Abstract
Background
The prevalence of food allergy is rising and etiologic factors remain uncertain. Evidence implicates a role of vitamin D in the development of atopic diseases. Based on seasonal patterns of UVB exposure (and consequent vitamin D status), we hypothesized that food allergy patients are more often born in fall or winter.
Objective
Investigate whether season of birth is associated with food allergy.
Methods
We performed a multicenter chart review of all patients presenting to three Boston emergency departments (EDs) for food-related acute allergic reactions between 1/1/01 and 12/31/06. Months of birth among food allergy patients were compared to those of patients visiting the ED for reasons other than food allergy.
Results
We studied 1,002 food allergy patients. Among younger children with food allergy (age <5 years) – but not among older children or adults – 41% were born in spring/summer compared to 59% in fall/winter (P=0.002). This approximately 40/60 ratio differed from birth season of children treated in the ED for non-food allergy reasons (P=0.002). Children <5 years old born in fall/winter had a 53% higher odds of food allergy compared to controls. This finding was independent of the suspected triggering food and allergic comorbidities.
Conclusions
Food allergy is more common in Boston children who were born in the fall and winter seasons. We propose that these findings are mediated by seasonal differences in UVB exposure. These results add support to the hypothesis that seasonal fluctuations in sunlight and perhaps vitamin D may be involved in the pathogenesis of food allergy.
Keywords: Food allergy, season of birth, epidemiology, UVB, vitamin D
Key Messages.
Birth in fall or winter is more common among Boston children with food allergy
Low UVB exposure and vitamin D insufficiency may contribute to the pathogenesis of food allergy in early childhood.
Introduction
Atopic diseases are a growing problem worldwide.1 Food allergy (FA), for example, currently affects 2-6% of children and 1-3% of adults,2, 3 and appears to be rising, based on epidemiologic studies in the UK, USA and Australia.4-7 In general terms, atopic diseases are the consequence of maladaptive immune responses to environmental substances – such as pollens, fungal spores, dust mites or foods. Some of the common pathologic features include an imbalance of the immune system with a Th2 bias and production of antigen-specific IgE antibodies. Developmentally, the first signs of atopic disease are usually atopic dermatitis and FA which present in early childhood and may persist for decades. There is growing evidence that programming of the atopic phenotype is influenced in the antenatal, perinatal and infant periods.8, 9 Nevertheless, and similar to other atopic conditions, a complete model of the risk factors and pathogenesis of FA remains elusive. Some of the risk factors for the development of FA include genetic predisposition, atopic diathesis and acidity of the gut.10, 11
In 2007, Camargo and colleagues proposed that vitamin D status might also influence risk of FA/anaphylaxis after making the observation of a strong north-south gradient in EpiPen prescription rates in the United States.12 A similar finding has recently been confirmed in Australia13 and a similar latitudinal gradient identified in hypoallergenic formula prescriptions in that country.14 Several lines of evidence link ultraviolet light B (UVB) sunlight exposure and/or vitamin D status to atopic disorders.15-19 Because UVB exposure and vitamin D status are strongly related to season at higher latitudes,20 with lowest levels of both observed during the late fall and winter seasons, we hypothesized that a higher percentage of Boston patients with FA would be born in the fall and winter seasons. To examine this novel hypothesis, we investigated whether (1) season of birth (SoB) might be associated with FA in a broad group of patients presenting to three Boston emergency departments (EDs), and (2) if such an association were identified, would it differ by age at presentation, suspected triggering food allergen, or presence of co-morbid allergic conditions.
Methods
Study design
We created a cohort of patients by reviewing all cases of FA presenting to the EDs of Brigham and Women's Hospital (adults), Children's Hospital Boston (children) and Massachusetts General Hospital (adults and children) between January 1, 2001 and December 31, 2006. Patients with FA were initially identified by screening hospital billing databases with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes indicative of food-related allergic reactions. The codes used were 995.60 (anaphylactic shock due to unspecified food), 995.61-995.69 (anaphylactic shock due to specified food), 995.0 (other anaphylactic shock), 693.1 (dermatitis due to food), 995.7 (adverse food reaction, not otherwise classified), 558.3 (allergic gastroenteritis), and 692.5 (contact dermatitis due to food). In addition, random samplings of the codes 995.3 (allergy, unspecified), 995.1 (angioedema) and 708.X (urticaria) were reviewed to identify possible additional cases of food-related acute allergic reactions within these more non-specific allergy codes. These ICD-9-CM diagnosis codes have been identified by our group as effective in capturing FA cases.21 The study was approved by the Institutional Review Boards (IRB) of all three institutions and granted a waiver of consent/authorization for this administrative database study.
