at the time of reperfusion and studied the effects on infarct size. Intra-cardiac administration of HNG (2 mg/kg) at the time of reperfusion reduced infarct size by 47% (p < 0.01, Figure 2C). The infarct size compared to AAR was 48 ± 4% in controls compared to 26 ± 3% in HNG treated animals (p < 0.01). When infarct size is expressed as percent of left ventricle (LV), it was 32 ± 3% among controls compared to 17 ± 2% in HNG (p < 0.01) demonstrating a significant reduction in infarct size following treatment with HNG. Representative photomicrographs from vehicle and HNG treated animals are shown in Figure 2B.
HNG Results in Improved Cardiac Function post MI-R
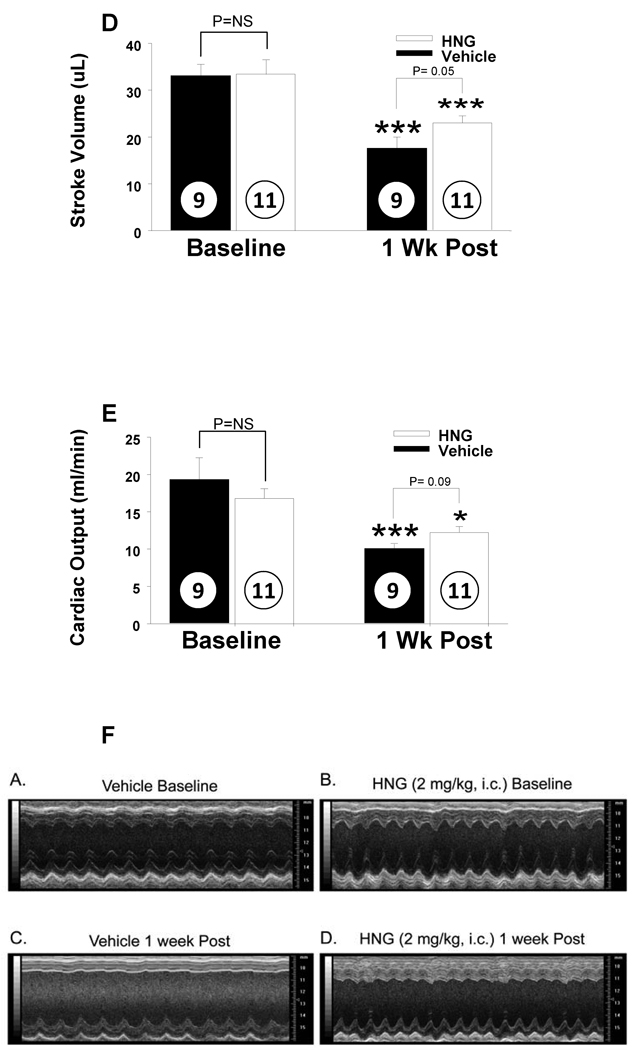
The effects of HNG on left ventricular structure and function following 45 min of ischemia and 7 days of reperfusion were assessed by in vivo transthoracic echocardiography. These data are depicted in Figures 3A–3E. Post MI-R, there was a significant increase in left ventricular end systolic diameter (LVESD) (from baseline) in the vehicle treated groups but not in the HNG treated group (2.1 ± 0.1 to 4.2 ± 0.4 mm in I/R + vehicle, p < 0.001; 2.3 ± 0.1 to 2.7 ± 0.2 mm in I/R + HNG, Figure 3A). Similarly, left ventricular end diastolic diameter (LVEDD) was increased in vehicle (compared to baseline) but was unchanged in HNG (3.6 ± 0.1 to 4.6 ± 0.3 mm in I/R + vehicle p < 0.001; 3.7 ± 0.1 to 3.7 ± 0.1 mm in I/R + HNG p = NS; Figure 3B). Ejection fraction (EF) was significantly decreased in both vehicle and HNG treated groups (78.0 ± 2.2 to 25.23 ± 3.2 % in vehicle and from 75.5 ± 1.6 to 56 ± 3.8 % in HNG, p < 0.0001; Figure 3C). Stroke volume decreased from 33.3 ± 2.0 to 18.1 ± 1.9 mL/min in I/R + vehicle group and from 34.7 ± 2.5 to 22.8 ± 1.4 ml/min in I/R + HNG (p<0.001, Figure 3D). Cardiac outputs were significantly decreased post MI-R in both groups with a decrease from 19.2 ± 1.4 to 10.1 ± 1.0 mL/min in I/R + vehicle group and a decrease from 17.2 ± 1.1 to 12.4 ± 0.8 mL/min in I/R + HNG (p<0.001, Figure 3E).
Figure 3.
