Abstract
Study Objectives:
Because the maternal environment can affect several physiological functions of the newborn, the aim of the present study was to examine the impact of sleep restriction during pregnancy on renal morphology and function in young offspring.
Design:
Female 3-month-old Wistar rats were divided in 2 experimental groups: C (control) and SR (sleep restriction between the 14th and 20th day of pregnancy). Pregnancy was confirmed by vaginal smear. SR females were subjected to sleep restriction by the multiple platform technique for 20 h daily. After birth, only male litters (6 for each mother) were selected and designated OC (offspring from C) and OSR (offspring from SR). At 2 months of age, blood pressure (BP) was measured by tail plethysmography; at 3 months the renal plasma flow (RPF), glomerular filtration rate (GFR), glomerular area, and number of glomeruli per mm3 were evaluated.
Measurements and Results:
Offspring from SR had higher systolic blood pressure than OC. In this group (OSR), we also observed significant increase in RPF and GFR, enlarged glomeruli diameter, and reduced number of glomeruli per mm3 of renal tissue.
Conclusions:
Our data suggest that sleep restriction during pregnancy is able to modify renal development, resulting in morphologic and functional alterations in young offspring.
Citation:
Thomal JT; Palma BD; Ponzio BF; Franco MCP; Zaladek-Gil F; Fortes ZB; Tufik S; Gomes GN. Sleep restriction during pregnancy: hypertension and renal abnormalities in young offspring rats. SLEEP 2010;33(10):1357-1362.
Keywords: Sleep, renal function, glomerular hypertrophy, hypertension, fetal programming
ALTERATIONS IN THE MATERNAL ENVIRONMENT CAN AFFECT EMBRYONIC AND FETAL LIFE, PREDISPOSING AN INDIVIDUAL TO CARDIOVASCULAR, metabolic, and endocrine diseases in adult life. This has been known as perinatal programming (or the Barker hypothesis)1,2 and has been demonstrated both in epidemiologic and experimental research.3,4 Studies from our laboratory have shown that alterations in the maternal environment, such as undernutrition and diabetes mellitus, are capable of causing renal morphologic and functional impairment, as well as hypertension in the offspring.5–8
Franco et al.9 demonstrated that nutritional restriction during pregnancy is able to promote renal impairment and hypertension associated with endothelial dysfunction in the offspring. Similarly, Rocha et al.7 showed that diabetes mellitus during pregnancy in addition to functional and morphologic alterations in kidneys from the offspring led to the development of hypertension in the animals after 2 months of age.
Numerous studies suggest that sleep deprivation disrupts vital biological processes necessary for physical health and leads to problems such as reduced glucose tolerance,10 increased blood pressure,11,12 activation of the sympathetic nervous system,10 and reduced leptin levels.13 Some sleep disorders have been associated with a larger body mass index (BMI) and the prevalence of obesity.11,14 Recent data from the National Sleep Foundation have suggested that women are more susceptible to biological and lifestyle factors than men.15 During pregnancy, there are hormonal, physiological, and behavioral changes that may affect the sleep pattern and duration, increasing the risk for developing sleep disorders such as insomnia,16 restless legs syndrome, and breathing disorders (snoring and obstructive sleep apnea).17,18 Although sleep restriction has achieved much attention in the last decade, there are few studies evaluating the effect of sleep debt during pregnancy on fetal development. Sleep deprivation as a stress stimulus in pregnancy has been shown to alter maternal and fetal outcomes19 and also promote changes in offspring during adulthood such as emotional response, sexual behavior and biochemical alterations,20–22 but the impact of this sleep debt on renal development remains unclear.
The aim of this study was to examine the impact of sleep restriction during pregnancy on renal morphology and function in young offspring rats.
MATERIALS AND METHODS
All experiments in this study were approved by the Ethical Research Committee of the Universidade Federal de São Paulo - UNIFESP (1627/07) and followed international guidelines for the care of research animals.