Data collection
A structured chart review was performed using a data abstraction form which included birth date, date of visit, age, sex, race/ethnicity, past medical history, patient-reported co-morbid allergic conditions (prior allergic reactions, asthma, hay fever, atopic dermatitis, hives, and angioedema), suspected triggering food allergen(s) (peanut, tree nuts, seeds, fruits and vegetables, crustaceans, fish, food additives, milk products, eggs, wheat, other), clinical signs and symptoms, ED presentation, and treatment in the ED. All charts were reviewed by physicians and verified by an allergist/immunologist for both accuracy and internal consistency. A food-related acute allergic reaction was defined as an acute episode of IgE-mediated symptoms (such as urticaria, bronchospasm, hypotension, vomiting, etc.) in which the onset was uniquely and temporally related to exposure to a known or suspected food allergen. Cases not consistent with FA (such as those with inadequate documentation of history and/or physical exam, recent initiation of a new medication, concurrent infectious illnesses, non-urticarial rashes or prolonged symptoms >24hrs after consumption of suspected foods) were excluded from this study. A diagnosis of anaphylaxis was assigned if documentation was consistent with the criteria outlined by the National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium.22
Age determination and season of birth
The first documented ED visit was used to calculate age, as some patients had multiple ED visits over the 6-year study period. Subsequent presentations were not included in the final dataset (i.e., each person contributed once to the association between season of SoB and FA). SoB were categorized as spring (March, April, May), summer (June, July, August), fall (September, October, November) and winter (December, January, February). For sensitivity analyses, data were combined into pre-specified sunlight exposure groups of light (spring and summer) and dark (fall and winter). Age groups were pre-specified prior to analysis.
Control groups
Three control groups were used for comparison with the FA cohort: 1) all children with non-FA-related ED visits at Children's Hospital Boston and Massachusetts General Hospital; 2) all births in Boston; and 3) all births in Massachusetts. For each control group, data were obtained from 2004 because it is mid-way through the 6-year study period. If a patient had more than one visit during 2004, only the first visit was included to match the methodology used in the compilation of cases.
Statistical Analysis
Data analysis was performed using STATA 10.0 (StataCorp, College Station, Texas). Subjects were included in our analysis only if they had an ICD-9 code indicative of an acute food-related allergic reaction. To identify potential cases of FA we used a stratified sampling method reflecting the population of patients treated in the ED with general ICD-9 codes (995.1 – angioedema, 995.3 anaphylaxis and 708.X). Using the survey module in STATA 10.0, sample weights were assigned to account for unequal probabilities of selection, over-sampling, and non-response.
Data are expressed as mean (standard deviation [SD]), median (interquartile range [IQR]), and proportion (95% confidence interval [CI]). Comparisons across SoB and between the ED population and the general population were evaluated using Chi-square tests. Odds ratios (OR) comparing these populations are presented with binomial 95%CI. A two-sided P <0.05 was considered statistically significant.
Results
Season of birth is associated with food allergy in children
We studied 1,121 patient visits for documented food-related acute allergic reactions presenting to the three Boston hospital EDs. Of these 1,002 were unique patients. The age range of the patients was 2 months to 94 years, with a median age of 15 years (IQR, 3 - 31 years). Half of the cohort was female and 48% were white. 47% of patients had a known allergy to the suspected offending allergen. Eighty eight percent of patients arrived to the ED within six hours after exposure to a suspected food allergen. The most common suspected triggers of the presenting reaction were peanuts (22%), tree nuts (19%), and shellfish (14%). Of these patients, 60% met criteria for anaphylaxis, and 62% had at least one or more documented co-morbid allergic condition at presentation (e.g., prior allergic reactions 61%, history of asthma 44%, atopic dermatitis 23%, hay fever 15%).
