Abstract
Objective
To study the sex-specific association of angina pectoris with mortality in community-dwelling older adults with and without diabetes.
Methods
Baseline prevalence of angina was evaluated in 822 men and 1184 postmenopausal women aged 50–89 years at the 1984–1987 Rancho Bernardo Study clinic visit, when an oral glucose tolerance test (OGTT) and the Rose angina questionnaire were administered. All-cause and coronary heart disease (CHD) mortality were assessed after an average follow-up period of 13.2 years. Sex-specific Cox proportional hazard models were used to examine the independent association of angina with mortality by glucose tolerance category.
Results
At baseline, average age was 71 years for both sexes; 61 men (7.4%) and 142 women (12.0%) had angina. Overall, 129 men (15.9%) and 130 women (11.0%) had type 2 diabetes; 228 men (27.7%) and 357 women (30.2%) had impaired glucose tolerance (IGT). During follow-up, 485 men (59%) and 557 women (47%) died, of whom 103 men (21.2%) and 104 women (18.7%) had fatal CHD. Women with diabetes and angina had a 3–4-fold greater risk of dying from CHD than women with diabetes but without angina, independent of covariates. Women with angina and IGT had twice the risk of CHD mortality compared with women with IGT but without angina. A smaller increased risk of fatal CHD in men was not statistically significant.
Conclusions
Angina was associated with an increased risk of dying from CHD among women, especially among those who also had IGT or diabetes.
Introduction
Coronary heart disease (CHD) is the leading cause of death in both men and women in the United States, accounting for approximately one in every five deaths.1 Diabetes mellitus is an established risk factor for cardiac morbidity and mortality, independently increasing the risk of fatal CHD 2–3-fold, with a higher odds of death in women than men.1–3 In a meta-analysis comparing angina by Rose questionnaire in 74 cohorts, angina pectoris was almost always more common in women than men,4 although its prognostic implications in women are less clear.
The association between angina and CHD mortality has been studied in populations, such as that of Framingham, which reported that angina was less predictive of future coronary events in women than in men,5–7 a finding confirmed elsewhere.8–10 Many studies used small clinic-based or hospital-based samples with short follow-up periods or did not examine the interaction between sex and diabetes status on long-term outcomes of angina.7,9,11–15 Additionally, many of these studies captured diabetes by self-report or a history of fasting plasma glucose levels alone, potentially missing many undiagnosed cases. One recent registry study in Finland reported that, compared with women in the general population, women with chronic, stable angina had increased cardiac mortality and nonfatal myocardial infarctions (MI), which was particularly high in women with both diabetes and angina.16 To our knowledge, however, no other population-based studies report the long-term mortality risk in people who have both angina and diabetes, and there are no published population-based studies of this comorbidity and cardiovascular outcome in the United States.
The purpose of this study was to examine the influence of diabetes status on the mortality risk associated with angina, independent of other cardiovascular risk factors, in a population-based sample of older, community-dwelling men and women.
Materials and Methods
Study population
The Rancho Bernardo Study enrolled 82% of a geographically defined, middle-class, mainly Caucasian adult community in Southern California between 1972 and 1974.17–19 Participants have been followed ever since by annual mailed questionnaires and periodic clinic visits. In 1984–1987, 82% of all surviving community-dwelling participants aged ≥40 attended a follow-up visit (n = 2479). After excluding 105 people who exceeded the age range of 50–89 years, 21 premenopausal women, 7 participants with type 1 diabetes, 20 who could not be classified by diabetes status, and 320 with history of MI, ischemic resting electrocardiographic (ECG) abnormalities, or coronary artery bypass graft (CABG) surgery, there remained 2006 participants (822 men and 1184 postmenopausal women) who are the focus of this report. The study protocol was approved by the Human Subjects Protections Program of the University of California, San Diego; all participants gave written informed consent before participation.
Procedures
Participants were seen in the Rancho Bernardo Study clinic by trained nurses and interviewers between 7:00 and 11:00 am after a requested 12–16-hour fast. Fasting blood was drawn from the antecubital vein for measurement of fasting plasma glucose, lipids, and lipoproteins. An oral glucose tolerance test (OGTT) (75 g load) was administered, and blood was collected 2 hours later for assessment of postchallenge glucose. Blood pressure was measured in fasting subjects according to the Hypertension Detection and Follow-up Program protocol using a standard mercury sphygmomanometer after the participant had been seated quietly for at least 5 minutes20 and was calculated as the mean value of two measurements taken at least 1 minute apart. Categorical hypertension was defined as average systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of hypertensive medication.
Height, weight, hip girth, and waist girth were measured in the clinic with participants wearing light clothing and no shoes. Body mass index (BMI) calculated as weight (kilograms) divided by height (meters)2, waist-to-hip ratio (WHR) calculated as waist circumference at the bending point divided by maximum hip circumference (centimeters/centimeters), and waist circumference alone were used as estimates of central adiposity. Current medication use was verified by prescriptions, pills, or containers brought to the clinic for this purpose.
