Abstract
Study Objectives:
Sleep deprivation negatively affects memory consolidation, especially in the case of hippocampus-dependent memories. Studies in rodents have shown that 5 hours of sleep deprivation immediately following footshock exposure selectively impairs the formation of a contextual fear memory. In these studies, both acquisition and subsequent sleep deprivation were performed in the animals' main resting phase. However, in everyday life, subjects most often learn during their active phase.
Design:
Here we examined the effects of sleep deprivation on memory consolidation for contextual fear in rats when the task was performed at different times of the day, particularly, at the beginning of the resting phase or right before the onset of the active phase.
Measurements and Results:
Results show that sleep deprivation immediately following training affects consolidation of contextual fear, independent of time of training. However, in the resting phase memory consolidation was impaired by 6 hours of posttraining sleep deprivation, whereas, in the active phase, the impairment was only seen after 12 hours of sleep deprivation. Since rats sleep at least twice as much during the resting phase compared with the active phase, these data suggest that the effect of sleep deprivation depends on the amount of sleep that was lost. Also, control experiments show that effects of sleep deprivation were not related to the amount of stimulation the animals received and were therefore not likely an indirect effect of the sleep-deprivation method.
Conclusion:
These results support the notion that sleep immediately following acquisition, independent of time of day, promotes memory consolidation and that sleep deprivation may disrupt this process depending on the amount of sleep that is lost.
Citation:
Hagewoud R; Whitcomb SN; Heeringa AN; Havekes R; Koolhaas JM; Meerlo P. A time for learning and a time for sleep: the effect of sleep deprivation on contextual fear conditioning at different times of the day. SLEEP 2010;33(10):1315-1322.
Keywords: Sleep restriction, memory formation, hippocampus, fear conditioning, circadian rhythmicity, glucocorticoids
THERE IS GROWING EVIDENCE THAT SLEEP PLAYS A ROLE IN LEARNING AND MEMORY PROCESSES.1–5 A COMMON APPROACH TO STUDY THE ROLE OF SLEEP in learning and memory processes is the use of sleep deprivation. This approach is important not only from a fundamental perspective, but also from a social and clinical perspective, since disrupted and restricted sleep are major problems in our society.6,7 Numerous studies have shown that sleep deprivation after acquisition of a learning task has a negative effect on memory consolidation and subsequent performance in both humans8–11 and animals.12–19
A well-known learning task to study the role of sleep in memory and the effects of sleep deprivation in rodents is the fear-conditioning paradigm. In this task, animals learn to associate a specific context (the test environment) or a conditioned stimulus (for example, a tone cue) with an aversive unconditioned stimulus (footshock). When the animals are later exposed to the same context or cue, aversive learning is expressed, and animals will exhibit a fear-related freezing response.20,21 Both contextual and cued fear learning involve the amygdala. However, contextual fear learning also depends on the hippocampus.22–24 Studies in mice have examined the effect of 5 hours of sleep deprivation immediately following training in a fear-conditioning paradigm and have shown that sleep deprivation selectively impairs memory consolidation for contextual fear but not cued fear.14,19 This demonstrates that sleep deprivation has a negative effect on memory consolidation, particularly when it involves the hippocampus. This finding is in line with several other studies, using spatial and nonspatial versions of the Morris water maze, showing that sleep deprivation selectively affects consolidation of hippocampus-dependent spatial memory and does not affect performance in the hippocampus-independent nonspatial versions of the task.12,13,16 Altogether, these studies suggest that sleep plays a critical role in hippocampus functioning.
Importantly, the fear-conditioning studies in mice showed that the formation of contextual fear memory was disrupted by 5 hours of sleep deprivation immediately after the acquisition but not by delayed sleep deprivation (5-10 hours after acquisition).14 This finding that sleep deprivation immediately following training affects memory consolidation, whereas delayed sleep deprivation does not, has also been reported in several other studies with a variety of learning paradigms.12,13,15,17 It suggests there is a critical time window immediately following training during which memory consolidation is sensitive to sleep loss and indicates that the timing of sleep after learning might be important for memory consolidation.
Most experimental studies that have examined the role of sleep in memory consolidation performed the task near or in the main resting phase and sleep deprive the subjects immediately following training. However, in real life, subjects do not only learn right before they go to sleep, but often learn during their active phase. In the present study, we examined the effects of sleep deprivation on memory consolidation for contextual fear in rats, not only when the task was performed at the beginning of the resting (light) phase, but also right before the onset of the active (dark) phase. The nocturnal rat spends about 65% to 80% of the light phase asleep and about 20% to 35% of the dark phase.25–28 If, indeed, sleep plays a role in memory consolidation, how does it fulfill this role in case learning takes place at the start of the dark phase when it normally is followed by very little and often fragmented sleep?