Experimental Groups
Pregnant female rats
For the study, we used 3-month-old female and male Wistar rats from our colony, weighing 200-250 g and 300-350 g, respectively. The virgin females in proestrus were caged overnight with a male, and vaginal smears were taken the following morning. A positive smear was subsequently defined as day 0 of gestation. The pregnant females were divided in 2 groups: C (control mothers, n = 12) and SR (sleep restriction mothers, n = 12). Both groups had free access to food and water for all the experimental period. The animals were maintained in a room with controlled temperature and humidity (21 ± 2°C, 60%) on a 12:12 h light/dark cycle with lights on at 07:00, until the 12th day of pregnancy. After this period, they were transferred to a sleep restriction room, under the same conditions. During pregnancy, body weight of C and SR animals was measured once a week.
Sleep restriction
The sleep restriction technique was based on the muscle atonia that accompanies paradoxical sleep.23 Briefly, 18 narrow circular platforms (6.5 cm in diameter), were placed inside a tiled tank (123 x 44 x 44 cm) filled with water to within 1 cm of the upper border of the platform. The SR animals (n = 12) were placed on the platforms (exceeding number) in an arrangement that allowed them to move inside the tank, jumping from one platform to the other. Two days before the beginning of the study, the animals were adapted to the water tank for a period of 1 h to avoid unnecessary drops in the water. On the 14th day of pregnancy, the SR animals were placed into the tank at 14:00. On the following day, at 10:00, they were placed back in their home cages where they could sleep freely, with free access to food and water. This procedure was repeated until the 20th day of pregnancy. At 10:00 on the 20th day of pregnancy, the rats were placed back in their home cages. All groups had free access to food and water throughout the study. The animals of C group remained in their home cages (4 animals/ cage) until the 20th day of pregnancy in the same room where deprivation took place. Six animals from each group were randomly chosen and sacrificed by decapitation on the 20th day of pregnancy (corresponding to the end of sleep restriction) for analysis of adrenal weight. The remainder was kept in their home cages for spontaneous parturition and weaning of the offspring.
Offspring Groups
After birth, the litters, consisting of 6 male rats, were left with the mother for 28 days. After weaning, the rats were placed in individual cages and were divided into 2 groups: (OC) offspring from control mothers; (OSR) offspring from sleep restricted mothers. The groups had water and food ad libitum. OC and OSR body weights were evaluated from birth until 3 months of age.
Measurement of Arterial Blood Pressure
At 2 and 3 months of age, systolic blood pressure was determined in conscious rats by an indirect tail cuff method (plethysmography, IITC Life Science, Inc.).
Renal Function Evaluation
Renal function was studied in 3-month-old offspring (groups OC and OSR).
The animals were anesthetized with sodium thiopental (30 mg/kg) and placed on a warm table to maintain body temperature at 37°C. A tracheotomy was performed, followed by insertion of polyethylene catheters into the jugular vein for infusions and into the carotid artery for blood sampling. After the catheter insertion into the carotid artery, a sample of blood was collected for the analysis of pH, pCO2,HCO-3,Na+, and K+(i-STAT1 Analyser; Abbot, USA). Urine was collected from a catheter inserted into the bladder. After the surgical procedure, a one-hour stabilization period was allowed before the beginning of 4 collection periods. The animals were primed with 1 mL of saline containing inulin (300 mg/Kg) and sodium para-aminohippurate (PAH, 6.66 mg/Kg) and then underwent continuous infusion of a saline solution containing inulin (5 mg/ min/ Kg) and PAH (1.33 mg/ min/ Kg) at 0.1 mL/min. Plasma and urine inulin and PAH concentrations were measured by colorimetry for estimation of glomerular filtration rate (GFR) and renal plasma flow (RPF). Titratable acid (TA) in urine was measured by microtitration with 0.01 M sodium hydroxide, and the excreted amount of ammonium (EANH4) was evaluated by colorimetry as described previously.24
Morphometric Analysis
Morphometric analysis was performed in 3-month-old offspring (groups OC and OSR) by the following method: kidneys from the rats of each group were dissected out rapidly after sacrifice, cleaned of connective tissue, weighed, and fixed in Bouin Liquid. Kidneys were longitudinally cut, wax embedded, and histological sections (5 μm width) near renal hilus were prepared. Sections were stained with hematoxylin and eosin stain for morphological analysis. Glomerular area and diameter were measured using the image analysis program Image-Pro Plus. Images were acquired through a Leica DMLA microscope connected to a microcomputer by a Sony video camera. Twenty different images (fields) in each slide were analyzed for each group. Each image had an area of 277,000 μm2. Glomeruli were counted and expressed as the number of glomeruli/mm3 (n), calculated by the formula n = G/ (Fx Ax (D+T)), where G = total glomeruli number in the examined fields, F = number of fields, A = area of the examined fields, D = mean glomerular diameter, and T = the thick section in millimeters7 (magnification 200x).