Standard questionnaires were used to obtain personal and family history of diabetes mellitus, history of cigarette smoking (never/past/current), current physical activity (exercise ≥3 times/week, yes/no), and alcohol intake (number of drinks of beer, wine, and hard liquor consumed in the past week, converted to grams of alcohol). Angina was based on responses to a modified Rose angina questionnaire,21 which asks: Have you ever had any pain or discomfort in your chest? Do you get this pain or discomfort when you walk uphill or hurry? Do you get it when you walk at an ordinary pace on the level? What do you do if you get it while you are walking? If you stand still, what happens to it? (If relieved), how soon? Did you see a doctor because of this pain or discomfort? (If yes), what did the doctor say it was?
According to the Rose questionnaire protocol, a diagnosis of angina required pain that occurred while walking uphill or hurrying, was relieved in ≤10 minutes after exertion, and was located in any level of the sternum or in the left anterior chest and left arm. Classification was further graded as grade 1 if the pain was evoked while walking at an ordinary pace on the level; otherwise, angina classification was grade 2. For this study, angina pectoris was defined as meeting the Rose questionnaire criteria for angina (grade 1 or 2), self-reported physician diagnosis, or use of angina medication.
Total cholesterol and triglyceride levels were measured by enzymatic techniques using a ABA-200 biochromatic analyzer (Abbott Labortories, Irving, TX). High-density lipoprotein cholesterol (HDL-C) level was measured after precipitation of the other lipoproteins with heparin and manganese chloride. Determination of plasma glucose was performed in a research laboratory using a glucose oxidase method.
Type 2 diabetes and impaired glucose tolerance (IGT) were defined using the 1999 World Health Organization (WHO) criteria, where a diagnosis of type 2 diabetes requires a fasting plasma glucose level (FPG) ≥126 mg/dL or a 2-hour postchallenge glucose level (PCG) ≥200 mg/dL or a history of type 2 diabetes diagnosed by a physician or treatment with an oral hypoglycemic agent or insulin.22 A diagnosis of IGT requires an FPG 110–<126 mg/dL and PCG<200, or FPG<110 and PCG 140–<200, without a prior diagnosis or treatment of diabetes. All others were classified as having normal glucose tolerance.
Classification of MI and ischemic resting ECG abnormalities for baseline exclusion were based on history, physician diagnosis, or ECG criteria. ECG changes were subcategorized into ECG coronary probable (major Q or QS wave [Minnesota Code 1.1, 1.2] and complete left bundle branch block [LBBB] [Minnesota Code 7.1.1]) and ECG coronary possible (small Q or QS wave [Minnesota Code 1.3], ST depression [Minnesota Code 4.1–4.3], and flattened or T waves [Minnesota Code 5.1–5.3]). This algorithm is based on Whitehall criteria as applied in the WHO Multinational Study of Diabetes and Vascular Disease.23 Participants were followed through 2004 for vital status, with death certificates obtained for decedents. A certified nosologist coded underlying cause of death using the International Classification of Diseases, Ninth Revision (ICD-9).
Statistical analysis
HDL-C and triglyceride levels were not normally distributed and were log-transformed for analyses. Comparisons of age, measures of obesity (i.e., BMI, WHR, waist circumference), lipid levels, and other characteristics by angina status and sex used two-sample independent t tests for continuous variables and chi-square analyses for categorical variables. Age-adjusted comparisons of obesity and lipids by angina status within men and women were performed with analysis of covariance (ANCOVA). The association of angina with CHD and all-cause mortality was examined with sex-specific Cox proportional hazards regression models. All models met the proportional hazards assumption and were assessed by visualization of log-log plots. The varying impact of angina on mortality risk by sex and diabetes status was verified using an interaction term (i.e., gender × diabetes status) in the regression models. These interactions were significant (p < 0.0001), therefore, hazard ratios (HR) stratified by sex and diabetes status are presented. To avoid collinearity, BMI, waist circumference, and WHR were tested in individual regression models. Only WHR is presented; models using BMI or waist circumference yielded similar results. To assess confounding, 10% change in the beta-estimate approach was used. Confounding effects were assessed individually with age and WHR for all covariates of interest in the general proportional hazards model.
All tests except for the Cox proportional hazards regression models were two-tailed, with p < 0.05 indicating statistical significance. The Cox models were conducted using a one-tailed analysis based on the assumption that having angina pectoris would not decrease the risk of CHD mortality. Data were analyzed using SAS version 9.1 (SAS Institute Inc, Cary, NC) and Intercooled STATA 9 (StataCorp LP, College Station, TX).
