Abstract
Background
The use of selective agonists of the thyroid hormone receptor isoform β (TRβ) has been linked to metabolic improvement in animal models of diet-induced obesity, nonalcoholic liver disease, and genetic hypercholesterolemia.
Methods
To identify potential target tissues of such compounds, we exposed primary murine brown adipocytes and skeletal myocytes for 24 hours to 50 nM GC-24, a highly selective TRβ agonist. GC-24 (17 ng/[g BW·day] for 36 days) was also tested in a mouse model of diet-induced obesity.
Results
While the brown adipocytes responded to GC-24, with 17%–400% increases in the expression of 12 metabolically relevant genes, the myocytes remained largely unresponsive to GC-24 treatment. In control mice kept on chow diet, GC-24 treatment accelerated energy expenditure by about 15% and limited body weight gain by about 50%. However, in the obese animals the GC-24-mediated reduction in body weight gain dropped to only 20%, while energy expenditure remained unaffected. In addition, an analysis of gene expression in the skeletal muscle, brown adipose tissue, and liver of these obese animals failed to identify a conclusive GC-24 transcriptome footprint.
Conclusion
Feeding a high-fat diet impairs most of the beneficial metabolic effects associated with treatment with TRβ-selective agonists.
Introduction
Thyroid hormone is a highly metabolically active molecule that accelerates energy expenditure and reduces serum lipid concentrations at physiological concentrations (1). Nevertheless, its generalized use as a strategy to improve metabolic homeostasis has not been possible given its pleiotropic nature, with substantial effects in the heart, skeletal muscle, bone, and central nervous system to name a few. This concept has been evolving and frequently revisited, particularly in light of the understanding that thyroid hormone signaling is mediated through two thyroid hormone receptor isoforms (2), thyroid hormone receptor isoform α and β (TRα and TRβ), which conveniently exhibit diverse tissue distribution (3). While TRα expression predominates in the heart, skeletal muscle, bone, and brain, TRβ is preferentially expressed in the liver, with the adipose tissue expressing both TR isoforms.
The development of a TRβ-selective agonist (4) has prompted a number of studies addressing whether such molecules could be used to trigger the metabolic effects of thyroid hormones while preserving the TRα-expressing tissues (5–8). To a large extent, the findings have been encouraging, with a series of studies indicating that the use of TRβ-selective agonists can prevent or improve metabolic parameters and/or complications resulting from high-fat feeding, nonalcoholic liver disease (9), or genetic hypercholesterolemia (10). Because many of the biological effects attributed to TRβ-selective agonists are linked to lipid metabolism, it is well accepted that the liver is a major target of such molecules. In fact, tissue distribution analyses suggest that these molecules achieve TR selectivity by virtue of being concentrated predominantly in the liver, a tissue in which TRβ predominates (5).
At the same time, thyroid hormone is known for accelerating energy expenditure and decreasing the size of the white adipose tissue depot (1); thus, some beneficial effects of TRβ-selective agonists could be due to a decrease in adiposity. In this regard, treatment with GC-1, a TRβ-selective agonist, was shown to accelerate energy expenditure in rats (11), but the cellular and molecular basis underlying this metabolic effect remains unresolved. Given that the uncoupling protein 1 (Ucp1) expression in the brown adipose tissue (BAT) is highly sensitive to triiodothyronine (T3) (12), it is thus possible that TRβ agonists act to stimulate BAT. In fact, in an early study UCP1 expression was shown to be induced by GC-1 (13). BAT is the main site of adaptive thermogenesis in small mammals, and recently its presence has been well documented in adult humans (14). BAT has the thyroid-hormone-activating type 2 deiodinase (D2), which is several fold stimulated during cold exposure, increasing tissue T3 concentration and the expression genes encoding key thermogenic proteins (15). Accordingly, mice with targeted disruption of the D2 gene are cold intolerant and shivering is activated to sustain thermal homeostasis (16,17).
Studies with GC-24, a highly selective TRβ agonist, indicate that BAT was the only clear GC-24 metabolic target identified in a rat model of diet-induced obesity, with only minimal alterations in gene expression observed in liver, white adipose tissue, and skeletal muscle (18). Thus, in this study we used an in vitro approach to evaluate the metabolic actions of GC-24 and specifically test whether this molecule can modify gene expression in primary cultures of murine brown adipocytes and skeletal myocytes. Our data indicate that while a number of metabolically relevant genes are rapidly upregulated in the brown adipocytes by GC-24, skeletal myocytes remain largely unresponsive under similar conditions. At the same time, while treatment with GC-24 accelerated energy expenditure and limited body weight gain in chow-fed mice, a similar treatment only slightly minimize body weight gain and did not affect energy expenditure in a mouse model of high-fat feeding. In addition, we failed to detect a significantly measurable mRNA footprint in liver, skeletal muscle, or BAT of the obese animals. We conclude that although brown adipocytes in culture constitute an important metabolic target of TRβ-selective agonists, in a mouse model of diet-induced obesity their effects are much less prominent and a major metabolic target tissue of these compounds remains to be identified.