HNG treatment improves cardiac function post MI-R: Cardiac function post MIR was evaluated by Echocardiography. Mice treated with HNG had significantly improved cardiac function as evidenced by effects on A) LVESD B) LVEDD C) Ejection fraction D) Stroke Volume and E) Cardiac Output. F) Representative baseline and post MIR M-mode images of the LV from vehicle and HNG treated mice. Values are means ± SE. **p <0.01; ***p <0.001 vs. baseline.
When one week post MI-R cardiac function was compared between the vehicle and HNG treated groups, LVESD (4.1 ± 0.4 vs.2.7 ± 0.2 mm in I/R + vehicle vs. I/R + HNG respectively, p < 0.001; Figure 3A) and LVEDD were significantly lower in the HNG treated group compared to the vehicle treated animals (4.6 ± 0.3 vs.3.7 ± 0.1 mm, I/R + vehicle vs. I/R + HNG respectively, p < 0.001; Figure 3B). The animals that received HNG treatment demonstrated a significantly higher EF as compared to the vehicle group (57 ± 3% in the HNG treated group as compared to 24 ± 2% in the vehicle group, p = 0.000; Figure 3C). Stroke volume was increased in the HNG treated group (22.7 ± 1.4 vs. 18.0 ± 1.9 ml/min in I/R + HNG vs. I/R + vehicle respectively, p=0.05; Figure 3D) with a tendency towards increased cardiac output (12.4 ± 0.8 vs. 10 ± 1.0 ml/min in I/R + HNG vs. I/R + vehicle respectively, p=0.09; Figure 3E). Representative baseline and post MI-R M-Mode images from vehicle and HNG treated animals are shown in Figure 3F.
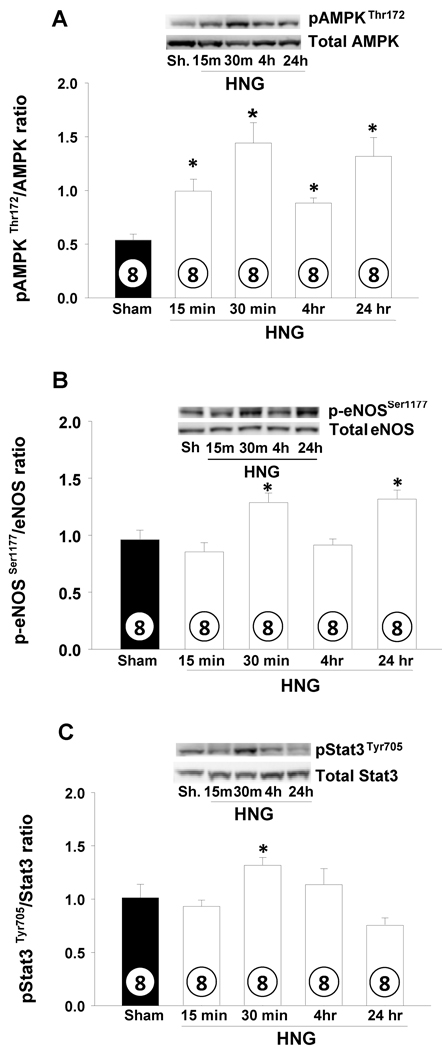
HNG activates AMPK-eNOS signaling in heart
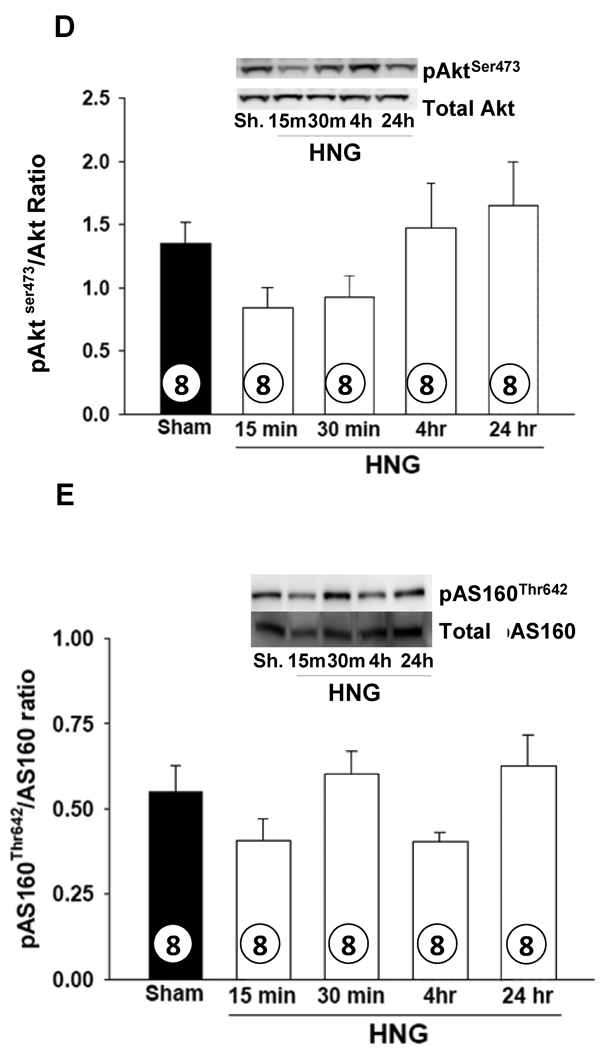
To understand mechanisms through which treatment with HNG improved cardiac survival, we studied changes in signaling pathways relevant to energy metabolism and cell survival in the heart following a single i.p. dose of HNG (2 mg/kg) by Western blot analysis. There was a significant activation of AMPK in the heart within 15 min of administration of HNG administration as evidenced by the increased phosphorylation of AMPK (pAMPKThr172) and increased pAMPKThr172/AMPK ratio. This activation persisted up to 24 hrs (p<0.001; Figure 4A). Phosphorylation of eNOS (p-eNOSSer1177) was significantly higher at 30 min and 24 hr (p< 0.01; Figure 4B) in response to HNG. Activation of STAT-3 (pSTAT-3Tyr705 normalized to total STAT-3) tended to be elevated at 30 min compared to Sham but returned to normal at 4 hr (p = 0.07; Figure 4C). The ratio of pAKTSer473 to total Akt protein tended to decrease, but not statistically significant at 15 min after HNG and returned to normal by 4 hr (Figure 4D). There were no differences in activation of pAS160Thr642 (Figure 4E), mTOR or MAPK pathways following HNG treatment.
Figure 4.
HNG activates cardiac AMPK and eNOS signaling pathways.Effects of HNG or vehicle over a time course on A) pAMPKThr172 B) phospho-eNOSSer1177 C) pSTAT-3Tyr705 D) pAktS473 and E) pAS160Thr642 levels. Values are means ± SE. * p< 0.05, significantly different from SHAM.
To test the relevance of these pathways in cardio-protective effects of HNG following ischemia-reperfusion, signaling pathways were assessed post MI-R at different time points. Following MI-R, there was a significant increase in AMPK activation in both vehicle and HNG-treated groups. However the vehicle and HNG treated post MI-R groups were not different from each other, although a tendency for higher levels of AMPK activation with HNG was observed at 12 hrs (Supplemental Figure 1, P=0.07). We also observed increased levels of pSTAT-3 and eNOS (Supplemental Figure 2) following MI-R in both groups. There were no significant changes in eNOS, Akt, STAT-3, MAPK or VEGF between the vehicle and HNG-treated groups.
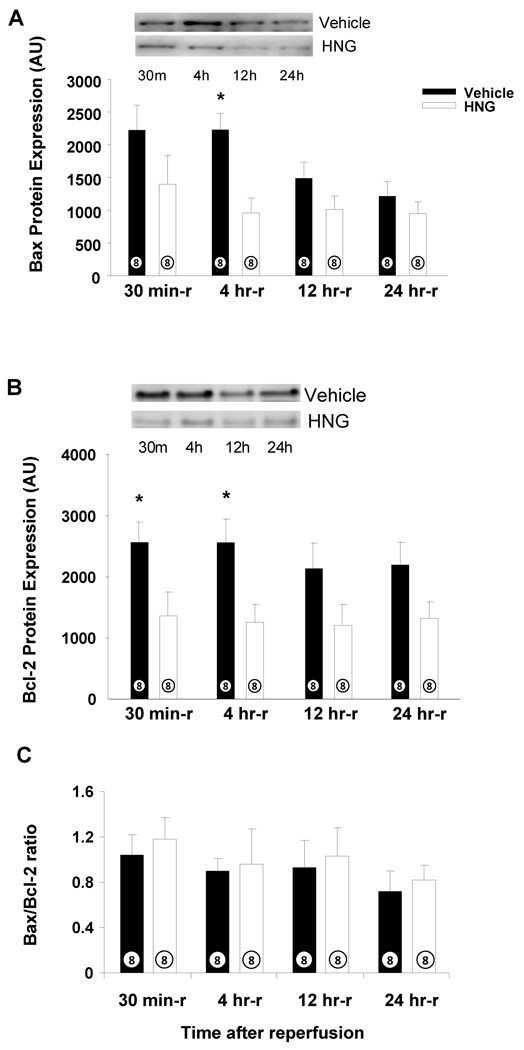
HNG attenuates expression of Bax after Ischemia and Reperfusion
As determined by Western blot, there was a significant increase in Bax with MI-R in saline-treated animals at 30 min and 4 hrs post reperfusion (Figure 5A). In parallel with an increase in Bax, Bcl2 expression was also increased up to 24 hrs (Figure 5B). In the HNG-treated group, Bax and Bcl2 expression were significantly attenuated compared to vehicle-treated groups (Figure 5A and B, p < 0.05). Interestingly, there were no significant differences in the ratio of Bcl-2 to Bax in both the groups (Figure 5C). Furthermore, no changes in cleaved caspase 3 levels were observed between the groups (data not shown).