Glucose Tolerance Test
This test was performed in 3-month-old OC and OSR groups, fasted for 12 hours. The rats were anesthetized with Fentanyl (Virbac) and Zoletil (Janseen-Cilag) (20 mg/kg and 0.025 mg/kg, respectively) and placed on a heated table to maintain body temperature at 37°C. A sample of blood from the tail was collected to measure the glucose basal level (time 0), using an Accu-Check Advantage glucose meter. After this procedure, the glucose solution was injected into the penile vein (1 g/kg). Blood samples were collected after 4 min from the beginning of the infusion and in the following periods of 4 min each until 44 min, to measure the glucose level.
Effect of Maternal Sleep Restriction on Vascular Reactivity in Adult Offspring
In the offspring, the vascular reactivity was assayed at 16 weeks of age as described previously.25 Isolated fragments of the descending thoracic aorta (4 mm in length) were connected to a force transducer to record the isometric force and placed in organ baths filled with 15 mL Krebs solution (37°C; 94% O2/6% CO2; pH 7.4). Concentration-dependent response curves to acetylcholine (ACh) (10-9 – 10-5 M) and sodium nitroprusside (SNP) (10−9 – 10-6 M) were cumulatively obtained during submaximal contractions of phenylephrine (PE) (10-7M) (a concentration that induces 60% to 80% of the maximum effect). In some experiments, the endothelium was removed by gently rubbing the intimal surface with a stainless-steel rod. The effectiveness of endothelium removal was confirmed by the absence of relaxation induced by acetylcholine (10–6mol/L). Isometric tension was recorded by using an isometric force displacement transducer connected to a data acquisition system (PowerLab 8/S, AD Instruments Pty Ltd, Castle Hill, Australia).
Statistical Analyses
All values are expressed as means ± SD. Prisma (Graph Pad Software) was used for all statistical analysis. Data were analyzed by the unpaired Student t-test or 2-way ANOVA followed by Bonferroni post-test. P ≤ 0.05 was considered an indicative of significant difference.
RESULTS
Pregnant Female Rats
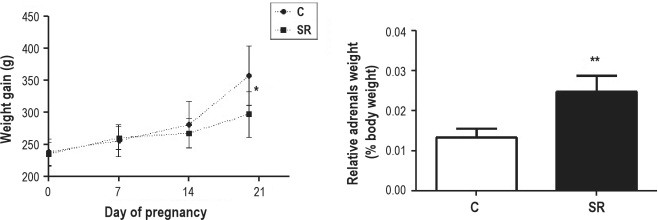
We observed that animals subjected to sleep restriction during pregnancy presented a decrease in weight gain when compared to control group (C). In this group, we also observed an increase in the relative adrenal weight, suggesting indirectly that sleep restriction was a stressful stimulus (Figure 1).
Figure 1.
Weight gain during pregnancy and relative adrenal weight from mothers SR (sleep restricted) and C (control mothers). *P ≤ 0.05 vs. group C (Two-way ANOVA, Bonferroni post-test); **P ≤ 0.0001 vs. group C (Student t-test).
Offspring
Body weight, kidney weight, and relative kidney weight
We did not observe differences in body weight during the time period from birth until 2 months of age. However, in OSR rats, at 3 months of age, body weight (BW) and kidney weight (KW) were decreased in comparison to the OC group. No differences were found in relative kidney weight (RKW – normalized per 100 g of body weight) among the studied groups (BW: OC: 384.67 ± 4.43; OSR: 361.83 ± 8.38 g, KW: OC: 1.57 ± 0.03; OSR: 1.43 ± 0.03 g, RKW: OC: 0.41 ± 0.01; OSR: 0.39 ± 0.01%).