Materials and Methods
Animals and treatment
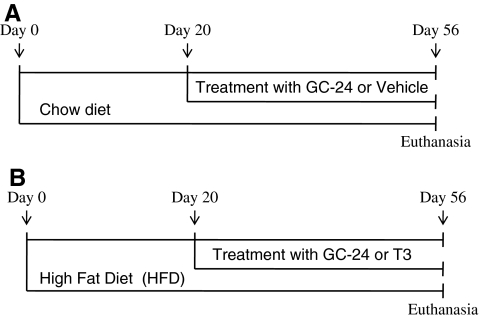
Male C57BL/J6 mice about 5–6 weeks old were purchased from Jackson Laboratory. Mice were kept at 21°C ± 1°C, with a 12-hour dark–light cycle starting at 06:00 hours, and housed in standard plastic cages with four mice per cage. All procedures described were approved by the local Institutional Animal Care and Use Committee. Animals were fed either chow diet (3.3 kcal/g, Teklad 7001; Harlan Teklad) or high-fat diet (HFD; 4.5 kcal/g, TD 95121; Harlan Teklad). After 20 days on the chow or the HFD, the animals started receiving daily subcutaneous injections of vehicle, T3 (30 ng/[g BW·day]) or equimolar doses of GC-24 (17 ng/[g BW·day]) for 36 days as indicated (Fig. 1). Food consumption and body weight were measured daily. Animals were subsequently euthanized using carbon dioxide (CO2). Tissue samples were obtained and immediately snap frozen for further analyses.
FIG. 1.
Experimental design. (A) Group of mice kept on Chow diet; (B) Group of mice kept on high-fat diet (HFD).
Indirect calorimetry
Mice were individually housed and acclimated to the calorimeter cages for 2 days followed by 2 days of data collection of gas exchanges and food intake. Indirect calorimetry was performed with a computer-controlled open circuit calorimetry system (Oxymax; Columbus Instruments) comprised of six respiratory chambers equipped with a stainless steel elevated wire floor, water bottle, and food tray connected to a balance. Oxygen (O2) consumption and CO2 production were measured for each mouse at 14-minute intervals, and outdoor air reference values were determined after every 10 measurements. Gas sensors were calibrated daily with primary gas standards containing known concentrations of O2, CO2, and N2 (Airgas). A mass flow meter was used to measure and control airflow. O2 was measured by an electrochemical sensor based on a limited-diffusion metal air battery. CO2 was measured with a spectrophotometric sensor. The respiratory exchange rate was calculated as the ratio between CO2 production (liters) over O2 consumption (liters). Energy expenditure was calculated using the following formula: (3.815 + 1.232 × VCO2/VO2) × VO2.
Primary cell cultures
Interscapular BAT and skeletal muscle (gastrocnemius) cells were immediately processed, and precursor cells were differentiated in vitro as previously described (19,20). Briefly, tissues were surgically removed from mice (8–10 mice per group) killed by CO2 asphyxiation. The dissected tissues were pooled, minced, and digested with collagenase (Sigma-Aldrich) dissolved in the medium containing Dulbecco's modified Eagle's medium, 10 mM HEPES, and antibiotics (25 μg/mL streptomycin, 25 μg/mL tetracycline, 25 μg/mL ampicillin, and 0.8 μg/mL Fungizone). Cells were strained to remove tissue debris, plated in BD 75-cm2 T-flasks (BD Biosciences), and incubated (37°C, 5% CO2) for 5–6 days in the same medium plus 10% (v/v) fetal bovine serum and 3 nM insulin. Differentiation of preadipocytes into mature brown adipocytes was confirmed by the presence of multilocular lipid droplets in the cytosol by light microscopy. Cells were treated for 24 hours with 50 nM of T3 or GC-24, and dimethyl sulfoxide was used as vehicle. Subsequently, cells were harvested and processed for RNA isolation, as described.
mRNA analysis
Total RNA was extracted from adipose tissue samples using the RNeasy kit (Qiagen) as previously described (21). The extracted RNA was analyzed by a NanoDrop spectrophotometer, and 2.5 μg of total RNA was reverse transcribed into cDNA by using High Capacity cDNA reverse Transcription Kit (Applied Biosystem). Genes of interest were measured by RT-qPCR (BioRad iCycler iQ Real-Time PCR Detection System) using the iQ SYBR Green Supermix (BioRad) with the following conditions: 15 minutes at 94°C (Hot Start), 30–50 seconds at 94°C, 30–50 seconds at 55–60°C, and 45–60 seconds at 72°C for 40 cycles. A final extension at 72°C for 5 minutes was performed as well as the melting curve protocol to verify the specificity of the amplicon generation. Standard curves consisting of four to five points of serial dilution of mixed experimental and control group cDNA were prepared for each assay. Cyclophilin A was used as a housekeeping internal control gene. The coefficient of correlation (r2) was >0.98 for all standard curves, and the amplification efficiency ranged between 80% and 110%. Results are expressed as ratios of test mRNA/cyclophilin mRNA. The mRNA levels of the following genes were measured: nuclear respiratory factor 1 (Nrf-1); sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (Serca); phospholamban (Pnl); myosin heavy chain alpha (Mhcα); myosin heavy chain beta (Mhcβ); hyperpolarization-activated cyclic nucleotide-gated channel (Hcn); estrogen-related receptor (Errα); uncoupling protein 3 (Ucp3); glucose transporter (Glut 4); forkhead box protein O1 (Fox O1); cyclophilin A (Cyclo A); peroxisome proliferator-activated receptor γ coactivator 1α (Pgc-1α); peroxisome proliferator-activated receptor γ coactivator 1β (Pgc-1β); carnitine palmitoyl transferase-1 (Cpt-1β); acetyl-coenzyme A carboxylase (Acc); murine medium-chain acyl-CoA-dehydrogenase (mMcad); murine long-chain acyl-CoA-dehydrogenase (mLcad); peroxisome proliferator-activated receptors (Ppar α); peroxisome proliferator-activated receptor δ (Ppar δ); cytochrome oxidase (Cox); ATP synthase, H+ transporting, mitochondrial F0 complex, subunit c-1 (Atp5g1); uncoupling protein 1 (Ucp1); superoxide dismutase 1 (Sod-1); superoxide dismutase 2 (Sod-2); scavenger receptor class B (Sr-b1); cholesterol 7a-hydroxylase (Cyp7a); type I deiodinase (D1); small heterodimer partner (Shp).