METHODS
Animals and Housing Conditions
All experiments were performed with adult male Wistar rats (Harlan, Horst, The Netherlands), weighing 300 to 340 g at the start of the experiment. Animals were individually housed in standard macrolon cages (42.5 × 26 × 15.5 cm), and a layer of sawdust served as bedding. Food and water were provided ad libitum. Animals were maintained on a 12-hour light:12-hour dark cycle. Light intensity in the light phase was 45 lux. The dark phase consisted of dim red light conditions (1-2 lux). All procedures described in the present study were approved by the Animal Experiment Committee of the University of Groningen in compliance with Dutch law and regulations.
Experiment Set-up
Several experiments were performed to examine the effects of sleep deprivation on memory consolidation for contextual fear when training was performed at 2 different time points of the day. In the first experiment, we examined whether sleep deprivation affects memory consolidation when training is performed at the beginning of the light phase (i.e., resting phase) when rats sleep about 65% to 80% of the time.25–28 This first experiment was aimed to replicate earlier studies in mice showing that sleep deprivation adversely affects the formation of contextual fear memory.14,19 In the following experiments, we examined if and how sleep deprivation affects memory consolidation when training is performed immediately before the onset of the dark phase (i.e., active phase) when rats sleep only 20% to 35% of the time.25–28 In both conditions, the training and test sessions of the contextual fear-conditioning paradigm were carried out in the light (45 lux), either at the beginning of the light phase or at the end, right before the onset of the dark phase. In the second case, we chose to perform the test at the end of the light phase rather than the beginning of the dark phase to avoid differences in light conditions as an additional factor that might affect the strength of the conditioning. Testing was always performed 24 hours after training. In all experiments, 2 groups of animals were used, a group subjected to 6 or 12 hours of sleep deprivation after training and a non-sleep-deprived control group.
Contextual Fear Conditioning
One week prior to the start of the fear-conditioning experiments, all animals were handled daily. Contextual fear conditioning was performed in a black quadrilateral Plexiglas chamber (40 × 40 × 40 cm), which was located in a separate experiment room. Background noise (60 dB) was present in the room. During training, an animal was placed in the chamber and exposed to the conditioning context for 3 minutes followed by a mild electric footshock (0.7 mA, 2 sec) delivered through a stainless-steel grid floor. The animal was removed from the chamber and returned to its home cage 30 seconds after the shock. The chamber was thoroughly cleaned with 70% ethanol between subjects. Twenty-four hours later, the animal was placed in the same chamber for 5 minutes without shock presentation. Contextual memory was tested by assessing freezing behavior, defined as complete lack of movement except for respiration. Behavior was recorded on videotapes, which were analyzed afterward by an experimenter who was unaware of the treatment of the animals. The amount of time the animals displayed freezing behavior was expressed as a percentage of the total test time.
Sleep Deprivation
Animals were sleep deprived after training for either 6 hours during the light or for 6 or 12 hours during the dark phase. Sleep deprivation was accomplished by mild stimulation, which involved tapping on the cage, gently shaking the cage, or, when this was not sufficient to keep the animals awake, disturbing the sleeping nest.29,30 Previous studies have shown that this procedure is effective in keeping rodents awake for several hours, as established by electroencephalographic recordings,31 without being a major stressor.29,30,32
Plasma Corticosterone Levels
To assess effects of sleep deprivation by mild stimulation on plasma levels of the stress hormone corticosterone under the current experimental conditions, separate groups of animals were sleep deprived for either 6 hours in the light phase or 6 or 12 hours in the dark phase; the results were compared with home-cage control animals. Blood samples were taken from the tail at the end of the sleep-deprivation period and collected in precooled plastic centrifuge tubes containing 0.01% ethylenediaminetetraacetic acid (EDTA) as anticoagulant and antioxidant. Blood was centrifuged at 4°C for 15 minutes at 2600 g, and plasma was stored at −80°C until further processing. Corticosterone levels were determined by radioimmunoassay (MP Biomedicals, Orangeburg, NY).
Statistical analysis
Behavioral freezing responses and plasma levels of corticosterone in all experiments were analyzed using an independent-samples t test. The total number of stimulations per hour needed to keep animals awake was analyzed using a repeated-measures analysis of variance with a between-subject factor Treatment (6-h sleep deprivation light/6-h sleep deprivation dark) and a within-subject factor Time (1-h blocks). Data are expressed as mean ± SEM in all figures, and P < 0.05 was considered as significant.