Arterial blood pressure
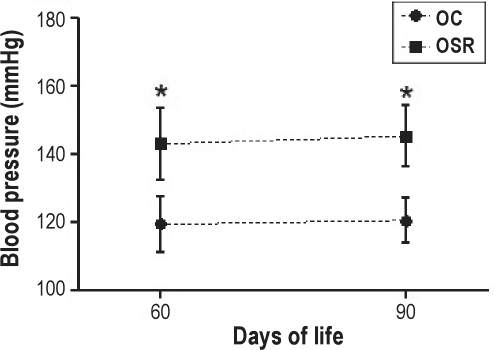
As shown in Figure 2, arterial blood pressure (BP) was significantly increased in OSR in comparison to the OC group at 2 and 3 month of age.
Figure 2.
Arterial blood pressure of the offspring. OC refers to offspring from control mothers; OSR, offspring from sleep-restricted mothers. *P ≤ 0.001 vs. group OC (Two-way ANOVA, Bonferroni post-test).
Morphological parameters
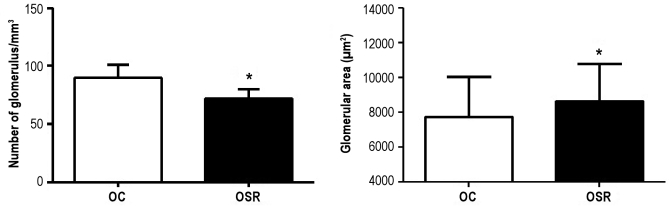
In the OSR group, the number of glomeruli was significantly decreased in comparison to the OC group. Moreover, the glomerular area was increased in the OSR group in comparison to OC (OC: 7716.85 ± 144.63; OSR: 8628.68 ± 146.79 μm2 (Figure 3), suggesting a compensatory hypertrophy.
Figure 3.
Morphometric renal parameters of the studied groups.OC refers to offspring from control mothers; OSR, offspring from sleep-restricted mothers. *P ≤ 0.0001 vs. group OC (Student t-test).
Renal function parameters
Renal plasma flow (RPF) and glomerular filtration rate (GRF) were increased in the OSR group when compared to OC rats. No significant changes in the urinary titratable acidity or in the excreted amount of ammonium (normalized by GFR) were observed in OSR compared to OC (Table 1), in addition to pH, pCO2,HCO-3,Na+, and K+ in arterial blood among the studied groups (data not shown).
Table 1.
Renal function parameters in the studied groups
| Group | V (mL/min/kg) | GFR (mL/min/kg) | RPF (mL/min/kg) | FF% | TA/GFR (μEq/mL) | EANH4/GFR (μEq/mL) |
|---|---|---|---|---|---|---|
| OC | 0.09 ± 0.04 (34/09) | 7.40 ± 1.40 (34/09) | 22.79 ± 5.32 (34/09) | 33.44 ± 6.60 (34/09) | 0.14 ± 0.10 (34/09) | 0.34 ± 0.59 (34/09) |
| OSR | 0.11 ± 0.04** (45/12) | 9.58 ± 1.87** (45/12) | 26.90 ± 5.83* (45/12) | 35.82 ± 5.36 (45/12) | 0.14 ± 0.06 (45/12) | 0.32 ± 0.10 (45/12) |
Data are reported as means ± SD. The number of measurements/number of animals is indicated in parentheses. OC refers to offspring from control mothers; OSR, offspring from sleep restricted mothers; V, urinary flow; GFR, glomerular filtration rate; RPF, renal plasma flow; FF%, filtration fraction (RPF/GFR%); TA; titratable acid; EANH4, excreted amount of ammonium.
P ≤ 0.05 vs. group OC;
P ≤ 0.001 vs. group OC (Student t-test).
