Abstract
Study Objectives:
We determined if sleep deprivation would amplify the effect of negative emotional distracters on working memory.
Design:
A crossover design involving 2 functional neuroimaging scans conducted at least one week apart. One scan followed a normal night of sleep and the other followed 24 h of sleep deprivation. Scanning order was counterbalanced across subjects.
Setting:
The study took place in a research laboratory.
Participants:
24 young, healthy volunteers with no history of any sleep, psychiatric, or neurologic disorders.
Interventions:
N/A
Measurements and Results:
Study participants were scanned while performing a delayed-response working memory task. Two distracters were presented during the maintenance phase, and these differed in content: highly arousing, negative emotional scenes; low-arousing, neutral scenes; and digitally scrambled versions of the pictures. Irrespective of whether volunteers were sleep deprived, negative emotional (relative to neutral) distracters elicited greater maintenance-related activity in the amygdala, ventrolateral prefrontal cortex, and fusiform gyri, while concurrently depressing activity in cognitive control regions. Individuals who maintained or increased distracter-related amygdala activation after sleep deprivation showed increased working memory disruptions by negative emotional distracters. These individuals also showed reduced functional connectivity between the amygdala and the ventromedial and dorsolateral prefrontal cortices, regions postulated to mediate cognitive control against emtional distraction.
Conclusions:
Increased distraction by emotional stimuli following sleep deprivation is accompanied by increases in amygdala activation and reduced functional connectivity between the amygdala and prefrontal cognitive control regions. These findings shed light on the neural basis for interindividual variation in how negative emotional stimuli might distract sleep deprived persons.
Citation:
Chuah LYM; Dolcos F; Chen AK; Zheng H; Parimal S; Chee MWL. Sleep deprivation and interference by emotional distracters. SLEEP 2010;33(10):1305-1313.
Keywords: Amygdala, prefrontal cortex, working memory, emotion, attention, sleep deprivation
EMOTION IS A DOUBLE-EDGED SWORD THAT CAN ENHANCE1–3 OR HINDER COGNITION,4–6 DEPENDING ON THE CONTEXT IN WHICH EMOTIONAL STIMULI are encountered. The emotive content of pictures may enhance our attention and memory, particularly when task-relevant.7–9 However, task-irrelevant, emotion-laden stimuli are also potent distracters, often reducing the recall of neutral memoranda presented earlier.10,11
Distraction by negative emotional events is postulated to result from the interaction of two neural systems, a “hot, affective” ventral system and a “cold, executive” dorsal system.10,12,13 Negative emotional stimuli increase maintenance-related activity within the ventral system, which includes the amygdala and the ventrolateral prefrontal cortex (vlPFC). Concurrently, these stimuli attenuate activation of the dorsal executive system, which includes the dorsolateral prefrontal cortex (dlPFC) and the lateral parietal cortex (LPC).
Sleep deprivation can also impair attention and memory,14–19 and its growing prevalence,20 especially among “knowledge workers,” motivates a detailed exploration of its impact on human behavior. Several behavioral and imaging studies have examined the effects of sleep deprivation on attention,17,18,21 memory,22–24 and emotion processing.25,26 However, less is known about how these elements interact with each other and, in particular, how emotional stimuli can affect memory by serving as distracters in sleep deprived persons. An understanding of the neural mechanisms underpinning this effect could help reduce adverse outcomes in health care settings, shift work, and military operations.
Given earlier observations concerning the greater efficacy of negative emotional distracters compared to neutral distracters,10,11 it is possible that emotional distracters could also have a greater impact on working memory in sleep deprived persons. This would underscore the value of maintaining emotional neutrality in workplaces where critical decisions are made by sleep deprived individuals, for example, in intensive care units.
In studying the neural mechanisms underlying the effects of negative distraction on memory, we focused on the amygdala, as it is known to mediate the effect of emotional stimuli on attention and memory.27–29 Additionally, sleep deprivation has been shown to elevate amygdala activation in response to aversive emotional targets26 as well as reduce the functional connectivity between the amygdala and a medial frontal region associated with emotional regulation. Analyses of functional connectivity between the amygdala and other neural structures also suggest that emotional stimuli may be processed in a manner that facilitates more robust recollection in spite of sleep deprivation.30
In light of these findings, we predicted that (1) working memory would be impaired following sleep deprivation and that emotional distracters would be more effective than neutral distracters; (2) declines in the ability to maintain neutral memoranda in the presence of distracting negative emotional stimuli following sleep deprivation would be associated with elevated amygdala activity; and (3) an increase in emotional distractibility would be associated with reduced psychophysiological interaction (PPI) between the amygdala and brain regions responsible for cognitive control and emotion regulation.
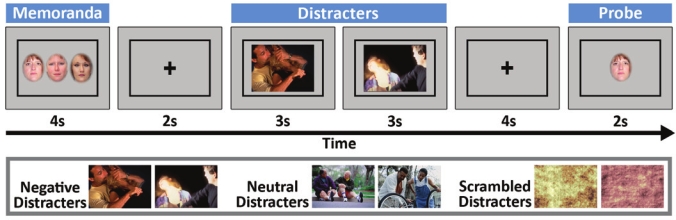
To test these predictions, healthy young adults underwent functional neuroimaging (fMRI) after a night of normal sleep and also following 24 h of total sleep deprivation. We assessed the effect of emotional distraction by evaluating short-term memory for neutral faces after 1 of 3 types of distracters (negative emotional, neutral and scrambled patterns) was presented during a maintenance interval (Figure 1).10 We also evaluated how sleep deprivation interacted with different distracters to modulate fMRI signal magnitude in the “hot, affective” ventral system and “cold, executive” dorsal systems during the maintenance period. Finally, we evaluated how sleep loss altered task-related changes in functional connectivity, using psychophysiological interaction (PPI) analyses.
Figure 1.
A schematic of the working memory task. Participants were instructed to view and remember the faces, view the distracters and to indicate if the probe face had been presented earlier. Distracters could be negative, neutral, or scrambled pictures.
