Abstract
Study Objectives:
We evaluated the influence of maternal self-reported habitual sleep duration during early pregnancy on blood pressure (BP) levels and risk of hypertensive disorders of pregnancy.
Design:
Prospective cohort study.
Setting:
Clinic-based study.
Participants:
A cohort of 1,272 healthy, pregnant women.
Measurements and Results:
We abstracted maternal antenatal BP values from medical records and estimated mean BP differences across hours of sleep categories in regression models, using generalized estimating equations. Odds ratios (OR) and 95% confidence intervals (95% CIs) for pregnancy induced hypertension (PIH) and preeclampsia (PE) in relation to long and short sleep duration were estimated. Mean 1st and 2nd trimester systolic (S) and diastolic (D) BP values were similar among women reporting to be short sleepers (≤ 6 h) vs. women reporting to sleep 9 hours. However, both short and long sleep duration in early pregnancy were associated with increased mean 3rd trimester SBP and DBP. For example, mean 3rd trimester SBP was 3.72, and 2.43 mm Hg higher for women reporting ≤ 6 h and 7-8 h sleep, respectively, compared with women reporting 9 h of sleep. Mean 3rd trimester SBP was 4.21 mm Hg higher for women reporting long sleep (≥ 10 h) vs. the reference group. Short and long sleep durations were associated with increased risks of PIH and PE. The ORs for very short (< 5 h) and long (≥ 10 h) sleepers were 9.52 (95% CI 1.83 to 49.40) and 2.45 (95% CI 0.74 to 8.15) for PE.
Conclusions:
Our findings are consistent with a larger literature that documents elevated blood pressure and increased risks of hypertension with short and long sleep duration.
Citation:
Williams MA; Miller RS; Qiu C; Cripe SM; Gelaye B; Enquobahrie D. Associations of early pregnancy sleep duration with trimester-specific blood pressures and hypertensive disorders in pregnancy. SLEEP 2010;33(10):1363-1371.
Keywords: Blood pressure, sleep duration, hypertension, preeclampsia, pregnancy
SLEEP LOSS AND SLEEP DISORDERS ARE THOUGHT TO BE AMONG THE MOST COMMON YET FREQUENTLY OVERLOOKED AND READILY TREATABLE HEALTH problems.1 It is estimated that some 50 to 70 million Americans chronically suffer from a disorder of sleep and wakefulness, hindering daily functioning and adversely affecting health1; and it is likely that an equal or greater number of Americans voluntarily restrict their sleep in order to watch television or use the internet.2 The impact of chronic sleep loss and sleep related disorders can be seen in virtually every key indicator of public health: mortality, morbidity, work performance, accidents and injuries, functioning and quality of life, family well-being, and health care utilization.3
Several studies have implicated insufficient sleep as a risk factor for elevated blood pressure and hypertension in men and non-pregnant women.4–9 Although causal mechanisms underlying these associations have yet to be empirically demonstrated, evidence from experimental studies suggest that insufficient sleep results in metabolic and neuroendocrine alterations that may contribute to hypertension and cardiovascular disease.10–17 Most sleep studies, however, have excluded pregnant women; hence very little is known about how insufficient sleep during gestation contributes to increased risks of medical complications of pregnancy, including hypertensive disorders and other perinatal outcomes.
To the best of our knowledge, no studies have examined the association between insufficient sleep and blood pressure among pregnant women. In this report, we examine the relationship between trimester-specific mean systolic (S), diastolic (D), and mean arterial pressure (MAP) blood pressure levels with maternal self-reported habitual sleep duration during early pregnancy. On the basis of available literature from non-pregnant women5–9 adolescents and children,18,19 we hypothesized that short and long sleep duration would be associated with increased mean blood pressures across all 3 trimesters of pregnancy. We also hypothesized that the risks for incident pregnancy-induced hypertension (without proteinuria) and preeclampsia (pregnancy induced hypertension with proteinuria) would be elevated among women who reported habitual short and long sleep duration during early pregnancy.
METHODS
Study Population and Setting
This analysis is based on data collected from a cohort of healthy women attending prenatal care clinics affiliated with Swedish Medical Center in Seattle, Washington. Eligible women started prenatal care before 20 weeks gestation, were 18 years of age or older, could speak and read English, and planned to carry the pregnancy to term and to deliver at either hospital. At 14 weeks gestation, on average, participants reported sociodemographic, behavioral, and health characteristics in a structured interview. After delivery, study personnel abstracted data from participants' hospital labor and delivery medical records and clinic records. Medical record abstractors recorded information on maternal blood pressures and weight measured at routine prenatal care visits. Between December 2003 and July 2006, 1,393 (82%) of 1,685 approached women consented to participate. We sequentially excluded 12 women with early pregnancy losses, 52 who were lost to follow-up, and 5 with missing information on early pregnancy sleep duration. We also excluded 52 women with pre-gestational chronic hypertension. Pre-gestational chronic hypertension was defined based on physician diagnosis as recorded in the medical record. Thus, 1,272 women remained for analysis. All study procedures were approved by the Institutional Review Board of Swedish Medical Center. All participants provided written informed consent.
