Abstract
Background
The thyroidal response of pregnant patients with established Hashimoto's thyroiditis remains poorly described. The aim of this study was to determine the impact of pregnancy on Hashimoto's thyroiditis as revealed by changes in postpregnancy levothyroxine requirements.
Methods
We performed a retrospective study of 799 hypothyroid patients in a university hospital. We reviewed the clinical records and selected a group of well-documented pregnant (n = 34) and nonpregnant (n = 32) hypothyroid women for study. We reviewed levothyroxine intake and serum thyrotropin (TSH) levels during three consecutive 9-month time intervals that were immediately before, during, and after pregnancy. We compared the percent change in levothyroxine dose between the prepregnancy level and each trimester during and after pregnancy.
Results
There were two patterns of levothyroxine supplementation during gestation. In pattern 1 (n = 11) there was either no change or a single levothyroxine dose increase with no subsequent changes in each trimester (T1 = T2 = T3). In pattern 2 (n = 18), multistep levothyroxine dose increases were required throughout pregnancy (T1 < T2 < T3) to maintain desired TSH levels (<2.0 mU/L). Women with pattern 2 had mean TSH levels during gestation that differed significantly from pattern 1 (2.8 ± 0.5 vs. 1.3 ± 0.1 mU/L respectively; p < 0.03). Further, in multivariate logistic regression, women with pattern 2 were 62 times more likely than women with pattern 1 to have a levothyroxine dose at least 20% above baseline at 3 months postpartum (p = 0.04).
Conclusions
We showed that >50% of hypothyroid women with Hashimoto's thyroiditis experienced an increase in levothyroxine requirements in the postpartum compared to pregestational doses. This pattern of enhanced levothyroxine need was most likely dependent on the preexisting thyroid functional reserve and postpartum progression of autoimmune destruction.
Introduction
The leading cause of hypothyroidism in the United States is Hashimoto's autoimmune thyroiditis (1). There is a large literature on the influence of pregnancy on autoimmune thyroid disease and on the influence of autoimmune thyroid disease on pregnancy, both of which impact clinical management (2–9). This is evidenced by the changing levels of autoantibodies to thyroid peroxidase and thyroglobulin, which usually decrease throughout pregnancy in such women, and by the well-documented improvement in autoimmune hyperthyroidism (Graves' disease) caused by decreasing levels of thyroid-stimulating antibodies (10,11).
Pregnancy is also a time when thyroid gland activity is increased to cope with the enlarging distribution volume and serum thyroid hormone binding capacity, increased iodine clearance, and fetal requirements (12). The stress placed on the thyroid gland in pregnancy reveals any compromise in thyroid functional reserve and is evidenced by a rising serum thyrotropin (TSH) level. Studies have shown that the majority of the pregnant women with increased serum TSH, in the absence of iodine deficiency, are thyroid antibody positive, revealing that autoimmune thyroid disease is the primary reason for their lack of thyroid reserve (13). Presumably, there is just not enough time for the thyroid gland to recover from the thyroiditis-induced damage or it is incapable of doing so. Alternatively, this logic may be faulty and autoimmune thyroiditis, unlike Graves' disease, may not improve during pregnancy, but exacerbate as seen with systemic lupus erythematosus (14,15). This would imply that the thyroid antibody levels may drop, but the T-cell-mediated destruction continues.
The normal maternal immune system returns slowly to baseline after delivery but first appears to pass through a period of enhanced immune reactivity (7). Although long recognized, interest in the aggravation of autoimmune thyroid disease in the postpartum period was reawakened 25 years ago by Amino et al. (16). Since then many studies, including from our own group, have showed both the exacerbation and de novo occurrence of autoimmune thyroid disease in the postpartum period (17,18). Studies have also shown that more of the women with preexisting thyroid antibodies develop postpartum thyroid dysfunction (30%–50%), which reflects the key role of autoimmune susceptibility in these postpartum immune changes (19,20).
Despite our improved understanding of the relationship between pregnancy and autoimmune thyroid disease, the impact of pregnancy on established Hashimoto's thyroiditis remains speculative and poorly documented. Previous studies have shown that up to 56% of hypothyroid women need to change their prepregnancy levothyroxine requirements during the postpartum period (21). We, therefore, developed the simple hypothesis that any changes in established Hashimoto's thyroiditis secondary to changes in immune function during pregnancy and the postpartum should be revealed by changes in maternal requirements for levothyroxine replacement during pregnancy and the postpartum when compared with prepregnancy dosing. Such levothyroxine supplementation often needs to be increased by 30%–50% in such patients during pregnancy because of their tenuous thyroid reserve and the increased thyroid demands. The thyroid reserve in hypothyroid patients may vary considerably depending on how much thyroid function remains, but it has long been assumed that levothyroxine dosing should be restored to pregestational amounts after delivery (22,23). While other exogenous factors may influence the difference between prepregnancy and postpregnancy dosing, such as weight changes, ingestion of drugs that interfere with levothyroxine absorption or metabolism, breastfeeding, or compliance, these are likely to be either obvious or minor contributors. However, it is also likely that pregnant women with established Hashimoto's thyroiditis may experience a clinical exacerbation in the postpartum period with yet further deterioration in their thyroid reserve. This would be revealed as a long-term need for more levothyroxine after delivery when compared with prepregnancy requirements.