Results
At the 1984–1987 clinic visit, there were 2006 participants aged 50–89 (mean 70.9 ± 9.7 years for men, 70.5 ± 9.1 years for women). A total of 61 men (7.4%) and 142 (12.0%) women had angina at baseline. By 2004, after an average follow-up of 13.2 years, 59% of men (485) and 47% of women (557) had died; of these, 21.2% of the deaths in men (103) and 18.7% of the deaths in women (104) were attributed to CHD. Among the 61 men with angina, a total of 7 of 33 (21.1%) with normal glucose, 2 of 18 (11.1%) with IGT, and 4 of 10 (40.0%) with diabetes died of CHD during follow-up; among the 761 men without angina, 52 of 432 (12.0%) with normal glucose, 18 of 210 (8.6%) with IGT, and 20 of 119 (16.8%) with diabetes died of CHD. Among the 142 women with angina, 9 of 66 (13.6%) with normal glucose, 9 of 54 (16.7%) with IGT, and 7 of 22 (31.8%) with diabetes died of CHD; among the 1042 women without angina, 39 of 631 (6.2%) with normal glucose, 31 of 303 (10.2%) with IGT, and 9 of 108 (8.3%) with diabetes died of CHD.
At baseline, women had lower BMI, WHR, waist circumference (p < 0.0001) and higher HDL-C levels (p < 0.0001) and were more likely to have IGT but less likely to have type 2 diabetes (p = 0.008) than men (Table 1). Women were also less likely to exercise three or more times per week, reported lower rates of having ever smoked and daily alcohol consumption and less formal education (p < 0.01). Approximately 30% of women reported current estrogen use.
Table 1.
Baseline Characteristics Among 822 Men and 1184 Women Aged 50–89: The Rancho Bernardo Study, 1984–1987
| Characteristic | Men (n = 822) | Women (n = 1184) | p valuea |
|---|---|---|---|
| Age (years)b | 70.9 ± 9.7 | 70.5 ± 9.1 | 0.61 |
| BMI (kg/m2)b | 25.9 ± 3.3 | 24.2 ± 3.8 | <0.0001 |
| Waist/hip ratio (cm/cm)b | 0.9 ± 0.05 | 0.8 ± 0.06 | <0.0001 |
| Waist circumference (cm)b | 94.1 ± 9.0 | 78.7 ± 9.7 | <0.0001 |
| Triglycerides (mg/dL)c | 99.0 (69.0–148.0) | 99.0 (69.0–142.0) | 0.54 |
| HDL-C (mg/dL)c | 52.0 (44.0–62.0) | 67.0 (56.0–80.0) | <0.0001 |
| Hypertensiond | |||
| Yes | 219 (26.6) | 349 (29.5) | 0.17 |
| Diabetesd | 0.008 | ||
| Normal | 465 (56.6) | 697 (58.9) | |
| Impaired glucose tolerance | 228 (27.7) | 357 (30.2) | |
| Type 2 diabetes | 129 (15.7) | 130 (11.0) | |
| Exercise (≥3 times/week)d | |||
| Yes | 698 (84.9) | 933 (78.8) | 0.0006 |
| Smoking statusd | |||
| Current smoker | 88 (10.7) | 171 (14.4) | <0.0001 |
| Alcohol consumption (daily average)d | <0.0001 | ||
| None | 234 (28.5) | 498 (42.1) | |
| 1–30 g/day (1–2 drinks) | 449 (54.6) | 595 (50.3) | |
| ≥30 g/day (≥3 drinks) | 139 (16.9) | 91 (7.7) | |
| Educationd | |||
| College graduate | 426 (52.0) | 304 (25.9) | <0.0001 |
| Estrogen Used | |||
| Current | — | 1139 (29.8) | — |
All variables had <1% missing except estrogen use in women (3.8%).
For comparisons between men and women (independent t and chi-square tests). Log-transformed values used for independent t tests for triglycerides and HDL variables.
Mean ± SD.
Median (25%–75%).
n (%).
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; SD, standard deviation.
Table 2 presents sex-specific comparisons of covariates in participants with and without angina. Men with angina were significantly older, had lower HDL-C levels, and were less likely to consume ≥3 alcoholic drinks/day than men without angina (p < 0.05). Women with angina were significantly older, had greater waist circumferences, higher triglycerides, lower HDL-C levels, and higher rates of hypertension than women without angina (p < 0.05). Angina was associated with a nonsignificantly lower risk of all-cause mortality for both sexes before stratification by diabetes status (data not shown).
Table 2.
