Abstract
Background
Breast density tends to decrease when women stop taking hormone therapy (HT). Some women find HT cessation difficult to tolerate, possibly because of fluctuations in endogenous hormone levels and vasomotor symptoms. We hypothesized that women with dense breasts might have lower tolerance for short-term HT suspension than do women with fatty breasts.
Methods
As part of the Radiologic Evaluation And breast Density (READ) trial, we randomly assigned 881 women aged 45–80 with a prior screening (index) mammogram to suspend HT for 1 or 2 months before their next screening (study) mammogram. We measured continuous breast density on index mammograms using computer-assisted thresholding. At study mammograms, women indicated tolerance for stopping HT from 1 (extremely difficult) to 7 (very easy). Using linear regression, we evaluated the association between index breast density and tolerance after cessation, adjusting for age, body mass index (BMI), HT type, randomization group, and vasomotor symptoms.
Results
A higher percentage of breast density was associated with lower unadjusted mean tolerance scores (tolerance 4.27, 95% confidence interval [CI] 3.77-4.77 for women with ≥50% density, and 4.73, 95% CI 4.45-5.01 for women with <10% density, not a statistically significant difference). In adjusted analyses, neither percent breast density nor dense breast area was associated with tolerance for HT suspension.
Conclusions
Although HT use affects breast density, tolerance for suspending HT is not associated with breast density. Women with dense breasts have the greatest potential for decreases in density after HT cessation; they should tolerate stopping HT as well as women with fatty breasts.
Introduction
Breast density is among the strongest risk factors for breast cancer, with studies showing risk 3–5-fold higher for women whose breasts comprise >75% dense tissue than for women with <10% density.1–6 Breast density is associated with many hormonal and reproductive factors, including postmenopausal hormone therapy (HT, estrogen with or without progestin).7,8 HT use, particularly estrogen plus progestin, increases breast density by an average of 6% in 1 year in some postmenopausal women.9 Studies have also shown that suspending HT for as little as 4 weeks can decrease breast density.10–12
Current guidelines recommend that women engage in shared decision making with their providers about whether to use HT, and for how long, to minimize risks of breast cancer and cardiovascular disease (CVD).13–16 Despite risks associated with taking HT and potential benefits from stopping, some women are still reluctant to stop using hormones.17,18 A few previous studies have shown that tolerance for stopping HT is inversely correlated with the presence and severity of menopausal symptoms.17–19 In women who are unwilling to stop taking HT permanently, some clinicians have recommended suspending HT for a short time before mammography to improve breast cancer detection, but no evidence supports this recommendation.12
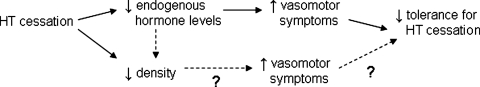
We thought tolerance for stopping HT might be related to breast density and hypothesized that women with greater breast density would have lower tolerance for HT suspension. We based this hypothesis on the pathway Figure 1 describes. First, stopping HT can decrease breast density10–12 and endogenous hormone levels.20,21 Fluctuating estrogen and progestin levels have been associated with an increase in vasomotor symptoms (hot flashes and night sweats),22,23 which may lead to lower tolerance for stopping HT. Women with dense breasts have the greatest potential for a decrease in density after stopping HT. Women with dense breasts may also have higher endogenous hormone levels to begin with24–26 and may experience larger fluctuations in endogenous hormone levels after stopping HT compared with women with fatty breasts. This could lead to more frequent or severe vasomotor symptoms and less tolerance for HT suspension among women with dense breasts. We know of no studies that have evaluated whether breast density is related to either tolerance or vasomotor symptoms.
FIG. 1.
Hypothesized pathway describing association among hormone therapy (HT) cessation, breast density, and tolerance for HT cessation. Tolerance for ceasing HT may be decreased through two different pathways. The first, through lowered levels of endogenous hormones and increased vasomotor symptoms, seems likely. The second, through decreased breast density and increased vasomotor symptoms, is more suggestive.
We conducted this analysis within a randomized clinical trial (RCT) designed to study the effects of short-term HT suspension (1–2 months before a mammogram) on recall for an abnormal finding on a mammogram and mammographic breast density.12 We evaluated the association between mammographic breast density among women currently taking HT and tolerance for short-term HT suspension. We accounted for vasomotor symptoms to determine if density was associated with tolerance independent of women's symptom experiences.
Materials and Methods
The Radiological Evaluation And breast Density (READ) RCT was designed to examine the effect of short-term HT suspension on mammographic breast density and mammography recall for an abnormal finding on an examination.12 The study was undertaken based on strong evidence suggesting that HT cessation caused improved mammography performance.27,28 The READ study methods and results have been described previously.12 Briefly, the study included 1704 women aged 45–80 randomly assigned to one of three groups: group 0 continued taking HT before their mammogram (n = 567), group 1 stopped HT 1 month before their mammogram (n = 570), and group 2 stopped HT 2 months before their mammogram (n = 567). All women were enrolled in the Breast Cancer Screening Program (BCSP)29 at Group Health Cooperative, a nonprofit integrated healthcare delivery system in Washington State. As part of this program, breast cancer risk factors are collected and updated at each routine mammogram for women aged ≥40.