Hence, the aim of this study was to examine the risk of exacerbated hypothyroidism during and after pregnancy in women with Hashimoto's thyroiditis and to define the characteristics of those women most impacted, if any. Such information would aid us in diagnosis and treatment strategies for autoimmune thyroid disease in pregnancy and the postpartum.
Materials and Methods
Participants
We conducted a retrospective analysis of all autoimmune hypothyroid patients seen by R.S.H. and T.F.D. in the Endocrine Faculty Practice of the Mount Sinai School of Medicine in New York City during a 3-year period (2004–2006). The study was approved by the Mount Sinai School of Medicine Institutional Review Board. The criteria for being included in the pregnancy study group were (a) childbearing age 20–45 years; (b) previous diagnosis of autoimmune hypothyroidism requiring levothyroxine supplementation (the diagnosis of hypothyroidism was established according to biochemical criteria regarding serum TSH levels above the normal range with a normal or below normal range serum thyroxine (T4) level and confirmed by the presence of thyroid antibodies); (c) availability of systematic clinical and biochemical medical records from 9 months before until 9 months after pregnancy including at least one thyroid function test (TSH or free T4 [FT4]) in every 9-month period; and (d) had good compliance as evidenced by regular follow-up and prescription requests. Control patients had all the same criteria except item (c) but required a total minimum follow-up period of 36 months after their last pregnancy. The information collected included age, weight, number and date of pregnancies, date of the hypothyroid diagnosis, serum TSH and total T4 or FT4, thyroid antibody levels, length of disease treatment, and doses of levothyroxine supplementation with time. Ultrasound data were not a requirement and this information was not available in all the clinical records. Similarly, data relative to breast feeding or resumption of menses after delivery were documented inconsistently. However, we routinely advise pregnant women not to take drugs and multivitamin supplements at the same time as levothyroxine, making it unlikely that this could have influenced levothyroxine absorption.
Laboratory determinations
The measurements of serum T4, FT4, and TSH were performed by the Clinical Endocrinology Laboratory of the Mount Sinai Hospital using assays from commercial vendors (Corning). Thyroid peroxidase antibody and thyroglobulin antibody were measured by radioimmunoassay (Kronus, Inc.) and >1.0 mU/L was considered a positive result. Reference ranges in nonpregnant women for the tests used in the laboratory were as follows: T4, 4.50–12.50 μg/dL; FT4, 0.89–1.76 ng/dL; TSH, 0.30–4.60 mU/L. Laboratory test reference ranges in pregnant women were trimester specific, according to gestational age as previously published by Panesar et al. (24).
Study design and statistical analysis
The clinical aim in the care of these patients was to maintain TSH levels between 0.3 and 2.0 mU/L during the pregnancy. Daily levothyroxine doses (μg) and thyroid function test measurements were extracted from medical records for each individual during three consecutive 9-month time intervals, termed period 1, period 2, and period 3. For group A (the pregnant patients), the study period included 9 months immediately before pregnancy, during pregnancy, and immediately after delivery. For group B (nonpregnant controls), three consecutive 9-month intervals for which levothyroxine and TSH data were available comprised the study period.
For 9-month windows during which an individual's levothyroxine dosage changed, the average daily dose for the period was calculated. Changes in serum TSH levels and in daily levothyroxine supplement doses were calculated for each individual between period 3 and period 1 (Δ3−1), period 2 and period 1 (Δ2−1), and period 3 and period 2 (Δ3−2). For each measure in each pair of periods, a and b, the distribution of Δb−a was tested for deviation from normality using the Shapiro–Wilk test (25). Each measurement and period was tested in each of the two groups separately and in the entire sample. For each measure in each group, the mean percentage change from period a to period b (mean Δb−a) was calculated as
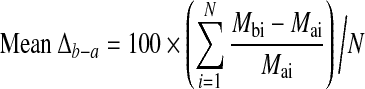 |
where Mai and Mbi are the values of the measure (either TSH or levothyroxine) for individual i in study period a and b, respectively (1 ≤ a ≤ 2; a < b ≤ 3) and N is the number of individuals in the group.
Two nonparametric tests, the Wilcoxon signed rank test and the binomial sign test, were also used to compare patients' levothyroxine requirements and TSH levels between study periods. For the binomial sign test, the null hypothesis was that median change between periods was zero. All nonzero changes between periods were recorded as positive or negative and treated as independent Bernoulli trials with p = 0.5, for which the expected distribution about zero is symmetrical under H0. Two-sided alternative hypotheses were used for all tests.