Statistical analysis
All data were analyzed using PRISM software (GraphPad Software) and are expressed as mean ± standard error of the mean. One-way analysis of variance was used to compare more than two groups, followed by the Student–Newman–Keuls test to detect differences between two groups. The Student's t-test was used to compare the differences between two groups. p < 0.05 was used to reject the null hypothesis.
Results
Effects of GC-24 on gene expression in mouse primary myocytes and brown adipocytes
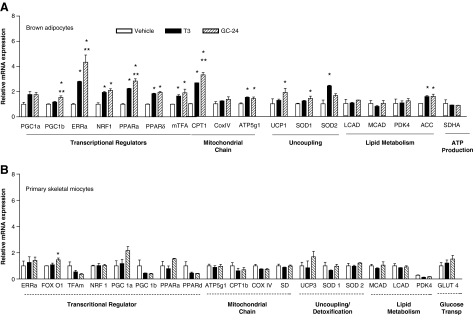
To evaluate the gene expression profile induced by the TRβ-selective agonist GC-24, brown adipocytes and primary skeletal myocytes were exposed in vitro to 50 nM GC-24 for 24 hours. Brown adipocytes were particularly sensitive to this molecule, with increases of 17%–400% observed in the expression of multiple genes, including Pgc-1β, Erra, Nrf-1, Ppar α and Ppar δ, mTfa, Cpt1, Atp5g1, Ucp1, Sod-1, and Acc (all p < 0.01; Fig. 2A). Other genes were not affected by treatment with GC-24, including Pgc-1a, CoxIV, Lcad, Mcad, Pdk-4, and SdhaA (Fig. 2A). As a comparison, other brown adipocyte cultures were treated with equimolar amounts of T3 and similar responses were observed, although less pronounced (Fig. 2A). On the other hand, in skeletal myocytes the changes in gene expression were minimal across 19 genes studied (Errα, Tfam, Ucp3, Nrf-1, Pgc-1α, Pgc-1β, Cpt-1β, Acc, Mcad, Lcad, Ppar α, Ppar δ, CoxIV, Atp5g1, Sod-1, Sod-2, Sd, Glut 4, and Pdk-4), with only Fox O1 increasing about 40% (p < 0.05; Fig. 2B). Treatment with T3 at the same concentration did not affect gene expression at all in these skeletal myocytes cells (Fig. 2B).
FIG. 2.
Gene expression profile of primary brown adipocytes (A) and skeletal myocytes (B) exposed to vehicle, 50 nM T3 or 50 nM GC-24, for 24 hours. Results are expressed as ratios of test mRNA/cyclophilin mRNA and normalized to the levels observed in the vehicle-treated cells. The genes analyzed are grouped in different sets as indicated. Values are the mean ± SEM of three independent samples; gene abbreviations are as indicated in the Materials and Methods section; *p < 0.01 versus vehicle-treated cells; **p < 0.01 versus T3-treated cells. T3, triiodothyronine; SEM, standard error of the mean.