All women in this trial had to have (1) at least one previous screening mammogram within the past 2 years (index mammogram), (2) a BCSP questionnaire that indicated they were using HT at the time of that mammogram, and (3) confirmation of continued HT dispensings from Group Health's electronic pharmacy data at the time of recruitment. We block randomized by index mammogram breast density (based on Breast Imaging Reporting and Data System [BI-RADS®] categories) and HT type (combination estrogen plus progestin [EPT] or estrogen only (ET]) to ensure equal distributions across randomization groups.30 We did not include women with a BI-RADS density category 1 (almost entirely fat) because these women were unlikely to have a clinically important decrease in breast density. We determined HT type (ET or EPT) using automated pharmacy dispensing data. Women had to have two estrogen dispensings in the 6 months before recruitment; HT type was distinguished by the presence of one or more progestin dispensings in those 6 months.31 All study procedures were compliant with the Health Information Portability and Accountability Act and approved by the Group Health and Department of Defense Institutional Review Boards.
Study population
This analysis included 1137 of the 1704 women from the READ study who were randomly assigned to stop HT (groups 1 and 2). We excluded 204 women who withdrew from the study before the study mammogram. Among 933 women who completed the study, 40 did not return their follow-up questionnaire or respond to the question about tolerance for stopping HT, 2 were missing breast density from the index mammogram, and 10 had no information on body mass index (BMI). After excluding these 52 women for these analyses, the final sample was 881 women: 445 in group 1 (1 month HT suspension) and 436 in group 2 (2 months HT suspension).
Predictor and outcome assessment
Our main predictor of interest was mammographic breast density on the index mammogram. We chose the index rather than study mammogram because we were interested in the effect of a woman's density on tolerance while she was still taking HT. HT cessation influenced the density measures from the study mammograms.12 We measured continuous breast density using a computer-assisted interactive thresholding method (Cumulus).32 We digitized the left breast craniocaudal projection from the index mammogram using a Kodak Lumisys 85 scanner. Small films were scanned at 87 μm/pixel and large films at 116 μm/pixel and were converted to square centimeters for dense area and breast area using the following conversion factors: 7.554 × 10−5cm2/pixel (small films) and 1.350 × 10−4cm2/pixel (large films). A single reader (E.A.B.) interpreted percent density, dense area, and total breast area for each mammogram. Mammograms were read in batches of 50 films each and included 10% interbatch repeat films for quality assurance. The reader was blinded to information on randomization group, HT type, and date of the mammogram. The mean absolute difference (standard error, SE) in quality control samples was 3.3% (0.2%) for percent breast density, 4.7 (0.4) cm2 for dense area, and 2.5 (0.2) cm2 for breast area. The concordance correlation between intrabatch repeats was 0.96 for percent breast density, 0.95 for dense area, and 1.0 for breast area.
We collected information on baseline covariates, including age, BMI, race/ethnicity, education, and history of HT use via self-report on the BCSP questionnaire at the index mammogram. Women self-reported information on several self-reported menopausal symptoms from a mailed study questionnaire completed at randomization. This report includes frequency and severity (mild, moderate, or severe) of vasomotor symptoms (hot flashes or night sweats) reported before HT cessation. Symptoms were reassessed after HT cessation but are not included in this report as they occurred concomitantly with HT cessation and could not be used as a predictor of tolerance. The occurrence of symptoms after cessation has been reported previously.12
The main outcome, tolerance for stopping HT, was assessed from a mailed follow-up questionnaire that women completed when they had their study mammogram after HT cessation. We asked women to indicate what it was like to try to stay off HT before their mammogram. Tolerance was self-reported on a scale from 1 to 7, with 1 being “extremely difficult/couldn't stay off” and 7 being “very easy/not a problem.”
Statistical analyses
We included all 881 women in the analyses regardless of their adherence to study recommendations to suspend HT use. Using linear regression, we modeled the effects of breast density on tolerance score. We categorized percent breast density (<10%, 10–<25%, 25–<50%, and >50%), and dense area (<10 cm2, 10–<30 cm2, 30–<50 cm2, and >50 cm2) for ease of interpretation. Tolerance was modeled continuously and presented and reported as adjusted means with 95% confidence intervals (CI) for each category of percent density or dense area. We tested for linear trend by including the categorical density variables as continuous variables in the model and present the F statistic and p values for the results. We adjusted for HT type, age group, BMI, randomization group, and intensity of vasomotor symptoms at baseline, as these confounders were significantly (p < 0.05) associated with tolerance in univariable models. Given the high correlation between intensity and frequency of vasomotor symptoms, we included only intensity in the final adjusted models. In secondary analyses, we assessed interactions between density and HT type, age group, BMI, and randomization group. We used linear regression to explore whether intensity or frequency of vasomotor symptoms had any relation to percentage of breast density or dense area. All analyses were conducted in STATA, v.10.0 (StataCorp, College Station, TX) with two-sided p values.