Age and Age-Adjusted Baseline Characteristics Among 822 Men and 1184 Women Aged 50–89 by Angina Status: The Rancho Bernardo Study, 1984–1987
| |
Men |
Women |
||||
|---|---|---|---|---|---|---|
| |
Angina |
|
Angina |
|
||
| |
Yes |
No |
|
Yes |
No |
|
| n = 61 | n = 761 | p valuea | n = 142 | n = 1042 | p valuea | |
| Age (years) | 74.5 ± 8.7 | 70.4 ± 9.8 | 0.002 | 73.0 ± 8.8 | 70.2 ± 9.1 | 0.0004 |
| BMI (kg/m2) | 25.8 ± 0.4 | 25.9 ± 0.1 | 0.75 | 24.8 ± 0.3 | 24.1 ± 0.1 | 0.06 |
| Waist/hip ratio (cm/cm) | 0.91 ± 0.007 | 0.92 ± 0.002 | 0.75 | 0.80 ± 0.005 | 0.80 ± 0.0001 | 0.33 |
| Waist circumference (cm) | 93.1 ± 1.2 | 94.2 ± 0.3 | 0.36 | 80.3 ± 0.8 | 78.4 ± 0.3 | 0.03 |
| Triglycerides (mg/dL) | 102.2 ± 1.0 | 103.9 ± 1.1 | 0.82 | 97.9 ± 1.0 | 123.7 ± 1.0 | <0.0001 |
| HDL-C (mg/dL) | 48.2 ± 1.0 | 52.4 ± 1.0 | 0.02 | 61.2 ± 1.0 | 67.2 ± 1.0 | 0.0002 |
| Hypertension (present) | 20.9% | 26.8% | 0.35 | 39.5% | 27.1% | 0.002 |
| Diabetes (present) | 13.6% | 15.0% | 0.69 | 13.2% | 9.8% | 0.19 |
| Exercise (≥3 times/week) | 83.5% | 85.0% | 0.77 | 77.9% | 79.0% | 0.82 |
| Smoking status (current) | 7.1% | 10.0% | 0.38 | 10.3% | 14.1% | 0.25 |
| Alcohol consumption (≥3 drinks/day) | 5.2% | 17.6% | 0.01 | 9.4% | 7.4% | 0.46 |
| Education (college graduate) | 40.5% | 52.9% | 0.07 | 26.2% | 25.8% | 0.88 |
| Estrogen (current) | — | — | — | 40.4% | 39.8% | 0.99 |
Data are expressed as age-adjusted mean ± SE or %.
For comparisons between angina and no angina (independent t and chi-square tests). Log transformed values used for ANCOVA for triglycerides and HDL-C variables and transformed back for adjusted means.
SE, standard error; ANCOVA, analysis of covariance.
Sex-specific, multivariate hazard models comparing the time to CHD mortality stratified by angina and diabetes status are shown in Table 3. Each risk factor variable was found to confound the estimate in at least one strata. Therefore, HRs are presented for three models: an age-adjusted model, an age and biological risk factors model, and a fully adjusted model with age as well as biological and behavioral risk factors. In the age-adjusted analysis (model 1), women with diabetes who also had angina had a 3.87 times greater risk of dying from CHD compared with women without angina (p = 0.01). Among women who had normal glucose or IGT, women with angina had a 2-fold greater risk of CHD mortality compared with women without angina (normal glucose: HR = 1.99, p = 0.03; IGT: HR = 1.98, p = 0.04). Model 2 shows that after adjustment for age, WHR, hypertension, triglycerides, and HDL-C levels, there was also a significantly increased risk of CHD mortality in women with angina and IGT (HR = 2.10, p = 0.03) or type 2 diabetes (HR = 3.01, p = 0.03). Adjustment for all biological and behavioral risk factors yielded similar results (Table 3). No significant association was observed between angina and CHD mortality among men after adjustment for covariates and stratification by glycemic status.
Table 3.
Sex-Specific Multivariate Cox Proportional Hazards for Angina and Coronary Heart Disease Mortality by Glucose Tolerance Category: The Rancho Bernardo Study, 1984–2004
| |
Normal glucose |
Impaired glucose tolerance |
Type 2 diabetes |
||||||
|---|---|---|---|---|---|---|---|---|---|
| CHD mortality | HR | 95% CI upper bounda | p valuea | HR | 95% CI upper bounda | p valuea | HR | 95% CI upper bounda | p valuea |
| Menb | |||||||||
| Model 1 | 1.68 | (3.27) | 0.10 | 0.82 | (2.82) | 0.39 | 1.93 | (4.83) | 0.12 |
| Model 2 | 2.21 | (4.39) | 0.03 | 1.00 | (3.70) | 0.50 | 1.63 | (4.23) | 0.20 |
| Model 3 | 1.07 | (4.01) | 0.47 | 1.65 | (4.42) | 0.20 | 1.36 | (3.27) | 0.28 |
| Womenb | |||||||||
| Model 1 | 1.99 | (3.67) | 0.03 | 1.98 | (3.71) | 0.04 | 3.87 | (9.34) | 0.01 |
| Model 2 | 1.64 | (3.13) | 0.10 | 2.10 | (3.98) | 0.03 | 3.01 | (8.02) | 0.03 |
| Model 3 | 1.91 | (3.83) | 0.06 | 2.35 | (4.59) | 0.02 | 3.55 | (11.18) | 0.03 |
Upper bound of 95% confidence interval (CI) and p values given for one-sided analysis.
Model 1, adjusted for age only; model 2, model 1 + WHR, hypertension, triglycerides, HDL-C; model 3, model 2 + physical activity, current smoking, alcohol intake, education, and current estrogen (women).
CHD, coronary heart disease; HR, hazard ratio.
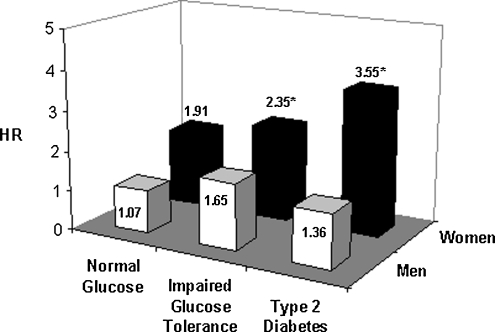
Results of the fully adjusted models are shown in Figure 1. Having both angina and IGT or type 2 diabetes was associated with a greater risk of CHD mortality among women but not men.