Metabolic effects of GC-24 in animals kept on a chow diet
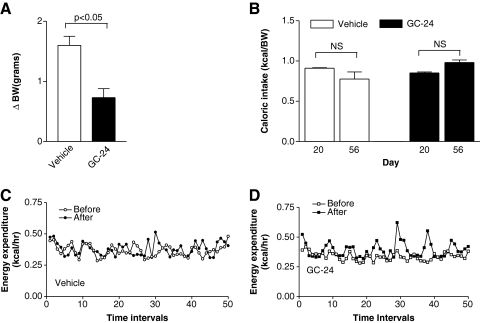
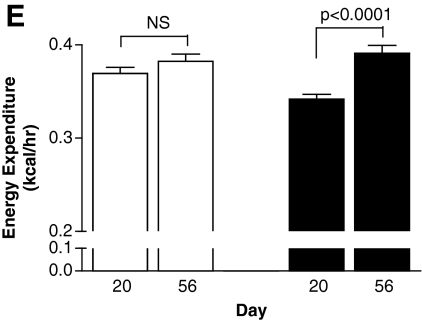
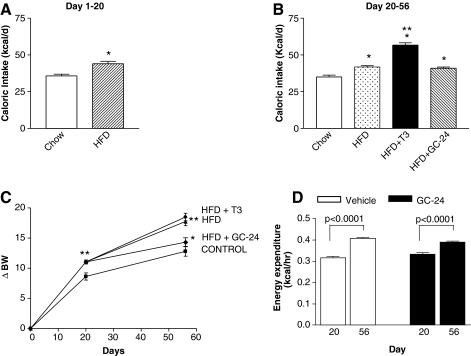
On the basis of these findings that brown adipocytes are metabolic targets of GC-24, we next tested the hypothesis that BAT activation in vivo by GC-24 would trigger metabolic effects in animals. Treatment with GC-24 (17 ng/[g BW·day]) for 36 days (Fig. 1A) cut by half the body weight gain (p < 0.05; Fig. 3A) while not affecting the daily caloric intake (Fig. 3B). At the same time, the rate of energy expenditure was accelerated by about 15% in GC-24-treated animals (p < 0.0001; Fig. 3C–E), which is compatible with the finding that brown adipocytes are activated by this molecule (Fig. 2A).
FIG. 3.
Metabolic profile of animals kept on a chow diet and treated with GC-24. (A) Animals were weighed daily and differences in body weight (ΔBW) are shown. (B) Caloric intake. Food intake was measured during a 48-hour period. (C) Energy expenditure in vehicle-treated mice before (day 20) and after treatment (day 56). (D) Same as (C), except that animals were treated with GC-24. (E) Energy expenditure shown as an average of the data in (C) and (D). Each entry is the mean ± SEM of three to four animals. NS, not significant.
Metabolic effects of GC-24 in animals kept on a HFD
Next, we tested the hypothesis that BAT activation achieved through treatment with GC-24 can minimize the metabolic consequences of feeding with a HFD. To this end, mice were placed on a HFD for 3 weeks to induce obesity, and subsequently treated with GC-24 (Fig. 1B). On a HFD alone, these animals exhibited an approximately 25% increase in caloric intake (Fig. 4A) that resulted in about 13% (p < 0.01) increase in body weight gain (Fig. 4C). Subsequently, daily treatment with GC-24 (17 ng/[g BW·day]) or T3 (30 ng/[g BW·day]) was started, remaining for the next 5 weeks (Fig. 1B). During the treatment phase, the T3-treated animals exhibited a further increase in caloric intake (∼23%; p < 0.001), whereas the same was not observed in the GC-24-treated animals (Fig. 4B). Notably, T3 treatment did not slow down the body weight gain associated with the high-fat feeding, whereas the effect of GC-24 dropped to only 20% that in control mice (p < 0.01; Fig. 4C). The body mass index (BMI) changed accordingly, increasing ∼15% with high-fat feeding (p < 0.01; Table 1). Notably, combination of high-fat feeding with GC-24 treatment resulted in only a ∼5% increase in BMI, whereas a similar combination with T3 was much less effective (11% increase in BMI; p < 0.01; Table 1). The individual analysis of heart, BAT, and kidney of these animals indicates that the combination of high-fat feeding and treatment with T3 increased their weights significantly by 11%–100% (p < 0.01), whereas GC-24 did not (Table 1). In the liver, both T3 and GC-24 had similar effects, decreasing organ weight when compared with high-fat feeding alone (Table 1). The indirect calorimetry indicates that between days 20 and 56 there was a significant acceleration in the rate of energy expenditure, compatible with continued feeding of a HFD. These rates were not affected by treatment with GC-24 (Fig. 4D).
FIG. 4.
Metabolic profile of animals kept on HFD and treated with GC-24. (A) Caloric intake. Food intake was measure daily. During the first 3 weeks of the experiment, animals were fed with either chow or HFD; *p < 0.001 versus chow diet. (B) HFD mice in (A) were split into three different groups and treated with GC-24 or T3 as indicated. Shown is the average of daily caloric intake for each animal group during the treatment period. Each entry is the mean ± SEM of eight animals; *p < 0.001 versus chow; **p < 0.001 versus HFD. (C) Body weight gain during the experimental period. Animals were weighed daily and body weight gain (ΔBW) is shown. Each entry is the mean ± SEM of eight animals; *p < 0.01 versus chow; **p < 0.001 versus HFD. (D) Energy expenditure of HFD animals treated with vehicle or GC-24.
Table 1.