Glucose tolerance test
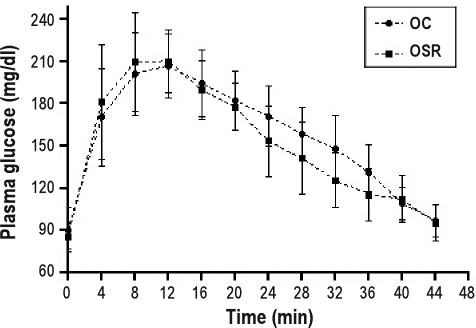
A Glucose tolerance test showed that glycemia remained at normal levels in the studied groups, suggesting no differences in glucose metabolism in the offspring from sleep-restricted mothers (Figure 4).
Figure 4.
Glucose tolerance test of the offspring. Data are reported as means ± SD. OC refers to offspring from control mothers; OSR, offspring from sleep-restricted mothers.
Vascular studies
Endothelium-dependent relaxation in response to acetylcholine:
No significant changes were observed in the cumulative concentration-effect curves for both Ach and SNP in aortic rings isolated from the studied groups (Table 2).
Table 2.
Vascular reactivity in the studied groups
| Ach Relaxation (n) | Rmax (%)a | pEC50b |
|---|---|---|
| OC (10) | 90.79 ± 4.16 | 6.99 (6.8–7.2) |
| OSR (10) | 88.78 ± 1.76 | 7.07 (6.9–7.1) |
| SNP Relaxation (n) | ||
| OC (E+) (10) | 101.2 ± 2.95 | 8.1 (7.9–8.3) |
| OC (E−) (10) | 107.8 ± .92 | 8.3 (7.7–8.8) |
| OSR (E+) (10) | 99.9 ± 2.47 | 8.1 (7.9–8.3) |
| OSR (E−) (10) | 99.1 ± 2.18 | 7.6 (7.9–8.3) |
n refers to number of observations; OC, offspring from control mothers; OSR, offspring from sleep restricted mothers; E+, with endothelium; E−, without endothelium.
Values are expressed as means ± SEM;
Mean (95% confidence interval).
DISCUSSION
In the present work, we observed that sleep restriction during pregnancy is able to modify renal development, resulting in morphologic and functional alterations in young offspring. Adequate daily sleep is an important part of a healthful and productive lifestyle; however, chronic sleep restriction in today's society is very common. There are some potential explanations for the high prevalence of sleep restriction. The demands of today's 24-hour society, including lifestyle, long work hours, and rotating night shift work, can lead to sleep restriction.26,27 Clinical and experimental studies have shown that insufficient sleep results in a variety of adverse physiologic effects, including hypertension, stress responses (such as activation of the sympathetic nervous system and sympathovagal balance),12, impairment of glucose control, and development of type 2 diabetes mellitus,10,28,29 obesity,11,14,29 and damage to the immune system.30,31 Moreover, during normal pregnancy, anatomic and physiologic changes occur, predisposing pregnant women to sleep disturbances, such as insomnia, snoring, obstructive sleep apnea, and restless legs syndrome.17, Consistent with the literature, we observed that sleep restriction was a stressful stimulus to pregnant rats, since the SR rats presented a decrease in weight gain and hypertrophy of adrenals glands, which are classic signs of stress.32 It is important to mention that sleep restriction/ deprivation involves, to variable degrees, imposition of nonspecific stress, which may interact with the effects attributable to sleep loss per se. Discrepancies in literature may be related at least in part to technical approaches and the duration of SR. It is difficult to compare results of different deprivation protocols and durations, but it is known that the principal approaches used in the literature (disk over water, platform, and gentle handling) in fact induce sleep deprivation. Despite these limitations, there is emerging evidence that in rodents, chronic sleep loss is more detrimental rather than acute sleep loss.
In a previous work from Martins et al.,33 a decrease in body weight gain in rats subjected to a similar sleep deprived model was shown to be due to a decrease in food intake in the first hours of sleep changes. As expected, in pregnant rats, the same effect on body weight gain induced by sleep restriction was observed during the second half of the gestational period. The impairment in body weight in 3-month-old offspring seems to indicate that long-term consequences are present in this model, confirming that the maternal environment exerts a fundamental role in offspring homeostasis.