FIG. 1.
Multivariate HRs for angina and CHD mortality by gender and diabetes status in 822 men and 1184 women aged 50–89: The Rancho Bernardo Study, 1984–2004. *p < 0.05. HR, hazard ratio.
Discussion
Although worldwide rates of CHD mortality in developed countries have declined by approximately 25% worldwide in the last three decades, CHD remains a significant public health issue.1,24–31 The decline reflects a reduction in both the incidence of MI and case fatality rates. The prevalence of angina, estimated at 8.9 million American adults, is substantial and is expected to increase with the aging United States population,1 causing a considerable clinical and economic burden associated with increased medical visits, medications, and expensive procedures.32,33 Direct costs due to angina have been estimated to range from $8.8 billion for chronic angina to upwards of $75 billion for coronary artery disease.34
To our knowledge, this is the first long-term prospective study to evaluate the association between angina pectoris and CHD mortality by glycemic status and sex in the United States. In this cohort of community-dwelling older men and women, a significantly increased risk of fatal CHD was observed in women with both angina and IGT or diabetes, an association not observed in men. Angina was also marginally associated with long-term risk of CHD mortality in women with normal glucose tolerance (p = 0.06).
A study in Finland using data from over 118,000 men and women identified as having “nitrate angina” or “test-positive angina,” based on reimbursement for prescription nitrate medications or abnormal invasive tests, indicated that the overall prognosis of angina did not differ in women and men.17 Increases in CHD mortality after a 4-year follow-up period were observed in both men and women with nitrate angina, although increases were higher among women with test-positive angina, and diabetes was strongly associated with cardiac events for either classification. However, this Finnish study reported the combined risk of fatal CHD and non-fatal MI and did not separately assess the impact of IGT, limiting comparison to the present study. Furthermore, angina and comorbid conditions were assessed via prescription reimbursements, which may not capture untreated or undiagnosed angina, hypertension, or diabetes. In contrast, in the Rancho Bernardo cohort, angina was assessed for all participants; diagnoses of hypertension, IGT, and diabetes were based on clinical criteria and blood tests; and participants were followed over 20 years, significantly longer than participants in the Finnish study.
The physiological mechanisms underlying increased cardiac mortality risk of women with angina and diabetes remain unclear. Type 2 diabetes, an important predictor of CHD risk and prognosis in men and women, may have greater prognostic implications in women over other traditional CHD risk factors.1,35 Women with diabetes have a substantially greater risk of CHD and CHD mortality than men.35–37 In a meta-analysis of type 2 diabetes and fatal CHD from 37 studies of over 447,000 people, the summary relative risk for CHD mortality was 3.5 (95% CI 2.70-4.53) in women and 2.06 (95% CI 1.81-2.34) in men.35
It has been proposed that women with diabetes have a greater risk of cardiovascular abnormalities than do men with diabetes.38–40 For example, women with diabetes have more hypertension and higher lipid levels than men with diabetes. Similar trends between men and women were also observed in the present study about angina. However, even after adjustment for hypertension, triglycerides, and HDL-C, women with angina and diabetes had a greater mortality risk than men.
Significant differences in the epidemiology, diagnosis, and treatment patterns of CHD between men and women may also explain the greater risk of CHD mortality associated with angina and diabetes in women. Women carry a greater risk factor burden for CHD and may experience different coronary disease manifestations from men, such as having more chest pain than a clearly defined event such as MI.9,41 A 26-year follow-up from the Framingham study indicates that in women, angina pectoris was more frequently uncomplicated (80%), whereas angina in men often occurs after an infarction (66%).6 The clinical presentation of angina between men and women also often differs, with women less likely to have typical angina but more likely to report more intense chest pain and more frequently having pain and other sensations in the neck and throat.42 However, women are less likely to be referred for diagnostic or aggressive therapeutic procedures than men 43,44 when experiencing either acute or chronic coronary symptoms.45–48 Moreover, women with diabetes or cardiovascular disease are less likely than men to receive aspirin, statins, or antihypertensive medications.49–52
Several potential limitations of this study were considered. The Rancho Bernardo cohort is predominantly white, educated, and middle class; thus, results may not apply to other ethnic and socioeconomic groups. Another limitation is the relatively small sample of participants, particularly men with angina. It is possible that many of the men with CHD excluded from the study sample may have been those with a history of angina as well, thus resulting in a smaller sample and limited power to observe a significant association. Of the 320 individuals excluded at baseline for CHD, more men were excluded than women (216 vs. 104, respectively, p < 0.001). Although this was expected because men have a higher risk of MI/CHD at an earlier age than women, this could explain the observed lack of an association in the study results for the men.