Effect of GC-24 or Triiodothyronine Treatment on Body Mass Index and Tissue Weighs
| Group | BMI (kg/m2) | Liver (g) | Heart (g) | BAT (g) | Kidney (g) |
|---|---|---|---|---|---|
| Control | 2.88 ± 0.01 | 1.23 ± 0.04 | 0.13 ± 0.01 | 0.08 ± 0.004 | 0.16 ± 0.01 |
| HF | 3.32 ± 0.04a | 1.45 ± 0.03a | 0.14 ± 0.01 | 0.08 ± 0.01 | 0.17 ± 0.01 |
| HF + T3 | 3.21 ± 0.11a | 1.36 ± 0.04 | 0.17 ± 0.01b | 0.17 ± 0.01b | 0.19 ± 0.01b |
| HF + GC-24 | 3.01 ± 0.08b | 1.26 ± 0.02b | 0.12 ± 0.01c | 0.09 ± 0.01c | 0.15 ± 0.01c |
Values are expressed as mean ± standard error of the mean of eight animals.
p < 0.05 versus control; bp < 0.05 versus HFD; cp < 0.05 versus T3.
BAT, brown adipose tissue; BMI, body–mass index; HFD, high-fat diet; T3, triiodothyronine.
Effects of GC-24 on gene expression in animal tissues
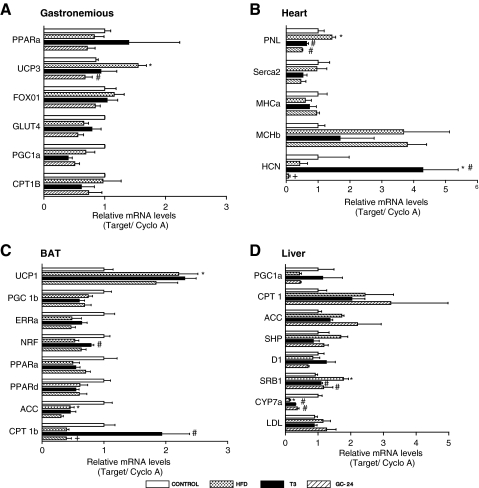
Given that brown adipocytes are a target of GC-24 and that treatment with GC-24 reduced body weight gain (Figs. 3A and 4C), we hypothesized that expression of metabolically relevant genes in the BAT of these animals would be upregulated by GC-24. In this regard, TRβ analogues are known to act in the liver and in the BAT while sparing the heart and bones (5,22). Thus, we looked at the expression of key genes in BAT, skeletal muscle, heart, and liver, finding that changes in gene expression in these tissues were very mild in the animals kept on a HFD (Fig. 5A–D). On the other hand, clear cut GC-24-triggered effects were documented in other tissues of the same animals. In the heart, HCN mRNA levels fell to almost undetectable levels in the animals treated with GC-24, whereas equimolar doses of T3 increased its mRNA levels by about fourfold (Fig. 5B), an effect that was reported previously (5). Similarly, PNL mRNA levels in the heart decreased with both GC-24 and T3 treatments (Fig. 5B). Notably, the only GC-24-induced change in mRNA levels detected in skeletal muscle was a decrease in Ucp3 mRNA levels (Fig. 5A), while in the BAT there was a marked decrease in Cpt1b mRNA (Fig. 5C). In the liver, the proposed main target of TRβ-selective agonists, treatment with GC-24 was limited to increasing Cyp7a mRNA levels by approximately threefold and reducing SRB1 mRNA levels by 50% (Fig. 5D). Despite these changes, it is difficult to ascertain how much of these effects are direct or due to an improvement in the overall metabolic profile of these animals caused by GC-24.
FIG. 5.
Gene expression profile in skeletal muscle (gastrocnemius), heart, liver, and BAT of mice placed on chow or HFD and treated with GC-24 or T3 as indicated. Each entry is the mean ± SEM of eight animals; gene abbreviations are as indicated in the Materials and Methods section; *p < 0.01 versus chow; #p < 0.01 versus HFD; +p < 0.01 versus T3-treated animals. BAT, brown adipose tissue.
Discussion
The use of TRβ analogs has been shown to have promising metabolic effects in animals fed chow diet (23) and in animal models of nonalcoholic liver disease (9) or genetic hypercholesterolemia (10), with only minimal repercussions in heart (5,23), bone (22), brain (24), or perturbations of thyroid hormone homeostasis (13). In this regard, a striking effect of TRβ-selective agonists is to accelerate the basal metabolic rate with a resulting decrease in adiposity and body weight (11), as also documented in the present study (Fig. 3). Thus, a logical next step and the scope of the present investigation was (i) to identify the site(s) where TRβ-selective agonists, for example, GC-24, are acting to produce their metabolic effects, and (ii) whether these effect can be harnessed to prevent obesity in animals kept on a HFD.
On the basis of data obtained with GC-1, another TRβ-selective agonist, one would expect that BAT be a primary target of GC-24 (13). In fact, brown adipocytes in culture respond to GC-24 by increasing expression of 11 metabolically relevant genes that were tested (Fig. 2A). Further, animals kept on chow diet had their energy expenditure rate accelerated by treatment with GC-24 (Fig. 3C–E) without affecting their caloric intake (Fig. 3B), limiting their body weight gain over time (Fig. 3A). However, in the present mouse model of obesity, expression of a number of BAT genes was not affected at all by treatment with a dose of GC-24 that is the molar equivalent to 10 times the physiological replacement dose of T3 (Fig. 5C). Despite this, a small reduction in body weight gain (Fig. 4C) and sizable modifications in gene expression in the heart of the same animals were observed (Fig. 5B). Interestingly, treatment with GC-24 did not affect gene expression in the skeletal muscle of the obese mice (Fig. 5A), nor did it in the primary cultures of skeletal myocytes (Fig. 2B), making it unlikely that muscle is the site at which TRβ-selective agonists trigger their main effects. In the liver, a bona fide target of TRβ-selective agonists (25), treatment with GC-24 only induced Cyp7a and lowered Srb1, whereas expression of other genes remained unaffected (Fig. 5D). Although induction of Cyp7a is compatible with the cholesterol-lowering effect of TRβ-selective agonists, it is puzzling the lack of a major foot print left by GC-24.