A larger percentage of children with FA had birthdates in fall or winter than spring or summer (Fig 1A). In children age <5 years with FA, the seasonal distribution of births was spring 20%, summer 21%, fall 28%, and winter 31% (P=0.01). When SoB was combined into pre-specified groups of higher and lower UVB exposure (i.e., spring/summer [light] and fall/winter [dark]), we found that 41% of children age <5 years with FA were born in light seasons compared to 59% in dark seasons, (Fig 2, P=0.002). A case-control analysis of patients age <5 years (in all years including 2004) revealed that fall/winter birth was associated with 53% higher odds of having FA (OR 1.53; 95%CI 1.22-1.92; P=0.002). Among older children and adults with FA, the seasonal association was not observed except in the subset of adults with FA and asthma (43% vs. 57%; P=0.09).
FIG 1.
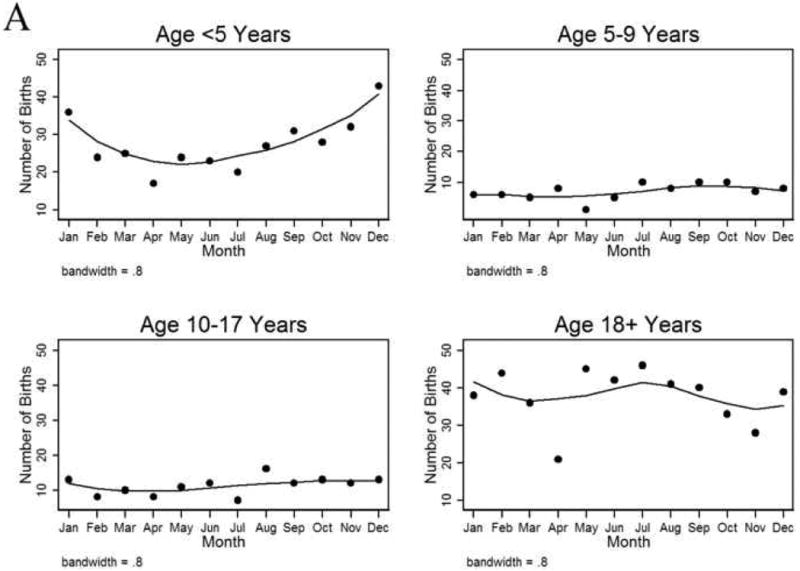
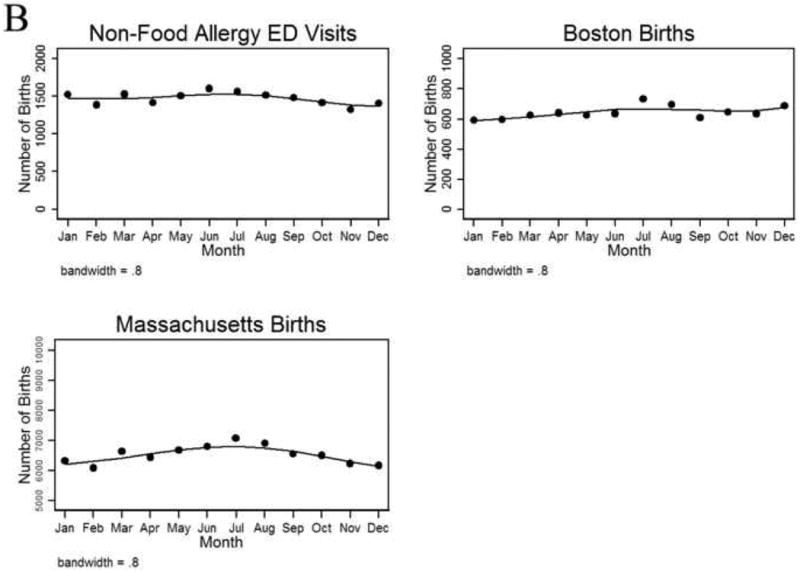
A, Seasonal variation in birth dates among food allergy patients treated in the emergency department (ED), by age group. B, Seasonal variation in birth dates of patients presenting to the ED for reasons other than food allergy (age <5 years), in all Boston births, and in all Massachusetts births.
FIG 2.