METHODS
Participants
Twenty-nine individuals completed this counterbalanced crossover study. Data from 5 individuals were discarded as they did not respond to at least 95% of all trials during the sleep deprivation session. This was to ensure that all subjects were awake and paying attention to the stimuli even when sleep deprived. Thus, the final sample consisted of 24 individuals (14 female; mean age, 22.33 years; SD, 1.34 years). All volunteers were right handed with self-reported regular sleeping habits (sleeping ≥ 6.5 h each night for the past month). The sleeping habits of all volunteers were monitored using wrist actigraphy to ascertain maintenance of regular sleep hours for the entire study duration (i.e., they slept no later than 01:00 and woke no later than 09:00). All volunteers declared that they had no history of psychiatric, sleep, or neurological disorders.
Design and Procedure
The experimental protocol was approved by the Singapore General Hospital IRB. Participants made 3 visits to the laboratory over 2 weeks. The first was a briefing session, during which informed consent was obtained. During the briefing, subjects were introduced to the in-scanner task and completed 12 practice trials. Subjects also completed the trait scale of the State Trait Anxiety Inventory (STAI)31 and the Positive and Negative Affect Schedule Extended Form (PANAS-X).32 At the end of this session, subjects were asked to wear an actiwatch and were reminded to maintain regular sleeping hours throughout the study duration. A week later, subjects came to the lab either for a rested wakefulness session or a sleep deprivation session; the final test session took place the week following. The order of the sessions (rested wakefulness, sleep deprivation) was counterbalanced across subjects.
For both sessions, prior to each scan, subjects signed informed consent to undergo fMRI and confirmed that they had not smoked or consumed any medications, stimulants, alcohol, or caffeine for ≥ 24 h prior to scanning. They were given task instructions and practiced 6 trials of the in-scanner task (2 of each condition) prior to entering the scanner. Karolinska Sleepiness Scale (KSS) scores were taken at the end of the practice trials and after each in-scanner run. The state component of the STAI was administered before and after scanning while the PANAS-X was administered once before each scan.
For the rested wakefulness session, participants were present at the lab at 07:30. Compliance to a regular sleep schedule was verified by checking the volunteer's sleep diary and wrist actigraph. Subjects then completed a 10-min trial from the psychomotor vigilance task (PVT), the PANAS-X, and the state component of the STAI. Scanning took place at ∼08:00.
For the sleep deprivation session, participants arrived at the laboratory no later than 19:00 on the test night, after staying awake the whole day. A check of their sleep habits was conducted as described for the rested wakefulness session. Participants were monitored throughout the night and were only allowed to engage in non-strenuous activities such as reading, working on a computer, and conversing. Every hour, from 20:00 to 05:00, participants completed a 10-min PVT trial and the KSS. Scanning took place at ∼05:30, close to, or at, the cognitive performance nadir for most persons.
The scanning times were chosen as they represent the start times of a regular workday and the low-point of cognitive performance after a night of sleep deprivation.33,34 However, we note that this schedule does not align the rested wakefulness and sleep deprivation sessions to the same circadian phase and does not allow a decomposition of the effects of sleep deprivation into “circadian” and “homeostatic” components.
Experimental Task
Subjects were scanned while performing a delayed-match-to-sample working memory task for faces,10 with distracters presented during the maintenance interval between the memoranda and probes. Memoranda consisted of sets of 3 faces, and the distracters consisted of pairs of novel pictures. The picture distracters were of 3 types: highly arousing, negative emotional scenes (mutilations, aggressive behaviors); low arousing neutral scenes (mundane activities); and digitally scrambled versions of the pictures (Figure 1). All the neutral and emotional distracters included people and were mostly obtained from the International Affective Picture System (IAPS),35 with supplementation from in-house sources. The average IAPS arousal/valence ratings for the emotional scenes were 5.84/2.49; ratings for the neutral scenes were 2.0/5.45. All stimuli (faces, scenes) were presented in color. There were 2 separate sets of stimuli (for both faces and distracters) for the rested wakefulness and sleep deprivation sessions, so that no stimulus was repeated within or across sessions. Care was also taken to ensure that average IAPS ratings of arousal and valence of the emotional and neutral distracters were similar across these 2 sets; their equivalence was verified by subsequent ratings made by the participants in this study (Supplementary Table 1, supplementary materials are available online only at www.journalsleep.org). The order of use of these 2 stimulus sets was counterbalanced across subjects within the different scanning orders.
Supplementary Table 1.
Mean ratings (standard deviation) of distractibility and emotional intensity on the neutral and emotional scenes
| Distractibility | Rested Wakefulness |
Sleep Deprivation |
||
|---|---|---|---|---|
| Neutral | Emotional | Neutral | Emotional | |
| A/B | 1.83 (0.67) | 4.95 (1.10) | 1.89 (1.05) | 4.98 (1.10) |
| RWA-SDB (n = 11) | 1.93 (1.10) | 4.82 (1.05) | 1.94 (1.35) | 4.69 (0.98) |
| RWB-SDA (n = 13) | 1.75 (0.65) | 5.06 (1.18) | 1.85 (0.77) | 5.23 (1.17) |
| Emotional Intensity | ||||
| A/B | 1.62 (0.62) | 4.84 (1.15) | 1.71 (0.67) | 4.97 (0.99) |
| RWA-SDB (n = 11) | 1.49 (0.55) | 4.68 (1.09) | 1.77 (0.68) | 4.83 (0.97) |
| RWB-SDA (n = 13) | 1.73 (0.67) | 4.98 (1.23) | 1.66 (0.68) | 5.08 (1.03) |
There were 2 stimulus sets (A and B) to ensure that no stimulus was repeated across the 2 testing sessions, and the order of their use was counterbalanced across subjects and their scan order. Eleven subjects were presented with stimulus set A during their rested wakefulness session and stimulus set B during their sleep deprivation session (RWA-SDB), and vice versa for the remaining 13 participants (RWB-SDA). There were significant differences only in ratings between neutral and emotional scenes. N = 24.
There were a total of 120 trials for each scanning session, 40 for each condition, presented over 10 runs of 12 trials (4 emotional, 4 neutral, 4 scrambled). Each run started with 12 s of fixation, of which 4 s was discarded to allow for steady state magnetization, and ended with at least 20 s of fixation, lasting a total of 6.2 min. The order of the trials was pseudorandomized so that no more than 2 trials of any condition were presented consecutively. For each trial (Figure 1), subjects were presented with a memoranda set of 3 faces for 4 s (subtending a visual angle of 10° × 8.5°). Following a 2-s fixation, 2 distracters were presented consecutively for 3 s each. Each distracter stimulus subtended a maximum visual angle of 19.5° × 14.5°. Following 4 s of fixation, a single face probe (visual angle 6° × 8.5°) was presented, and participants indicated if the face had been presented earlier or if it was new. Subjects were instructed to look at the distracters while maintaining their focus to perform accurately, and to respond as quickly as possible to the probe. Half of the probes were old. Random intertrial intervals of fixation lasting 8, 10, 12, and 14 s were used. All intertrial intervals occurred with equal frequency.