Description of Covariates and Trimester-Specific Blood Pressure Assessment
At the time of enrollment in the study, a 45- to 60-min structured questionnaire was administered by a trained interviewer. Information on medical and reproductive histories and sociodemographic and lifestyle characteristics including average number of hours of sleep before and during early pregnancy was collected. Maternal average nightly sleep duration before and during pregnancy was ascertained by asking women the following questions: (1) “During the year prior to this pregnancy, how many hours per night did you sleep?” and (2) “Since becoming pregnant, how many hours per night do you sleep?” Responses were recorded as integers. We limited questions to early pregnancy because this period precedes any clinical manifestation of hypertensive disorders of pregnancy. As a result of this restriction, subjects are unlikely to report changes in nightly sleep duration because of symptoms related to preeclampsia and other hypertensive disorders of pregnancy. Pre-pregnancy weight and height were also based on self-reports made during the interview. Pre-pregnancy body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Our measure of weight gain during pregnancy was the weight gain between pre-pregnancy and 18 to 22 weeks gestation (henceforth referred to as early pregnancy). This measure of early pregnancy weight gain is not likely to be influenced by preeclampsia-associated edema, dietary modifications motivated by diagnosis of pregnancy complications, or decreased energy expenditure prompted by prescribed bed rest. We calculated early pregnancy weight gain by subtracting pre-pregnancy weight from weight at 18 to 22 weeks gestation. Early pregnancy weight gain values are expressed as kilograms. Gestational age of pregnancy was determined using maternal self-reported last normal menstrual period and this date was confirmed by ultrasound exams completed prior to 20 weeks gestation. Pregnancy induced hypertension (PIH) and preeclampsia,20 and gestational diabetes mellitus21 were defined according to published diagnostic criteria. We used the definition in the literature22 to define first, second, and third trimesters as follows: first trimester < 15 weeks; second trimester 15-28 weeks; and third trimester ≥ 29 weeks gestation.
All pregnancy-associated blood pressure measurements (i.e., systolic and diastolic blood pressures) along with the date and gestational age when the blood pressure was taken were abstracted from participants' clinical and hospital medical records. For the purposes of this study we primarily used antepartum clinical blood pressures taken and recorded during routine visits. Blood pressures taken upon admission for inpatient observation, or admission to the emergency room were considered only when blood pressure from an expected antepartum visit was unavailable. Blood pressures taken during active labor or during the postpartum period were not considered in these analyses.
As would be expected in any antepartum population, a subgroup of women, particularly those with a complicated pregnancy, had more than the expected numbers of antepartum visits and recorded blood pressures. To prevent overrepresentation in the sample of such women, we randomly selected blood pressure readings from among appropriate gestational age categories if/when there was a larger than expected number of blood pressure recordings. In instances where blood pressures were taken twice (on the same day) to confirm an initial reading, we randomly selected one of the associated readings. An average of 12.2 (median: 13; interquartile range: 11–14) blood pressure values were recorded for each study participant. Details of the constitution of this database have been previously described.23
During the study period many different health care providers made blood pressure readings as part of routine clinical practice. Although the measures are not strictly standardized as would be in a clinical trial, blood pressures were taken using standard mercury sphygmomanometers (scaled to even numbers), and patients were rested and seated during examination. Mean arterial pressure (MAP), considered an integrated parameter of blood pressure, is known to be more reproducible than individual systolic (SBP) and diastolic (DBP) blood pressures.24 We therefore computed mean arterial pressures for each subject according to the following formula: MAP = diastolic + 1/3(systolic – diastolic).
Statistical Analyses
The blood pressure record (SBP, DBP, MAP) was the dependent variable and categorical sleep duration (h) was the primary covariate. We classified participants as short (≤ 6 h); intermediate (7-8 h); normal (9 h) and long (≥ 10 h) duration sleepers. We categorized the sleep duration variables because each additional hour of sleep was not associated with the same mean change in blood pressure level. These cut-points were based on a prior literature evaluating sleep duration and hypertension5–9 and on a literature describing generally longer sleep duration patterns among pregnant women.25–27 Linear regression models were fitted using generalized estimating equations to adjust for repeated BP measurements on the same woman.28 Based on exploratory investigation of the correlation between repeated measurements, an exchangeable correlation structure was assumed for all analyses. Robust estimates of the standard errors were used throughout. All models were fitted with trimester as an effect modifier. Test statistics were constructed as the ratio of the relevant point estimate to its robust standard error and associated P-values calculated from normal tables. Logistic regression procedures were used to estimate odds ratios (ORs) and 95% confidence intervals (95% CIs) of preeclampsia (PE) and pregnancy induced hypertension (PIH) risks in relation to categories of short and long sleep duration. These analyses were completed to address our secondary aim of evaluating the extent to which short and long sleep duration in early pregnancy are risk factors for PIH and PE. For these analyses, we classified participants as very short (< 5 h); short (5-6 h); reference (7-9 h), and long (≥ 10 h) duration sleepers. Statistical significance was defined at P-value < 0.05. Analyses were carried out using Stata Software, Version 9.2.29
RESULTS
The sociodemographic characteristics of the study cohort by sleep duration categories are presented in Table 1. Overall, participants included in this analysis tended to be Caucasian, well-educated, and married. First trimester SBP, DBP, and MAP values for the entire cohort were as follows (mean ± standard error): 111.8 ± 0.1 mm Hg, 66.6 ± 0.1 mm Hg, and 81.7 ± 0.1 mm Hg. Mean second trimester SBP (111.4 ± 0.1 mm Hg), DBP (65.8 ± 0.1 mm Hg), and MAP (81.0 ± 0.1 mm Hg) values were all slightly lower than the mean values for the first trimester (P < 0.0005). Mean third trimester SBP (114.1 ± 0.1 mm Hg), DBP (68.4 ± 0.1 mm Hg), and MAP (82.7 ± 0.1 mm Hg) values were statistically significantly higher than first and second trimester values, respectively (P-values < 0.0005). These somewhat J-shaped patterns of mean blood pressures across trimesters are consistent with reports in the literature.30
Table 1.