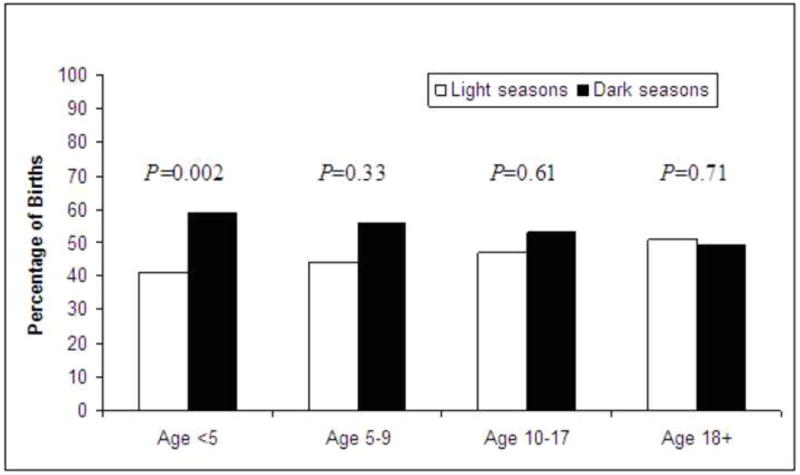
Distribution of food allergy patients according to birth in light (spring/summer) versus dark (fall/winter) periods, by age group.
Absence of fall/winter predominance in control groups
No fall/winter predominance in the birth months of patients presenting to the ED for non-FA-related reasons was identified among those age <18 years (P=0.87). There actually was a slight excess of summer births among the children age <5 years with a non-FA-related ED visit (Fig 1B, P<0.001). The summer predominance also was present for overall births in Boston (P=0.03) and Massachusetts (P<0.001) (Fig1B).
Seasonal association was not influenced by suspected food allergen trigger or comorbid allergic conditions
Although statistical power was limited, we observed a similar ratio of light/dark season births (∼40% vs. ∼60%) among patients age <5 years presenting with a suspected dietary trigger (Table 1), regardless of whether this was peanut, tree nut or egg. History of any allergic co-morbid condition (e.g., asthma, atopic dermatitis, or hay fever) at presentation also did not modify the SoB-FA association (Table 1).
TABLE 1.
Distribution of seasons of birth and odds ratios among children with food allergy age <5 years by suspected food allergen and co-morbid condition.
| Fall/winter | Spring/summer | OR* (95% CI) | |
|---|---|---|---|
| Food allergy cases (n=330) | 59% | 41% | 1.53 (1.22 – 1.92) |
| Non-food allergy controls (n=17,658) | 48% | 52% | -- |
| By Suspected Food Allergen | |||
| Peanut (n=102) | 60% | 40% | 1.59 (1.05 – 2.43) |
| Tree nut (n=58) | 59% | 41% | 1.52 (0.87 – 2.67) |
| Milk (n=62) | 61% | 39% | 1.69 (0.99 – 2.96) |
| Egg (n=31) | 58% | 42% | 1.48 (0.69 – 3.29) |
| By Comorbid Condition | |||
| Any allergic condition** (n=206) | 60% | 40% | 1.62 (1.21 – 2.17) |
| Asthma (n=64) | 58% | 42% | 1.47 (0.87 – 2.51) |
| No Asthma (n=142) | 61% | 39% | 1.69 (1.19 – 2.42) |
The odds ratio describes the odds of food allergy for children born in fall/winter (compared to spring/summer) using different case groups.
Includes history of prior allergic reactions, asthma, hay fever, atopic dermatitis, hives, and angioedema.
Comparing SoB among subgroups of FA children age <5 years (versus all children with a non-FA-related ED visit), we found consistent evidence that fall/winter birth was associated with ∼50% higher odds of having FA (Table 1).
Discussion
In an ED-based cohort of FA patients in Boston, we found that birth in fall or winter was associated with FA in children aged <5 years but not in older age groups. This rather simple but novel finding expands on the known risk factors associated with development of FA. In prior studies we have observed that a higher absolute latitude – in both northern and southern hemispheres – is a risk factor for FA/anaphylaxis as measured by rates of epinephrine autoinjector prescriptions,12, 13 anaphylaxis admissions,12, 13 and infant hypoallergenic formula prescriptions.14 Sheehan and colleagues recently expanded on this work by describing a similar association between latitude and food-related anaphylaxis admissions in US children.23 Taken together, these findings suggest a potential role for UVB exposure and/or vitamin D insufficiency in the pathogenesis of FA in children. Although we are confident of the SoB-FA association in our cohort, we readily acknowledge that other factors such as infections, maternal and infant dietary patterns, and exposure to indoor pollutants may contribute to FA pathogenesis and the observed seasonal patterns. Lessening the likelihood of these factors being the dominant explanation for the influence of SoB is that unlike vitamin D, none has been as closely linked to risk of atopic diseases. Additional studies will be necessary to address this specifically.