After scanning, participants rated the emotional and neutral stimuli, using a Likert scale of 1 to 9, on their distractibility and emotional intensity. The ratings were made over 2 separate consecutive runs.
Imaging Procedure
Subjects viewed the stimuli using MR-compatible LCD visual goggles (Resonance Technologies, California) and responded using a button box held in their right hand. Foam padding was used to restrict head motion. Images were acquired on a 3T Siemens Tim TRIO system (Siemens, Erlangen). A single-shot gradient-echo EPI was used (186 volumes, TR 2000 ms, TE 30 ms, flip angle 90°, FOV 192 × 192 mm, matrix 64 × 64). Parallel imaging (GRAPPA, acceleration factor 2) was enabled. Thirty-six oblique axial slices (3 mm thick with 0.3 mm interslice gap) approximately parallel to the intercommisural plane were acquired. High resolution coplanar T1 anatomical images were also obtained. For the purpose of image display on Talairach space, further 3D high-resolution anatomical reference images were acquired using a T1-weighted MP-RAGE (magnetization-prepared rapid acquisition gradient echo) sequence (TR 2530 ms, TI = 1200 ms, flip angle 7°, FOV 256 × 192 mm, resulting voxel dimensions 1 × 1 × 1 mm).
DATA ANALYSIS
Behavioral Data
Performance was indexed using corrected recognition, computed separately for each condition as the difference between hit rate and false alarm rate (% Hits - % False Alarms). Effects of state and condition were investigated using a 2 (State: rested wakefulness, sleep deprivation) by 3 (Condition: scrambled, neutral, emotional) repeated-measures ANOVA, and significant effects were further explored using paired-samples t-tests. Average distractibility and emotional intensity ratings were computed separately for the emotional and neutral distracters at each state and analyzed using 2 (State: rested wakefulness, sleep deprivation) by 2 (Condition: neutral, emotional) repeated-measures ANOVA. We also investigated if sleep deprivation alters self-report of anxiety and mood on the STAI and PANAS-X, and if state-related changes on these scales may account for performance change on the working memory task.
Imaging Data
Functional images were preprocessed using Brain Voyager QX version 1.10.4 (Brain Innovation, Maastricht). Data preprocessing included inter-slice timing correction using trilinear and then sinc interpolation, linear trend removal, and temporal high-pass filtering of period 184 s to remove low-frequency non-linear drifts ≤ 2 cycles per run. Three-dimensional rigid-body motion correction across runs was performed using, as the reference image, the first image of the functional run that was acquired immediately before the anatomical coplanar T1-weighted image. Spatial smoothing was performed using an 8-mm Gaussian kernel (full-width at half-maximum). Functional slices were co-registered to the MPRAGE anatomical volume and transformed into Talairach space.
The BOLD signal for each run was first normalized to baseline (specified as all fixation time points preceding the first trial and fixation time points that fall between 28 s post trial onset and the onset of a new trial). The functional data was then selectively averaged in each subject as a function of trial type and time point (16 time points from the onset of each trial), using customized in-house software. All trials were included in these analyses. The individual averaged functional data was then submitted to a second-level, mixed-effects general linear modeling (GLM) analysis.36 To identify the neural regions differentially affected by emotional distracters, we conducted a 2 (State: rested wakefulness, sleep deprivation) by 2 (Condition: neutral, emotional) by 16 (Time-on-trial) repeated-measures ANOVA, with subjects as a random factor.37 Of particular interest were regions that showed a Condition by Time-on-trial interaction, and a State by Condition by Time-on-trial interaction. Percent signal changes were extracted from these regions of interest (defined as a cube of side 9 mm surrounding the peak voxel) and subjected to post hoc analyses. Possible time-on-task effects were also investigated (see Supplementary results at www.journalsleep.org).
To test our hypothesis of an association between state-related change in amygdala activation and emotional distractibility following sleep deprivation, we first identified the amygdala regions of interest (ROI) that showed a Condition by Time-on-trial interaction. Signal changes within the right and left amygdala were averaged across the time points 12, 14, and 16 s post-trial onset. These time points were established by previous research to show peak effects of distraction during the maintenance interval.10,11 We then correlated state-related change in activation in these ROI with state-related change in task performance separately for the neutral and emotional conditions.
To identify the regions which show different functional connectivity in the emotional distracter relative to the neutral conditions, a psychophysiological interaction (PPI) analysis38,39 between an amygdala seed region and the rest of the brain was conducted separately for each state. A cube of side 9 mm within the right amygdala was chosen as the seed region as it showed the largest Condition by Time-on-trial interaction (Table 1). Psychophysiological interaction analysis for each volunteer was conducted as follows: The design matrix of regressors included the BOLD signal time course in the right amygdala seed region, regressors coding for the temporal ordering of task conditions, and finally, a PPI term, which was the product of the deconvolved time course in the amygdala with a vector representing the order of the psychological variables of interest (emotional vs. neutral). This product was subsequently re-convolved with a canonical hemodynamic function.39 This analysis was restricted to the maintenance interval. To explore the hypothesis that state-related change in functional connectivity between the amygdala and prefrontal control regions may be related to emotional distractibility following sleep deprivation, we correlated state-related change in amygdala connectivity and state-related change in performance. This was conducted separately for the emotional and neutral distracter conditions. An outlier who inflated the correlations between state-related change in amygdala connectivity and performance was removed.
Table 1.