Characteristics of the study cohort (N = 1,272) by categories of first-trimester nightly sleep hours, Seattle, Washington, 2003-2006
| Study Groups: | ≤ 6 h N = 174 | 7-8 h N = 702 | 9 h N = 261 | ≥ 10 h N = 135 | |
|---|---|---|---|---|---|
| Characteristics | % | % | % | % | P-value |
| Maternal age (y) | |||||
| < 25 | 5.2 | 1.1 | 1.2 | 5.2 | < 0.001 |
| 25-34 | 43.7 | 58.3 | 65.1 | 65.2 | |
| ≥ 35 | 51.2 | 40.6 | 33.7 | 29.6 | |
| Maternal race/ethnicity | |||||
| Non-Hispanic White | 83.3 | 87.2 | 91.2 | 91.9 | 0.001 |
| African American | 4.6 | 1.3 | 0.4 | 0.7 | |
| Asian | 9.8 | 8.7 | 4.6 | 2.2 | |
| Other | 2.3 | 2.9 | 3.8 | 5.2 | |
| Multiparous | 51.2 | 42.7 | 30.3 | 24.4 | < 0.001 |
| < 12 years education | 4.6 | 1.9 | 2.7 | 3.7 | 0.001 |
| Smoked during pregnancy | 7.5 | 3.7 | 6.1 | 7.6 | 0.071 |
| Alcohol use during pregnancy | 10.3 | 12.8 | 15.3 | 16.3 | 0.331 |
| No exercise during pregnancy | 5.8 | 5.8 | 7.3 | 14.1 | 0.006 |
| No prenatal vitamin | 4.6 | 1.9 | 1.5 | 3.1 | 0.146 |
| Pre-pregnancy body mass index (kg/m2) | |||||
| < 18.5 | 5.2 | 4.8 | 5.4 | 2.2 | 0.089 |
| 18.5-24.99 | 62.6 | 72.8 | 74.3 | 69.6 | |
| 25-29.99 | 21.8 | 16.0 | 15.7 | 22.2 | |
| ≥ 30 + | 10.3 | 6.4 | 4.6 | 5.9 | |
| Pregnancy weight gain (kg) | 7.2 ± 3.4 | 6.6 ± 3.6 | 7.0 ± 3.4 | 6.7 ± 3.9 | 0.461 |
| Gestational age at delivery | |||||
| < 28* | 1.1 | 0.3 | 0.4 | 0.8 | 0.404 |
| 28-36 | 9.2 | 9.8 | 9.2 | 11.9 | |
| 37-40 | 79.3 | 76.6 | 72.4 | 75.4 | |
| > 40 | 10.3 | 13.3 | 18.0 | 11.9 | |
| Incident pregnancy induced | |||||
| hypertension | 6.9 | 3.7 | 5.0 | 6.7 | 0.201 |
| Incident preeclampsia | 3.5 | 0.6 | 2.3 | 3.0 | 0.011 |
Includes pregnancies ending in miscarriage, induced abortion, or fetal demise.
The relationships between trimester-specific mean SBP and early pregnancy sleep duration are summarized in Table 2. Mean first trimester SBP in each sleep duration category was slightly elevated relative to that of women who reporting an average of 9 h per night during early pregnancy. Using those women who reported sleeping 9 h per night as the reference group, we noted that mean first trimester SBP values were 1.24, 0.20, and 0.71 mm Hg higher for among women who reported short (≤ 6 h), intermediate (7-8 h), and long (≥ 10 h) sleep duration in early pregnancy. However, the difference in first trimester SBP was not statistically significant for short vs. normal duration sleepers. The differences in first trimester mean SBP for short vs. normal duration sleepers increased (Δ = 1.62; 95% CI 0.00 to 3.2) after we adjusted for confounding by maternal age, race/ethnicity, parity, educational attainment, and marital status. Further adjustment for maternal pre-pregnancy BMI greatly attenuated the difference (Δ = 0.87; 95% CI to 0.6 to 2.4). Second trimester mean SBP values were not associated with maternal early sleep duration. However, there was evidence that early pregnancy short and long sleep duration were positively associated with mean SBP in the third trimester. After adjusting for maternal age, race/ethnicity, parity, educational attainment, marital status, and pre-pregnancy BMI, we noted that short (3.72 mm Hg), intermediate (2.43 mm Hg), and long (4.21 mm Hg) duration sleepers had statistically significant higher mean third trimester SBP than normal duration sleepers.
Table 2.