Men typically have higher rates of MI and coronary artery disease than women, although women have at least an equal prevalence of angina. This study suggests that the association between angina and CHD mortality is particularly high for women in the presence of diabetes or IGT. Earlier studies have failed to identify an increased risk of coronary events after angina because they did not examine CHD mortality risk by glucose status. An association in women with IGT or diabetes may not be evident if the prevalence of IGT or diabetes is low in the population. Further long-term population-based studies are needed to confirm this association.
A recent study compared two populations of women, one with nonobstructive coronary artery disease and a community-based population of women who were asymptomatic with no history of heart disease. Although not exactly comparable with our study, the study by Gulati et al.53 indicates more cardiovascular events in the population with signs and symptoms suggestive of ischemia compared with the asymptomatic population, further highlighting the significant adverse outcomes of CHD observed in women.
Results of this study support the clinical importance of taking into account the copresence of angina and diabetes in the care of women with either of these conditions. Women with symptoms of angina and diabetes should be evaluated for risk of subsequent coronary events, with treatment to control or reduce current risk factors for CHD considered.
Acknowledgments
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant DK-31801 and National Institute on Aging grant AG-07181. Data from this article were previously presented at the 2007 San Diego Epidemiology Research Exchange.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Rosamond W. Flegal K. Friday G, et al. Heart disease and stroke statistics—2007 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Wingard DL. Barrett-Connor E. Heart disease and diabetes. In: Harris MI, editor; Cowie CC, editor; Stern MP, editor; Boyko EJ, editor; Reiber GE, editor; Bennett PH, editor. Diabetes in America. 2nd. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; 1995. pp. 429–448. [Google Scholar]
- 3.Kanaya AM. Grady D. Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: A meta-analysis. Arch Intern Med. 2002;162:1737–1745. doi: 10.1001/archinte.162.15.1737. [DOI] [PubMed] [Google Scholar]
- 4.Hemingway H. Langenberg C. Damant J. Frost C. Pyorala K. Barrett-Connor E. Prevalence of angina in women versus men: A systematic review and meta-analysis of international variations across 31 countries. Circulation. 2008;117:1526–1536. doi: 10.1161/CIRCULATIONAHA.107.720953. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB. Feinleib M. Natural history of angina pectoris in the Framingham study. Prognosis and survival. Am J Cardiol. 1972;29:154–163. doi: 10.1016/0002-9149(72)90624-8. [DOI] [PubMed] [Google Scholar]
- 6.Lerner DJ. Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: A 26-year follow-up of the Framingham population. Am Heart J. 1986;111:383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 7.Murabito JM. Evans JC. Larson MG. Levy D. Prognosis after the onset of coronary heart disease. An investigation of differences in outcome between the sexes according to initial coronary disease presentation. Circulation. 1993;88:2548–2555. doi: 10.1161/01.cir.88.6.2548. [DOI] [PubMed] [Google Scholar]
- 8.Weinblatt E. Shapiro S. Frank CW. Prognosis of women with newly diagnosed coronary heart disease—A comparison with course of disease among men. Am J Public Health. 1973;63:577–593. doi: 10.2105/ajph.63.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orencia A. Bailey K. Yawn BP. Kottke TE. Effect of gender on long-term outcome of angina pectoris and myocardial infarction/sudden unexpected death. JAMA. 1993;269:2392–2397. [PubMed] [Google Scholar]
- 10.Elveback LR. Connolly DC. Coronary heart disease in residents of Rochester, Minnesota. V. Prognosis of patients with coronary heart disease based on initial manifestation. Mayo Clin Proc. 1985;60:305–311. doi: 10.1016/s0025-6196(12)60537-0. [DOI] [PubMed] [Google Scholar]
- 11.Hochman JS. McCabe CH. Stone PH, et al. Outcome and profile of women and men presenting with acute coronary syndromes: A report from TIMI IIIB. TIMI Investigators. Thrombolysis in Myocardial Infarction. J Am Coll Cardiol. 1997;30:141–148. doi: 10.1016/s0735-1097(97)00107-1. [DOI] [PubMed] [Google Scholar]