Neural regions whose activity showed significant (P < 0.001, corrected) effects in a 2 (State: rested wakefulness, sleep deprivation) by 2 (Condition: neutral, emotional) by 16 (Time-on-trial) repeated-measures ANOVA. Of particular interest were regions which showed a significant interaction of Condition by Time-on-trial.
| Neural region | BA | Side | Talairach |
F value | P | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| State × Time-on-trial | df 15, 345 | ||||||
| Superior Parietal Cortex | 7 | R | 18 | −61 | 43 | 7.20 | 8.62 e-14 |
| L | 18 | −79 | 40 | 7.86 | 3.13 e-15 | ||
| Middle Temporal Gyrus | 37 | R | 42 | −58 | 4 | 7.45 | 2.40 e-14 |
| L | −48 | −61 | 7 | 4.55 | 6.39 e-08 | ||
| Fusiform Gyrus | 19 | R | 21 | −61 | −11 | 7.16 | 1.03 e-13 |
| L | −27 | −55 | −11 | 7.28 | 5.78 e-14 | ||
| Superior Occipital Gyrus | 19 | R | 21 | −76 | 31 | 8.49 | 1.39 e-16 |
| L | −27 | −79 | 19 | 7.62 | 1.05 e-14 | ||
| Condition × Time-on-trial | df 15, 345 | ||||||
| Amygdala | R | 18 | −7 | −8 | 12.06 | 5.94 e-24 | |
| L | −24 | −4 | −11 | 10.97 | 9.23 e-22 | ||
| Insula/Lentiform Nucleus | R | 21 | 8 | 4 | 9.51 | 9.61 e-19 | |
| L | −21 | −1 | −5 | 10.64 | 4.32 e-21 | ||
| *Caudate Nucleus | R | 12 | 17 | 13 | 13.28 | 2.37 e-26 | |
| L | −15 | 17 | 4 | 10.66 | 3.84 e-21 | ||
| Inferior Frontal Gyrus | 45 | R | 48 | 29 | 13 | 18.37 | 1.21 e-35 |
| L | −51 | 26 | 16 | 8.35 | 2.79 e-16 | ||
| Inferior Frontal Gyrus | 47 | R | 36 | 29 | −5 | 6.97 | 2.70 e-13 |
| L | −39 | 26 | −5 | 5.82 | 9.69 e-11 | ||
| Middle Frontal Gyrus | 6/9 | R | 42 | 2 | 34 | 19.71 | 6.20 e-38 |
| L | −45 | −1 | 31 | 9.23 | 3.62 e-18 | ||
| *Middle Frontal Gyrus | 10 | R | 30 | 53 | 13 | 27.58 | < 0.00000 |
| L | −33 | 50 | 10 | 32.25 | < 0.00000 | ||
| *Middle Frontal Gyrus | 46 | R | 36 | 35 | 28 | 20.25 | 7.75 e-39 |
| *Middle Frontal Gyrus | 6/8 | R | 33 | 17 | 49 | 14.44 | 1.48 e-28 |
| L | −36 | 17 | 46 | 23.70 | 2.38 e-44 | ||
| Medial Prefrontal Gyrus | 9 | 0 | 59 | 28 | 15.90 | 2.87 e-31 | |
| *Anterior Cingulate | 32 | R | 6 | 32 | 25 | 7.46 | 2.28 e-14 |
| L | −9 | 29 | 34 | 8.42 | 1.98 e-16 | ||
| 24/32 | R | 9 | 41 | 4 | 8.75 | 3.78 e-17 | |
| Superior Temporal Gyrus | 22/42 | R | 51 | −7 | 7 | 15.00 | 1.30 e-29 |
| L | −60 | −19 | 10 | 14.85 | 2.52 e-29 | ||
| Superior Parietal Cortex | 7 | R | 24 | −52 | 46 | 14.07 | 7.28 e-28 |
| L | −27 | −55 | 49 | 10.60 | 5.14 e-21 | ||
| *Inferior Parietal Cortex | 40 | R | 48 | −58 | 40 | 28.97 | < 0.00000 |
| L | −51 | −58 | 37 | 38.43 | < 0.00000 | ||
| *Precuneus | 18 | L | −6 | −73 | 28 | 24.70 | < 0.00000 |
| Occipital Gyrus | 19 | R | 33 | −79 | 19 | 26.70 | < 0.00000 |
| L | −30 | −88 | 19 | 18.99 | < 0.00000 | ||
| Middle Occipital Gyrus | 19 | R | 42 | −55 | −5 | 26.54 | < 0.00000 |
| L | −45 | −61 | −2 | 26.70 | < 0.00000 | ||
No regions showed significant main effects of State or Condition, or significant State by Condition or State by Condition by Time-on-trial interactions. All regions marked with an asterisk
show reducedactivity for emotional distracters relative to neutral distracters.
RESULTS
Behavioral Findings
Working memory was impaired by sleep deprivation
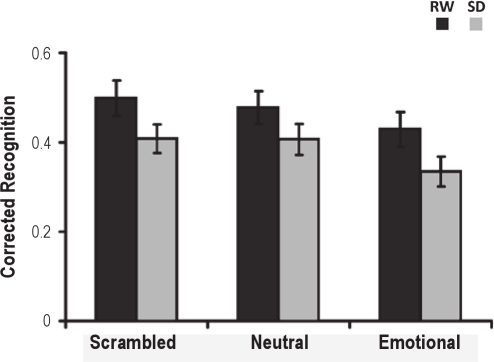
Consistent with previous findings,10 working memory for faces (hit rate – false alarm rate) was poorest for the emotional distracter condition, F2,46 = 4.59, P = 0.02 (Figure 2, Supplementary Table 2). There was significant decline in performance following sleep deprivation, F1,23 = 14.45, P = 0.001. However, the interaction between state and condition was not significant, F2,46 = 0.08, P = 0.92.
Figure 2.
Sleep deprivation impaired working memory. Corrected recognition was significantly lowered with emotional distracters and showed a trend for decline with neutral and scrambled pictures. Error bars indicate ± 1 SE. (RW: rested wakefulness; SD: sleep deprivation).
Supplementary Table 2.
Mean (standard deviation) working memory performance as a function of condition (scrambled, neutral, emotional scenes) and state (rested wakefulness, sleep deprivation). N = 24
| Corrected Recognition (Hits Rate – FA Rate) | Rested Wakefulness | Sleep Deprivation |
|---|---|---|
| Scrambled | 0.499 (0.195) | 0.408 (0.155) |
| Neutral | 0.478 (0.181) | 0.407 (0.169) |
| Emotional | 0.429 (0.193) | 0.335 (0.164) |
| Reaction Time (ms) | ||
| Scrambled | 1215 (191) | 1303 (238) |
| Neutral | 1237 (214) | 1312 (245) |
| Emotional | 1234 (192) | 1302 (235) |
| Non-responses (%) | ||
| Scrambled | 0.29 (0.62) | 0.92 (1.01) |
| Neutral | 0.13 (0.34) | 0.21 (0.42) |
| Emotional | 0.25 (0.44) | 0.63 (0.82) |
Additional analyses were conducted to investigate possible effects of time of task on performance (see Supplementary results). While performance did decline with increased time on task, there were no interactions with state and condition.