Unadjusted and adjusted mean (SE) systolic blood pressure (SBP), by trimester and nightly hours of sleep during early pregnancy, Seattle, Washington, 2003-2006
| First Trimester |
Second Trimester |
Third Trimester |
||||
|---|---|---|---|---|---|---|
| Sleep group h/night | Unadjusted Mean [SE] | Δ (95% CI) | Unadjusted Mean [SE] | Δ (95% CI) | Unadjusted Mean [SE] | Δ (95% CI) |
| 9 h | 111.46 [0.53] | 111.62 [0.49] | 114.00 [0.56] | |||
| ≤ 6 h | 112.70 [0.82] | 1.24 (−0.4 to 2.8) | 112.12 [0.82] | 0.50 (−1.1 to 2.1) | 118.05 [0.86] | 4.05 (2.4 to 5.7) |
| 7-8 h | 111.66 [0.63] | 0.20 (−1.0 to 1.4) | 111.08 [0.60] | −0.54 (−1.7 to 0.6) | 116.27 [0.61] | 2.27 (1.1 to 3.5) |
| ≥ 10 h | 112.17 [0.90] | 0.71 (−1.1 to 2.5) | 112.74 [0.82] | 1.12 (−0.5 to 2.7) | 118.90 [0.92] | 4.90 (3.1 to 6.7) |
| MODEL 1 | ||||||
| Sleep group h/night | 1Adjusted Mean [SE] | Δ (95% CI) | 1Adjusted Mean [SE] | Δ (95% CI) | 1Adjusted Mean [SE] | Δ (95% CI) |
| 9 h | 111.99 [0.55] | 112.16 [0.49] | 114.53 [0.56] | |||
| ≤ 6 h | 113.61 [0.82] | 1.62 (0.0 to 3.2) | 113.02 [0.82] | 0.86 (−0.8 to 2.5) | 118.94 [0.86] | 4.41 (2.7 to 6.1) |
| 7-8 h | 112.50 [0.63] | 0.51 (−0.7 to 1.8) | 111.93 [0.60] | −0.23 (−1.4 to 0.9) | 117.11 [0.61] | 2.58 (1.4 to 3.8) |
| ≥ 10 h | 112.50 [0.89] | 0.51 (−1.2 to 2.3) | 113.08 [0.81] | 0.92 (−0.7 to 2.5) | 119.23 [0.91] | 4.70 (2.9 to 6.5) |
| MODEL 2 | ||||||
| Sleep group h/night | 2Adjusted Mean [SE] | Δ (95% CI) | 2Adjusted Mean [SE] | Δ (95% CI) | 2Adjusted Mean [SE] | Δ (95% CI) |
| 9 h | 110.71 [0.53] | 110.89 [0.49] | 113.24 [0.56] | |||
| ≤ 6 h | 111.58 [0.76] | 0.87 (−0.6 to 2.4) | 111.06 [0.76] | 0.17 (−1.4 to 1.7) | 116.96 [0.80] | 3.72 (2.1 to 5.3) |
| 7-8 h | 111.07 [0.60] | 0.36 (−0.8 to 1.5) | 110.51 [0.56] | −0.38 (−1.5 to 0.7) | 115.67 [0.58] | 2.43 (1.3 to 3.6) |
| ≥ 10 h | 110.71 [0.86] | 0.00 (−1.7 to 1.7) | 111.33 [0.76] | 0.44 (−1.0 to 1.9) | 117.45 [0.83] | 4.21 (2.6 to 5.8) |
Adjusted for maternal age, race/ethnicity, parity, and educational attainment. The adjusted mean values reported for MODEL 1 are from a regression model based on the entire study cohort (N = 1,272) with indicators variables specified to represent Non-Hispanic white, nulliparous, college educated women 25-34 years of age.
Adjusted for maternal age, race/ethnicity, parity, educational attainment, and pre-pregnancy body mass index. The other covariates in Table 1 were not confounders. The adjusted mean values reported for MODEL 2 are from a regression model based on the entire cohort (N = 1,272) with indicator variables specified to represent Non-Hispanic white, nulliparous, college educated women 25-34 years of age, with a pre-pregnancy BMI < 25 kg/m2.
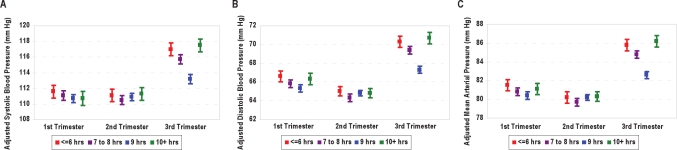
Similar associations of maternal early pregnancy sleep duration and with trimester specific mean DBP and MAP were also observed (Tables 3 and 4, respectively). Mean first trimester DBP, before adjusting for confounders, was lowest for normal duration sleepers (66.00, standard error [SE] 0.36, mm Hg) and highest for short (67.70, SE 0.59 mm Hg) and long (67.49, SE 0.66 mm Hg) duration sleepers. As can been seen in the third part of Table 3, mean first-trimester DBP values remained statistically significantly higher for short duration sleepers compared with normal duration sleepers after we controlled for potential confounders, including maternal pre-pregnancy BMI (Δ = 1.32; 95% CI 0.2 to 2.4). Short, intermediate, and long duration sleepers had increased mean third trimester DBP values (3.04, 2.13, and 3.43 mm Hg) compared with their counterparts who reported normal sleep durations. We noted this similar U-shaped pattern for the integrated measure of maternal blood pressure, MAP for the third trimester (Table 4). Short, intermediate, and long duration sleepers had increased mean third trimester MAP values (3.18, 2.20, and 3.65 mm Hg) compared with normal duration sleepers. A graphical summary of the associations between maternal trimester-specific mean SBP, DBP, and MAP values and sleep duration is presented in Figure 1. Summaries of the associations of trimester-specific MAP with sleep duration after stratification by parity (Table 5) and pre-pregnancy overweight status (Table 6) are provided. There was no evidence of effect modification (P-values all > 0.05) by either covariate, though maternal sleep duration appeared to be more strongly associated with third-trimester blood pressure values among nulliparous women than among multiparous women (Table 5). Associations of blood pressure values with sleep duration were similar for lean and overweight women (Table 6).
Table 3.