To examine the robustness of our finding, we examined the SoB-FA association in several subgroups. Although statistical power was limited, we identified a consistent association in patients <5 years presenting with an acute allergic reaction attributed to a suspected food allergen trigger. We recognize that children classified under a specific allergen (e.g., milk) might also be allergic to another food (e.g., concurrent peanut allergy) and this may biologically underly the presenting food allergic reaction. Confirmatory testing was not a feasible and part of this study and the present study cannot rule-out that the SoB-FA finding is more (or less) important for particular food allergens. The presence of co-morbid allergic conditions did not influence the SoB-FA ratio of ∼40% vs. ∼60% of light/dark season births in patients <5 years.
Interestingly, the only association of SoB in adult FA patients was in the group reporting a concomitant diagnosis of asthma. This raises the possibility that there may be an extended influence of UVB exposure and/or vitamin D insufficiency on the pathogenesis of lifelong asthma per se12, 13 – a topic that goes beyond the scope of this report. FA can develop during adulthood and it is possible that late-onset FA has a different pathogenesis than that seen in children. However, the general absence of a SoB-FA association in adults does not exclude a role for UVB/vitamin D in development of FA in adulthood.
An association of SoB and risk of disease years later is not unprecedented. For example, SoB has been linked with risk of several immune-mediated diseases including multiple sclerosis and Crohn's disease in adults.25, 26 In addition, risks of multiple sclerosis and Crohn's disease have also been associated with latitude, further implicating a role of UVB and vitamin D in these conditions.25, 27 Birth month has been associated with risk of atopic dermatitis, recurrent wheezing and aeroallergen sensitization in childhood, suggesting that exposure to seasonal allergens in an early developmental period may contribute to the development of atopic disease.28-31 Of particular relevance to our study is an association between birth in fall or winter and food allergen-specific IgE in children less than one year of age.32, 33 In a population of infants at high risk for atopic diseases fall/winter birth was associated with elevated cord blood total IgE levels, and increased IgE levels in cord blood were in turn associated with a > 3-fold incidence of urticaria due to FA by twelve months of age.34
We speculate that vitamin D may be the factor that mediates the observed association between SoB and childhood FA as there is inadequate UVB intensity for synthesis of active vitamin between the months of November and April in Boston.35 Studies have observed associations between vitamin D receptor polymorphisms and risk of atopic disease.36, 37 The mechanisms by which vitamin D could contribute to the prevention of FA include the promotion of maturation from the natural Th2 bias of the newborn, development of the adaptive immune system, effective management of infections, and healing of inflamed tissues. Vitamin D is known to have immunomodulatory effects on both Th1 and Th2 responses and affect production of the tolerogenic cytokine IL-10.18, 38 Birth in low UVB exposure months has been associated with lower cord blood levels of vitamin D and the tolerogenic cytokine IL-10.39, 40
Our study has several potential limitations. The analysis was cross-sectional and not longitudinal which limited the amount of information available to us. With >1,000 total subjects, and a P value of 0.002 for light/dark seasons in the primary group of interest (children <5 years), we believe that the findings are not due to chance. The absence of a detectable association in many patients (FA patients >5 years), and absence of a fall/winter finding in three control groups, reinforces that our methods are sound and that the results are not due a systematic study error.
Similar to other cross-sectional studies of FA, this study was not designed to gather confirmatory information on specificity of food-allergen trigger or actual number of specific food allergies in patients. Such information could be gathered in future studies by evaluation of specific IgE antibodies, skin prick testing and double blind-placebo controlled challenges. It is possible that a fraction of cases were in actuality not food-related but these would have been a minority and their inclusion would be expected to obscure, rather than create, an association. Our approach identified a relatively “pure” cohort of FA cases but information in the medical record was subject to limitations related to documentation (such as the condition of asthma is more likely to documented by an ED provider than, for example, hay fever).
The slight excess of summer births in the control groups was consistent with a national trend of slightly more births in the summer.41 Combining month of birth into seasons and light/dark groups was necessary for statistical analysis. We acknowledge that these are broad time periods but implemented grouping based on actual seasonal UVB exposure in the Boston area.42 It would be expected that any artifactual impact of such grouping would be to mask an association rather than expose one. It is possible that the pre-specified age groups may obscure additional information but doing so was necessary to permit sufficiently powered calculations and, as noted for the grouping of birth months and seasons, are unlikely to create an association.