It is well established that the prenatal environment exerts a profound influence on the development of the organism, and that stressful events during pregnancy can cause long-term physiological and behavioral alterations in offspring.4,21,34–36 Regarding kidney development, it has been shown that several factors other than fetal undernutrition can adversely affect nephrogenesis,5,37 and this effect can be transferred and perpetuated across generations.38 In both animal and human studies, it has been shown that environmental changes have the greatest impact if they occur during the early periods of nephrogenesis. Singh et al. demonstrated that acute fetal exposure to corticosterone, which represents the most important endocrine response to stress, promotes a decrease in nephron number and hypertension in the animals at 4 month of age.39 Our results suggest that maternal sleep restriction during the beginning of metanephrogenesis,40 besides acting as stressful stimuli, is able to influence this intrauterine process, resulting in a decreased nephron number. It is not clear how a stressful stimuli may cause a reduction in nephron formation, however, different hypotheses have been formulated, including changes in DNA methylation, increased apoptosis in the developing kidney, alterations in renal renin–angiotensin system activity, and increased fetal glucocorticoid exposure.41–43 Molecular mechanism(s) through which fetal programming exerts its effects on different organs, including the kidney, may also contribute to this effect.41,42
In addition to reduction in number of nephrons, we observed that offspring from sleep restricted mothers developed hypertension. This alteration was detected when measurements of blood pressure were performed at 2 and 3 months of age. However, it is possible that changes in blood pressure have happened before this period. Because OSR rats exhibited hypertension, we tested the vascular reactivity in the offspring. Our experiments showed that the maternal stressful condition studied in this work did not affect aortic vascular reactivity in 3-month-old offspring. However, the effect of hypertension on microvessels, as in the mesenteric bed, remains to be further elucidated, as well as the long-term effect of hypertension on endothelial function. It is also possible that a lack of sleep may increase circulating norepinephrine, which mediates peripheral vasoconstriction via α-adrenergic receptors, leading to increased blood pressure and heart rate.In 3-month-old offspring, renal morphologic and functional alterations, such as enlarged glomeruli, increased renal plasma flow, and glomerular filtration rate, were observed in OSR. According to Brenner's theory,44 the reduction in nephron number could result in decreased sodium excretion and therefore in the development of hypertension, especially with increased sodium ingestion. Moreover, once the nephron number is reduced, the remaining glomeruli undergo compensatory hypertrophy and hyperfiltration (increasing the single nephron filtration rate) to maintain adequate renal function.44
Our results show that maternal sleep restriction during nephrogenesis was able to increase global GFR. This effect is probably related to the increase in RPF observed in these animals and may be due to changes in the autoregulation of the glomerular filtration rate. In a classical model of reduced nephron number by subtotal nephrectomy, Kimura et al.45 observed changes in the resistance of renal arterioles, suggesting that hyperfiltration in this model could be the consequence of a dysfunction in the autoregulatory mechanism, which is also observed in the course of diabetic nephropathy.
Lucas et al.5 showed that 3-month-old intrauterine food-restricted rats, born with a reduced nephron number, presented a decline in renal function and compensatory glomerular hypertrophy; these group also observed that glomerulosclerosis was accelerated in 18-month-old rats.24 Further studies investigating our sleep-restricted model are required to determine if the alterations observed in 3-month-old offspring will worsen with age, exacerbating the nephron loss.
To our knowledge, this is the first study to report that maternal sleep restriction results in a nephron deficit, renal functional alterations, and the development of hypertension in offspring. These results may have important implications for pregnant women who exhibit sleep disturbances that may result in a sleep-deprived condition.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Miss Maria de Fátima Cavanal for her expert technical assistance. Research support was provided by Conselho Nacional de Desenvolvimento Cient́ifico e Tecnológico (CNPq) and by Associação Fundo de Incentivo a Psicofarmacologia (AFIP).