Unadjusted and adjusted mean (SE) diastolic blood pressure (DBP), by trimester and nightly hours of sleep during early pregnancy, Seattle, Washington, 2003 - 2006
| First Trimester |
Second Trimester |
Third Trimester |
||||
|---|---|---|---|---|---|---|
| Sleep group h/night | Unadjusted Mean [SE] | Δ (95% CI) | Unadjusted Mean [SE] | Δ (95% CI) | Unadjusted Mean [SE] | Δ (95% CI) |
| 9 h | 66.00 [0.36] | 65.50 [0.33] | 68.01 [0.38] | |||
| ≤ 6 h | 67.70 [0.59] | 1.70 (0.5 to 2.9) | 66.01 [0.58] | 0.51 (−0.6 to 1.6) | 71.39 [0.62] | 3.38 (2.2 to 4.6) |
| 7-8 h | 66.41 [0.42] | 0.41 (−0.4 to 1.2) | 64.97 [0.40] | −0.53 (−1.3 to 0.2) | 70.08 [0.42] | 2.07 (1.3 to 2.9) |
| ≥ 10 h | 67.49 [0.66] | 1.49 (0.2 to 2.8) | 65.98 [0.58] | 0.48 (−0.7 to 1.6) | 71.89 [0.66] | 3.88 (2.6 to 5.2) |
| MODEL 1 | ||||||
| Sleep group h/night | 1Adjusted Mean [SE] | Δ (95% CI) | 1Adjusted Mean [SE] | Δ (95% CI) | 1Adjusted Mean [SE] | Δ (95% CI) |
| 9 h | 66.19 [0.37] | 65.69 [0.33] | 68.19 [0.38] | |||
| ≤ 6 h | 68.04 [0.61] | 1.85 (0.7 to 3.0) | 66.35 [0.58] | 0.66 (−0.5 to 1.8) | 71.71 [0.63] | 3.52 (2.3 to 4.7) |
| 7-8 h | 66.76 [0.43] | 0.57 (−0.3 to 1.4) | 65.32 [0.40] | −0.37 (−1.2 to 0.4) | 70.43 [0.42] | 2.24 (1.4 to 3.1) |
| ≥ 10 h | 67.58 [0.65] | 1.39 (0.1 to 2.7) | 66.07 [0.58] | 0.38 (−0.8 to 1.5) | 71.97 [0.65] | 3.78 (2.5 to 5.1) |
| MODEL 2 | ||||||
| Sleep group h/night | 2Adjusted Mean [SE] | Δ (95% CI) | 2Adjusted Mean [SE] | Δ (95% CI) | 2Adjusted Mean [SE] | Δ (95% CI) |
| 9 h | 65.29 [0.35] | 64.80 [0.33] | 67.29 [0.38] | |||
| ≤ 6 h | 66.61 [0.57] | 1.32 (0.2 to 2.4) | 64.97 [0.54] | 0.17 (−0.9 to 1.2) | 70.33 [0.59] | 3.04 (1.9 to 4.2) |
| 7-8 h | 65.75 [0.40] | 0.46 (−0.3 to 1.2) | 64.32 [0.37] | −0.48 (−1.2 to 0.3) | 69.42 [0.40] | 2.13 (1.4 to 2.9) |
| ≥ 10 h | 66.32 [0.61] | 1.03 (−0.2 to 2.2) | 64.84 [0.54] | 0.04 (−1.0 to 1.1) | 70.72 [0.60] | 3.43 (2.3 to 4.6) |
Adjusted for maternal age, race/ethnicity, parity, and educational attainment. The adjusted mean values reported for MODEL 1 are from a regression model based on the entire study cohort (N = 1,272) with indicators variables specified to represent Non-Hispanic white, nulliparous, college educated women 25-34 years of age.
Adjusted for maternal age, race/ethnicity, parity, educational attainment, and pre-pregnancy body mass index. The other covariates in Table 1 were not confounders. The adjusted mean values reported for MODEL 2 are from a regression model based on the entire cohort (N = 1,272) with indicator variables specified to represent Non-Hispanic white, nulliparous, college educated women 25-34 years of age, with a pre-pregnancy BMI < 25 kg/m2.
Table 4.
Unadjusted and adjusted mean (SE) mean arterial pressure (MAP), by trimester and nightly hours of sleep during early pregnancy, Seattle, Washington, 2003-2006
| First Trimester |
Second Trimester |
Third Trimester |
||||
|---|---|---|---|---|---|---|
| Sleep group h/night | Unadjusted Mean [SE] | Δ (95% CI) | Unadjusted Mean [SE] | Δ (95% CI) | Unadjusted Mean [SE] | Δ (95% CI) |
| 9 h | 81.16 [0.36] | 80.91 [0.32] | 83.34 [0.38] | |||
| ≤ 6 h | 82.71 [0.60] | 1.55 (0.4 to 2.7) | 81.38 [0.60] | 0.47 (−0.7 to 1.6) | 86.94 [0.63] | 3.60 (2.4 to 4.8) |
| 7-8 h | 81.49 [0.44] | 0.33 (−0.5 to 1.2) | 80.38 [0.45] | −0.53 (− 1.3 to 0.3) | 85.48 [0.43] | 2.14 (1.3 to 3.0) |
| ≥ 10 h | 82.39 [0.66] | 1.23 (−0.1 to 2.5) | 81.58 [0.59] | 0.67 (− 0.5 to 1.8) | 87.55 [0.69] | 4.21 (2.9 to 5.5) |
| MODEL 1 | ||||||
| Sleep group h/night | 1Adjusted Mean [SE] | Δ (95% CI) | 1Adjusted Mean [SE] | Δ (95% CI) | 1Adjusted Mean [SE] | Δ (95% CI) |
| 9 h | 81.46 [0.38] | 81.21 [0.32] | 83.64 [0.38] | |||
| ≤ 6 h | 83.23 [0.62] | 1.77 (0.6 to 3.0) | 81.90 [0.60] | 0.69 (−0.5 to 1.9) | 87.46 [0.64] | 3.82 (2.6 to 5.1) |
| 7-8 h | 82.00 [0.44] | 0.54 (−0.3 to 1.4) | 80.89 [0.41] | −0.32 (−1.1 to 0.5) | 85.99 [0.43] | 2.35 (1.5 to 3.2) |
| ≥ 10 h | 82.55 [0.66] | 1.09 (−0.2 to 2.4) | 81.75 [0.59] | 0.54 (−0.6 to 1.7) | 87.72 [0.67] | 4.08 (2.8 to 5.4) |
| MODEL 2 | ||||||
| Sleep group h/night | 2Adjusted Mean [SE] | Δ (95% CI) | 2Adjusted Mean [SE] | Δ (95% CI) | 2Adjusted Mean [SE] | Δ (95% CI) |
| 9 h | 80.42 [0.36] | 80.17 [0.32] | 82.59 [0.38] | |||
| ≤ 6 h | 81.51 [0.57] | 1.09 (0.0 to 2.2) | 80.22 [0.56] | 0.05 (−1.0 to 1.1) | 85.77 [0.59] | 3.18 (2.0 to 4.3) |
| 7-8 h | 80.81 [0.40] | 0.39 (−0.4 to 1.2) | 79.70 [0.38] | −0.47 (−1.2 to 0.3) | 84.79 [0.40] | 2.20 (1.4 to 3.0) |
| ≥ 10 h | 81.06 [0.61] | 0.64 (−0.6 to 1.8) | 80.29 [0.54] | 0.12 (−0.9 to 1.2) | 86.24 [0.61] | 3.65 (2.5 to 4.8) |
Adjusted for maternal age, race/ethnicity, parity, and educational attainment. The adjusted mean values reported for MODEL 1 are from a regression model based on the entire study cohort (N = 1,272) with indicators variables specified to represent Non-Hispanic white, nulliparous, college educated women 25-34 years of age.