We identified a large number of FA patients seeking care in the ED and believe them to be representative of FA patients in general. Even though a large percentage of patients met criteria for food-related anaphylaxis, the magnitude of the association of SoB was similar to that observed in patients that did not meet criteria for anaphylaxis. To more broadly apply our findings, it will be helpful to replicate this study in other populations and at different global latitudes. Our vitamin D-FA hypothesis assumes that patients in this study born in fall or winter have lower vitamin D levels or predicts a trajectory of low vitamin D levels. Additional studies will need to pursue this specifically. The strong influence of season on vitamin D status in Boston20 suggests that our inference is sound. With regards to vitamin D contributing to the trend of increasing FA, data in adults (which is likely reflective of children) suggests that vitamin D insufficiency in the U.S. has become more common over the past 20 years.43 Examination of the role of vitamin D could be addressed by future studies designed to measure serum 25-hydroxyvitamin D levels at different developmental time periods and make assessments of food-specific IgE antibodies, skin prick testing, clinical FA and ex vivo immune system function.
In summary, we found that birth in fall/winter was 50% more common among children <5 years with FA compared to birth in spring/summer. This finding adds to an increasing body of evidence that UVB exposure and/or vitamin D insufficiency may be involved in the pathogenesis of FA. Our findings, combined with the current understanding of risks of atopy, suggest that dysfunction during critical periods of immune system development may have persistent consequences. The model of UVB/vitamin D insufficiency contributing to development of an atopic phenotype suggests a potential opportunity for primary prevention. However, until results are available from prospective randomized controlled trials that formally test the vitamin D-FA hypothesis, it would be premature for parents to disregard current policies regarding safe sun exposure or to increase vitamin D intake for the specific prevention of food allergy.
Acknowledgments
The authors thank: Rose Xu (Knowledge Management Information Systems Department, Children's Hospital Boston); Stacey Duey and the Research Patient Data Registry group (Partners Health Care); and James K. West (Massachusetts Department of Public Health), for their help with the identification of cases and controls.
Declaration of funding: Dr. Vassallo was supported by T32 AI-60548, and Dr. Rudders by T32 AI-007512, from the National Institutes of Health (Bethesda, MD). Dr. Camargo was supported, in part, by the Massachusetts General Hospital Center for D-receptor Activation Research (Boston, MA).
Abbreviations
- CI
confidence interval
- ED
emergency department
- FA
food allergy
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- SE
standard error
- SoB
season of birth
- UVB
ultraviolet light B
Footnotes
Author Contributions:
Dr. Vassallo: data collection, data analysis, manuscript preparation
Dr. Banerji: data collection, manuscript preparation
Dr. Rudders: data collection, manuscript preparation
Dr. Clark: statistical analysis, manuscript preparation
Dr. Mullins: manuscript preparation
Dr. Camargo: project design, manuscript preparation
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.Venter C, Pereira B, Voigt K, Grundy J, Clayton CB, Higgins B, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008;63:354–9. doi: 10.1111/j.1398-9995.2007.01570.x. [DOI] [PubMed] [Google Scholar]
- 3.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–46. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Sheikh A, Strachan DP, Anderson HR. Time trends in allergic disorders in the UK. Thorax. 2007;62:91–6. doi: 10.1136/thx.2004.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin RY, Anderson AS, Shah SN, Nurruzzaman F. Increasing anaphylaxis hospitalizations in the first 2 decades of life: New York State, 1990 -2006. Ann Allergy Asthma Immunol. 2008;101:387–93. doi: 10.1016/S1081-1206(10)60315-8. [DOI] [PubMed] [Google Scholar]