Figure 5.
HNG down-regulates Bax following MI-R: Effects of HNG or vehicle on A) Bax levels post MI-R. B) Bcl-2 levels post MI-R. C) Bax/Bcl-2 ratio post MI-R. Values are means ± SE. * p < 0.05, significantly different from vehicle.
HNG improves cardiomyocyte survival and decreases apoptosis in vitro
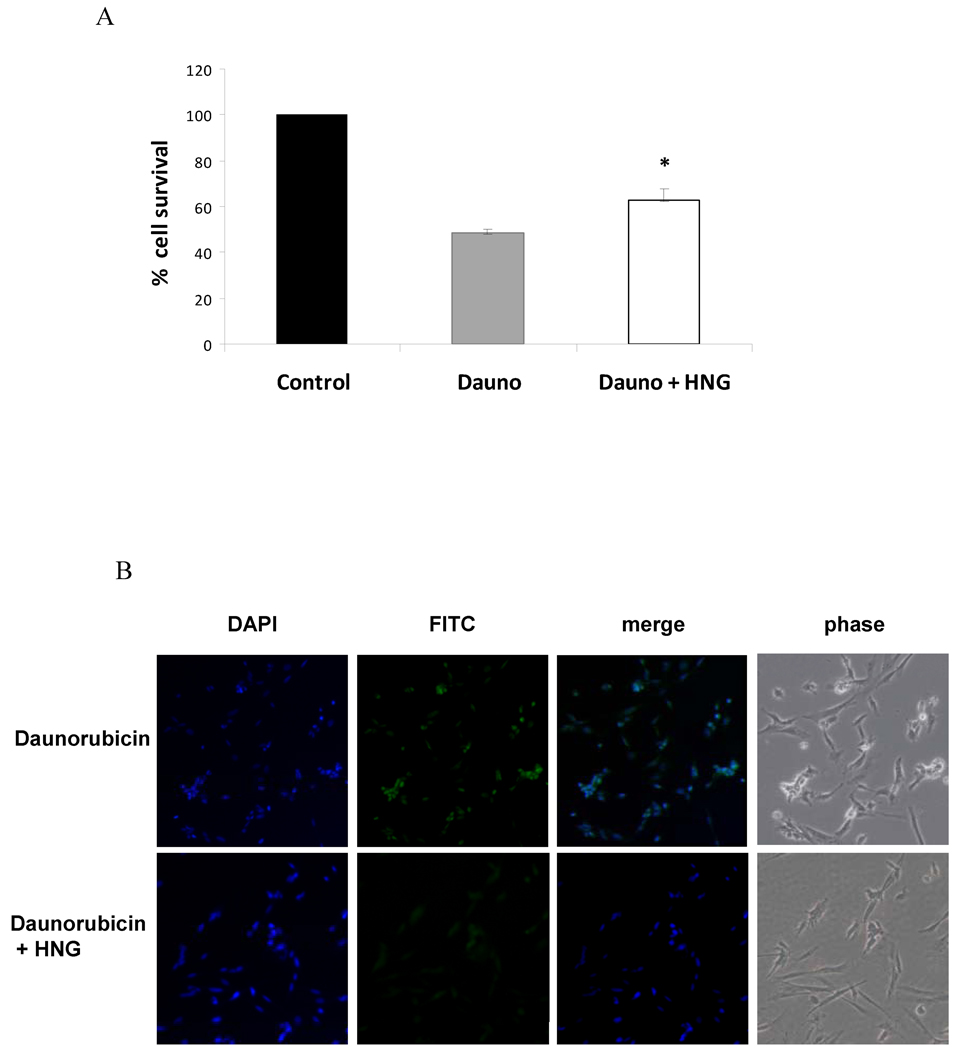
In vitro, a cell viability assay showed that HNG conferred a 15% survival protection against daunorubicin induced apoptosis in cardiomyocytes. Daunorubicin alone, at 1µM concentration killed 52% (48% survival) of cells. In presence of HNG, 37% cells were killed (63% survival) (Figure 6A). CaspASE FITC -VAD-FMK in situ apoptosis assay substantiated the effect of HNG on apoptosis in H9c2 cells. In the presence of HNG, there was a significant decrease in FITC stained apoptotic cells as compared to cells exposed to daunorubicin alone (Figure 6B). This was also evident in phase contrast images where cells looked normal and viable in the presence of HNG+ Daunorubicin while daunorubicin-only treated cells were visibly stressed and apoptotic.