RESULTS
Experiment 1: Training and 6 Hours of Sleep Deprivation in the Light Phase
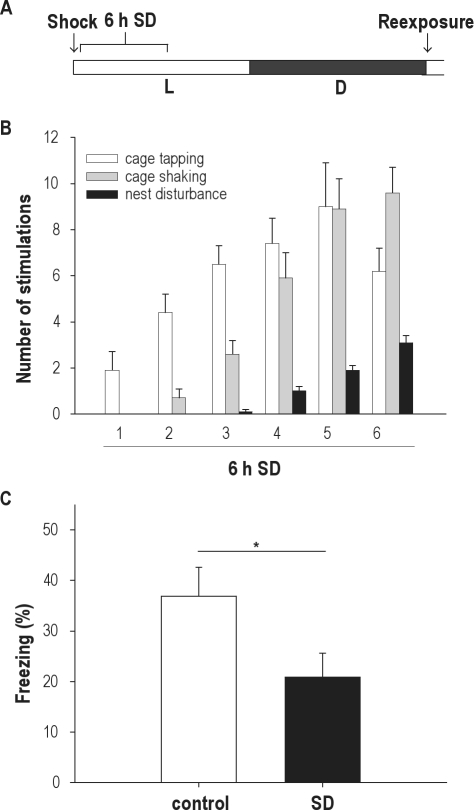
The first experiment examined whether 6 hours of sleep deprivation immediately following contextual fear conditioning at the beginning of the light phase would affect consolidation of contextual fear memory in rats (Figure 1A). The number of stimulations that was needed to keep the animals awake during the 6-hour sleep-deprivation period is shown in Figure 1B. Initially, the animals required little stimulation and spent most of their time exploring their cage. The number of stimulations needed to keep the animals awake gradually increased during ongoing sleep deprivation (F5,45 = 31,273, P < 0.001), suggesting an increased drive for sleep. Rats that were sleep deprived for 6 hours immediately following training showed reduced freezing upon reexposure to the shock box 24 hours after training, compared with control animals (20.8% ± 4.8% vs 36.9% ± 5.7%, respectively; n = 10 in both groups; t18 = 2.165, P = 0.044; Figure 1C). The results are thus in agreement with previous studies in mice showing impairments of fear memory when sleep deprivation is performed immediately after training in the light phase.14,19
Figure 1.
(A) Scheme of the contextual fear-conditioning paradigm in Experiment 1 with training at the beginning of the resting (light) phase. Half of the animals were subjected to sleep deprivation (SD) for 6 h immediately after the training; 24 h after training, all animals were tested for contextual fear during a 5-min test phase. (B) Mean number and type of stimulations needed to keep the animals awake for 6 h during the first half of the light phase. The number of stimulations needed to keep the animals awake gradually increased in the course of the SD period, suggesting an increased drive for sleep. (C) Animals sleep deprived for 6 h immediately following training (n = 10) displayed significantly less freezing behavior in response to the shocked context than did control animals (n = 10). Data are expressed as mean ± SEM. *P < 0.05.
Experiment 2: Training and 6 Hours of Sleep Deprivation During the Dark Phase
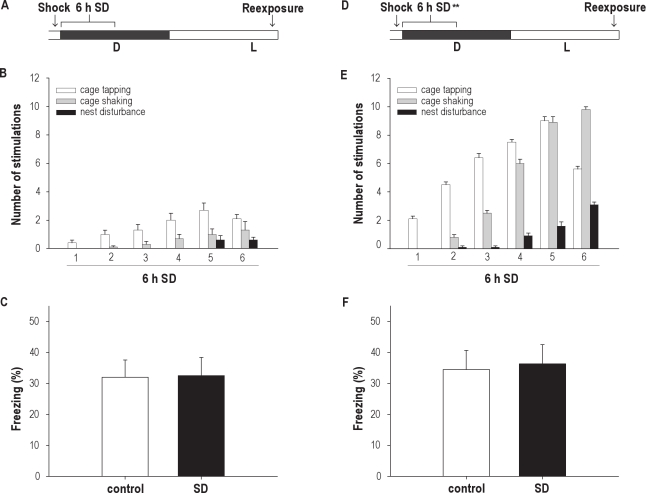
Experiment 2 addressed the question whether the memory-impairing effect of sleep deprivation is independent of the actual time of training. Animals were exposed to a footshock right before the onset of the dark phase, and half of the animals were sleep deprived for 6 hours immediately afterward (Figure 2A). Since rats spontaneously sleep far less during the first 6 hours of the dark phase, in comparison with the first 6 hours of the light phase, significantly fewer stimulations were needed to keep the animals awake in this experiment, compared with the first experiment (compare Figure 2B with Figure 1B; effect of treatment and interaction effect: F1,18 = 185.060, F5,90 = 17.143, P < 0.001 in both cases). Upon reexposure to the shock box 24 hours after training, the animals subjected to 6 hours of sleep deprivation immediately following training showed a freezing response similar to that of control animals (32.5% ± 5.9% vs 32.0% ± 5.6%, respectively; n = 10 in both groups; t18 = −0.051, P > 0.9; Figure 2C). The data suggest that 6 hours of sleep deprivation immediately following training does not negatively affect memory consolidation for contextual fear conditioning performed right before the onset of the dark phase.
Figure 2.