Univariate and multivariate logistic regression were used to identify factors associated with a sustained increase in levothyroxine supplementation needs in the postpartum period. Daily levothyroxine doses at 3 months postpartum were compared to daily doses during the prepregnancy (baseline) period. Women with 3 month postpartum doses that were at least 20% above baseline were considered to have a potential transient hypothyroidism in the context of postpartum thyroiditis (PPT) or a sustained and increased need for supplementation. The area covariate refers to the area under the levothyroxine supplementation curve during pregnancy. Vertical axes for the calculations were percent change in dose relative to prepregnancy level and the horizontal axis was time (months). The shape of the curve was approximated as a trapezoid when dosage changed between periods.
All statistical analyses were performed using Stata 8 (26) or R (27). Results are reported as mean ± standard error of the mean (range) unless otherwise stated.
Results
Participant groups
In the selected study period, a total of 799 hypothyroid patients were reviewed. Patients were identified through a computer register, searching for those with the diagnosis of hypothyroidism or Hashimoto's thyroiditis. Six hundred ninety-seven (87%) of the sample were women of whom 320 (46%) were of childbearing age (20–45 years) at the time of the first visit. Two hundred fifty of these young women did not fulfill the inclusion criteria, leaving a total of 70 autoimmune hypothyroid women selected for the study. Of these women, 38 had a history of pregnancy during the study period (group A). Six women in this group were pregnant twice and one patient was pregnant thrice. The remaining 32 women constituted the control group (group B).
Group A patients with a TSH >5.0 at the time of pregnancy (n = 4) were excluded from the analysis, leaving 34 women in this group. The characteristics of patients from groups A and B who were included in the study are shown in Table 1. There were no statistically significant differences between the groups except that group B, the nonpregnant controls, were heavier than group A (p < 0.05). Of note, there was no difference in mean weights before and after pregnancy (period 1 vs. period 3) in group A (63.9 ± 12.7 kg vs. 66.2 ± 13.2 kg) nor in the controls (73.3 ± 18.6 kg vs. 72.0 ± 18.6 kg).
Table 1.
Characteristics of the Groups
| Group A | Group B | p-Value | |
|---|---|---|---|
| n | 34 | 32 | |
| Mean age (years) | 34.6 ± 0.8 (27–45) | 33.6 ± 1.3 (21–44) | ns |
| Median age | 34 | 36 | ns |
| Caucasian ethnic origin (%) | 94.1 | 87.5 | ns |
| Time from initial diagnosis (months) | 76.8 ± 18.1 (6–252) | 54.7 ± 13.6 (1–240) | ns |
| Gestations per women | 1.2 (1–3) | NA | — |
| Mean weight (kg) | 63.9 ± 12.7 | 73.3 ± 18.6 | <0.05 |
This table summarizes the characteristics of both group A (pregnant patients) and group B (controls). Data are expressed as mean ± SEM (range). Time from initial diagnosis refers to the elapsed time from diagnosis of hypothyroidism to the study inclusion. There were no statistically significant differences between the groups except in weight.
ns, not significant; SEM, standard error of the mean; NA, not available.
In 29 (76%) of the women from group A there was a complete data set available for serum TSH levels and levothyroxine doses at least during the 3 months before pregnancy (BP-3), in each trimester (defined in 14 week blocks from the date of delivery) (T1, T2, and T3), and during the postpartum period at 3, 6, and 9 months (PP3, PP6, and PP9). In these women, the percent change in levothyroxine dose was compared between prepregnancy, pregnancy, and the postpartum. Nine of the women in group B had a history of pregnancy but at least 36 months earlier.
Thyroid function testing
All the subjects in control group B were, initially, adequately supplemented with levothyroxine. Their normal mean serum TSH level was within the normal range (1.8 ± 0.2 mU/L) in the arbitrarily chosen period 1 (Table 2). The group A mean serum TSH levels during period 1, before pregnancy, was also within the normal range (1.9 ± 0.2 mU/L). Thirty-one (91.1%) of the group A women were euthyroid during period 1 (mean TSH: 2.2 ± 0.2 mU/L), whereas 3 (8.8%) patients in this group were oversupplemented (mean TSH: 0.04 ± 0.01 mU/L) before pregnancy. Mean TSH serum levels during period 3 were 2.0 ± 0.4 and 2.1 ± 0.5 mU/L for groups A and B respectively, indicating an adequate supplementation in both groups at the end of the study (Table 2).
Table 2.