Figure 6.
HNG improves cardiomyocyte survival in vitro and decreases apoptosis in response to Daunorubicin. Effect of HNG on A) Cardiomyocyte survival as assessed by cell viability assay, * p < 0.05 Dauno compared to Dauno+HNG, and B) early markers of apoptosis as assessed by CaspASE FITC -VAD-FMK in situ apoptosis assay.
Discussion
In a series of experiments, we demonstrate that HNG offers cardioprotection in a mouse model of MI-R injury, including a decrease in infarct size by 50% and significant improvement in LV function. We demonstrate that this protective effect of HNG is dose dependent, with a maximum effect at 2 mg/kg, is independent of the route of administration (intra-peritoneally or intracardiac) and is elicitable independent of the timing of delivery (1 hr pre-treatment or at the time of reperfusion).
HN protein is endogenously expressed in the heart with the greatest levels found in cardiomyocytes. Furthermore, we show that the expression of the protein is regulated, with levels increasing in the left ventricle in response to a severe stress such as MI-R. This increase is consistent with its role in conferring robust cytoprotection in animal models of Alzheimer’s disease, serum deprivation and stroke(5; 6; 11; 20; 27). In studies described here, we demonstrate for the first time, a potential role for HNG in offering protection against MI-R injury in vivo in a clinically relevant model system. Considering that HNG is a potent analog of HN (1,000 times more potent than native HN), we propose that augmenting the physiological response (an increase in endogenous HN in response to MI-R) by administration of exogenous HNG leads to enhanced cardioprotection.
It is especially interesting that the beneficial effects are elicitable when given 1 hr prior to MI-R or during reperfusion; this suggests that the treatment with HNG initiates protective signaling cascades that aid survival when exposed to a severe insult (i.e., cardiac ischemia). Moreover, the demonstration that HNG affords cardioprotection even when given during reperfusion highlights clinical therapeutic potential in acute coronary ischemia and myocardial infarction. Treatment with HNG resulted in preservation of left ventricular dimensions post MI-R and resulted in significant improvement in cardiac function as evidenced by better ejection fraction, stroke volume and cardiac output. The improvements in cardiac function seen in HNG-treated animals could be directly related to the smaller size of infarct.
HN has been shown to act thru CNTFR-α/gp130/WSX-1 receptor complex in offering protection against apoptosis (11); many downstream signaling pathways such as MAPK, PI3K and Jak-STAT could be activated in response to signaling through this complex (28). In fact, HN has been shown to induce its cytoprotective effects through activation of Jak-Stat, Akt, JNK and p38 MAPK signaling pathways (15; 16; 29). All components of this receptor and signaling complex are present in the heart and skeletal muscle and signaling through gp 130 receptors has been shown to activate MAPK and Jak-STAT in the heart (28) and induce AMPK activation in skeletal muscle (30).
In our study, significant activation of AMPK was noticed in the heart within 15 min of treatment with HNG and persisted up to 24 hr. This could be potentially important as activation of AMPK can work through multiple mechanisms which include: 1) offering a metabolic advantage through switch to an energy conservation mode by stimulating energy-yielding processes such as promoting glucose transport and accelerating glycolysis while inhibiting anabolic processes such as triglyceride and protein synthesis, and 2) activating additional cardioprotective pathways such as endothelial nitric oxide synthase (eNOS) which promotes vasodilation, decreases oxidative stress and increases peroxisome proliferator-activated receptor-γ coactivator (PGC)α, an important regulator of mitochondrial biogenesis and function (21; 22), and 3) decreasing apoptosis as has been shown in cardiomyocytes, endothelial cells, thymocytes, astrocytes via improved glucose utilization and inhibition of cytochrome C release from the mitochondria (31–33). Indeed, we demonstrate that HNG treatment is associated with phosphorylation of eNOS at serine residue 1177 (eNOSSer1177). Phosphorylation of this residue is a critical requirement for eNOS activation and has been reported to be mediated by AMPK during myocardial ischemia and AMPK-eNOS signaling has been shown to mediate the cardioprotective effects of metformin (22). The increased generation of nitric oxide by eNOS could offer cardioprotection through its effects on vasodilation, inhibition of oxidative stress, platelet aggregation, leukocyte chemotaxis and apoptosis (22). Concerns have been raised regarding the increased fatty acid oxidation with activation of this pathway, which could exacerbate acidosis during the reperfusion phase. Nevertheless, the role of AMPK in this process still seems likely beneficial as exemplified in AMPK-DN mice subjected to MI-R, which demonstrate a larger infarct size, more apoptosis, and worse cardiac function than wild type (34).