These observations raise two important questions: (i) Is thyroid hormone (or GC-24) signaling reduced in obesity and/or models of high-fat feeding? (ii) What is the mechanism by which treatment with GC-24 prevents body weight gain in the present mouse model of obesity? Addressing the first question, recent studies indicate that thyroid hormone signaling is likely to be impaired in humans with fatty liver, after a large gene set of positively regulated T3-responsive genes was found to be downregulated in surgical liver biopsies from obese subjects (26). Further, T3-induced expression of this set of genes in the mouse liver was abolished by feeding a HFD, indicating that impaired thyroid hormone action contributes to altered patterns of gene expression in fatty liver. This study supports these recent observations to the extent that the GC-24-induced acceleration of energy expenditure in mice fed a chow diet was diminished in the obese animals (Fig. 3C–E vs. Fig. 4D), as was the reduction in body weight gain (Fig. 3A vs. Fig. 4C). In addition, BAT and liver of high-fat-fed mice did not respond to treatment with GC-24 (Fig. 5C, D). The impairment in TRβ-mediated thyroid hormone signaling was quite remarkable perhaps because treatment with GC-24 started after the animals had been on a HFD for 3 weeks (Fig. 1B). A less pronounced impairment in GC-24 signaling by high-fat feeding was also observed in a rat model in which the administration of GC-24 was split into two daily injections versus one single injection in the present study (18). A mechanistic explanation for such impairment in thyroid hormone (GC-24) signaling is unknown, but as discussed by Pihlajamäki et al. (27), it possibly involves a reduction in the Pgc-1 levels, a well-known TR coactivator.
These observations have important clinical implications given that the development of TRβ-selective agonists is aimed at treating the metabolic consequences of obesity and dyslipidemia. If confirmed in a clinical setting, the present findings would indicate that relatively higher doses of TRβ-selective agonists should be used in individuals on a HFD, obese or with liver steatosis. It is possible that feeding a HFD somehow accelerates thyroid hormone/GC-24 catabolism, which would explain the decreased efficiency of these molecules under such settings. However, this hypothesis is unlikely as seen by the modifications in gene expression in the heart of the present animals (Fig. 5B), indicating that GC-24 and T3 maintain their biological effects in certain tissues despite the high-fat feeding.
The second point raised by the present findings has to do with the site of action of GC-24, which remains poorly characterized. Our present data confirm that brown adipocytes are a target of GC-24 (Fig. 2A) and that energy expenditure is accelerated in nonobese animals (Fig. 3C–E). However, the lack of acceleration in energy expenditure in obese animals (Fig. 4D) and the fact that BAT gene expression was not affected by treatment with GC-24 (Fig. 5C) indicate otherwise. Of course, it is possible that BAT activation is so limited that cannot be detected by the present analysis, or it does not involve aerobic pathways, or it takes place through a different metabolic pathway not involving the eight key genes studied. The first possibility is more likely given that in obese rats treated with GC-24, some gene induction was observed in BAT (18). Similar arguments could be used to analyze the involvement of other metabolically relevant tissues, including skeletal muscle, liver, and adipose tissue. While we presently found no evidence that skeletal muscle is a target of GC-24, there are other studies indicating that TRβ-selective agonists spare the skeletal muscle tissue (28). At the same time, white adipose tissue is unlikely to be a target of GC-24 in a setting that is similar to the present studies (18).
A novel observation brought to light with our data is that treatment with GC-24 does not increase food intake, a well-known effect of T3 (28). In fact, administration of GC-24 failed to increase further the caloric intake of mice placed on a HFD (Fig. 4B). It is well accepted that T3 increases caloric intake as a result of direct actions in the medial-basal hypothalamus (28) and also indirectly, as a result of the increased energy expenditure (29). At face value, the present observation indicates that this T3 pathway is mediated via a TRα mechanism. Given that the brain is a predominantly TRα-expressing tissue (30), it is indeed likely that these central (metabolic) effects of T3 are mediated by a TRα-dependent mechanism. At the same time, it is unknown whether the blood–brain barrier could be playing a role in this pathway selectivity as well. In the periphery, given that tissues such as BAT, skeletal muscle, and liver were not activated in the GC-24-treated animals, it would seem unlikely that indirect effects triggered by GC-24 could increase caloric intake.