Results
Table 1 shows participant characteristics stratified by HT type. Of 881 women, 556 (63%) were ET users and 325 (37%) were EPT users. ET users had a slightly lower median percent breast density (19.8%, range 0.4%–69.1%) compared with EPT users (24.4%, range 0.8%–73.7%); dense area was similar between the two groups. ET users tended to be older, have a slightly higher BMI, and be more likely to have used ET for ≥10 years compared with EPT users. ET users were less likely to report having vasomotor symptoms, and EPT users were more likely to report having moderate or severe and more frequent vasomotor symptoms at baseline.
Table 1.
Characteristics of Radiologic Evaluation and Breast Density Trial Study Participants at Baseline Mammogram by Hormone Therapy Type
| |
ET |
EPT |
Total |
|---|---|---|---|
| |
n = 556 |
n = 325 |
n = 881 |
| n (%) | n (%) | n (%) | |
| Index % breast density | |||
| Mean (SD) | 22.9 (14.8) | 26.5 (15.2) | 24.2 (15.1) |
| Median | 19.8 | 24.4 | 21.9 |
| Index % breast density | |||
| <10 | 121 (21.8) | 53 (16.3) | 174 (19.8) |
| 10–<25 | 220 (39.6) | 111 (34.2) | 331 (37.6) |
| 25–<50 | 176 (31.7) | 137 (42.2) | 313 (35.5) |
| 50+ | 39 (7.0) | 24 (7.4) | 63 (7.2) |
| Index dense breast area (cm2) | |||
| Mean (SD) | 30.9 (21.5) | 31.8 (21.8) | 31.2 (21.6) |
| Median | 26.0 | 27.1 | 26.5 |
| Index dense breast area (cm2) | |||
| <10 | 56 (10.1) | 33 (10.2) | 89 (10.1) |
| 10–<30 | 276 (49.6) | 147 (45.2) | 423 (48.0) |
| 30–<50 | 135 (24.3) | 100 (30.8) | 235 (26.7) |
| 50+ | 89 (16.0) | 45 (13.9) | 134 (15.2) |
| Age group (years) | |||
| <55 | 118 (21.2) | 76 (23.4) | 194 (22.0) |
| 55–64 | 270 (48.6) | 197 (60.6) | 467 (53.0) |
| 65+ | 168 (30.2) | 52 (16.0) | 220 (25.0) |
| BMI | |||
| <25 | 177 (31.8) | 145 (44.6) | 322 (36.6) |
| 25–<30 | 175 (31.5) | 111 (34.2) | 286 (32.5) |
| 30+ | 204 (36.7) | 69 (21.2) | 273 (31.0) |
| Caucasian race | |||
| No | 62 (11.2) | 20 (6.2) | 82 (9.4) |
| Yes | 492 (88.8) | 302 (93.8) | 794 (90.6) |
| Education | |||
| ≤High school | 97 (17.6) | 35 (10.8) | 132 (15.1) |
| Some college or technical school | 234 (42.5) | 110 (34.0) | 344 (39.3) |
| College graduate | 101 (18.3) | 78 (24.1) | 179 (20.5) |
| Postgraduate degree | 119 (21.6) | 101 (31.2) | 220 (25.1) |
| History of estrogen use (years) | |||
| <5 | 47 (8.8) | 54 (18.0) | 101 (12.1) |
| 5–9 | 96 (18.1) | 96 (32.0) | 192 (23.1) |
| 10+ | 389 (73.1) | 150 (50.0) | 539 (64.8) |
| Type of HT | |||
| ET | – | – | 556 (63.1) |
| EPT | 325 (36.9) | ||
| Randomization group | |||
| 1-month suspension | 277 (49.8) | 168 (51.7) | 445 (50.5) |
| 2-month suspension | 279 (50.2) | 157 (48.3) | 436 (49.4) |
| Intensity of vasomotor symptoms | |||
| None | 310 (56.8) | 145 (46.2) | 455 (52.9) |
| Mild | 42 (7.7) | 25 (8.0) | 67 (7.8) |
| Moderate | 92 (16.9) | 77 (24.5) | 169 (19.7) |
| Severe | 102 (18.7) | 67 (21.3) | 169 (19.7) |
| Frequency of vasomotor symptoms | |||
| None | 310 (56.7) | 145 (46.2) | 455 (52.9) |
| <1 per day | 117 (21.4) | 80 (25.5) | 197 (22.9) |
| 1–2 per day | 73 (13.4) | 51 (16.2) | 124 (14.4) |
| 3–4 per day | 31 (5.7) | 26 (8.3) | 57 (6.6) |
| >4 per day | 16 (2.9) | 12 (3.8) | 28 (3.3) |
BMI, body mass index; EPT, estrogen plus progestin therapy; ET, estrogen only therapy; HT, hormone therapy; SD, standard deviation.