Activation of STAT-3 has been shown to be a crucial step in the neuroprotective effects (15) as well as the insulin-sensitizing effects of HN (3). A protective role of STAT-3 activation following ischemia/reperfusion injury has been shown earlier (35; 36). Indeed, activation of STAT-3 promotes cardiomyocyte survival and hypertrophy as well as cardiac angiogenesis in response to various pathophysiological stimuli. Mice with cardiomyocyte-restricted deletion of STAT-3 (cardiac specific STAT3-KO) show enhanced susceptibility to injury caused by myocardial ischemia (36), further highlighting the role for this pathway in cardiac survival post-MI-R. Activation of Akt has been shown to be important in the protective role of HN in stroke (20). However, we demonstrate that there is no significant change in pAkt or pSTAT-3 in the heart suggesting that the cardioprotective effects of HNG in this model are not mediated through these pathways.
Cardiac myocyte death during MI-R occurs through necrosis, as well as apoptosis (37; 38). The activation of AMPK as well as the ability of HN to inactivate pro-apoptotic peptides such as Bax, Bid, Bim suggested that modulation of apoptosis pathway could be a mechanism through which HNG offers cardio-protection(13; 14). Indeed, Bax was significantly down regulated in the myocardium of HNG treated animals. The sustained rise of Bax seen in controls following MI-R was significantly attenuated in animals treated with HNG suggesting Bax down-regulation as another potential mechanism through which HNG offers better survival. This is similar to the mechanism implicated in cardio-protection via ischemic pre-conditioning and intermittent hypoxia where down regulation of Bax decreases myocardial apoptosis and therefore infarct size (39). A role for Bax in regulating cardiac apoptosis following M-IR is illustrated in Bax−/− mice which demonstrate significantly smaller infarct size compared to wild type, while Bax+/− demonstrate an intermediate infarct size following MI-R(40). Along with up-regulation of Bax, there is also up-regulation of the anti-apoptotic Bcl-2 protein in the vehicle-treated animals that underwent MI-R. In the HNG treated animals, the rise in Bcl-2 levels is also attenuated resulting in a similar Bax/Bcl-2 ratio as controls. This is not consistent with studies that showed favorable change in Bax/Bcl2 ratio as one of the mechanisms that mediates cardiac survival (41). In addition, we observed no differences in cleaved caspase-3 expression between vehicle and HNG-treated groups. A role of HNG in attenuating apoptosis is also demonstrated in vitro where cardiomyocytes exposed to daunorubicin, an anthracycline chemotherapeutic drug known to induce apoptosis, show significantly decreased apoptosis and improved survival in the presence of HNG.
The lack of a significant difference in AMPK, eNOS between controls and HNG treated animals post MI-R is intriguing especially considering the significant activation of AMPK and eNOS in the heart in acute signaling experiments. It is possible that the activation of these signaling pathways in response to a severe stressor such as MI-R overwhelms the difference attributable to HNG. It is also plausible that we may have missed a response because of the time points we chose or potential differences in these and other members of the apoptosis pathway may become apparent if this data could be interpreted in the context of the size of the infarct. It also raises the point that in addition to the proposed intermediate pathways, other mechanisms may be involved in the cardio-protection offered by humanin. Future studies in Bax−/− and AMPK DN mice should shed light on the relative role of each pathway in offering cardio-protection in response to HNG.
In summary, these studies demonstrate a novel role for HNG in offering cardioprotection in a mouse model of MI-R. Our data suggest that this protection may be mediated through activation of AMPK-eNOS signaling as well as alteration in pro-apoptotic factors. The significant decrease in infarct size accompanied by improvement in cardiac function following a single treatment with HNG demonstrates clinical utility in the treatment for acute myocardial ischemia. Considering that diabetes mellitus increases the risk for cardiovascular disease and exacerbates the severity of acute myocardial infarction and our recent work showing the salubrious role of HN and analogs on insulin sensitivity and diabetes, future studies may reveal a role for HNG in the treatment of ischemic heart disease in the setting of diabetes.