REFERENCES
- 1.Barker DJP. In utero programming of chronic disease. Clin Sci. 1998;95:115–28. [PubMed] [Google Scholar]
- 2.Barker DJP, Bagby SP, Hanson MA. Mechanisms of disease: in utero programming in the pathogenesis of hypertension. Nature Clin Prac Nephrology. 2002;2:700–7. doi: 10.1038/ncpneph0344. [DOI] [PubMed] [Google Scholar]
- 3.Aerts L, Van Assche A. Intra-uterine transmission of disease. Placenta. 2003;24:905–11. doi: 10.1016/s0143-4004(03)00115-2. [DOI] [PubMed] [Google Scholar]
- 4.Roseboom TJ, van der Meullen JH, Ravelli AC, et al. Coronary heart disease after prenatal exposure to the Dutch famine, 1944-45. Heart. 2000;84:595–8. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucas SRR, Costa-Silva VL, Zaladek-Gil F. Effects of intrauterine undernutrition on the renal function of the progeny. Brazilian J Med Biol Res. 1989;22:1303–6. [PubMed] [Google Scholar]
- 6.Lucas SRR, Costa-Silva VL, Miraglia SM, Zaladek-Gil F. Functional and morphometric evaluation of offspring kidney after intrauterine undernutrition. Pediatr Nephrol. 1997;11:719–23. doi: 10.1007/s004670050374. [DOI] [PubMed] [Google Scholar]
- 7.Rocha SO, Gomes GN, Forti Al, do Carmo Pinho Franco M, Fortes ZB, de Fátima Cavanal M, Gil FZ. Long-term effects of maternal diabetes on vascular reactivity and renal function in rat male offspring. Pediatr Res. 2005;58:1274–9. doi: 10.1203/01.pdr.0000188698.58021.ff. [DOI] [PubMed] [Google Scholar]
- 8.Magaton A, Gil FZ, Casarini DE, Cavanal M de F, Gomes GN. Maternal diabetes mellitus – early consequences for the offspring. Pediatr Nephrol. 2007;22:37–43. doi: 10.1007/s00467-006-0282-4. [DOI] [PubMed] [Google Scholar]
- 9.Franco MC, Ponzio BF, Gomes, et al. Micronutrient perinatal supplementation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009;85:327–33. doi: 10.1016/j.lfs.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 11.Bjorvatn B, Sagen IM, Oyane N, et al. The association between sleep duration, body mass index and metabolic measures in the Hordaland Health Study. J Sleep Res. 2007;16:66–76. doi: 10.1111/j.1365-2869.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- 12.Gangwisch J, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief Communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels and increased hunger an appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 14.Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165:25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- 15.National Sleep Foundation Sleep In America Poll. 2007 [Google Scholar]
- 16.Lopes EA, Coin de Carvalho LB, Costa Seguro da PB, et al. Sleep disorders in pregnancy. Arq Neuropsiquiatr. 2004; 62:217–21. doi: 10.1590/s0004-282x2004000200005. [DOI] [PubMed] [Google Scholar]
- 17.Pien GW, Scwab J. Sleep disorders during pregnancy. Sleep. 2004;27:1405–17. doi: 10.1093/sleep/27.7.1405. [DOI] [PubMed] [Google Scholar]
- 18.Sahota PK, Jain SS, Dhand R. Sleep disorders in pregnancy. Curr Opin Pulm Med. 2003;9:477–83. doi: 10.1097/00063198-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Chang JJ, Pien GW, Duntley SP, Macones GA. Sleep deprivation during pregnancy and maternal and fetal outcomes: is there a relationship? Sleep Med Rev. 2010;14:107–14. doi: 10.1016/j.smrv.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velazquez-Moctezuma J, Dominguez Salazar E, Cruz Rueda ML. The effect of prenatal stress on adult sexual behavior in rats depends on the nature of the stressor. Physiol Behav. 1992;53:443–8. doi: 10.1016/0031-9384(93)90137-5. [DOI] [PubMed] [Google Scholar]
- 21.Suchecki D, Palermo Neto J. Prenatal Stress and emotional response of adult offspring. Physiol Behav. 1990;49:423–6. doi: 10.1016/0031-9384(91)90259-q. [DOI] [PubMed] [Google Scholar]
- 22.Calegare BFA, et al. Biochemical, biometrical and behavioral changes in male offspring of sleep deprived mice. Psychoneuroendocrinology. 2010;35:775–84. doi: 10.1016/j.psyneuen.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Jouvet D, Vimont P, Delorme F, Jouvet M. Study of selective deprivation of the paradoxical sleep phase in the cat. C R Seances Soc Biol Fil. 1964;158:756–9. [PubMed] [Google Scholar]