Table 2 shows the unadjusted mean tolerance scores for each breast density category. Mean tolerance scores were lower for women with higher percent breast density; however, the CIs overlapped. There were no differences in tolerance by dense breast area. Tolerance scores were significantly higher among ET users who were >65 years (mean 4.85, 95%CI 4.52–5.18) compared with <55 (mean 4.24, 95%CI 3.93–4.55), but the tolerance scores were not higher among women 55–64 years old. Tolerance scores were lower for women who used ET compared with EPT, but the difference was not statistically significant. Women randomly assigned to 2 months of HT cessation had significantly lower tolerance compared with those assigned to 1 month. Frequency and severity of baseline vasomotor symptoms were both significantly associated with tolerance (p for trend <0.01). Women with more frequent or severe symptoms had lower tolerance for cessation. When we evaluated tolerance scores separately by randomization group (rather than HT type), there were no differences in the associations between density and tolerance (data not shown).
Table 2.
Univariate Associations Between Breast Density at Baseline (Index) Mammogram and Tolerance for Hormone Therapy Suspension at Follow-Up
| |
Tolerance for HT suspension |
|||||
|---|---|---|---|---|---|---|
| ET Mean tolerance (95% CI) | pa | EPT Mean tolerance (95% CI) | pa | Total Mean tolerance (95% CI) | pa | |
| Index % breast density | ||||||
| <10 | 4.58 (4.24-4.92) | 0.08 | 5.08 (4.60-5.55) | 0.23 | 4.73 (4.45-5.01) | 0.05 |
| 10–<25 | 4.50 (4.25-4.76) | 4.52 (4.16-4.88) | 4.51 (4.30-4.72) | |||
| 25–<50 | 4.27 (3.99-4.56) | 4.60 (4.28-4.91) | 4.42 (4.20-4.63) | |||
| 50+ | 4.10 (3.43-4.77) | 4.54 (3.77-5.31) | 4.27 (3.77-4.77) | |||
| Index dense breast area (cm2) | ||||||
| <10 | 4.27 (3.77-4.76) | 0.60 | 5.00 (4.42-5.58) | 0.93 | 4.54 (4.16-4.92) | 0.62 |
| 10–<30 | 4.41 (4.19-4.64) | 4.45 (4.13-4.77) | 4.43 (4.24-4.61) | |||
| 30–<50 | 4.47 (4.13-4.82) | 4.85 (4.50-5.20) | 4.63 (4.38-4.88) | |||
| 50+ | 4.45 (4.04-4.86) | 4.58 (4.00-5.16) | 4.49 (4.16-4.83) | |||
| Age group (years) | ||||||
| <55 | 4.24 (3.93-4.55) | <0.01 | 4.61 (4.25-4.97) | 0.28 | 4.40 (4.17-4.64) | 0.01 |
| 55–64 | 4.30 (4.07-4.53) | 4.55 (4.27-4.83) | 4.40 (4.22-4.57) | |||
| 65+ | 4.85 (4.52-5.18) | 5.15 (4.58-5.72) | 4.92 (4.64-5.20) | |||
| BMI (kg/m2) | ||||||
| <25 | 4.28 (3.98-4.58) | 0.16 | 4.62 (4.31-4.93) | 0.27 | 4.43 (4.21-4.65) | 0.14 |
| 25–29.9 | 4.41 (4.14-4.68) | 4.45 (4.11-4.79) | 4.42 (4.21-4.63) | |||
| ≥30 | 4.55 (4.29-4.82) | 5.01 (4.58-5.45) | 4.67 (4.44-4.90) | |||
| Caucasian race | ||||||
| No | 4.26 (3.74-4.78) | 0.50 | 5.10 (4.29-5.91) | 0.26 | 4.46 (4.02-4.91) | 0.87 |
| Yes | 4.43 (4.26-4.60) | 4.61 (4.40-4.82) | 4.50 (4.37-4.63) | |||
| Education | ||||||
| ≤High school | 4.44 (4.03-4.85) | 0.33 | 4.63 (4.02-5.23) | 0.57 | 4.49 (4.15-4.83) | 0.39 |
| Some college | 4.53 (4.29-4.78) | 4.78 (4.43-5.13) | 4.61 (4.41-4.81) | |||
| College graduate | 4.25 (3.87-4.63) | 4.51 (4.06-4.96) | 4.36 (4.07-4.65) | |||
| Postgraduate degree | 4.31 (3.96-4.66) | 4.59 (4.24-4.95) | 4.44 (4.19-4.69) | |||
| History of estrogen use | ||||||
| <5 years | 4.04 (3.46-4.62) | 0.12 | 4.72 (4.22-5.22) | 0.60 | 4.41 (4.02-4.79) | 0.68 |
| 5–9 years | 4.25 (3.88-4.62) | 4.65 (4.27-5.02) | 4.45 (4.18-4.71) | |||
| 10+ years | 4.45 (4.26-4.64) | 4.57 (4.27-4.88) | 4.48 (4.32-4.65) | |||
| Type of HT use | ||||||
| ET | – | – | 4.42 (4.26-4.58) | 0.09 | ||
| EPT | 4.65 (4.44-4.85) | |||||
| Randomization group | ||||||
| 1-month suspension | 4.64 (4.41-4.86) | <0.01 | 5.03 (4.75-5.31) | <0.01 | 4.78 (4.61-4.96) | <0.01 |
| 2-month suspension | 4.20 (3.97-4.44) | 4.24 (3.95-4.53) | 4.22 (4.03-4.40) | |||
| Intensity of vasomotor symptoms | ||||||
| None | 4.64 (4.42-4.86) | <0.01 | 5.10 (4.80-5.40) | <0.01 | 4.79 (4.61-4.97) | <0.01 |
| Mild | 4.62 (4.10-5.14) | 4.20 (3.46-4.94) | 4.46 (4.04-4.89) | |||