(A and D) Scheme of the contextual fear conditioning paradigm in Experiment 2 and 3 with training right before the onset of the active (dark) phase. (A) In Experiment 2, half of the animals were subjected to sleep deprivation (SD) for 6 h immediately following training; 24 h after training, animals were tested for contextual fear during a 5-min test phase. (B) The mean number and type of stimulations needed to keep the animals awake during the first half of the dark phase. (C) SD animals (n = 10) did not differ in the amount of freezing in response to the shocked context, compared with control animals (n = 10). (D) In Experiment 3, half of the animals were subjected to SD for 6 h immediately following training but, in this case, with the same amount and type of stimulation as needed to keep animals awake for 6 h during the light phase; 24 h after training, animals were tested for contextual fear during a 5-min test phase. (E) Number and type of stimulations used during the 6-h SD period (matched with the number and type of stimulations given during the 6 h of SD in the light phase, as shown in Figure 1B). (F) SD animals (n = 10) did not differ in the amount of freezing in response to the shocked context, compared with control animals (n = 10). Data are expressed as mean ± SEM. **Indicates SD period with increased number of stimulations.
Experiment 3: Training and 6 Hours of Sleep Deprivation With High-Intensity Stimulation During the Dark Phase
One possible explanation for the finding that 6 hours of sleep deprivation during the light phase in Experiment 1 impaired memory formation, whereas 6 hours of sleep deprivation during the dark phase in Experiment 2 did not, is the fact that animals that are sleep deprived during the light phase received far more stimulations to keep them awake. In other words, the memory impairment may have been, in part, a consequence of the interfering stimulation rather than sleep loss per se. To test this possibility, we repeated the preceding experiment with 6 hours of sleep deprivation in the dark phase (Figure 2D); however, we now subjected the animals to the same number of stimulations that was needed to keep animals awake for a 6-hour period at the beginning of the light phase (compare Figure 2E with Figure 1B). The results show that memory for contextual fear was not affected by a higher number of stimulations. Upon reexposure to the shock box the next day, animals subjected to 6 hours of sleep deprivation during the dark phase with high-intensity stimulation displayed amounts of freezing similar to that of control animals (36.3% ± 6.2% vs 34.5% ± 6.1%, respectively; n = 10 in both groups; t18 = −0.206, P > 0.8; Figure 2F). These results suggest that the sleep deprivation-induced effects during the light phase in the first experiment were not likely to be due to the number of stimulations animals received during the sleep-deprivation procedure. If the effect was a consequence of the amount of stimulation, we would have expected to have seen an effect of it during the dark phase as well.
Experiment 4: Training and 12 Hours of Sleep Deprivation During the Dark Phase
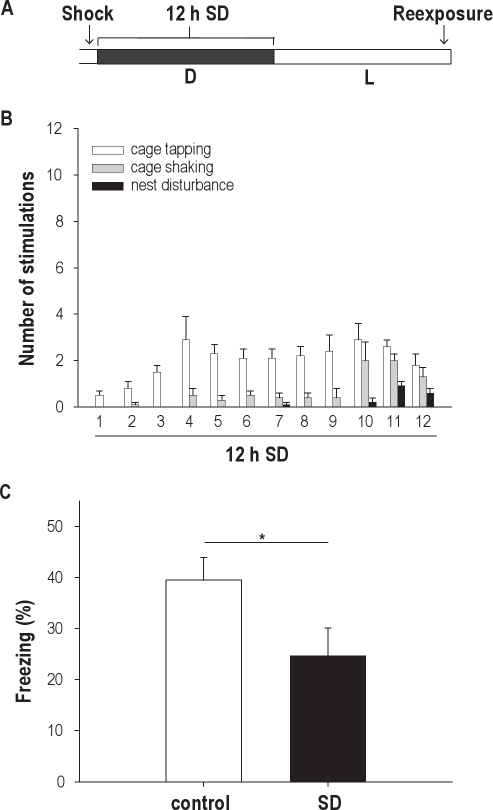
An alternative explanation for the finding that 6 hours of sleep deprivation during the dark phase failed to affect the formation of fear memory might be the fact that the actual sleep loss was far less, as compared with the amount lost with 6 hours of sleep deprivation during the light phase. To induce a similar amount of sleep loss as during the 6-hour period in the light phase, animals should be sleep deprived over a longer period. For this reason, we performed an experiment in which a group of animals was sleep deprived immediately following fear conditioning during the entire dark phase (12-h sleep deprivation; Figure 3A). Results show that animals sleep deprived from 0 to 12 hours after training, on average, showed less freezing behavior in the shocked context, compared with control animals. However, due to large variation in the sleep-deprivation group, this trend did not reach statistical significance (sleep-deprived animals, 25.0% ± 6.9%; Control, 43.5% ± 6.1%; n = 10 in both groups; t18 = 1.766, P = 0.09). We therefore repeated the experiment to increase the sample size, and the combined data indeed show that 12 hours of sleep deprivation immediately following training, during the complete dark phase, results in significantly less freezing behavior, compared with control animals (24.6% ± 5.5% vs 39.5% ± 4.4% with n = 20 and n = 19, respectively; t37 = 2.137, P = 0.039, Figure 3C). The number of stimulations needed to keep the animals awake over the 12-hour period in the dark phase is shown in Figure 3B. Despite the longer period of sleep deprivation, the total number of stimulations that was required to keep the animals awake for the 12-hour dark phase was still lower (33.7 ± 2.0) than during the 6 hours of sleep deprivation at high-stimulus intensity (68.9 ± 0.8) in the previous experiment. Yet, only the 12-hour sleep-deprivation period in the dark phase affected contextual fear memory, which suggests that this effect is not dependent on the amount of stimulation but, rather, on the total amount of sleep that was lost.