Emotional scenes were perceived as more distracting and emotionally more intense
Consistent with prior work,10 emotional scenes were rated as more distracting, F1,23 = 254.54, P < 0.001, and more emotionally intense, F1,23 = 340.26, P < 0.001, relative to neutral distracters (Supplementary Table 1). Sleep deprivation did not influence these ratings (smaller P = 0.33), and there was no significant interaction between state and distracter type for either of the 2 scales (smaller P = 0.77).
State anxiety increased following sleep deprivation but did not correlate with state-related change in working memory
Participants reported greater anxiety on the STAI state scale when sleep deprived relative to when they were well rested, t23 = 7.97, P < 0.001. There were no significant differences between the 2 test sessions for the Positive and Negative Affect scales of the PANAS-X (Supplementary Table 3). Also, there were no correlations between state-related changes in self-ratings of distractibility, emotionality and mood, and change in working memory performance for any of the 3 conditions.
Supplementary Table 3.
Mean (standard deviation) of ratings on the State and Trait Anxiety Inventory (STAI) and the Positive and Negative Affect Schedule Extended Form (PANAS-X) as a function of test session.
| Briefing | Rested Wakefulness | Sleep Deprivation | |
|---|---|---|---|
| STAI | 38.00 (6.38) | 34.60 (7.25)# | 45.25 (8.60)* |
| PANAS-X (Positive Affect) | 34.29 (6.41) | 32.46 (7.18) | 29.29 (8.77) |
| PANAS-X (Negative Affect) | 18.21 (5.12) | 16.88 (5.57) | 17.08 (6.26) |
The trait component of the STAI was administered at briefing while the state scale was administered during the scanning sessions. The STAI scores at rested wakefulness (RW) and following sleep deprivation were averaged across the 2 test points (pre and post scan). N = 24.
Briefing differed from Rested Wakefulness, P < 0.05;
Rested Wakefulness differed from Sleep Deprivation, P < 0.05
Neuroimaging Findings
Emotional distracters elevated activity in the ventral “affective” system while simultaneously depressing activity in the dorsal “cognitive” system
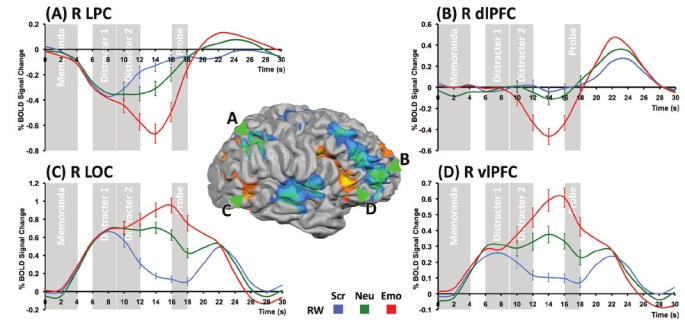
Activation in several regions showed a Condition by Time-on-trial interaction driven primarily by differences during the maintenance interval (Figure 3, Table 1). Consistent with previous studies,10,12,37 emotional distracters resulted in elevated activity in several brain regions of the ventral affective system, including the amygdala, the vlPFC, and in the occipital cortex (Figure 3; Table 1). Activation in these regions was driven by the presence of meaningful distracters with further significant elevation of activity by negative emotional content (Figure 3).
Figure 3.
Dissociable effects of emotional distraction in dorsal and ventral neural systems at rested wakefulness (RW). There were dissociable patterns of activity during the maintenance phase in (A) lateral parietal cortex (LPC), (b) dorsolateral prefrontal cortex (dlPFC), (C) lateral occipital complex (LOC) and (D) ventrolateral PFC (vlPFC). Emotional distracters resulted in elevated activity in vlPFC and LOC while simultaneously reducing activity in the dlPFC and LPC to below prestimulus baseline levels. Error bars indicate ± 1 SE. (Scr: Scrambled; Neu: Neutral; Emo: Emotional).
In contrast, emotional distracters reduced activation below baseline in the dlPFC and the LPC (Figure 3; Table 1). Decreases in maintenance related activity also occurred in the cingulate gyrus and bilateral superior temporal gyri. These effects can be attributed specifically to the emotional nature of the distracters and not mere distraction, as they were observed in the contrast between emotional and neutral distracters but not between neutral distracters and scrambled pictures (Figure 3).
Individuals vulnerable to emotional distraction following sleep deprivation had elevated amygdala activation
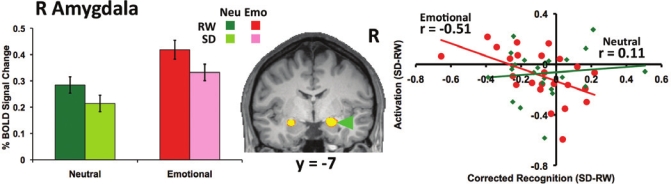
No region manifested a significant effect of State, a State by Condition interaction, or a State by Condition by Time interaction (Table 1, Supplementary Figure 1). However, supporting our second hypothesis, increased amygdala activation to emotional distracters following sleep deprivation correlated with decreases in working memory (right: r = −0.51; P = 0.01; Figure 4; left: r = −0.57, P = 0.004, Supplementary Figure 2). These correlations were selective to the emotional condition.
Figure 4.
Increased amygdala activity was associated with greater emotional distractibility following sleep deprivation (SD). Right amygdala activity (averaging the signal at time points 12, 14 and 16 s post trial onset) was elevated in response to emotional distracters relative to neutral distracters at rested wakefulness (RW). A State (rested wakefulness, sleep deprivation) by Condition (Neutral, Emotional) repeated-measures ANOVA conducted on averaged activation within this region of interest indicated decreases in amygdala activation following sleep deprivation, F1,23 = 5.44, P = 0.03, but this did not vary with condition, F1,23 = 0.17, P = 0.69. Critically, state-related change in amygdala activation correlated with the corresponding alteration of emotional distractibility (P = 0.01), while no parallel effect was present for neutral distracters. A similar effect was present for the left amygdala (Supplementary Figure 2). Error bars indicate ± 1 SE. (Neu: Neutral; Emo: Emotional).