Adjusted for maternal age, race/ethnicity, parity, educational attainment, and pre-pregnancy body mass index. The other covariates in Table 1 were not confounders. The adjusted mean values reported for MODEL 2 are from a regression model based on the entire cohort (N = 1,272) with indicator variables specified to represent Non-Hispanic white, nulliparous, college educated women who are 25-34 years of age, with a pre-pregnancy BMI < 25 kg/m2.
Figure 1.
Association between adjusted maternal trimester-specific mean (A) systolic, (B) diastolic and (C) mean arterial blood pressure, according to categories of maternal average sleep duration during early pregnancy (short duration, red ' 6 h; intermediate, purple 7-8 h; normal duration, blue 9 h; and long duration, green, ≥ 10 h). The vertical lines represent ± standard error. The adjusted mean values are for Non-Hispanic white, nulliparous, college educated women 25-34 years of age.
Table 5.
Adjusted mean (SE) mean arterial pressure (MAP), by trimester and nightly hours of sleep during early pregnancy, after stratification by parity, Seattle, Washington, 2003 - 2006
| First Trimester |
Second Trimester |
Third Trimester |
||||
|---|---|---|---|---|---|---|
| All Women (N = 1,272) | ||||||
| Sleep group h/night | 1Adjusted Mean [SE] | Δ (95% CI) | 1Adjusted Mean [SE] | Δ (95% CI) | 1Adjusted Mean [SE] | Δ (95% CI) |
| 9 h | 80.42 [0.36] | 80.17 [0.32] | 82.59 [0.38] | |||
| ≤ 6 h | 81.51 [0.57] | 1.09 (0.0 to 2.2) | 80.22 [0.56] | 0.05 (−1.0 to 1.1) | 85.77 [0.59] | 3.18 (2.0 to 4.3) |
| 7-8 h | 80.81 [0.40] | 0.39 (−0.4 to 1.2) | 79.70 [0.38] | −0.47 (−1.2 to 0.3) | 84.79 [0.40] | 2.20 (1.4 to 3.0) |
| ≥ 10 h | 81.06 [0.61] | 0.64 (−0.6 to 1.8) | 80.29 [0.54] | 0.12 (−0.9 to 1.2) | 86.24 [0.61] | 3.65 (2.5 to 4.8) |
| Nulliparous Women (N = 771) | ||||||
| Sleep group h/night | 2Adjusted Mean [SE] | Δ (95% CI) | 2Adjusted Mean [SE] | Δ (95% CI) | 2Adjusted Mean [SE] | Δ (95% CI) |
| 9 h | 79.64 [0.62] | 76.78 [0.93] | 82.08 [0.86] | |||
| ≤ 6 h | 81.45 [0.84] | 1.81 (0.2 to 3.4) | 75.68 [1.24] | −1.10 (−3.5 to 1.3) | 86.62 [1.05] | 4.55 (2.5 to 6.6) |
| 7-8 h | 80.28 [0.68] | 0.66 (−0.7 to 2.0) | 72.78 [0.78] | −4.00 (−5.5 to 2.5) | 84.50 [0.70] | 2.42 (1.0 to 3.8) |
| ≥ 10 h | 81.07 [0.94] | 1.44 (−0.4 to 3.3) | 74.88 [1.11] | −1.90 (−4.1 to 0.3) | 87.01 [0.89] | 4.93 (3.2 to 6.7) |
| Multiparous Women (N = 501) | ||||||
| Sleep group h/night | 3Adjusted Mean [SE] | Δ (95% CI) | 3Adjusted Mean [SE] | Δ (95% CI) | 3Adjusted Mean [SE] | Δ (95% CI) |
| 9 h | 80.17 [0.65] | 75.32 [1.17] | 79.63 [0.80] | |||
| ≤ 6 h | 80.26 [1.04] | 0.11 (−1.9 to 2.1) | 69.70 [1.37] | −5.63 (−8.3 to 2.9) | 80.48 [1.04] | 0.95 (−1.1 to 3.0) |
| 7-8 h | 80.13 [0.71] | −0.04 (−1.4 to 1.4) | 71.62 [0.78] | −3.70 (−5.2 to 2.2) | 79.75 [0.73] | 0.22 (−1.2 to 1.7) |
| ≥ 10 h | 80.07 [1.22] | −0.10 (−2.5 to 2.3) | 72.77 [1.58] | −2.55 (−5.6 to 0.5) | 81.56 [1.12] | 2.22 (−0.2 to 4.2) |
Adjusted for maternal age, race/ethnicity, parity, educational attainment, and pre-pregnancy body mass index. The adjusted mean values reported are from a regression model based on the entire study cohort (N = 1,272) with indicators variables specified to represent Non-Hispanic white, nulliparous, college educated women 25-34 years of age, with a pre-pregnancy BMI < 25 kg/m2.