Conclusions
Administration of the TRβ-selective agonist GC-24 failed to accelerate energy expenditure (Fig. 4D) and activate gene expression in BAT, skeletal muscle, and liver of obese mice (Fig. 5), although significant changes were noticed in nonobese mice (Fig. 3C–E) and cultures of brown adipocytes (Fig. 2A). These data would indicate that the model of high-fat feeding results in impaired thyroid hormone (GC-24) signaling, disrupting the ability of such molecules to promote metabolic homeostasis as reported in other animal models, including humans. An objective explanation for the small effect of GC-24 on preventing body weight gain is still lacking. Perhaps very important is the observation that, as opposed to T3, GC-24 did not increase food consumption in this animal model. The metabolic impact of this observation should be evaluated in further studies since it could very well represent a major mechanism by which TRβ-selective agonist mediate metabolic homeostasis.
Acknowledgments
The authors are grateful to Ms. Jessica Hall for critically reviewing this article. This work was supported in part by the National Institute for Diabetes and Digestive and Kidney Diseases (DK65055).
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Bianco AC. Maia AL. da Silva WS. Christoffolete MA. Adaptive activation of thyroid hormone and energy expenditure. Biosci Rep. 2005;25:191–208. doi: 10.1007/s10540-005-2885-6. [DOI] [PubMed] [Google Scholar]
- 2.Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 3.Brent GA. Tissue-specific actions of thyroid hormone: insights from animal models. Rev Endocr Metab Disord. 2000;1:27–33. doi: 10.1023/a:1010056202122. [DOI] [PubMed] [Google Scholar]
- 4.Chiellini G. Apriletti JW. Yoshihara HA. Baxter JD. Ribeiro RC. Scanlan TS. A high-affinity subtype-selective agonist ligand for the thyroid hormone receptor. Chem Biol. 1998;5:299–306. doi: 10.1016/s1074-5521(98)90168-5. [DOI] [PubMed] [Google Scholar]
- 5.Trost SU. Swanson E. Gloss B. Wang-Iverson DB. Zhang H. Volodarsky T. Grover GJ. Baxter JD. Chiellini G. Scanlan TS. Dillmann WH. The thyroid hormone receptor-beta-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology. 2000;141:3057–3064. doi: 10.1210/endo.141.9.7681. [DOI] [PubMed] [Google Scholar]
- 6.Bryzgalova G. Effendic S. Khan A. Rehnmark S. Barbounis P. Boulet J. Dong G. Singh R. Shapses S. Malm J. Webb P. Baxter JD. Grover GJ. Anti-obesity, anti-diabetic, and lipid lowering effects of the thyroid receptor beta subtype selective agonist KB-141. J Steroid Biochem Mol Biol. 2008;111:262–267. doi: 10.1016/j.jsbmb.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Erion MD. Cable EE. Ito BR. Jiang H. Fujitaki JM. Finn PD. Zhang BH. Hou J. Boyer SH. van Poelje PD. Linemeyer DL. Targeting thyroid hormone receptor-beta agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc Natl Acad Sci U S A. 2007;104:15490–15495. doi: 10.1073/pnas.0702759104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyabara EH. Aoki MS. Soares AG. Saltao RM. Vilicev CM. Passarelli M. Scanlan TS. Gouveia CH. Moriscot AS. Thyroid hormone receptor-beta-selective agonist GC-24 spares skeletal muscle type I to II fiber shift. Cell Tissue Res. 2005;321:233–241. doi: 10.1007/s00441-005-1119-3. [DOI] [PubMed] [Google Scholar]
- 9.Perra A. Simbula G. Simbula M. Pibiri M. Kowalik MA. Sulas P. Cocco MT. Ledda-Columbano GM. Columbano A. Thyroid hormone (T3) and TRbeta agonist GC-1 inhibit/reverse nonalcoholic fatty liver in rats. FASEB J. 2008;22:2981–2989. doi: 10.1096/fj.08-108464. [DOI] [PubMed] [Google Scholar]
- 10.Grover GJ. Mellstrom K. Malm J. Therapeutic potential for thyroid hormone receptor-beta selective agonists for treating obesity, hyperlipidemia and diabetes. Curr Vasc Pharmacol. 2007;5:141–154. doi: 10.2174/157016107780368271. [DOI] [PubMed] [Google Scholar]
- 11.Villicev CM. Freitas FR. Aoki MS. Taffarel C. Scanlan TS. Moriscot AS. Ribeiro MO. Bianco AC. Gouveia CH. Thyroid hormone receptor beta-specific agonist GC-1 increases energy expenditure and prevents fat-mass accumulation in rats. J Endocrinol. 2007;193:21–29. doi: 10.1677/joe.1.07066. [DOI] [PubMed] [Google Scholar]