Serum Thyrotropin Levels
| Group | n | Period 1 | Period 2 | First trimester | Second trimester | Third trimester | Period 3 | PP3 | PP6 | PP9 |
|---|---|---|---|---|---|---|---|---|---|---|
| A (Pregnant) | 34 | 1.9 ± 0.2 | 2.4 ± 0.4 | 2.0 ± 0.4 | ||||||
| Pattern 1 | 11 | 2.1 ± 0.3 | 1.3 ± 0.1 | 1.3 ± 0.2 | 1.7 ± 0.2 | 1.2 ± 0.2 | 1.5 ± 0.3 | 0.4 ± 0.2 | 1.3 ± 0.3 | 2.1 ± 0.5 |
| Pattern 2 | 18 | 1.9 ± 0.5 | 2.8 ± 0.5 | 4.2 ± 0.8 | 4.0 ± 1.5 | 1.7 ± 0.4 | 2.3 ± 0.6 | 1.3 ± 0.5 | 2.9 ± 0.8 | 2.5 ± 0.9 |
| B (Control) | 32 | 1.8 ± 0.2 | 2.9 ± 0.7 | 2.1 ± 0.5 |
Serum TSH levels (mU/L) are shown during the consecutive measurement periods. Data are expressed as mean ± SEM. In pregnant patients (group A) TSH was measured during the 9-month period of gestation (period 2), and the 9-month period immediately before pregnancy (period 1) and after delivery (period 3). We were able to obtain data on thyroid function during each trimester in 29 patients from group A. These patients were divided into pattern 1 (no increase or one-step increase in levothyroxine dose) and pattern 2 (a continuous increase in levothyroxine dose during pregnancy). In these patients the follow-up was for 3, 6, and 9 months after delivery (PP3, PP6, and PP9). Group B corresponded to nonpregnant Hashimoto's thyroiditis women (controls). In group B, TSH determinations were measured during three consecutive 9-month periods (period 1, period 2, and period 3).
PP, postpartum period; TSH, thyrotropin.
Lack of normal distribution in serum TSH and levothyroxine doses
To determine the appropriate type of analyses to perform, we tested the distributions of individual differences in levothyroxine dosage and TSH levels for deviation from normality. The individual differences in TSH levels were not normally distributed among pregnant patients (group A) or controls (group B) for period 2 versus period 1 and among controls (group B) for period 3 versus period 2 (p < 0.05). The individual differences in TSH for period 3 versus period 1 did not deviate from normality in any group. However, individual levothyroxine dose differences were also not normally distributed among pregnant patients (group A), controls (group B), or in the total sample (groups A + B) for period 3 versus period 1, period 2 versus period 1, and period 3 versus period 2 (p < 0.01).
Levothyroxine dosing in nonpregnant women with Hashimoto's thyroiditis
In theory, Hashimoto's thyroiditis is an ongoing disease until the thyroid gland is completely destroyed. In practice, many patients appear to reach an equilibrium with some long lasting thyroid reserve. While there is anecdotal evidence for increasing levothyroxine requirements with time, unrelated to pregnancy, there has been little documentation of this phenomenon once a patient is stabilized on levothyroxine. From the information collected from nonpregnant, group B control women, regarding changes in their dose over time (mean Δ3−1), we concluded that the pace of dose increments in reproductive age hypothyroid nonpregnant women with Hashimoto's thyroiditis was slow, about 3.5% (or 2.1 μg) per year. There were no significant differences in mean levothyroxine requirements between period 3 and period 1, period 2 and period 1, or period 3 and period 2 in the control group (Table 3).
Table 3.
Daily Dose (in Micrograms) of Levothyroxine Supplementation
| Group | n | Period 1 | Period 2 | Period 3 | Mean Δ2−1 | Mean Δ3−1 | Mean Δ3−2 |
|---|---|---|---|---|---|---|---|
| A | 32 | 100.3 ± 7.2 | 112.2 ± 8.1 | 109.7 ± 8.4 | 12.3%a | 10.0%a | −7.5% |
| Pattern 1 | 11 | 83.9 ± 10.5 | 88.6 ± 11.2 | 79.6 ± 9.6 | 5.4% | −3.8% | −8.2%a |
| Pattern 2 | 18 | 113.8 ± 10.1 | 130.7 ± 11.8 | 129.3 ± 11.4 | 18.6%a | 17.6%a | 1.5% |
| B | 32 | 93.1 ± 7.5 | 94.3 ± 7.7 | 97.8 ± 7.6 | 1.6% | 8.0% | 5.7% |
Mean percent change in daily levothyroxine dosing between periods: period 2 and period 1 (mean Δ2−1), period 3 and period 1 (mean Δ3−1), and period 3 and period 2 (mean Δ3−2) are indicated. The results are provided in micrograms as mean ± SEM.
p < 0.05 (two sided) for both nonparametric tests.