Figure 3.
(A) Scheme of the contextual fear-conditioning paradigm in Experiment 4 with training right before the onset of the active (dark) phase. Half of the animals were subjected to sleep deprivation (SD) for 12 h immediately following training; 24 h after training, animals were tested for contextual fear during a 5-min test phase. (B) Mean number and type of stimulations needed to keep the animals awake for 12 h during the entire dark phase. (C) Animals sleep deprived for 12 h (n = 20) during the dark phase displayed significantly less freezing behavior in response to the shocked context than did control animals (n = 19). Data are expressed as mean ± SEM. *P < 0.05.
Since 12 hours of sleep deprivation covering the entire dark phase impaired consolidation of contextual fear memory, whereas 6 hours of sleep deprivation during the first half of the dark phase did not, it might, in theory, be that the effect of the 12-hour sleep deprivation was caused by sleep deprivation during the second half of the dark phase. Although this seems unlikely, we nevertheless performed an additional experiment to exclude this possibility. We subjected a new batch of animals to the fear-conditioning paradigm right before the onset of the dark phase and subjected half of them to a delayed 6-hour sleep-deprivation (6-12 h) period. No significant differences were found in freezing response between sleep-deprived animals and control animals (35.2% ± 5.8% vs 30.0% ± 4.9%, respectively; n = 10 in both groups; t18 = 0.690, P > 0.4). Thus, this confirms that, with training near the start of the dark phase, consolidation of fear memory is only disrupted with 12 hours of sleep deprivation.
Experiment 5: Sleep Deprivation and Plasma Corticosterone Levels
It is often suggested that the effects of sleep deprivation on learning and memory processes in animals may be related to stress induced by the sleep-deprivation procedure rather than to sleep loss per se. To examine whether sleep deprivation by our mild sensory-stimulation method increases plasma corticosterone levels, we performed an experiment in which separate groups of animals were sleep deprived for 6 hours in the light phase or for 6 or 12 hours in the dark phase and compared these results with those of home-cage control animals. At the end of the sleep-deprivation period, blood was collected. Results show that 6 hours of sleep deprivation in the light phase had no effect on plasma corticosterone levels (animals subjected to 6 hours of sleep deprivation light: 3.7 ± 0.4 μg/dL vs control animals: 4.2 ± 0.6 μg/dL; n = 10 and n = 9 respectively; t17 = 0.627, P > 0.5), nor did 6 hours or 12 hours of sleep deprivation in the dark phase (6-h sleep-deprivation-dark: 8.8 ± 0.9 μg/dL vs control animals: 7.9 ± 0.9 μg/dL with n = 10 in each group; 12-h sleep-deprivation-dark: 4.3 ± 1.1 μg/dL vs control animals: 2.5 ± 1.0 μg/dL with n = 10 in each group; P > 0.4 in both cases). These data show that our sleep-deprivation method by mild stimulation, independent of time of day and sleep-deprivation length, does not lead to persistent increases in plasma levels of corticosterone.
DISCUSSION
Previous studies in mice have shown that sleep selectively promotes the formation of contextual fear memory and that sleep deprivation immediately after acquisition selectively impairs the formation of this memory.14,19,33 We continued on from these findings and examined the effect of sleep deprivation in rats after training in a contextual fear-conditioning paradigm at different times of the day, particularly the beginning of the resting phase or right before the onset of the active phase. We show that 6 hours of sleep deprivation immediately following training in the light phase, the main resting phase, impairs consolidation of contextual fear memory in rats. Memory for contextual fear conditioning performed right before the onset of the dark phase was affected when training was immediately followed by 12 hours of sleep deprivation in the dark phase, but not by 6 hours of sleep deprivation.