- 6.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112:1203–7. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 7.Mullins RJ, Dear KB, Tang ML. Characteristics of childhood peanut allergy in the Australian Capital Territory, 1995 to 2007. J Allergy Clin Immunol. 2009;123:689–93. doi: 10.1016/j.jaci.2008.12.1116. [DOI] [PubMed] [Google Scholar]
- 8.Brockow I, Zutavern A, Hoffmann U, Grubl A, von Berg A, Koletzko S, et al. Early allergic sensitizations and their relevance to atopic diseases in children aged 6 years: results of the GINI study. J Investig Allergol Clin Immunol. 2009;19:180–7. [PubMed] [Google Scholar]
- 9.Ege MJ, Herzum I, Buchele G, Krauss-Etschmann S, Lauener RP, Roponen M, et al. Prenatal exposure to a farm environment modifies atopic sensitization at birth. J Allergy Clin Immunol. 2008;122:407–12. doi: 10.1016/j.jaci.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Cochrane S, Beyer K, Clausen M, Wjst M, Hiller R, Nicoletti C, et al. Factors influencing the incidence and prevalence of food allergy. Allergy. 2009;64:1246–55. doi: 10.1111/j.1398-9995.2009.02128.x. [DOI] [PubMed] [Google Scholar]
- 11.Untersmayr E, Vestergaard H, Malling HJ, Jensen LB, Platzer MH, Boltz-Nitulescu G, et al. Incomplete digestion of codfish represents a risk factor for anaphylaxis in patients with allergy. J Allergy Clin Immunol. 2007;119:711–7. doi: 10.1016/j.jaci.2006.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camargo CA, Jr, Clark S, Kaplan MS, Lieberman P, Wood RA. Regional differences in EpiPen prescriptions in the United States: the potential role of vitamin D. J Allergy Clin Immunol. 2007;120:131–6. doi: 10.1016/j.jaci.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 13.Mullins RJ, Clark S, Camargo CA., Jr Regional variation in epinephrine autoinjector presecriptions in Australia: more evidence for the vitamin D-anaphylaxis hypothesis. Ann Allergy Asthma Immunol. doi: 10.1016/S1081-1206(10)60265-7. in press. [DOI] [PubMed] [Google Scholar]
- 14.Mullins RJ, Clark S, Camargo CA., Jr Regional variation in infant hypoallergenic formula prescriptions in Australia: Support for the vitamin D-food allergy hypothesis. Pediatr Allergy Immunol. doi: 10.1111/j.1399-3038.2009.00962.x. in press. [DOI] [PubMed] [Google Scholar]
- 15.Krutmann J. Phototherapy for atopic dermatitis. Clin Exp Dermatol. 2000;25:552–8. doi: 10.1046/j.1365-2230.2000.00700.x. [DOI] [PubMed] [Google Scholar]
- 16.Zittermann A, Tenderich G, Koerfer R. Vitamin D and the adaptive immune system with special emphasis to allergic reactions and allograft rejection. Inflamm Allergy Drug Targets. 2009;8:161–8. doi: 10.2174/187152809788462644. [DOI] [PubMed] [Google Scholar]
- 17.Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippila C, Ahonen S, Nevalainen J, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp Allergy. 2009;39:875–82. doi: 10.1111/j.1365-2222.2009.03234.x. [DOI] [PubMed] [Google Scholar]
- 18.Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol. 2009;70:345–52. doi: 10.1016/j.humimm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Sidbury R, Sullivan AF, Thadhani RI, Camargo CA., Jr Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: a pilot study. Br J Dermatol. 2008;159:245–7. doi: 10.1111/j.1365-2133.2008.08601.x. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr. 1995;61:638S–45S. doi: 10.1093/ajcn/61.3.638S. [DOI] [PubMed] [Google Scholar]
- 21.Clark S, Gaeta TJ, Kamarthi GS, Camargo CA. ICD-9-CM coding of emergency department visits for food and insect sting allergy. Ann Epidemiol. 2006;16:696–700. doi: 10.1016/j.annepidem.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–7. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 23.Sheehan WJ, Graham D, Ma L, Baxi S, Phipatanakul W. Higher incidence of pediatric anaphylaxis in northern areas of the United States. J Allergy Clin Immunol. 2009;124:850–2. doi: 10.1016/j.jaci.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sicherer SH, Furlong TJ, Munoz-Furlong A, Burks AW, Sampson HA. A voluntary registry for peanut and tree nut allergy: characteristics of the first 5149 registrants. J Allergy Clin Immunol. 2001;108:128–32. doi: 10.1067/mai.2001.115755. [DOI] [PubMed] [Google Scholar]