- 24.Gil FZ, Lucas SR, Gomes GN, Cavanal Mde F, Coimbra TM. Effects of intrauterine food restriction and long-term dietary supplementation with L-arginine on age-related changes in renal function and structure of rats. Pediatr Res. 2005;57:724–31. doi: 10.1203/01.PDR.0000159514.06939.7E. [DOI] [PubMed] [Google Scholar]
- 25.Franco MC, Arruda RM, Dantas AP, et al. Inauterine undernutrition: expression and activity of the endothelial nitric oxide synthase in male and female adult offspring. Cardiovasc Res. 2002;56:145–53. doi: 10.1016/s0008-6363(02)00508-4. [DOI] [PubMed] [Google Scholar]
- 26. National Sleep Foundation – Sleep Survey, 2001. [Google Scholar]
- 27.Akerstedt T. Shift work and disturbed sleep. Occup Med (Lond) 2003;53:89– 94. doi: 10.1093/occmed/kqg046. [DOI] [PubMed] [Google Scholar]
- 28.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 29.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–78. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA. 2002;288:141–2. doi: 10.1001/jama.288.12.1471-a. [DOI] [PubMed] [Google Scholar]
- 31.Lange T, Perras B, Fehrn HL, Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychossom Med. 2003;65:831–5. doi: 10.1097/01.psy.0000091382.61178.f1. [DOI] [PubMed] [Google Scholar]
- 32.Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:2. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 33.Martins PJ, Nóbrega JN, Tufik S, D′Almeida V. Sleep deprivation-induced gnawing-relashionship to changes in feeding behavior in rats. Physiol Behav. 2008;93:229–34. doi: 10.1016/j.physbeh.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 34.Igosheva N, Matta S, Glover V. Prenatal stress alters cardiovascular responses in adult rats. J Physiol. 2004;557:273–85. doi: 10.1113/jphysiol.2003.056911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int. 2001;59:238–45. doi: 10.1046/j.1523-1755.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- 36.Battista MC, Oligny LL, St-Louis J, Brochu M. Intrauterine growth restriction in rats is associated with hypertension and renal dysfunction in adulthood. Am J Physiol Endocrinol Metab. 2002;283:E124–31. doi: 10.1152/ajpendo.00004.2001. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert JS, Lang AL, Nijland MJ. Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. J Physiol. 2005;565:137–47. doi: 10.1113/jphysiol.2005.084202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrison M, Langley-Evans SC. Intergenerational programming of impaired nephrogenesis and hypertension in rats following maternal protein restriction during pregnancy. Br J Nutr. 2009;101:1020–30. doi: 10.1017/S0007114508057607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh RR, Cullen-McEwen LA, Kett MM, et al. Prenatal corticosterone exposure results in altered AT1/AT2, nephron deficit and hypertension in the rat offspring. J Physiol. 2007;579:503–13. doi: 10.1113/jphysiol.2006.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moritz KM, Wintour EM. Functional development of the meso- and metanephros. Pediatr Nephrol. 1999;13:171–8. doi: 10.1007/s004670050587. [DOI] [PubMed] [Google Scholar]
- 41.Welham SJM, Wade A, Woolf AS. Protein restriction in pregnancy is associated with increased apoptosis of mesenchymal cells at the start of rat metanephrogenesis. Kidney Int. 2002;61:1231–42. doi: 10.1046/j.1523-1755.2002.00264.x. [DOI] [PubMed] [Google Scholar]
- 42.Dickinson H, Walker DW, Wintour EM, Moritz K. Maternal dexamethasone treatment at midgestation reduces nephron number and alters gene expression in the fetal spiny mouse. Am J Physiol Regul Integr Comp Physiol. 2007;292:R453–61. doi: 10.1152/ajpregu.00481.2006. [DOI] [PubMed] [Google Scholar]
- 43.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–7. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335–47. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 45.Kimura K, Tojo A, Nanba S, Matsuoka H, Sugimoto T. Morphometric analysis of arteriolar diameters in experimental nephropathies: application of microvascular casts. Virchows Archiv A Pathol Anat. 1990;471:319–23. doi: 10.1007/BF01605783. [DOI] [PubMed] [Google Scholar]