Comparison of levothyroxine requirements in pregnant women
In group A (pregnant hypothyroid patients) there were significant differences in mean daily levothyroxine requirements (μg) between period 3 and period 1 (p < 0.05) and between period 2 and period 1 (p < 0.05), but not between period 3 and period 2 when the entire group was considered (Table 3). These data indicated that levothyroxine dose requirements increased during pregnancy and remained high in the postpartum. These results also indicated that pregnancy induced a sustained increase in levothyroxine dosing (p < 0.05) in the postpartum period, whereas any change over this time in the controls (group B) was not significant. The increase in levothyroxine doses was most likely related to impaired thyroid function in the postpartum since the TSH levels remained optimized with the increased levothyroxine doses (Table 2). Mean TSH values at 3, 6, and 9 months after delivery for group A were 1.1 ± 0.4, 2.5 ± 0.6, and 2.5 ± 0.6 mU/L, respectively. TSH values broken down for these postpartum periods are depicted in Table 2. However, these mean values did not reveal the variability in thyroid status, which precipitated changes in levothyroxine dosing. During the first 3 months after delivery 55% of the patients had a mean TSH serum level under 0.4 mU/L and 15% had a mean TSH over 2.0 mU/L. In contrast, in the third 3 months after delivery 16% had low TSH levels and 16% had increased TSH values.
Multiple pregnancies
Two consecutive pregnancies were recorded in five women and provided additional and unique information. Mean daily levothyroxine doses in the three consecutive periods (period 1, period 2, and period 3) during the first pregnancy were 102.6, 157.0, and 131.1 μg/day, respectively. In contrast, the data for levothyroxine supplementation (period 1, period 2, and period 3) for the second pregnancy were 137.4, 163.3, and 146.4 μg. This represented an increase of ∼40% after a mean of 2 years and illustrated the pregnancy and postpregnancy increases seen in group A as a whole. Mean serum TSH levels before the first pregnancies was 4.4 ± 1.6 mU/L and before the second pregnancies was 3.7 ± 2.7 mU/L.
Patterns of levothyroxine supplementation
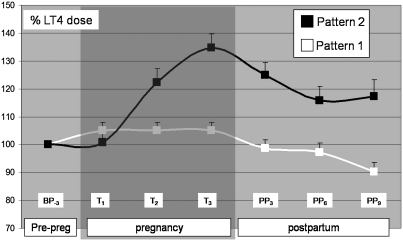
Overall, 26 (76%) of the group A pregnant patients in this study had their levothyroxine doses increased. When we analyzed the pattern of levothyroxine needed during pregnancy and the postpartum in the 29 pregnant Hashimoto's thyroiditis patients in whom the information was most complete, we identified two different patterns. The patients with the first pattern (pattern 1) showed either no need for an increased dose or only a one-step increase in the first trimester with no subsequent changes in levothyroxine dosing during gestation (T1 = T2 = T3). In contrast, the second pattern (pattern 2) depicted multistep dose increases in levothyroxine requirements (T1 < T2 < T3) (Table 3 and Fig. 1). Eleven (37.9%) women followed pattern 1, and 18 (62.0%) followed pattern 2. We did not find any difference between these women when comparing age, time since diagnosis, mean prepregnancy levothyroxine dose, mean TSH level before pregnancy or in the postpartum period, or changes in body weight. However, mean TSH during gestation differed between the groups (1.3 ± 0.1 vs. 2.8 ± 0.5 mU/L; p < 0.05), also reflecting the continued need for increased levothyroxine after delivery (Table 2). Mean serum TSH levels were above the target range during the first and second trimesters in pattern 2, confirming the need for increased dosing of these women. In contrast, the women with pattern 1 had mean TSH serum levels within the target range throughout pregnancy without any further changes in their dose requirements (Table 2). In multivariate logistic regression, women with pattern 2 were 62 times more likely than women with pattern 1 to have a levothyroxine dose at least 20% above baseline at 3 months postpartum (p = 0.005). Each additional microgram of levothyroxine in the prepregnancy dose was associated with a 3% reduction in subsequent risk of needing more (p = 0.04) (Table 4). It appeared that the >50% of hypothyroid pregnant women with Hashimoto's thyroiditis who experienced long-term exacerbation of their disease were discernible during pregnancy by a distinct pattern of increasing levothyroxine dosing.
FIG. 1.
Levothyroxine supplementation before pregnancy, during pregnancy, and in the postpartum period in Hashimoto's thyroiditis patients. White line represents women who required no supplementation or a one-step supplementation pattern (pattern 1) during pregnancy (n = 11), whereas the black line represents women who followed a multiple-step supplementation pattern (pattern 2) during pregnancy (n = 18). Lines illustrate the percentage variation in mean ± standard error of the mean of the levothyroxine dose in regard to the prepregnancy (BP-3) levothyroxine mean dose. The mean dose was 83.9 ± 10.5 μg/day for pattern 1, and 113.8 ± 10.1 μg/day for pattern 2. T1, T2, and T3 correspond to the consecutive trimesters during pregnancy (shaded area). The follow-up was for 9 months in the postpartum period (PP3, PP6, and PP9).
Table 4.