Supplementary Material
Acknowledgements
This study was supported by a grant from the National Institutes of Health (NIH) K08 AG027462 to R.H.M, D.M.H. is supported by a T32 Training Grant (T32AG23475), 2 RO1 HL-060849-08 and the American Diabetes Association (7–04-RA-59) to D.J.L. and by a grant from the American Diabetes Association (7-09-BS-26) to J.W.C. D.J.L, J.W.C, M.L., and B.L.P. are all supported by the Carlyle Fraser Heart Center (CFHC) at Emory.
Abbreviations
- HN
humanin
- HNG
analog of HN
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
R.H.M. and D.J.L. are both Co-Inventors on a U.S. Patent (PCT 2008-006720) for Treatment of Type 2 Diabetes Mellitus, Metabolic Syndrome, Myocardial Injury, and Neurodegeneration with Humanin and Analogs Thereof.
References
- 1.Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56:1–120. [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 3.Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, Budagov T, Cui L, Einstein FH, Poduval A, Hwang D, Barzilai N, Cohen P. Humanin: a novel central regulator of peripheral insulin action. PLoS One. 2009;4:e6334. doi: 10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung SS, Van Nostrand WE. Humanin rescues human cerebrovascular smooth muscle cells from Abeta-induced toxicity. J Neurochem. 2003;84:266–272. doi: 10.1046/j.1471-4159.2003.01524.x. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, Sobue G, Koide T, Tsuji S, Lang J, Kurokawa K, Nishimoto I. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc Natl Acad Sci U S A. 2001;98:6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sponne I, Fifre A, Koziel V, Kriem B, Oster T, Pillot T. Humanin rescues cortical neurons from prion-peptide-induced apoptosis. Mol Cell Neurosci. 2004;25:95–102. doi: 10.1016/j.mcn.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Kariya S, Takahashi N, Hirano M, Ueno S. Humanin improves impaired metabolic activity and prolongs survival of serum-deprived human lymphocytes. Mol Cell Biochem. 2003;254:83–89. doi: 10.1023/a:1027372519726. [DOI] [PubMed] [Google Scholar]
- 8.Kariya S, Hirano M, Furiya Y, Sugie K, Ueno S. Humanin detected in skeletal muscles of MELAS patients: a possible new therapeutic agent. Acta Neuropathol (Berl) 2005;109:367–372. doi: 10.1007/s00401-004-0965-5. [DOI] [PubMed] [Google Scholar]
- 9.Kariya S, Hirano M, Furiya Y, Ueno S. Effect of humanin on decreased ATP levels of human lymphocytes harboring A3243G mutant mitochondrial DNA. Neuropeptides. 2005;39:97–101. doi: 10.1016/j.npep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Kariya S, Takahashi N, Ooba N, Kawahara M, Nakayama H, Ueno S. Humanin inhibits cell death of serum-deprived PC12h cells. Neuroreport. 2002;13:903–907. doi: 10.1097/00001756-200205070-00034. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M. Humanin Inhibits Neuronal Cell Death by Interacting with a Cytokine Receptor Complex or Complexes Involving CNTF Receptor {alpha}/WSX-1/gp130. Mol Biol Cell. 2009 doi: 10.1091/mbc.E09-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 13.Zhai D, Luciano F, Zhu X, Guo B, Satterthwait AC, Reed JC. Humanin binds and nullifies Bid activity by blocking its activation of Bax and Bak. J Biol Chem. 2005;280:15815–15824. doi: 10.1074/jbc.M411902200. [DOI] [PubMed] [Google Scholar]
- 14.Luciano F, Zhai D, Zhu X, Bailly-Maitre B, Ricci JE, Satterthwait AC, Reed JC. Cytoprotective peptide humanin binds and inhibits proapoptotic Bcl-2/Bax family protein BimEL. J Biol Chem. 2005;280:15825–15835. doi: 10.1074/jbc.M413062200. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto Y, Suzuki H, Aiso S, Niikura T, Nishimoto I, Matsuoka M. Involvement of tyrosine kinases and STAT3 in Humanin-mediated neuroprotection. Life Sci. 2005;77:3092–3104. doi: 10.1016/j.lfs.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto Y, Tsuji O, Niikura T, Yamagishi Y, Ishizaka M, Kawasumi M, Chiba T, Kanekura K, Yamada M, Tsukamoto E, Kouyama K, Terashita K, Aiso S, Lin A, Nishimoto I. Involvement of c-Jun N-terminal kinase in amyloid precursor protein-mediated neuronal cell death. J Neurochem. 2003;84:864–877. doi: 10.1046/j.1471-4159.2003.01585.x. [DOI] [PubMed] [Google Scholar]
- 17.Tajima H, Niikura T, Hashimoto Y, Ito Y, Kita Y, Terashita K, Yamazaki K, Koto A, Aiso S, Nishimoto I. Evidence for in vivo production of Humanin peptide, a neuroprotective factor against Alzheimer's disease-related insults. Neurosci Lett. 2002;324:227–231. doi: 10.1016/s0304-3940(02)00199-4. [DOI] [PubMed] [Google Scholar]