| Moderate | 4.11 (3.74-4.48) | 4.52 (4.12-4.92) | 4.30 (4.03-4.57) | |||
| Severe | 3.94 (3.57-4.31) | 4.01 (3.56-4.47) | 3.97 (3.68-4.26) | |||
| Frequency of vasomotor symptoms | ||||||
| None | 4.64 (4.42-4.86) | <0.01 | 5.10 (4.80-5.40) | <0.01 | 4.79 (4.61-4.97) | <0.01 |
| <1 per day | 4.38 (4.06-4.71) | 4.61 (4.19-5.03) | 4.48 (4.22-4.73) | |||
| 1–2 per day | 3.95 (3.51-4.38) | 4.10 (3.67-4.53) | 4.01 (3.70-4.32) | |||
| 3–4 per day | 3.84 (3.19-4.49) | 4.08 (3.25-4.90) | 3.95 (3.44-4.46) | |||
| >4 per day | 3.81 (2.78-4.85) | 3.17 (2.45-3.89) | 3.54 (2.87-4.20) | |||
p value for test of linear trend for ordinal variables and for the t test for binary variables.
CI, confidence interval.
Tolerance had no statistically significant associations with percent breast density or dense breast area after adjusting for age, BMI, HT type, randomization group, and intensity of vasomotor symptoms (Table 3). There were no significant interactions between percent density or dense breast area and age group, BMI category, HT type, or randomization group (data not shown). Finally, intensity or frequency of vasomotor symptoms had no relation with percentage of density or dense area (data not shown).
Table 3.
Adjusted Associations Between Percent Breast Density, Dense Breast Area, and Tolerance for Hormone Therapy Suspension
| |
|
Test for linear trend |
|
|---|---|---|---|
| Baseline density | Adjusted mean tolerancea(95% CI) | F statistic | p value |
| Index % breast density | |||
| <10 | 4.68 (4.39-4.97) | 1.45 | 0.23 |
| 10–<25 | 4.48 (4.28-4.69) | ||
| 25–<50 | 4.46 (4.25-4.68) | ||
| 50+ | 4.35 (3.87-4.83) | ||
| Index dense breast area (cm2) | |||
| <10 | 4.48 (4.08-4.87) | 0.23 | 0.63 |
| 10–<30 | 4.45 (4.27-4.63) | ||
| 30–<50 | 4.63 (4.39-4.87) | ||
| 50+ | 4.48 (4.16-4.80) | ||
Adjusted for age, BMI, randomization group, type of HT, and intensity of vasomotor symptoms.
Discussion
Breast density is among the strongest risk factors for breast cancer.1–6 Use of HT, particularly EPT, is one of the few modifiable factors that influence breast density and breast cancer risk.9,26 Although the prevalence of HT use has decreased dramatically since 200231,33,34 when the Women's Health Initiative Estrogen Plus Progestin Study findings were published,35 some women are still reluctant to stop taking HT.17–19 We hypothesized that tolerance would be associated with breast density because stopping HT decreases breast density in some women. Presumably, this decrease in breast density reflects diminished circulating estrogen and progestin concentrations. Fluctuating serum concentrations of estrogens and progestins are associated with an increase in vasomotor symptoms, which may cause lower tolerance for stopping HT.22,23 However, our results showed that neither initial percent breast density nor dense area was associated with tolerance for short-term HT suspension among EPT or ET users, regardless of the presence of vasomotor symptoms. Further, density was not associated with the intensity or frequency of vasomotor symptoms. This suggests that tolerance for HT cessation is not influenced by a pathway involving breast density.
Current evidence states that women, especially those with such breast cancer risk factors as dense breasts and family history, should take HT only to relieve menopausal symptoms for the shortest possible time.13,14 However, some women are reluctant to stop taking HT for fear of vasomotor symptoms returning; this was true in our trial, as previously reported.18,19 These women may keep being screened regularly for breast cancer, but increased density may hinder cancer detection on mammography.7 If women with very dense breasts have low tolerance for stopping HT, they may not be able to comply with cessation. Therefore, short-term HT cessation before mammography would have little impact on improving detection for these women.