Sleep deprivation can alter the functional connectivity between the amygdala and cognitive control regions
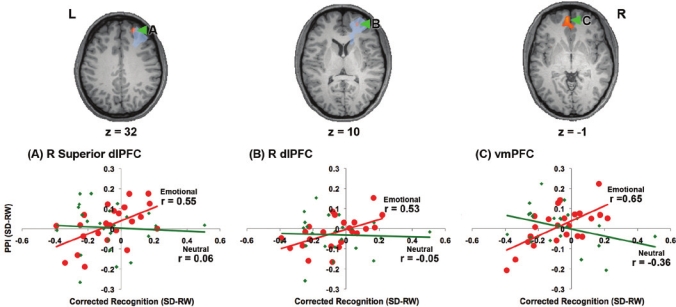
Increased distraction by emotional pictures following sleep deprivation also correlated with state-related decreases in the PPI between the amygdala and the dlPFC and vmPFC (Figure 5), whereas no effect was found in relation to neutral distracters anywhere in the brain. Thus, being able to maintain memoranda in working memory despite emotional distraction was associated with maintained or increased functional connectivity between the amygdala and frontal regions that have been shown in other studies to mediate cognitive control and regulate emotions.40–46
Figure 5.
Reduction in functional connectivity between the amygdala and brain regions known to mediate cognitive control, namely the dorsolateral prefrontal cortex (dlPFC) and ventromedial prefrontal cortex (vmPFC), was associated with greater distractibility by negative emotional stimuli during sleep deprivation. These correlations were computed following the removal of an influential outlier which inflated the correlations (N = 23). The region within the dorsolateral prefrontal cortex which showed a significant (P < 0.001, corrected) Condition by Time-on-trial interaction (Figure 3; Table 1) is shaded in blue. (RW: rested wakefulness; SD: sleep deprivation).
DISCUSSION
The present study generated two main findings: First, an increase in distractibility by negative emotional pictures following sleep deprivation was associated with state-related increases in amygdala activity. Second, individual differences in the ability to maintain performance in the presence of emotional distraction following sleep deprivation also correlated with state-related increases in the psychophysiological interaction (PPI) between the amygdala and brain regions associated with cognitive/emotional control, namely the dorsolateral (dlPFC) and ventromedial prefrontal cortex (vmPFC).
Emotional Distraction, Amygdala Activity, Attentional Resources and Sleep Deprivation—The Role of Individual Variation
Consistent with previous studies,10–12 and in both states, task-irrelevant emotional distracters elicited increased activity in the amygdala, ventrolateral prefrontal cortex (vlPFC), and occipital cortex, while reducing activation in the dorsolateral prefrontal cortex (dlPFC) and lateral parietal cortex.
Individuals prone to distraction by emotional stimuli when sleep deprived showed increased amygdala response towards negative emotional pictures, as well as reduced functional connectivity between the amygdala and frontal cognitive control regions. As correlation does not imply causation, further research is needed to further characterize the nature of this relationship. Nevertheless, connectivity analyses in studies concerned with interactions between emotion and cognition have helped uncover relationships between brain and behavior that were not apparent from analyses of activation magnitude or locus alone.47–51 Recent studies using connectivity analyses have also yielded further insights into the role of sleep in memory consolidation.24,30,52
The dlPFC, identified here as modulating the effect of amygdala activation on emotional distracter processing is known to support executive functions and working memory.53,54 However, this frontal region has also been implicated in the emotional modulation of episodic memory55 and in emotion regulation.56,57 Relevant to the current data, the dlPFC has also been shown to function as a “connectivity hub” for emotion-cognition interactions.13,27,58
The data presented here provide further support for the hypothesis that the outcome of task-irrelevant, emotional distraction depends on the interaction between a ventral, affective, “hot” system, and a dorsal, cognitive, “cold” system.10,13 On the one hand, emotional stimuli, being salient, have a known bottom-up, “competitive” advantage compared to neutral stimuli.9,59 Their appearance may therefore result in reallocation of cognitive resources in a task-irrelevant manner.10 On the other hand, the disadvantageous outcomes from the impact of emotional distraction can be mitigated by “top-down” intervention from cold cognitive control regions such as the dlPFC.60 As such, our present findings contribute to a better understanding of how hot and cold neural systems interact, extending the context under which this model can be applied to the setting of sleep deprivation.
Compelling as our findings appear to be, there are a number of potential limitations that should be highlighted. First, we had no control over the different strategies that may have been employed in order to cope with emotional distracters. Examples of different coping strategies include suppression, reappraisal,43,61 or refocusing of attention.62 Thus, while the present findings suggest the neural mechanisms through which sleep deprived persons might resist emotional distraction, it remains to be studied whether individuals susceptible to distraction when sleep deprived might benefit from explicit instructions on how to do so. This question could be addressed in subsequent studies that use methods proven to facilitate emotion regulation.56,57
Secondly, our findings differ from a previous report,26 in which a generalized increase in amygdala activity following sleep deprivation was found. However, two substantial differences between the studies must be noted: (1) the negative images were the task-relevant targets in the study of Yoo et al., whereas they were distracters in the present paradigm; and (2) participants in this study were tested in a within-subjects fashion, while Yoo et al. employed a between-subjects design. We have already pointed out that different strategies might be engaged to cope with distracters.
Summary
With these caveats in place, we conclude that increased distraction by negative emotional pictures following sleep deprivation is associated with increased amygdala activation and with reduced functional connectivity between the amygdala and cognitive control regions in the dorsolateral and ventromedial prefrontal cortices.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was funded by grants awarded to Dr. Chee from the Defense Science and Technology Agency Singapore (POD0713897) and the National Medical Research Council Singapore (NMRC/STaR/0004/2008). Florin Dolcos was supported by a Young Investigator Award from the US-based National Alliance for Research on Schizophrenia and Depression and a CPRF Award from the Canadian Psychiatric Research Foundation. William R. Rekshan III and Michele Veldsman assisted in data collection.
Work for this study was performed at Cognitive Neuroscience Lab, Duke-NUS Graduate Medical School Singapore.
SUPPLEMENTARY RESULTS
Time on task effects
We additionally investigated if differential effects of sleep deprivation on the different conditions may emerge as a function of the duration of the task. To investigate possible effects of time on task on cognitive performance, we conducted 2 (State: rested wakefulness, sleep deprivation) by 3 (Condition: scrambled, neutral, emotional) by 2 (Time-on-task: first 5 runs, last 5 runs) repeated-measures ANOVA on 2 indices of behavior, corrected recognition and reaction time.