Our finding that 6 hours of sleep deprivation after training during the dark phase had no effect on memory, in contrast with 6 hours of sleep deprivation after training during the light phase, is in line with the results of other recent studies showing that 6 hours of sleep deprivation immediately following training in a novel object-recognition task near or in the beginning of the dark phase does not affect recognition memory in mice and rats.34,35 These studies suggested that sleep immediately following novel object-recognition training is not required for the memory per se. However, in the present study, we showed that a longer period of sleep deprivation following training does negatively affect memory consolidation. Since rats only sleep about 20% to 35% of the time during the dark phase, compared with 65% to 80% of the time during the light phase,25–28 the amount of sleep we deprived the animals of during the 6 hours of sleep deprivation period in the dark phase is far less than the 6 hours of sleep deprivation during the light phase. Thus, the different effects on memory consolidation may have been due to the different amount of sleep that was lost. Importantly, a delayed 6 hours of sleep deprivation during the second half of the active phase did not affect memory, showing that a full 12-hour sleep-deprivation period immediately following training during the dark phase is necessary to impair memory. Together, these findings suggest that not only the timing of sleep deprivation after training, but also the duration of sleep deprivation, is important for an effect on memory.
It is still a matter of debate whether sleep plays an active role in memory consolidation or merely a passive role by preventing waking interference.4 Waking interference and disruption of ongoing memory consolidation might result from mental activity related to sensory input and processing of new information. Particularly in animal studies on sleep and memory, which often rely on forced sleep deprivation, it is often argued that interference might occur as a consequence of the stimulations required to keep the animal awake. In our study, we considered this possibility; however, our findings are not in line with this view. Memory consolidation during the light phase (i.e., main resting phase) was disrupted by a 6-hour period of mild sensory stimulation, whereas memory formation during the dark phase (i.e., active phase) was not disrupted, even when the amount of stimulation was exactly matched. Also, memory consolidation in the dark phase was only significantly affected by a much longer period of 12 hours of mild stimulation even though, in that case, the total amount of stimulation was still lower than the amount of stimulation used during 6 hours of sleep deprivation in the light phase. Importantly, the 6 hours of sleep deprivation in the light phase and 12 hours of sleep deprivation in the dark phase are associated with comparable amounts of sleep loss. Thus, the disruption of memory formation is perhaps better explained by the amount of sleep that was lost than by the amount of stimulation the animals received.
Along the lines of the waking-interference concept, stress is another commonly proposed factor to mediate the effects of sleep deprivation. However, it is unlikely that stress induced by our sleep-deprivation method caused impairments in memory. First of all, one might expect that the strongest stimulation would lead to the greatest impairment, which, as we discussed in the previous paragraph, was not the case. Furthermore, we show that plasma levels of the stress hormone corticosterone after sleep deprivation in the resting and active phase were low and not different from those of control animals. This is in line with the results of previous studies using the same sleep-deprivation method of mild stimulation, showing no significant elevations in stress hormone levels.29,30 In fact, these levels are in the same range or even lower than after spontaneous waking activities such as feeding and grooming.36 In addition, contrary to the view that stress has adverse effects on learning and memory processes, it is well known that glucocorticoids contribute to contextual fear conditioning in a positive way. Indeed, administration of glucocorticoid-receptor antagonists immediately before or after training, as well as adrenalectomy, impairs the formation of contextual fear memory,37,38 whereas administration of glucocorticoids immediately following fear conditioning facilitates the formation of contextual fear.39 Therefore, if the sleep-deprivation–induced effect had been due to a small acute increase of glucocorticoids instead of sleep loss per se, we would likely have found results opposite of the present findings.
The mechanisms through which sleep deprivation affects hippocampus function and memory consolidation are largely unknown. Several studies have indicated discrete periods of time after training for fear conditioning that are sensitive to inhibitors of protein kinase A (PKA) and protein synthesis.40–42 For example, administration of a PKA inhibitor immediately after or 4 hours after training impairs the formation of contextual fear memory.41 Since it has been shown that sleep deprivation during a similar time period after training disrupts memory for contextual fear, it is suggested that sleep deprivation might act on memory consolidation via these mechanisms.14 Indeed, a recent study in mice showed that sleep deprivation selectively impairs hippocampal 3′, 5′- cyclic AMP (cAMP)- and PKA-signaling.19 By affecting these pathways, sleep deprivation may alter the activity of transcription factors and expression of genes involved in synaptic plasticity43–45 and, ultimately, influence memory storage.19 Thus, the molecular mechanism through which sleep deprivation following training in the light phase may affect memory is now partly unraveled. Here we show that sleep deprivation immediately following training near the onset of the dark or active phase impairs contextual fear memory as well. To our knowledge, the time course of different signal transduction pathways underlying memory consolidation when training occurs near or in the active phase and the time periods of sensitivity to inhibitors of protein synthesis and PKA are unknown. Therefore, in future research, it would be of great interest to identify the molecular processes underlying the role of sleep in memory consolidation for training performed in the active phase.
Our data suggest that, in rats, sleep immediately following training, independent of time of day, is involved in memory consolidation. This leaves us with the intriguing question of how this works in humans, who generally have a fairly monophasic sleep pattern with little or no sleep during the active phase. How do sleep and sleep deprivation affect memory consolidation when training is performed early in the active phase and is not immediately followed by a substantial amount of sleep? Could it be that some forms of learning in humans are less effective when acquisition takes place early in the active phase because it often is not followed by sleep? Indeed, in line with this thought, it has been shown that napping during the day, after learning during the first half of the day, improves memory compared with no napping.46–49 Further studies will be needed to unravel mechanisms of sleep-dependent memory formation and to address the question of optimal timing for learning and sleep.