- 12.Hueb W. Gersh BJ. Costa F, et al. Impact of diabetes on five-year outcomes of patients with multivessel coronary artery disease. Ann Thorac Surg. 2007;83:93–99. doi: 10.1016/j.athoracsur.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 13.Gerber Y. Weston SA. Killian JM. Jacobsen SJ. Roger VL. Sex and classic risk factors after myocardial infarction: A community study. Am Heart J. 2006;152:461–468. doi: 10.1016/j.ahj.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Peterson PN. Spertus JA. Magid DJ, et al. The impact of diabetes on one-year health status outcomes following acute coronary syndromes. BMC Cardiovasc Disord. 2006;6:41. doi: 10.1186/1471-2261-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mozaffarian D. Bryson CL. Spertus JA. McDonell MB. Fihn SD. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J. 2003;146:1015–1022. doi: 10.1016/S0002-8703(03)00436-8. [DOI] [PubMed] [Google Scholar]
- 16.Hemingway H. McCallum A. Shipley M. Manderbacka K. Martikainen P. Keskimaki I. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA. 2006;295:1404–1411. doi: 10.1001/jama.295.12.1404. [DOI] [PubMed] [Google Scholar]
- 17.The Lipid Research Clinics Program Epidemiology Committee. Plasma lipid distributions in selected North American populations: The Lipid Research Clinics Program Prevalence Study. Circulation. 1979;60:427–439. doi: 10.1161/01.cir.60.2.427. [DOI] [PubMed] [Google Scholar]
- 18.Criqui MH. Frankville DD. Barrett-Connor E. Klauber MR. Holdbrook MJ. Turner JD. Change and correlates of change in high and low density lipoprotein cholesterol after six years: A prospective study. Am J Epidemiol. 1983;118:52–59. doi: 10.1093/oxfordjournals.aje.a113616. [DOI] [PubMed] [Google Scholar]
- 19.Criqui MH. Barrett-Connor E. Austin M. Differences between respondents and nonrespondents in a population-based cardiovascular disease study. Am J Epidemiol. 1978;108:367–372. doi: 10.1093/oxfordjournals.aje.a112633. [DOI] [PubMed] [Google Scholar]
- 20.The Hypertension Detection and Follow-Up Program. Hypertension Detection and Follow-Up Program Cooperative Group. Prev Med. 1976;5:207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 21.Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull WHO. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Geneva: World Helath Organization; 1999. Definition, diagnosis and classification of diabetes mellitus and its complications: Part 1: Diagnosis and classification of diabetes mellitus. [Google Scholar]
- 23.Fuller JH. McCartney P. Jarrett RJ, et al. Hyperglycaemia and coronary heart disease: The Whitehall study. J Chronic Dis. 1979;32:721–728. doi: 10.1016/0021-9681(79)90051-1. [DOI] [PubMed] [Google Scholar]
- 24.McGovern PG. Pankow JS. Shahar E, et al. Recent trends in acute coronary heart disease—Mortality, morbidity, medical care, and risk factors. The Minnesota Heart Survey Investigators. N Engl J Med. 1996;334:884–890. doi: 10.1056/NEJM199604043341403. [DOI] [PubMed] [Google Scholar]
- 25.Rosamond WD. Chambless LE. Folsom AR, et al. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med. 1998;339:861–867. doi: 10.1056/NEJM199809243391301. [DOI] [PubMed] [Google Scholar]
- 26.Capewell S. Morrison CE. McMurray JJ. Contribution of modern cardiovascular treatment and risk factor changes to the decline in coronary heart disease mortality in Scotland between 1975 and 1994. Heart. 1999;81:380–386. doi: 10.1136/hrt.81.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuulasmaa K. Tunstall-Pedoe H. Dobson A, et al. Estimation of contribution of changes in classic risk factors to trends in coronary-event rates across the WHO MONICA Project populations. Lancet. 2000;355:675–687. doi: 10.1016/s0140-6736(99)11180-2. [DOI] [PubMed] [Google Scholar]
- 28.Capewell S. Beaglehole R. Seddon M. McMurray J. Explanation for the decline in coronary heart disease mortality rates in Auckland, New Zealand, between 1982 and 1993. Circulation. 2000;102:1511–1516. doi: 10.1161/01.cir.102.13.1511. [DOI] [PubMed] [Google Scholar]
- 29.Cooper R. Cutler J. Svigne-Nickens P, et al. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: Findings of the National Conference on Cardiovascular Disease Prevention. Circulation. 2000;102:3137–3147. doi: 10.1161/01.cir.102.25.3137. [DOI] [PubMed] [Google Scholar]
- 30.McGovern PG. Jacobs DR., Jr. Shahar E, et al. Trends in acute coronary heart disease mortality, morbidity, and medical care from 1985 through 1997: The Minnesota Heart Survey. Circulation. 2001;104:19–24. doi: 10.1161/01.cir.104.1.19. [DOI] [PubMed] [Google Scholar]
- 31.Ergin A. Muntner P. Sherwin R. He J. Secular trends in cardiovascular disease mortality, incidence, and case fatality rates in adults in the United States. Am J Med. 2004;117:219–227. doi: 10.1016/j.amjmed.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Zaher C. Goldberg GA. Kadlubek P. Estimating angina prevalence in a managed care population. Am J Manag Care. 2004;10:S339–S346. [PubMed] [Google Scholar]