Similarly, to investigate possible effects of time on task on brain activity, percent signal change was first extracted from key regions of interest that showed a Condition by Time-on-trial interaction, as illustrated in Supplementary Figure 1. This included bilateral amygdala, right ventrolateral prefrontal cortex (vlPFC), dorsolateral prefrontal cortex (dlPFC), lateral parietal cortex (LPC) and the lateral occipital complex (LOC). We then investigated time on task effects using a 2 (State: rested wakefulness, sleep deprivation) by 2 (Condition: neutral, emotional) by 2 (Time-on-task: first 5 runs, last 5 runs) by 16 (Time-on-trial) repeated-measures ANOVA.
Our behavioral findings suggest that while cognitive performance deteriorated in the latter half of the task, there were no interactions with State or Condition. Concerning brain activity, with the exception of the lateral occipital cortex, activation in the regions of interest did not vary significantly with time on task, nor were there any significant interactions between Time-on-task, State, and Condition. These findings are detailed below.
Corrected Recognition.
Recognition accuracy decreased following sleep deprivation, F1,23 = 14.59, P = 0.001, and was lowest for the emotional distracter condition, F2,46 = 3.93, P = 0.03 (Supplementary Table 4). While performance deteriorated in the latter half, F1,23 = 9.94, P = 0.004, there were no interactions with State or Condition (smallest P = 0.42).
Supplementary Table 4.
Mean (standard deviation) of working memory performance as a function of State (rested wakefulness, sleep deprivation), Condition (scrambled, neutral, emotional scenes) and Time-on-task (first 5 runs, last 5 runs). N = 24
| Corrected Recognition (Hit Rate – FA Rate) | Rested Wakefulness |
Sleep Deprivation |
|||
|---|---|---|---|---|---|
| 1st Half | 2nd Half | 1st Half | 2nd Half | ||
| Scrambled | 0.52 (0.23) | 0.48 (0.22) | 0.50 (0.18) | 0.32 (0.26) | |
| Neutral | 0.51 (0.24) | 0.44 (0.22) | 0.44 (0.26) | 0.37 (0.18) | |
| Emotional | 0.47 (0.24) | 0.40 (0.28) | 0.36 (0.24) | 0.30 (0.20) | |
| Reaction Time (ms) | |||||
| Scrambled | 1223 (208) | 1207 (194) | 1319 (248) | 1286 (240) | |
| Neutral | 1285 (250) | 1189 (203) | 1323 (253) | 1300 (262) | |
| Emotional | 1262 (232) | 1207 (179) | 1311 (224) | 1296 (279) | |
Reaction Time.
Participants responded more slowly when sleep deprived, F1,23 = 6.71, P = 0.02. There was a trend toward an effect of Time-on-task, F1,23 = 4.08, P = 0.06 and no effect of Condition, F2,46 = 0.78, P = 0.47 (Supplementary Table 4). There were also no significant interactions (smallest P = 0.11).
Neural activation.
There were no significant effects of (1) Time-on-task, and no (2) State by Time-on-task (3) Condition by Time-on-task and (4) State by Condition by Time-on-task interactions in any region of interest (smallest P for all effects = 0.17). With the exception of the right LOC (Supplementary Figure 3), no region of interest showed a significant State by Condition by Time-on-task by Time-on-trial interaction (P < 0.001 for the LOC, all other regions, P > 0.06). Post hoc State by Condition by Time-on-trial repeated-measures ANOVA indicated a significant 3-way interaction for activation in the first 5 runs, F15,345 = 5.68, P < 0.001, but not in the last 5 runs, F15,345 = 0.92, P = 0.54.
Brain regions where maintenance-related activity was modulated by Condition (neutral, emotional) and State (RW, rested wakefulness; SD, sleep deprivation). Activation in these regions showed significant interactions of Condition by Time-on-trial (P < 0.001, corrected, Table 1). (dlPFC, dorsolateral prefrontal cortex; vlPFC, ventrolateral prefrontal cortex; LPC, lateral parietal cortex; LOC, lateral occipital cortex).
Increased left amygdala activity (averaging the signal at time points 12, 14, and 16 s post trial onset) was associated with greater emotional distractibility following sleep deprivation (SD). There was a decrease in activation within this ROI following SD, F1,23 = 4.55, P = 0.04, but this did not vary as a function of condition, F1,23 = 0.43, P = 0.52. (RW, rested wakefulness; SD, sleep deprivation).
There was a significant State (rested wakefulness, sleep deprivation) by Condition (neutral, emotional) by Time-on-task (first 5 runs, last 5 runs) by Time-on-trial interaction within the lateral occipital cortex. Post hoc State by Condition by Time-on-trial repeated-measures ANOVA indicated a significant interaction for activation in the first 5 runs, but not in the last 5 runs. (RW, rested wakefulness; SD, sleep deprivation).