In summary, our experiments in rats show that sleep deprivation immediately following acquisition impairs the consolidation of contextual fear memory independent of time of training. The data further suggest that the deficit in memory depends on the amount of sleep that is lost after training. A 6-hour sleep-deprivation period immediately after training impairs memory consolidation for contextual fear conditioning in the resting phase. However, with training right before the onset of the active phase, a 12-hour sleep-deprivation period—but not a 6-hour sleep-deprivation period—results in impairment in contextual fear memory. We conclude that both timing and quantity of sleep after learning may be important in the process of memory consolidation in rats.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank Dr. Ted Abel for fruitful discussions in the course of this project. We also thank Michele Azzolini, Lilly Bultsma, Timur Cetin, Charlotte Demkes, Wanda Douwenga, Martijn de Groot, Maarten Lahr, Arianna Novati, and Bert Venema for their help with the sleep deprivation and Jan Bruggink for the corticosterone analysis. This work was supported by The Netherlands Organization for Scientific Research (NWO Vidi grant 84.04.002 to Peter Meerlo).
Footnotes
A commentary on this article appears in this issue on page 1277.
REFERENCES
- 1.Graves L, Pack A, Abel T. Sleep and memory: a molecular perspective. Trends Neurosci. 2001;24:237–43. doi: 10.1016/s0166-2236(00)01744-6. [DOI] [PubMed] [Google Scholar]
- 2.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–52. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 3.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 4.Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Curr Biol. 2006;16:1290–4. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–66. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet MH, Arand DL. We are chronically sleep deprived. Sleep. 1995;18:908–11. doi: 10.1093/sleep/18.10.908. [DOI] [PubMed] [Google Scholar]
- 7.Rajaratnam SM, Arendt J. Health in a 24-h society. Lancet. 2001;358:999–1005. doi: 10.1016/S0140-6736(01)06108-6. [DOI] [PubMed] [Google Scholar]
- 8.Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–82. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 9.Stickgold R, James L, Hobson JA. Visual discrimination learning requires sleep after training. Nat Neurosci. 2000;3:1237–8. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara M, Iaria G, De GL, et al. The role of sleep in the consolidation of route learning in humans: a behavioural study. Brain Res Bull. 2006;71:4–9. doi: 10.1016/j.brainresbull.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Mograss MA, Guillem F, Brazzini-Poisson V, Godbout R. The effects of total sleep deprivation on recognition memory processes: a study of event-related potential. Neurobiol Learn Mem. 2009;91:343–52. doi: 10.1016/j.nlm.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Smith C, Rose GM. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol Behav. 1996;59:93–7. doi: 10.1016/0031-9384(95)02054-3. [DOI] [PubMed] [Google Scholar]
- 13.Smith C, Rose GM. Posttraining paradoxical sleep in rats is increased after spatial learning in the Morris water maze. Behav Neurosci. 1997;111:1197–204. doi: 10.1037//0735-7044.111.6.1197. [DOI] [PubMed] [Google Scholar]
- 14.Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–76. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjorness TE, Riley BT, Tysor MK, Poe GR. REM restriction persistently alters strategy used to solve a spatial task. Learn Mem. 2005;12:352–9. doi: 10.1101/lm.84805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hairston IS, Little MT, Scanlon MD, et al. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–33. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- 17.Palchykova S, Winsky-Sommerer R, Meerlo P, Durr R, Tobler I. Sleep deprivation impairs object recognition in mice. Neurobiol Learn Mem. 2006;85:263–71. doi: 10.1016/j.nlm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Alvarenga TA, Patti CL, Andersen ML, et al. Paradoxical sleep deprivation impairs acquisition, consolidation, and retrieval of a discriminative avoidance task in rats. Neurobiol Learn Mem. 2008;90:624–32. doi: 10.1016/j.nlm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Vecsey CG, Baillie GS, Jaganath D, et al. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461:1122–5. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–5. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 21.Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–82. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 22.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 23.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Kim JJ, Thompson RF, Tonegawa S. Hippocampal lesions impair contextual fear conditioning in two strains of mice. Behav Neurosci. 1996;110:1177–80. doi: 10.1037//0735-7044.110.5.1177. [DOI] [PubMed] [Google Scholar]