Our results suggest that low tolerance of HT suspension is related to several other factors, including intensity and frequency of vasomotor symptoms while still taking HT. This is evidenced by statistically significant p values for trend and differences in the CIs across symptom categories in Table 2. These results are not surprising and jibe with previous studies that have evaluated tolerance for stopping HT.17–19 Also, tolerance was associated with duration of cessation. Women who stopped HT for 2 months had lower tolerance than those who stopped for 1 month. Although previous studies have not shown whether length of suspension influences tolerance, this result may be generalizable only to those women who plan to suspend HT temporarily. It is possible that women who plan to stop permanently may have better tolerance over a longer time.
We also found that women aged ≥65 had significantly higher tolerance scores compared with women aged <55. Possible explanations are that older women are farther from menopause, have lower circulating estrogen levels, and are less likely to be bothered by menopausal symptoms compared with younger women.23,36 Older women are also more likely to be long-term HT users; these results may be encouraging for long-term HT users who are willing to attempt stopping HT.
Among our study's limitations, we used density measures from index mammograms taken up to 2 years before stopping HT, although density changed very little during this time, as our main trial results showed.12 Women who were randomized to continue using HT had a 0.1% absolute change in percent density from index to study mammograms. Continuous breast density measures are known to be somewhat subjective and contain measurement error, which could have biased our results toward the null.37,38 We did, however, take several steps to minimize measurement error. All density measures were performed by a single reader with QA films systematically distributed within and between batches. Our concordance correlations were very similar to those reported in previous studies.38,39 In addition, our study population may not be representative of all women currently taking HT. Roughly 54% of women invited to participate in this study refused, and almost 60% of those who refused did so because they were unwilling to stop taking HT.40 Nonparticipants were older and had less education and lower BMI compared with participants (all p < 0.05).40 Therefore, the women who participated in this trial were those who were willing to stop taking HT and may have a different tolerance experience than those who were unwilling. Although this may have introduced selection bias into our sample, we tried to account for this using recruitment materials that reassured women that it was okay if they were not able to stop taking their HT as instructed. Finally, we excluded women with fatty breasts (BI-RADS density category 1) from the trial. Although this group comprises <10% of women,41 this does limit the generalizability of our results to women with fatty breasts, as they may have a different experience tolerating HT suspension compared with women with denser breasts.
Our study had several strengths. Despite limitations in our continuous breast density measures, our measures may be more sensitive to differences in density between groups than studies that have used BI-RADS categories.42,43 This is because continuous density, measured on a scale from 0 to 100 is sensitive to small changes not detected with BI-RADS categories. We collapsed our continuous density measures into categories for ease of interpretation. In addition, our study was population based and conducted within the context of a randomized trial, and the results should be generalizable to the majority of women willing to consider short-term HT suspension.
In this large RCT, mammographic breast density was not associated with tolerance for stopping HT for 1–2 months. Intensity of vasomotor symptoms, length of HT suspension, and women's age predicted tolerance much more strongly than did breast density. Among the many benefits to stopping HT is decreased breast density. Current guidelines recommend that women engage in shared decision making about whether to take HT to manage symptoms and when to stop. Women who are reluctant to stop taking HT should consult with their providers about the risks and benefits of short-term cessation before a mammogram. Using these results may help them make more informed decisions by taking into account the risks and benefits, as well as potential tolerance for cessation.
Acknowledgments
The study team had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This study could not have been completed without the assistance of Tammy Dodd, Linda Palmer, R.N., and Melissa Rabelhofer. We thank Juleann Gandara, M.D., for her participation as the expert radiologist. We also thank members of our advisory board: Hermien Watkins, Paula Hoffman, Deb Schiro, and Margrit Schubiger; members of the Data Safety and Monitoring Board: Susan Heckbert, M.D., Ph.D., Chair, University of Washington Department of Epidemiology; Ben Anderson, M.D., University of Washington; Mary Anne Rossing, D.V.M., Ph.D., Fred Hutchinson Cancer Research Center; Robert D. Rosenberg, M.D., University of New Mexico; Thomas Lumley, Ph.D., University of Washington Department of Biostatistics; and Elizabeth Lin, M.D., M.P.H., Group Health Permanente medical monitor. We thank Robert Karl, M.D., Donna White, M.D., and Jo Ellen Callahan for their support for implementing this trial at Group Health. We also acknowledge Stephen Taplin, M.D., M.P.H., for his collaboration in getting this study funded when he was an investigator at Group Health Cooperative.