Prediction of Increased Levothyroxine Need
| Predictor | Odds ratio | p-Value |
|---|---|---|
| Univariate logistic regression results | ||
| Age (years) | 0.96 | 0.60 |
| Baseline TSH | 2.6 | 0.10 |
| Baseline levothyroxine | 0.98 | 0.16 |
| Prior pregnancies | 0.21 | 0.17 |
| Supplementation pattern 2 | 15.7 | 0.02 |
| Area | 1.02 | 0.08 |
| Multivariate logistic regression | ||
| Supplementation pattern 2 | 62.0 | 0.005 |
| Baseline levothyroxine | 0.97 | 0.04 |
Outcome of interest was a 3-month postpartum levothyroxine dose at least 20% over baseline.
Area was the area under the levothyroxine supplementation curve during pregnancy. Pattern 2 was the supplementation pattern during pregnancy such that doses increased in each trimester as described in the text.
When patients with inadequate prepregnancy levothyroxine intake (n = 6) were eliminated from the analysis, the supplementation pattern during pregnancy remained a significant predictor of increased levothyroxine needs in the postpartum. Women with supplementation pattern 2 were seven times more likely than those with pattern 1 to have postpartum levothyroxine doses greater than prepregnancy (p = 0.05).
Discussion
The natural history of Hashimoto's thyroiditis is highly variable. The clinical presentation can vary from the presence of thyroid antibodies persisting for many years (the slow form) to the rapid onset of thyroid destruction and permanent hypothyroidism (the fast form). Similarly, the disease may present with a goiter (classical Hashimoto's thyroiditis) or with thyroid atrophy (still often referred to as primary myxedema). This variability in the clinical phenotype has made the disease difficult to study and, to date, no genetic correlation or environmental relationships exist with these different natural histories. We do know that over 20 or more years, patients who show early thyroid failure by small increases in their serum TSH levels, when accompanied by thyroid antibodies (the slow form), progress to overt disease at a rate of 3%–5% per year (28,29). This type of analysis ignores those patients presenting with the fast form of the disease. One well-recognized acute precipitator of the fast form is postpregnancy. While often termed PPT, the disease appears to be identical to a transient form of Hashimoto's thyroiditis, and can be predicted by the presence of thyroid antibodies before pregnancy and is considered secondary to an exacerbated autoimmune response following the loss of placenta-induced immune suppression (7,11). Nevertheless, PPT may not always be transient and can also appear as a slow or fast evolution of permanent thyroid failure. Recent studies have showed significantly higher serum TSH levels 12 months postpartum in thyroid-antibody-positive women than in thyroid-antibody-negative women, reflecting pregnancy-related thyroid damage (30). In fact, long-term follow-up of transient PPT patients indicates that at least 30% develop more classical Hashimoto's thyroiditis with time (31). The present data from the control women indicate that the natural evolution of the slow form of Hashimoto's thyroiditis is a very gradual tendency to progressive thyroid function failure. Our data indicated that the pace of this impairment was ∼3.5% of thyroid function loss every year, as manifested by the increase in levothyroxine requirements, but we had no way to precisely predict the total exhaustion of the thyroid gland reserve without dynamic testing studies being applied in the form of TSH stimulation.
As discussed earlier, autoimmune thyroid disease in the form of Graves' disease is known to ameliorate during pregnancy secondary to the onset of placenta-induced immunosuppression. Because of decreases in serum thyroid antibody levels during pregnancy, this logic has also been assumed to be relevant to Hashimoto's thyroiditis. Yet, the data to support this conclusion have been absent. The many causes of an increased need for thyroid hormones in pregnancy have precluded any easy analysis of a change in need secondary to improvement or worsening of the destructive thyroiditis characteristic of Hashimoto's thyroiditis. Women who already take levothyroxine before pregnancy may need to increase their daily dose by, on average, 30%–50% above their preconception amount, but not all patients have this need (21). Several reasons have been promulgated to explain the incremental thyroid hormone requirements, including the rapid rise in levels of T4 binding globulin secondary to estrogen-induced changes in its glycosylation, the increased distribution volume of thyroid hormones in the vascular, hepatic, and fetal-placental units, the enhanced renal clearance of iodine, and the increased placental transport and metabolism of maternal levothyroxine (23,32). None of this excludes autoimmunity itself as a factor contributing to the maternal levothyroxine increase. All we can conclude at this stage is that the natural pace of levothyroxine requirements in women with Hashimoto's thyroiditis was dramatically increased from the control value of 3.5% to >15% under the influence of the physiological changes induced by pregnancy. Our data, however, revealed longer term changes in the requirement for levothyroxine supplementation during the postpartum when compared with prepregnancy dosing, indicating that, at least in the postpartum period, there had been a highly significant exacerbation of Hashimoto's thyroiditis resulting in a fall in thyroid reserve. While all such studies have their limitations, including their retrospective nature, these were unlikely to have influenced this conclusion.