- 25.Borbély AA, Neuhaus HU. Sleep-deprivation: effects on sleep and EEG in the rat. J Comp Physiol. 1979;133:71–87. [Google Scholar]
- 26.Lancel M, Kerkhof GA. Effects of repeated sleep deprivation in the dark- or light-period on sleep in rats. Physiol Behav. 1989;45:289–97. doi: 10.1016/0031-9384(89)90130-3. [DOI] [PubMed] [Google Scholar]
- 27.Franken P, Dijk DJ, Tobler I, Borbely AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–R208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 28.Tang X, Yang L, Sanford LD. Individual variation in sleep and motor activity in rats. Behav Brain Res. 2007;180:62–8. doi: 10.1016/j.bbr.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van der Borght K, Ferrari F, Klauke K, et al. Hippocampal cell proliferation across the day: increase by running wheel activity, but no effect of sleep and wakefulness. Behav Brain Res. 2006;167:36–41. doi: 10.1016/j.bbr.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Hagewoud R, Havekes R, Novati A, Keijser JN, Van der Zee EA, Meerlo P. Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J Sleep Res. 2010;19:280–8. doi: 10.1111/j.1365-2869.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- 31.Meerlo P, de Bruin EA, Strijkstra AM, Daan S. A social conflict increases EEG slow-wave activity during subsequent sleep. Physiol Behav. 2001;73:331–5. doi: 10.1016/s0031-9384(01)00451-6. [DOI] [PubMed] [Google Scholar]
- 32.Meerlo P, Turek FW. Effects of social stimuli on sleep in mice: non-rapid-eye-movement (NREM) sleep is promoted by aggressive interaction but not by sexual interaction. Brain Res. 2001;907:84–92. doi: 10.1016/s0006-8993(01)02603-8. [DOI] [PubMed] [Google Scholar]
- 33.Cai DJ, Shuman T, Gorman MR, Sage JR, Anagnostaras SG. Sleep selectively enhances hippocampus-dependent memory in mice. Behav Neurosci. 2009;123:713–9. doi: 10.1037/a0016415. [DOI] [PubMed] [Google Scholar]
- 34.Halassa MM, Florian C, Fellin T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–9. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palchykova S, Winsky-Sommerer R, Tobler I. Sleep deprivation in the dark period does not impair memory in OF1 mice. Chronobiol Int. 2009;26:682–96. doi: 10.1080/07420520902926025. [DOI] [PubMed] [Google Scholar]
- 36.Shiraishi I, Honma K, Honma S, Hiroshige T. Ethosecretogram: relation of behavior to plasma corticosterone in freely moving rats. Am J Physiol. 1984;247:R40–R45. doi: 10.1152/ajpregu.1984.247.1.R40. [DOI] [PubMed] [Google Scholar]
- 37.Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav Neurosci. 1997;111:503–11. [PubMed] [Google Scholar]
- 38.Pugh CR, Fleshner M, Rudy JW. Type II glucocorticoid receptor antagonists impair contextual but not auditory-cue fear conditioning in juvenile rats. Neurobiol Learn Mem. 1997;67:75–9. doi: 10.1006/nlme.1996.3741. [DOI] [PubMed] [Google Scholar]
- 39.Abrari K, Rashidy-Pour A, Semnanian S, Fathollahi Y. Post-training administration of corticosterone enhances consolidation of contextual fear memory and hippocampal long-term potentiation in rats. Neurobiol Learn Mem. 2009;91:260–5. doi: 10.1016/j.nlm.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Bernabeu R, Bevilaqua L, Ardenghi P, et al. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci U S A. 1997;94:7041–6. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–74. [PMC free article] [PubMed] [Google Scholar]
- 42.Wallenstein GV, Vago DR, Walberer AM. Time-dependent involvement of PKA/PKC in contextual memory consolidation. Behav Brain Res. 2002;133:159–64. doi: 10.1016/s0166-4328(01)00476-4. [DOI] [PubMed] [Google Scholar]
- 43.Guzman-Marin R, Ying Z, Suntsova N, Methippara M, Bashir T, Szymusiak R, Gomez-Pinilla F, McGinty D. Suppression of hippocampal plasticity-related gene expression by sleep deprivation in rats. J Physiol. 2006;575:807–19. doi: 10.1113/jphysiol.2006.115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ribeiro S, Goyal V, Mello CV, Pavlides C. Brain gene expression during REM sleep depends on prior waking experience. Learn Mem. 1999;6:500–8. doi: 10.1101/lm.6.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribeiro S, Mello CV, Velho T, Gardner TJ, Jarvis ED, Pavlides C. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. J Neurosci. 2002;22:10914–23. doi: 10.1523/JNEUROSCI.22-24-10914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006;86:241–7. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lahl O, Wispel C, Willigens B, Pietrowsky R. An ultra short episode of sleep is sufficient to promote declarative memory performance. J Sleep Res. 2008;17:3–10. doi: 10.1111/j.1365-2869.2008.00622.x. [DOI] [PubMed] [Google Scholar]
- 49.Mednick SC, Cai DJ, Kanady J, Drummond SP. Comparing the benefits of caffeine, naps and placebo on verbal, motor and perceptual memory. Behav Brain Res. 2008;193:79–86. doi: 10.1016/j.bbr.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]