A population-based trial to assess the effects of short-term HT suspension on mammography assessments and breast density, the READ study was funded by the Department of Defense (PI, D. Buist, DAMD17-03-1-0447), registered clinical trial number NCT00117663. Study participants were recruited from the Group Health Breast Cancer Screening Program funded by the National Cancer Institute (PI, D. Buist, U01CA63731). S.D.R. and K.M.N. receive grant support from Pfizer.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Boyd NF. Guo H. Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 2.Harvey JA. Bovbjerg VE. Quantitative assessment of mammographic breast density: Relationship with breast cancer risk. Radiology. 2004;230:29–41. doi: 10.1148/radiol.2301020870. [DOI] [PubMed] [Google Scholar]
- 3.Boyd NF. Byng JW. Jong RA, et al. Quantitative classification of mammographic densities and breast cancer risk: Results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87:670–675. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 4.Yaffe MJ. Boyd NF. Byng JW, et al. Breast cancer risk and measured mammographic density. Eur J Cancer Prev. 1998;7(Suppl 1):S47–55. doi: 10.1097/00008469-199802001-00010. [DOI] [PubMed] [Google Scholar]
- 5.Barlow WE. White E. Ballard-Barbash R, et al. Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst. 2006;98:1204–1214. doi: 10.1093/jnci/djj331. [DOI] [PubMed] [Google Scholar]
- 6.Chen J. Pee D. Ayyagari R, et al. Projecting absolute invasive breast cancer risk in white women with a model that includes mammographic density. J Natl Cancer Inst. 2006;98:1215–1226. doi: 10.1093/jnci/djj332. [DOI] [PubMed] [Google Scholar]
- 7.Carney PA. Miglioretti DL. Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168–175. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 8.Vachon CM. Sellers TA. Vierkant RA. Wu FF. Brandt KR. Case-control study of increased mammographic breast density response to hormone replacement therapy. Cancer Epidemiol Biomarkers Prev. 2002;11:1382–1388. [PubMed] [Google Scholar]
- 9.McTiernan A. Martin CF. Peck JD, et al. Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women's Health Initiative randomized trial. J Natl Cancer Inst. 2005;97:1366–1376. doi: 10.1093/jnci/dji279. [DOI] [PubMed] [Google Scholar]
- 10.Harvey JA. Pinkerton JV. Herman CR. Short-term cessation of hormone replacement therapy and improvement of mammographic specificity. J Natl Cancer Inst. 1997;89:1623–1625. doi: 10.1093/jnci/89.21.1623. [DOI] [PubMed] [Google Scholar]
- 11.Colacurci N. Fornaro F. De Franciscis P. Mele D. Palermo M. del Vecchio W. Effects of a short-term suspension of hormone replacement therapy on mammographic density. Fertil Steril. 2001;76:451–455. doi: 10.1016/s0015-0282(01)01967-7. [DOI] [PubMed] [Google Scholar]
- 12.Buist DS. Anderson ML. Reed SD, et al. Short-term hormone therapy suspension and mammography recall: A randomized trial. Ann Intern Med. 2009;150:752–765. doi: 10.7326/0003-4819-150-11-200906020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Utian WH. Archer DF. Bachmann GA, et al. Estrogen and progestogen use in postmenopausal women: July 2008 position statement of The North American Menopause Society. Menopause. 2008;15:584–602. doi: 10.1097/gme.0b013e31817b076a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion No. 420, November 2008: Hormone therapy and heart disease. Obstet Gynecol. 2008;112:1189–1192. doi: 10.1097/AOG.0b013e31818e8782. [DOI] [PubMed] [Google Scholar]
- 15.Heiss G. Wallace R. Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036–1045. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 16.Chlebowski RT. Kuller LH. Prentice RL, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360:573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parente L. Uyehara C. Larsen W. Whitcomb B. Farley J. Long-term impact of the Women's Health Initiative on HRT. Arch Gynecol Obstet. 2008;277:219–224. doi: 10.1007/s00404-007-0442-1. [DOI] [PubMed] [Google Scholar]
- 18.Ness J. Aronow WS. Prevalence and causes of persistent use of hormone replacement therapy among postmenopausal women: A follow-up study. Am J Ther. 2006;13:109–112. doi: 10.1097/00045391-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Horner E. Fleming J. Studd J. A study of women on long-term hormone replacement therapy and their attitude to suggested cessation. Climacteric. 2006;9:459–463. doi: 10.1080/13697130601024629. [DOI] [PubMed] [Google Scholar]
- 20.Chan MF. Dowsett M. Folkerd E, et al. Past oral contraceptive and hormone therapy use and endogenous hormone concentrations in postmenopausal women. Menopause. 2008;15:332–339. doi: 10.1097/gme.0b013e31806458d9. [DOI] [PubMed] [Google Scholar]
- 21.Tworoger SS. Missmer SA. Barbieri RL. Willett WC. Colditz GA. Hankinson SE. Plasma sex hormone concentrations and subsequent risk of breast cancer among women using postmenopausal hormones. J Natl Cancer Inst. 2005;97:595–602. doi: 10.1093/jnci/dji099. [DOI] [PubMed] [Google Scholar]