In keeping with these results, Caixàs et al. (21) showed that a high proportion of pregnant hypothyroid women change their levothyroxine requirements at some point during the follow-up after delivery. This occurred in ∼60% of women with autoimmune hypothyroidism compared to ∼20% of women who had received thyroid ablation, and we found that ∼30% of women still had thyroid dysfunction at the 9 month visit after delivery. The suggestion that postpartum exacerbation of autoimmune thyroiditis may still occur in Hashimoto's patients (21) seems highly plausible, but manifestations of PPT may be easily masked in those patients who are already taking replacement medication. However, the question of the status of Hashimoto's thyroiditis during pregnancy itself remains unresolved. The different patterns of levothyroxine requirements revealed by this study strongly suggest that Hashimoto's thyroiditis may deteriorate during pregnancy itself in a subset of women, but we did not have a control group of women with total thyroid ablation to allow us to draw our own comparison of levothyroxine needs in pregnancy and be able to deduce the impact of the immune changes rather than the normal physiological regulation of thyroid hormone requirements.
Nevertheless, we speculate that glands from women with enough reserve will be able to cope with the demands of pregnancy (giving pattern 1 requirements), whereas those with deficient thyroids will need a continuous increase in external hormone supplementation (pattern 2). There are no diagnostic tools for the beginning of the pregnancy that are currently able to discriminate women that will follow each pattern, although those women who are fully replaced (>1.2 μg/Kg) may be more likely to follow pattern 2, adding to the findings of Loh et al. (33) that the magnitude of the replacement depends not just on the etiology of the thyroid failure but also on the degree of failure even within a single cause.
Whereas the common recommendation in clinical practice is to reduce the levothyroxine supplementation dose after delivery in patients with Hashimoto's thyroiditis to the pregestational dose (22,23), we and others (21) have found no support for this routine practice. To come to such a conclusion, postpartum follow-up needs to be lengthy and is often absent in such studies. The best practice scenario remains the careful monitoring of serum TSH levels in the postpartum and the adjustment of levothyroxine replacement according to individual results.
In conclusion, we found that pregnancy induced a postpartum increase of an average 21% in levothyroxine requirements in a subset of pregnant women with Hashimoto's thyroiditis and this effect was mostly manifested in the first 3 months after delivery. These data suggested that clinically significant numbers of women with Hashimoto's thyroiditis have exacerbation of their hypothyroidism after childbirth compared to nulliparous women. In addition, a pattern of gradual increases in levothyroxine supplementation during pregnancy predicted this exacerbation most likely secondary to a further loss of thyroid reserve.
Acknowledgments
This study was supported in part by grants from NIDDKD (DK069713), the VA Merit Award Program, and the David Owen Segal Fund (Dr. Davies) and the Clínica Universitaria, University of Navarra (Dr. Galofré).
Disclosure Statement
T.F.D. is on the Board of Kronus Corp. (Boise, ID), a distributor of thyroid antibody assays.
References
- 1.Hollowell JG. Staehling NW. Flanders WD. Hannon WH. Gunter EW. Spencer CA. Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 2.Abalovich M. Amino N. Barbour LA. Cobin RH. De Groot LJ. Glinoer D. Mandel SJ. Stagnaro-Green A. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007;92:S1–S47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]
- 3.Casey BM. Subclinical hypothyroidism and pregnancy. Obstet Gynecol Surv. 2006;61:415–420. doi: 10.1097/01.ogx.0000223331.51424.9b. [DOI] [PubMed] [Google Scholar]
- 4.Lazarus JH. Thyroid disease in pregnancy and childhood. Minerva Endocrinol. 2005;30:71–87. [PubMed] [Google Scholar]
- 5.Glinoer D. Management of hypo- and hyperthyroidism during pregnancy. Growth Horm IGF Res. 2003;13:S45–S54. doi: 10.1016/s1096-6374(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 6.Weetman AP. The immunology of pregnancy. Thyroid. 1999;9:643–646. doi: 10.1089/thy.1999.9.643. [DOI] [PubMed] [Google Scholar]
- 7.Davies TF. The thyroid immunology of the postpartum period. Thyroid. 1999;9:675–684. doi: 10.1089/thy.1999.9.675. [DOI] [PubMed] [Google Scholar]
- 8.Guleria I. Sayegh MH. Maternal acceptance of the fetus: true human tolerance. J Immunol. 2007;178:3345–3351. doi: 10.4049/jimmunol.178.6.3345. [DOI] [PubMed] [Google Scholar]
- 9.Shah MS. Davies TF. Stagnaro-Green A. The thyroid during pregnancy: a physiological and pathological stress test. Minerva Endocrinol. 2003;28:233–245. [PubMed] [Google Scholar]