- 33.Reynolds MW. Frame D. Scheye R, et al. A systematic review of the economic burden of chronic angina. Am J Manag Care. 2004;10:S347–S357. [PubMed] [Google Scholar]
- 34.Javitz HS. Ward MM. Watson JB. Jaana M. Cost of illness of chronic angina. Am J Manag Care. 2004;10:S358–S369. [PubMed] [Google Scholar]
- 35.Huxley R. Barzi F. Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: Meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuanetti G. Latini R. Maggioni AP. Santoro L. Franzosi MG. Influence of diabetes on mortality in acute myocardial infarction: Data from the GISSI-2 study. J Am Coll Cardiol. 1993;22:1788–1794. doi: 10.1016/0735-1097(93)90758-s. [DOI] [PubMed] [Google Scholar]
- 37.Kanaya AM. Herrington D. Vittinghoff E, et al. Impaired fasting glucose and cardiovascular outcomes in postmenopausal women with coronary artery disease. Ann Intern Med. 2005;142:813–820. doi: 10.7326/0003-4819-142-10-200505170-00006. [DOI] [PubMed] [Google Scholar]
- 38.Wingard DL. Barrett-Connor EL. Ferrara A. Is insulin really a heart disease risk factor? Diabetes Care. 1995;18:1299–1304. doi: 10.2337/diacare.18.9.1299. [DOI] [PubMed] [Google Scholar]
- 39.Fuller JH. Keen H. Jarrett RJ, et al. Haemostatic variables associated with diabetes and its complications. BMJ. 1979;2:964–966. doi: 10.1136/bmj.2.6196.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldschmid MG. Barrett-Connor E. Edelstein SL. Wingard DL. Cohn BA. Herman WH. Dyslipidemia and ischemic heart disease mortality among men and women with diabetes. Circulation. 1994;89:991–997. doi: 10.1161/01.cir.89.3.991. [DOI] [PubMed] [Google Scholar]
- 41.Kannel WB. Vokonas PS. Demographics of the prevalence, incidence, and management of coronary heart disease in the elderly and in women. Ann Epidemiol. 1992;2:5–14. doi: 10.1016/1047-2797(92)90031-k. [DOI] [PubMed] [Google Scholar]
- 42.Alexander KP. Shaw LJ. Shaw LK. Delong ER. Mark DB. Peterson ED. Value of exercise treadmill testing in women. J Am Coll Cardiol. 1998;32:1657–1664. doi: 10.1016/s0735-1097(98)00451-3. [DOI] [PubMed] [Google Scholar]
- 43.Lehmann JB. Wehner PS. Lehmann CU. Savory LM. Gender bias in the evaluation of chest pain in the emergency department. Am J Cardiol. 1996;77:641–644. doi: 10.1016/s0002-9149(97)89322-8. [DOI] [PubMed] [Google Scholar]
- 44.Scirica BM. Moliterno DJ. Every NR, et al. Differences between men and women in the management of unstable angina pectoris (The GUARANTEE Registry). The GUARANTEE Investigators. Am J Cardiol. 1999;84:1145–1150. doi: 10.1016/s0002-9149(99)00525-1. [DOI] [PubMed] [Google Scholar]
- 45.Jaglal SB. Slaughter PM. Baigrie RS. Morgan CD. Naylor CD. Good judgement or sex bias in the referral of patients for the diagnosis of coronary artery disease? An exploratory study. Can Med Assoc J. 1995;152:873–880. [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw LJ. Miller DD. Romeis JC. Kargl D. Younis LT. Chaitman BR. Gender differences in the noninvasive evaluation and management of patients with suspected coronary artery disease. Ann Intern Med. 1994;120:559–566. doi: 10.7326/0003-4819-120-7-199404010-00005. [DOI] [PubMed] [Google Scholar]
- 47.Goldberg R. Goff D. Cooper L, et al. Age and sex differences in presentation of symptoms among patients with acute coronary disease: The REACT Trial. Rapid Early Action for Coronary Treatment. Coronary Artery Dis. 2000;11:399–407. doi: 10.1097/00019501-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Roger VL. Jacobsen SJ. Pellikka PA. Miller TD. Bailey KR. Gersh BJ. Gender differences in use of stress testing and coronary heart disease mortality: A population-based study in Olmsted County, Minnesota. J Am Coll Cardiol. 1998;32:345–352. doi: 10.1016/s0735-1097(98)00229-0. [DOI] [PubMed] [Google Scholar]
- 49.Persell SD. Baker DW. Aspirin use among adults with diabetes: Recent trends and emerging sex disparities. Arch Intern Med. 2004;164:2492–2499. doi: 10.1001/archinte.164.22.2492. [DOI] [PubMed] [Google Scholar]
- 50.Cull CA. Neil HA. Holman RR. Changing aspirin use in patients with type 2 diabetes in the UKPDS. Diabetes Med. 2004;21:1368–1371. doi: 10.1111/j.1464-5491.2004.01328.x. [DOI] [PubMed] [Google Scholar]
- 51.Tonstad S. Rosvold EO. Furu K. Skurtveit S. Undertreatment and overtreatment with statins: The Oslo Health Study 2000–2001. J Intern Med. 2004;255:494–502. doi: 10.1111/j.1365-2796.2004.01315.x. [DOI] [PubMed] [Google Scholar]
- 52.Wexler DJ. Grant RW. Meigs JB. Nathan DM. Cagliero E. Sex disparities in treatment of cardiac risk factors in patients with type 2 diabetes. Diabetes Care. 2005;28:514–520. doi: 10.2337/diacare.28.3.514. [DOI] [PubMed] [Google Scholar]
- 53.Gulati M. Cooper-DeHoff RM. McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease. Arch Intern Med. 2009;169:843–850. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]