- 18.Mamiya T, Ukai M. [Gly(14)]-Humanin improved the learning and memory impairment induced by scopolamine in vivo. Br J Pharmacol. 2001;134:1597–1599. doi: 10.1038/sj.bjp.0704429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tajima H, Kawasumi M, Chiba T, Yamada M, Yamashita K, Nawa M, Kita Y, Kouyama K, Aiso S, Matsuoka M, Niikura T, Nishimoto I. A humanin derivative, S14G-HN, prevents amyloid-beta-induced memory impairment in mice. J Neurosci Res. 2005;79:714–723. doi: 10.1002/jnr.20391. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Chua CC, Gao J, Hamdy RC, Chua BH. Humanin is a novel neuroprotective agent against stroke. Stroke. 2006;37:2613–2619. doi: 10.1161/01.STR.0000242772.94277.1f. [DOI] [PubMed] [Google Scholar]
- 21.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R, Lefer DJ. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009;104:403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 23.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen Sulfide Mediates Cardioprotection Through Nrf2 Signaling. Circ Res. 2009 doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buettner C, Patel R, Muse ED, Bhanot S, Monia BP, McKay R, Obici S, Rossetti L. Severe impairment in liver insulin signaling fails to alter hepatic insulin action in conscious mice. J Clin Invest. 2005;115:1306–1313. doi: 10.1172/JCI23109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG, Jr, Rossetti L. Critical role of STAT3 in leptin's metabolic actions. Cell Metab. 2006:49–60. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto Y, Niikura T, Ito Y, Sudo H, Hata M, Arakawa E, Abe Y, Kita Y, Nishimoto I. Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer's disease-relevant insults. J Neurosci. 2001;21:9235–9245. doi: 10.1523/JNEUROSCI.21-23-09235.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer P, Hilfiker-Kleiner D. Role of gp130-mediated signalling pathways in the heart and its impact on potential therapeutic aspects. Br J Pharmacol. 2008;153 Suppl 1:S414–S427. doi: 10.1038/bjp.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D, Li H, Yuan H, Zheng M, Bai C, Chen L, Pei X. Humanin delays apoptosis in K562 cells by downregulation of P38 MAP kinase. Apoptosis. 2005;10:963–971. doi: 10.1007/s10495-005-1191-x. [DOI] [PubMed] [Google Scholar]
- 30.Watt MJ, Dzamko N, Thomas WG, Rose-John S, Ernst M, Carling D, Kemp BE, Febbraio MA, Steinberg GR. CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat Med. 2006;12:541–548. doi: 10.1038/nm1383. [DOI] [PubMed] [Google Scholar]
- 31.Blazquez C, Geelen MJ, Velasco G, Guzman M. The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS Lett. 2001;489:149–153. doi: 10.1016/s0014-5793(01)02089-0. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 34.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilfiker-Kleiner D, Hilfiker A, Drexler H. Many good reasons to have STAT3 in the heart. Pharmacol Ther. 2005;107:131–137. doi: 10.1016/j.pharmthera.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, Hillmer A, Schmiedl A, Ding Z, Podewski E, Poli V, Schneider MD, Schulz R, Park JK, Wollert KC, Drexler H. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res. 2004;95:187–195. doi: 10.1161/01.RES.0000134921.50377.61. [DOI] [PubMed] [Google Scholar]
- 37.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996;74:86–107. [PubMed] [Google Scholar]
- 38.Fliss H, Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res. 1996;79:949–956. doi: 10.1161/01.res.79.5.949. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura M, Wang NP, Zhao ZQ, Wilcox JN, Thourani V, Guyton RA, Vinten-Johansen J. Preconditioning decreases Bax expression, PMN accumulation and apoptosis in reperfused rat heart. Cardiovasc Res. 2000;45:661–670. doi: 10.1016/s0008-6363(99)00393-4. [DOI] [PubMed] [Google Scholar]
- 40.Hochhauser E, Cheporko Y, Yasovich N, Pinchas L, Offen D, Barhum Y, Pannet H, Tobar A, Vidne BA, Birk E. Bax deficiency reduces infarct size and improves long-term function after myocardial infarction. Cell Biochem Biophys. 2007;47:11–20. doi: 10.1385/cbb:47:1:11. [DOI] [PubMed] [Google Scholar]
- 41.Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM, Zhou ZN. Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression. Cell Res. 2003;13:385–391. doi: 10.1038/sj.cr.7290184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.