Adjusted for maternal age, race/ethnicity, educational attainment, and pre-pregnancy body mass index. The adjusted mean values reported are from a regression model based on nulliparous women only (N = 771) with indicators variables specified to represent Non-Hispanic white, college educated women 25-34 years of age, with a pre-pregnancy BMI < 25 kg/m2.
Adjusted for maternal age, race/ethnicity, educational attainment, and pre-pregnancy body mass index. The adjusted mean values reported are from a regression model based on multiparous women only (N = 501) with indicator variables specified to represent Non-Hispanic white, college educated women 25-34 years of age, with a pre-pregnancy BMI < 25 kg/m2.
Table 6.
Adjusted mean (SE) mean arterial pressure (MAP), according to trimester and nightly hours of sleep during early pregnancy, after stratification by maternal overweight status, Seattle, Washington, 2003-2006
| First Trimester |
Second Trimester |
Third Trimester |
||||
|---|---|---|---|---|---|---|
| All Women (N = 1,272) | ||||||
| Sleep group h/night | 1Adjusted Mean [SE] | Δ (95% CI) | 1Adjusted Mean [SE] | Δ (95% CI) | 1Adjusted Mean [SE] | Δ (95% CI) |
| 9 h | 80.42 [0.36] | 80.17 [0.32] | 82.59 [0.38] | |||
| ≤ 6 h | 81.51 [0.57] | 1.09 (0.0 to 2.2) | 80.22 [0.56] | 0.05 (−1.0 to 1.1) | 85.77 [0.59] | 3.18 (2.0 to 4.3) |
| 7-8 h | 80.81 [0.40] | 0.39 (−0.4 to 1.2) | 79.70 [0.38] | −0.47 (−1.2 to 0.3) | 84.79 [0.40] | 2.20 (1.4 to 3.0) |
| ≥ 10 h | 81.06 [0.61] | 0.64 (−0.6 to 1.8) | 80.29 [0.54] | 0.12 (−0.9 to 1.2) | 86.24 [0.61] | 3.65 (2.5 to 4.8) |
| Lean Women (BMI < 25 kg/m2) (N = 968) | ||||||
| Sleep group h/night | 2Adjusted Mean [SE] | Δ (95% CI) | 2Adjusted Mean [SE] | Δ (95% CI) | 2Adjusted Mean [SE] | Δ (95% CI) |
| 9 h | 80.45 [0.61] | 80.10 [0.62] | 82.49 [0.71] | |||
| ≤ 6 h | 81.98 [0.73] | 1.53 (0.1 to 3.0) | 80.67 [0.69] | 0.57 (−0.8 to 1.9) | 86.06 [0.73] | 3.57 (2.1 to 5.0) |
| 7-8 h | 80.78 [0.45] | 0.33 (−0.6 to 1.2) | 79.76 [0.42] | −0.42 (−1.2 to 0.5) | 84.70 [0.44] | 2.21 (1.3 to 3.1) |
| ≥ 10 h | 81.68 [0.74] | 1.23 (−0.6 to 2.4) | 80.26 [0.66] | 0.16 (−1.1 to 1.4) | 85.69 [0.72] | 3.20 (1.8 to 4.5) |
| Overweight Women (BMI ≥ 25 kg/m2) (N = 304) | ||||||
| Sleep group h/night | 3Adjusted Mean [SE] | Δ (95% CI) | 3Adjusted Mean [SE] | Δ (95% CI) | 3Adjusted Mean [SE] | Δ (95% CI) |
| 9 h | 86.86 [1.06] | 86.52 [1.12] | 89.14 [0.98] | |||
| ≤ 6 h | 88.65 [1.39] | 1.79 (−0.9 to 4.5) | 86.33 [1.34] | −0.19 (−2.7 to 2.4) | 92.70 [1.44] | 3.56 (0.7 to 6.4) |
| 7-8 h | 87.46 [1.15] | 0.60 (−1.7 to 2.9) | 86.43 [1.10] | −0.09 (−3.3 to 1.1) | 90.40 [1.12] | 1.26 (−0.9 to 3.4) |
| ≥ 10 h | 86.79 [1.42] | −0.07 (−2.9 to 2.7) | 86.15 [1.10] | −0.37 (−2.6 to 1.9) | 92.48 [1.29] | 3.34 (1.8 to 6.9) |
Adjusted for maternal age, race/ethnicity, parity, educational attainment, and pre-pregnancy body mass index. The adjusted mean values reported are from a regression model based on the entire study cohort (N = 1,272) with indicators variables specified to represent Non-Hispanic white, nulliparous, college educated women 25-34 years of age, with a pre-pregnancy BMI < 25 kg/m2.
Adjusted for maternal age, race/ethnicity, parity, and educational attainment. The adjusted mean values reported are from a regression model based on lean women only (N = 968) with indicators variables specified to represent Non-Hispanic white, nulliparous, college educated women 25-34 years of age.