- 25.Pierrot-Deseilligny C. Clinical implications of a possible role of vitamin D in multiple sclerosis. J Neurol. 2009;256:1468–79. doi: 10.1007/s00415-009-5139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angelucci E, Cocco A, Cesarini M, Crudeli A, Necozione S, Caprilli R, et al. Monthly and seasonal birth patterns and the occurrence of Crohn's disease. Am J Gastroenterol. 2009;104:1608–9. doi: 10.1038/ajg.2009.107. [DOI] [PubMed] [Google Scholar]
- 27.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 28.Kusunoki T, Asai K, Harazaki M, Korematsu S, Hosoi S. Month of birth and prevalence of atopic dermatitis in schoolchildren: dry skin in early infancy as a possible etiologic factor. J Allergy Clin Immunol. 1999;103:1148–52. doi: 10.1016/s0091-6749(99)70191-0. [DOI] [PubMed] [Google Scholar]
- 29.Knudsen TB, Thomsen SF, Ulrik CS, Fenger M, Nepper-Christensen S, Backer V. Season of birth and risk of atopic disease among children and adolescents. J Asthma. 2007;44:257–60. doi: 10.1080/02770900701246832. [DOI] [PubMed] [Google Scholar]
- 30.Ozasa K, Hama T, Dejima K, Watanabe Y, Hyo S, Terada T, et al. A 13-year study of Japanese cedar pollinosis in Japanese schoolchildren. Allergol Int. 2008;57:175–80. doi: 10.2332/allergolint.O-07-513. [DOI] [PubMed] [Google Scholar]
- 31.Gazala E, Ron-Feldman V, Alterman M, Kama S, Novack L. The association between birth season and future development of childhood asthma. Pediatr Pulmonol. 2006;41:1125–8. doi: 10.1002/ppul.20442. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson L, Bjorksten B, Hattevig G, Kjellman B, Sigurs N, Kjellman NI. Season of birth as predictor of atopic manifestations. Arch Dis Child. 1997;76:341–4. doi: 10.1136/adc.76.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuzume K, Kusu M. Before-birth climatologic data may play a role in the development of allergies in infants. Pediatr Allergy Immunol. 2007;18:281–7. doi: 10.1111/j.1399-3038.2006.00526.x. [DOI] [PubMed] [Google Scholar]
- 34.Kaan A, Dimich-Ward H, Manfreda J, Becker A, Watson W, Ferguson A, et al. Cord blood IgE: its determinants and prediction of development of asthma and other allergic disorders at 12 months. Ann Allergy Asthma Immunol. 2000;84:37–42. doi: 10.1016/S1081-1206(10)62738-X. [DOI] [PubMed] [Google Scholar]
- 35.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–8. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 36.Poon AH, Laprise C, Lemire M, Montpetit A, Sinnett D, Schurr E, et al. Association of vitamin D receptor genetic variants with susceptibility to asthma and atopy. Am J Respir Crit Care Med. 2004;170:967–73. doi: 10.1164/rccm.200403-412OC. [DOI] [PubMed] [Google Scholar]
- 37.Raby BA, Lazarus R, Silverman EK, Lake S, Lange C, Wjst M, et al. Association of vitamin D receptor gene polymorphisms with childhood and adult asthma. Am J Respir Crit Care Med. 2004;170:1057–65. doi: 10.1164/rccm.200404-447OC. [DOI] [PubMed] [Google Scholar]
- 38.Adams JS, Liu PT, Chun R, Modlin RL, Hewison M. Vitamin D in defense of the human immune response. Ann N Y Acad Sci. 2007;1117:94–105. doi: 10.1196/annals.1402.036. [DOI] [PubMed] [Google Scholar]
- 39.Zittermann A, Dembinski J, Stehle P. Low vitamin D status is associated with low cord blood levels of the immunosuppressive cytokine interleukin-10. Pediatr Allergy Immunol. 2004;15:242–6. doi: 10.1111/j.1399-3038.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan Dillie KT, Tisler CJ, Dasilva DF, Pappas TE, Roberg KA, Carlson-Dakes KT, et al. The influence of processing factors and non-atopy-related maternal and neonate characteristics on yield and cytokine responses of cord blood mononuclear cells. Clin Exp Allergy. 2008;38:298–304. doi: 10.1111/j.1365-2222.2007.02891.x. [DOI] [PubMed] [Google Scholar]
- 41.National Birth Statistics United States Center for Disease Control, National Center for Health Statistics. [12/01/09]; Available from http://www.cdc.gov/nchs/ Last accessed.
- 42.Holick MF. Vitamin D: a D-Lightful health perspective. Nutr Rev. 2008;66:S182–94. doi: 10.1111/j.1753-4887.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 43.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169:626–32. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]