- 10.Amino N. Miyai K. Postpartm autoimmune endocrine syndromes. In: Davies TF, editor. Autoimmune Endocrine Disease. Wiley; New York: 1983. 247. [Google Scholar]
- 11.Stagnaro-Green A. Roman SH. Cobin RH. el-Harazy E. Wallenstein S. Davies TF. A prospective study of lymphocyte-initiated immunosuppression in normal pregnancy: evidence of a T-cell etiology for postpartum thyroid dysfunction. J Clin Endocrinol Metab. 1992;74:645–653. doi: 10.1210/jcem.74.3.1740500. [DOI] [PubMed] [Google Scholar]
- 12.Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–433. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 13.Galofré JC. Davies TF. Thyroid dysfunction in pregnancy. Endocrinol Nutr. 2007;54:535–546. [Google Scholar]
- 14.Dhar JP. Essenmacher LM. Ager JW. Sokol RJ. Pregnancy outcomes before and after a diagnosis of systemic lupus erythematosus. Am J Obstet Gynecol. 2005;193:1444–1455. doi: 10.1016/j.ajog.2005.02.104. [DOI] [PubMed] [Google Scholar]
- 15.D'Cruz DP. Systemic lupus erythematosus. BMJ. 2006;332:890–894. doi: 10.1136/bmj.332.7546.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amino N. Mori H. Iwatani Y. Tanizawa O. Kawashima M. Tsuge I. Ibaragi K. Kumahara Y. Miyai K. High prevalence of transient post-partum thyrotoxicosis and hypothyroidism. N Engl J Med. 1982;306:849–852. doi: 10.1056/NEJM198204083061405. [DOI] [PubMed] [Google Scholar]
- 17.Stagnaro-Green A. Postpartum thyroiditis. Best Pract Res Clin Endocrinol Metab. 2004;18:303–316. doi: 10.1016/j.beem.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Benhaim Rochester D. Davies TF. Increased risk of Graves' disease after pregnancy. Thyroid. 2005;15:1287–1290. doi: 10.1089/thy.2005.15.1287. [DOI] [PubMed] [Google Scholar]
- 19.Lazarus JH. Premawardhana LD. Screening for thyroid disease in pregnancy. J Clin Pathol. 2005;58:449–452. doi: 10.1136/jcp.2004.021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Premawardhana LD. Parkes AB. John R. Harris B. Lazarus JH. Thyroid peroxidase antibodies in early pregnancy: utility for prediction of postpartum thyroid dysfunction and implications for screening. Thyroid. 2004;14:610–615. doi: 10.1089/1050725041692828. [DOI] [PubMed] [Google Scholar]
- 21.Caixàs A. Albareda M. García-Patterson A. Rodríguez-Espinosa J. de Leiva A. Corcoy R. Postpartum thyroiditis in women with hypothyroidism antedating pregnancy? J Clin Endocrinol Metab. 1999;84:4000–4005. doi: 10.1210/jcem.84.11.6144. [DOI] [PubMed] [Google Scholar]
- 22.Alexander EK. Marqusee E. Lawrence J. Jarolim P. Fischer GA. Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241–249. doi: 10.1056/NEJMoa040079. [DOI] [PubMed] [Google Scholar]
- 23.Mandel SJ. Larsen PR. Seely EW. Brent GA. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med. 1990;323:91–96. doi: 10.1056/NEJM199007123230204. [DOI] [PubMed] [Google Scholar]
- 24.Panesar NS. Li CY. Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem. 2001;38:329–332. doi: 10.1258/0004563011900830. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro SS. Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- 26.StataCorp. Stata Statistical Software: release 8. StataCorp LP, College Station; TX: 2003. [Google Scholar]
- 27.R Development Core 2006 Team R: a language, environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: [Google Scholar]
- 28.Vanderpump MP. Tunbridge WM. French JM. Appleton D. Bates D. Clark F. Grimley Evans J. Hasan DM. Rodgers H. Tunbridge F. The incidence of thyroid disease in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol. 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 29.Huber G. Staub JJ. Meier C. Mitrache C. Guglielmetti M. Huber P. Braverman LE. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002;87:3221–3226. doi: 10.1210/jcem.87.7.8678. [DOI] [PubMed] [Google Scholar]
- 30.Mamede da Costa S. Sieiro Netto L. Coeli CM. Buescu A. Vaisman M. Value of combined clinical information and thyroid peroxidase antibodies in pregnancy for the prediction of postpartum thyroid dysfunction. Am J Reprod Immunol. 2007;58:344–349. doi: 10.1111/j.1600-0897.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 31.Tachi J. Amino N. Tamaki H. Aozasa M. Iwatani Y. Miyai K. Long term follow-up and HLA association in patients with postpartum hypothyroidism. J Clin Endocrinol Metab. 1988;66:480–484. doi: 10.1210/jcem-66-3-480. [DOI] [PubMed] [Google Scholar]
- 32.Stagnaro Green A. Pregnancy and thyroid disease. Immunol Allergy Clin North Am. 1994;14:865–878. [Google Scholar]
- 33.Loh JA. Wartofsky L. Jonklaas J. Burman KD. The magnitude of increased levothyroxine requirements in hypothyroid pregnant women depends upon the etiology of the hypothyroidism. Thyroid. 2009;19:269–275. doi: 10.1089/thy.2008.0413. [DOI] [PubMed] [Google Scholar]