- 22.Al-Azzawi F. Palacios S. Hormonal changes during menopause. Maturitas. 2009;63:135–137. doi: 10.1016/j.maturitas.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Randolph JF Jr. Sowers M. Bondarenko I, et al. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab. 2005;90:6106–6112. doi: 10.1210/jc.2005-1374. [DOI] [PubMed] [Google Scholar]
- 24.Boyd NF. Stone J. Martin LJ, et al. The association of breast mitogens with mammographic densities. Br J Cancer. 2002;87:876–882. doi: 10.1038/sj.bjc.6600537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bremnes Y. Ursin G. Bjurstam N. Rinaldi S. Kaaks R. Gram IT. Endogenous sex hormones, prolactin and mammographic density in postmenopausal Norwegian women. Int J Cancer. 2007;121:2506–2511. doi: 10.1002/ijc.22971. [DOI] [PubMed] [Google Scholar]
- 26.Greendale GA. Palla SL. Ursin G, et al. The association of endogenous sex steroids and sex steroid binding proteins with mammographic density: Results from the Postmenopausal Estrogen/Progestin Interventions Mammographic Density Study. Am J Epidemiol. 2005;162:826–834. doi: 10.1093/aje/kwi286. [DOI] [PubMed] [Google Scholar]
- 27.Rutter CM. Mandelson MT. Laya MB. Seger DJ. Taplin S. Changes in breast density associated with initiation, discontinuation, and continuing use of hormone replacement therapy. JAMA. 2001;285:171–176. doi: 10.1001/jama.285.2.171. [DOI] [PubMed] [Google Scholar]
- 28.Laya MB. Larson EB. Taplin SH. White E. Effect of estrogen replacement therapy on the specificity and sensitivity of screening mammography. J Natl Cancer Inst. 1996;88:643–649. doi: 10.1093/jnci/88.10.643. [DOI] [PubMed] [Google Scholar]
- 29.Taplin SH. Ichikawa L. Buist DS. Seger D. White E. Evaluating organized breast cancer screening implementation: The prevention of late-stage disease? Cancer Epidemiol Biomarkers Prev. 2004;13:225–234. doi: 10.1158/1055-9965.epi-03-0206. [DOI] [PubMed] [Google Scholar]
- 30.American College of Radiology (ACR) ACR Breast imaging reporting and data system, breast imaging atlas. Reston, VA: American College of Radiology; 2003. ACR BI-RADS—Mammography. [Google Scholar]
- 31.Buist DS. Newton KM. Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104:1042–1050. doi: 10.1097/01.AOG.0000143826.38439.af. [DOI] [PubMed] [Google Scholar]
- 32.Byng JW. Boyd NF. Fishell E. Jong RA. Yaffe MJ. The quantitative analysis of mammographic densities. Phys Med Biol. 1994;39:1629–1638. doi: 10.1088/0031-9155/39/10/008. [DOI] [PubMed] [Google Scholar]
- 33.Wegienka G. Havstad S. Kelsey JL. Menopausal hormone therapy in a health maintenance organization before and after Women's Health Initiative hormone trials termination. J Womens Health. 2006;15:369–378. doi: 10.1089/jwh.2006.15.369. [DOI] [PubMed] [Google Scholar]
- 34.Hersh AL. Stefanick ML. Stafford RS. National use of postmenopausal hormone therapy: Annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 35.Rossouw JE. Anderson GL. Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 36.Col NF. Guthrie JR. Politi M. Dennerstein L. Duration of vasomotor symptoms in middle-aged women: A longitudinal study. Menopause. 2009;16:453–457. doi: 10.1097/gme.0b013e31818d414e. [DOI] [PubMed] [Google Scholar]
- 37.Stone J. Gunasekara A. Martin LJ. Yaffe M. Minkin S. Boyd NF. The detection of change in mammographic density. Cancer Epidemiol Biomarkers Prev. 2003;12:625–630. [PubMed] [Google Scholar]
- 38.Kelemen LE. Pankratz VS. Sellers TA, et al. Age-specific trends in mammographic density: The Minnesota Breast Cancer Family Study. Am J Epidemiol. 2008;167:1027–1036. doi: 10.1093/aje/kwn063. [DOI] [PubMed] [Google Scholar]
- 39.Caire-Juvera G. Arendell LA. Maskarinec G. Thomson CA. Chen Z. Associations between mammographic density and body composition in Hispanic and non-Hispanic white women by menopause status. Menopause. 2008;15:319–325. doi: 10.1097/gme.0b013e3181405b8a. [DOI] [PubMed] [Google Scholar]
- 40.Reed SD. Buist DS. Anderson ML, et al. Short-term (1–2 mo) hormone therapy cessation before mammography. Menopause. 2009;16:1125–1131. doi: 10.1097/gme.0b013e3181a5ce60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Cancer Institute: Breast Cancer Surveillance Consortium. breastscreening.cancer.gov/data/variables/freq_tables_pct.html#DENSITY. [Nov 25;2009 ]. breastscreening.cancer.gov/data/variables/freq_tables_pct.html#DENSITY
- 42.Martin KE. Helvie MA. Zhou C, et al. Mammographic density measured with quantitative computer-aided method: Comparison with radiologists' estimates and BI-RADS categories. Radiology. 2006;240:656–665. doi: 10.1148/radiol.2402041947. [DOI] [PubMed] [Google Scholar]
- 43.Kerlikowske K. Ichikawa L. Miglioretti DL, et al. Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst. 2007;99:386–395. doi: 10.1093/jnci/djk066. [DOI] [PubMed] [Google Scholar]