REFERENCES
- 1.Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–63. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- 2.Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci U S A. 2005;102:2626–31. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith AP, Stephan KE, Rugg MD, Dolan RJ. Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron. 2006;49:631–8. doi: 10.1016/j.neuron.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 4.Hurlemann R, Hawellek B, Matusch A, et al. Noradrenergic modulation of emotion-induced forgetting and remembering. J Neurosci. 2005;25:6343–9. doi: 10.1523/JNEUROSCI.0228-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strange BA, Hurlemann R, Dolan RJ. An emotion-induced retrograde amnesia in humans is amygdala- and beta-adrenergic-dependent. Proc Natl Acad Sci U S A. 2003;100:13626–31. doi: 10.1073/pnas.1635116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahill L. Similar neural mechanisms for emotion-induced memory impairment and enhancement. Proc Natl Acad Sci U S A. 2003;100:13123–4. doi: 10.1073/pnas.2335833100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolcos F, Denkova E. Neural correlates of encoding emotional memories: a review of functional neuroimaging research. Cellscience. 2008;5:78–122. [Google Scholar]
- 8.LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- 9.Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9:585–94. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26:2072–9. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morey RA, Dolcos F, Petty CM, et al. The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. J Psychiatr Res. 2009;43:809–17. doi: 10.1016/j.jpsychires.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolcos F, Diaz-Granados P, Wang L, McCarthy G. Opposing influences of emotional and non-emotional distracters upon sustained prefrontal cortex activity during a delayed-response working memory task. Neuropsychologia. 2008;46:326–35. doi: 10.1016/j.neuropsychologia.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci U S A. 2002;99:11447–51. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell-McGinty S, Habeck C, Hilton HJ, et al. Identification and differential vulnerability of a neural network in sleep deprivation. Cereb Cortex. 2004;14:496–502. doi: 10.1093/cercor/bhh011. [DOI] [PubMed] [Google Scholar]
- 15.Chee MW, Chuah LY, Venkatraman V, Chan WY, Philip P, Dinges DF. Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: Correlations of fronto-parietal activation with performance. Neuroimage. 2006;31:419–28. doi: 10.1016/j.neuroimage.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Chee MW, Chuah YM. Functional neuroimaging and behavioral correlates of capacity decline in visual short-term memory after sleep deprivation. Proc Natl Acad Sci U S A. 2007;104:9487–92. doi: 10.1073/pnas.0610712104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chee MW, Tan JC, Zheng H, et al. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci. 2008;28:5519–28. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–68. [PubMed] [Google Scholar]
- 19.Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–7. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 20.Colten HR, Akltevogt BM. Sleep disorders and sleep deprivation: An unmet public health problem. Washington, D.C.: National Academies Press; 2006. [PubMed] [Google Scholar]
- 21.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 22.Orban P, Rauchs G, Balteau E, et al. Sleep after spatial learning promotes covert reorganization of brain activity. Proc Natl Acad Sci U S A. 2006;103:7124–9. doi: 10.1073/pnas.0510198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peigneux P, Laureys S, Fuchs S, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–45. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–92. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 25.Hu P, Stylos-Allan M, Walker MP. Sleep facilitates consolidation of emotional declarative memory. Psychol Sci. 2006;17:891–8. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- 26.Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep--a prefrontal amygdala disconnect. Curr Biol. 2007;17:R877–8. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9:148–58. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- 28.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 29.Phelps EA, Anderson AK. Emotional memory: what does the amygdala do? Curr Biol. 1997;7:R311–4. doi: 10.1016/s0960-9822(06)00146-1. [DOI] [PubMed] [Google Scholar]
- 30.Sterpenich V, Albouy G, Boly M, et al. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biol. 2007;5:e282. doi: 10.1371/journal.pbio.0050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spielberger CD. Manual for the State Trait Anxiety Inventory. Palo Alto, California: Consulting Psychologists Press; 1983. [Google Scholar]
- 32.Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule - Expanded Form. Iowa City: The University of Iowa; 1994. [Google Scholar]
- 33.Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 34.Graw P, Krauchi K, Knoblauch V, Wirz-Justice A, Cajochen C. Circadian and wake-dependent modulation of fastest and slowest reaction times during the psychomotor vigilance task. Physiol Behav. 2004;80:695–701. doi: 10.1016/j.physbeh.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system. Gainesville, FL: NIMH Center for the Study of Emotion and Attention; 1997. [Google Scholar]
- 36.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1994;2:189–210. [Google Scholar]
- 37.Anticevic A, Repovs G, Barch DM. Resisting emotional interference: Brain regions facilitating working memory performance. Cogn Affect Behav Neurosci in press. doi: 10.3758/CABN.10.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 39.Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–7. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 40.Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, Heller W. Cognitive control mechanisms, emotion and memory: A neural perspective with implications for psychopathology. Neurosci Biohav Rev. 2009;33:613–30. doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hermann A, Schafer A, Walter B, Stark R, Vaitl D, Schienle A. Emotion regulation in spider phobia: role of the medial prefrontal cortex. Soc Cogn Affect Neurosci. 2009;4:257–67. doi: 10.1093/scan/nsp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 45.Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whalen PJ, Bush G, McNally RJ, et al. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44:1219–28. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 47.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–12. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chee MW, Tan JC, Parimal S, Zagorodnov V. Sleep deprivation and its effects on object-selective attention. Neuroimage. 2010;49:1903–10. doi: 10.1016/j.neuroimage.2009.08.067. [DOI] [PubMed] [Google Scholar]
- 49.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A. 2009;106:912–7. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 51.Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E. Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci. 2006;26:9264–71. doi: 10.1523/JNEUROSCI.1016-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takashima A, Petersson KM, Rutters F, et al. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A. 2006;103:756–61. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pessoa L, Gutierrez E, Bandettini P, Ungerleider L. Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron. 2002;35:975–87. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- 54.Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci. 2002;5:479–84. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- 55.Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage. 2004;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 56.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 57.Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 58.Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proc Natl Acad Sci U S A. 2002;99:4115–20. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci U S A. 2002;99:11458–63. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dolcos F, Kragel P, Wang L, McCarthy G. Role of the inferior frontal cortex in coping with distracting emotions. Neuroreport. 2006;17:1591–4. doi: 10.1097/01.wnr.0000236860.24081.be. [DOI] [PubMed] [Google Scholar]
- 61.Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–91. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- 62.van Reekum CM, Johnstone T, Urry HL, et al. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage. 2007;36:1041–55. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Brain regions where maintenance-related activity was modulated by Condition (neutral, emotional) and State (RW, rested wakefulness; SD, sleep deprivation). Activation in these regions showed significant interactions of Condition by Time-on-trial (P < 0.001, corrected, Table 1). (dlPFC, dorsolateral prefrontal cortex; vlPFC, ventrolateral prefrontal cortex; LPC, lateral parietal cortex; LOC, lateral occipital cortex).
Increased left amygdala activity (averaging the signal at time points 12, 14, and 16 s post trial onset) was associated with greater emotional distractibility following sleep deprivation (SD). There was a decrease in activation within this ROI following SD, F1,23 = 4.55, P = 0.04, but this did not vary as a function of condition, F1,23 = 0.43, P = 0.52. (RW, rested wakefulness; SD, sleep deprivation).
There was a significant State (rested wakefulness, sleep deprivation) by Condition (neutral, emotional) by Time-on-task (first 5 runs, last 5 runs) by Time-on-trial interaction within the lateral occipital cortex. Post hoc State by Condition by Time-on-trial repeated-measures ANOVA indicated a significant interaction for activation in the first 5 runs, but not in the last 5 runs. (RW, rested wakefulness; SD, sleep deprivation).