- 12.Branco M. Ribeiro M. Negrao N. Bianco AC. 3,5,3'-Triiodothyronine actively stimulates UCP in brown fat under minimal sympathetic activity. Am J Physiol. 1999;276:E179–E187. doi: 10.1152/ajpendo.1999.276.1.E179. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro MO. Carvalho SD. Schultz JJ. Chiellini G. Scanlan TS. Bianco AC. Brent GA. Thyroid hormone-sympathetic interaction and adaptive thermogenesis are thyroid hormone receptor isoform-specific. J Clin Invest. 2001;108:97–105. doi: 10.1172/JCI12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celi FS. Brown adipose tissue—when it pays to be inefficient. N Engl J Med. 2009;360:1553–1556. doi: 10.1056/NEJMe0900466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gereben B. Zavacki A. Ribich S. Kim B. Huang S. Simonides W. Zeöld A. Bianco A. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29:898–938. doi: 10.1210/er.2008-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jesus LA. Carvalho SD. Ribeiro MO. Schneider M. Kim SW. Harney JW. Larsen PR. Bianco AC. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001;108:1379–1385. doi: 10.1172/JCI13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christoffolete MA. Linardi CC. de Jesus L. Ebina KN. Carvalho SD. Ribeiro MO. Rabelo R. Curcio C. Martins L. Kimura ET. Bianco AC. Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes. 2004;53:577–584. doi: 10.2337/diabetes.53.3.577. [DOI] [PubMed] [Google Scholar]
- 18.Amorim BS. Ueta CB. Freitas BC. Nassif RJ. Gouveia CH. Christoffolete MA. Moriscot AS. Lancelloti CL. Llimona F. Barbeiro HV. de Souza HP. Catanozi S. Passarelli M. Aoki MS. Bianco AC. Ribeiro MO. A TRbeta-selective agonist confers resistance to diet-induced obesity. J Endocrinol. 2009;203:291–299. doi: 10.1677/JOE-08-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fasshauer M. Klein J. Kriauciunas KM. Ueki K. Benito M. Kahn CR. Essential role of insulin receptor substrate 1 in differentiation of brown adipocytes. Mol Cell Biol. 2001;21:319–329. doi: 10.1128/MCB.21.1.319-329.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rando TA. Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christoffolete MA. Ribeiro R. Singru P. Fekete C. da Silva WS. Gordon DF. Huang SA. Crescenzi A. Harney JW. Ridgway EC. Larsen PR. Lechan RM. Bianco AC. Atypical expression of type 2 iodothyronine deiodinase in thyrotrophs explains the thyroxine-mediated pituitary thyrotropin feedback mechanism. Endocrinology. 2006;147:1735–1743. doi: 10.1210/en.2005-1300. [DOI] [PubMed] [Google Scholar]
- 22.Freitas FR. Moriscot AS. Jorgetti V. Soares AG. Passarelli M. Scanlan TS. Brent GA. Bianco AC. Gouveia CH. Spared bone mass in rats treated with thyroid hormone receptor TR beta-selective compound GC-1. Am J Physiol Endocrinol Metab. 2003;285:E1135–E1141. doi: 10.1152/ajpendo.00506.2002. [DOI] [PubMed] [Google Scholar]
- 23.Grover GJ. Egan DM. Sleph PG. Beehler BC. Chiellini G. Nguyen NH. Baxter JD. Scanlan TS. Effects of the thyroid hormone receptor agonist GC-1 on metabolic rate and cholesterol in rats and primates: selective actions relative to 3,5,3'-triiodo-L-thyronine. Endocrinology. 2004;145:1656–1661. doi: 10.1210/en.2003-0973. [DOI] [PubMed] [Google Scholar]
- 24.Manzano J. Morte B. Scanlan TS. Bernal J. Differential effects of triiodothyronine and the thyroid hormone receptor beta-specific agonist GC-1 on thyroid hormone target genes in the brain. Endocrinology. 2003;144:5480–5487. doi: 10.1210/en.2003-0633. [DOI] [PubMed] [Google Scholar]
- 25.Amma LL. Campos-Barros A. Wang Z. Vennstrom B. Forrest D. Distinct tissue-specific roles for thyroid hormone receptors beta and alpha1 in regulation of type 1 deiodinase expression. Molecular Endocrinology. 2001;15:467–475. doi: 10.1210/mend.15.3.0605. [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro MO. Effects of thyroid hormone analogs on lipid metabolism and thermogenesis. Thyroid. 2008;18:197–203. doi: 10.1089/thy.2007.0288. [DOI] [PubMed] [Google Scholar]
- 27.Pihlajamäki J. Boes T. Kim EY. Dearie F. Kim BW. Schroeder J. Mun E. Nasser I. Park PJ. Bianco AC. Goldfine AB. Patti ME. Thyroid hormone-related regulation of gene expression in human fatty liver. J Clin Endocrinol Metab. 2009;94:3521–3529. doi: 10.1210/jc.2009-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong WM. Martin NM. Smith KL. Gardiner JV. Connoley IP. Stephens DA. Dhillo WS. Ghatei MA. Small CJ. Bloom SR. Triiodothyronine stimulates food intake via the hypothalamic ventromedial nucleus independent of changes in energy expenditure. Endocrinology. 2004;145:5252–5258. doi: 10.1210/en.2004-0545. [DOI] [PubMed] [Google Scholar]
- 29.Dhillo WS. Appetite regulation: an overview. Thyroid. 2007;17:433–445. doi: 10.1089/thy.2007.0018. [DOI] [PubMed] [Google Scholar]
- 30.Brent GA. The molecular basis of thyroid hormone action. N Engl J Med. 1994;331:847–853. doi: 10.1056/NEJM199409293311306. [DOI] [PubMed] [Google Scholar]