Adjusted for maternal age, race/ethnicity, parity, and educational attainment. The adjusted mean values reported are from a regression model based on overweight women only (N = 304) with indicator variables specified to represent Non-Hispanic white, college educated women 25-34 years of age.
The secondary aim of this analysis was to assess the risk of incident diagnoses of pregnancy induced hypertension (PIH) and preeclampsia (PE) among women in the cohort (Table 7). Approximately 6.3% of the women in the study cohort were diagnosed with either PIH (n = 60) or PE (n = 20). After adjustment for maternal age, parity, use of prenatal vitamins, alcohol consumption during pregnancy and pre-pregnancy BMI, both very short (OR = 1.67; 95% CI 0.33 to 8.59) and long (OR = 1.51; 95% CI 0.70 to 3.28) sleep duration were associated with increased risk of incident PIH, relative to the reference group (7-9 h), though neither association was statistically significant. Very short (OR = 9.52; 95% CI 1.83 to 49.40) and long (OR = 2.45; 95% CI 0.74 to 8.15) sleep duration were also associated with increased risks of PE, relative to the reference group, though only the former association was statistically significant. Maternal cigarette smoking, race/ethnicity, educational attainment, gestational weight gain, and participation in leisure time physical activity during pregnancy were not confounders of these associations.
Table 7.
Multivariable adjusted odds ratios (OR) and 95% confidence interval (CI) of pregnancy induced hypertension and preeclampsia according to nightly sleep duration Seattle, Washington, 2003 - 2006
| Nightly Sleep Duration (h) |
||||
|---|---|---|---|---|
| < 5 N = 22 | 5-6 N = 152 | 7-9 N = 963 | ≥ 10 N = 135 | |
| 1192 Normotensives n (%) | 18 (1.5) | 138 (11.6) | 914 (76.7) | 122 (10.2) |
| 60 Incident PIH n (%) | 2 (3.3) | 10 (16.7) | 39 (65.0) | 9 (15.0) |
| Association for PIH | ||||
| Unadjusted OR (95% CI) | 2.60 (0.58 to 11.60) | 1.70 (0.83 to 3.48) | 1.00 (REF) | 1.73 (0.82 to 3.66) |
| 1Adjusted OR (95% CI) | 1.79 (0.37 to 8.76) | 1.47 (0.70 to 3.09) | 1.00 (REF) | 1.61 (0.75 to 3.44) |
| 2Adjusted OR (95% CI) | 1.70 (0.33 to 8.65) | 1.48 (0.70 to 3.13) | 1.00 (REF) | 1.53 (0.71 to 3.31) |
| 3Adjusted OR (95% CI) | 1.67 (0.33 to 8.59) | 1.49 (0.70 to 3.17) | 1.00 (REF) | 1.51 (0.70 to 3.28) |
| 20 Incident PE n (%) | 2 (10.0) | 4 (20.0) | 10 (50.0) | 4 (20.0) |
| Unadjusted OR (95% CI) | 10.2 (2.07 to 49.70) | 2.65 (0.82 to 8.56) | 1.00 (REF) | 3.00 (0.93 to 9.73) |
| 1Adjusted OR (95% CI) | 7.56 (1.45 to 39.40) | 2.36 (0.72 to 7.72) | 1.00 (REF) | 2.80 (0.86 to 9.12) |
| 2Adjusted OR (95% CI) | 9.88 (1.91 to 50.80) | 2.93 (0.89 to 9.66) | 1.00 (REF) | 2.46 (0.74 to 8.18) |
| 3Adjusted OR (95% CI) | 9.52 (1.83 to 49.40) | 3.02 (0.91 to 9.98) | 1.00 (REF) | 2.45 (0.74 to 8.15) |
Adjusted for pre-pregnancy body mass index;
Adjusted for maternal pre-pregnancy body mass index, age, and parity;
Adjusted for maternal pre-pregnancy body mass index, age, parity, prenatal vitamin use and alcohol consumption. Maternal race/ethnicity, cigarette smoking status, pregnancy weight gain, educational attainment, and participation in leisure time physical activity during pregnancy were not confounders of these reported associations.
Because associations of pregnancy induced hypertension and preeclampsia with sleep duration may be modified by other covariates, we reexamined odds ratios after stratifying participants according to parity and pre-pregnancy overweight status. Although inferences from these analyses are hindered by our relatively small sample size (as illustrated by the wide 95% confidence intervals surrounding strata-specific odds ratios), observed associations did not differ much according to these variables (Table 8). Very short nightly sleep duration, however, did appear to be more strongly related with preeclampsia risk among overweight women (OR = 12.70; 95% CI 1.04 to 154.40) than for lean women (OR = 6.71; 95% CI 0.72 to 62.80), although the interaction P-value was not statistically significant.
Table 8.
Multivariable adjusted odds ratios (OR) and 95% confidence interval (CI) of pregnancy induced hypertension and preeclampsia according to nightly sleep duration, after stratification by maternal characteristics Seattle, Washington, 2003-2006
| Nightly Sleep Duration (h) |
||||
|---|---|---|---|---|
| Incident PIH | < 5 N = 22 | 5-6 N = 152 | 7-9 N = 963 | ≥ 10 N = 135 |
| Stratifying Variables | ||||
| Nulliparous | 2.83 (0.33 to 23.90) | 1.11 (0.36 to 3.39) | 1.00 (REF)1 | 1.39 (0.57 to 3.38) |
| Multiparous | 1.11 (0.10 to 12.90) | 1.94 (0.68 to 5.50) | 1.00 (REF)1 | 2.05 (0.43 to 9.72) |
| Lean (< 25 kg/m2) | † | 1.89 (0.69 to 5.17) | 1.00 (REF)2 | 1.87 (0.68 to 5.14) |
| Overweight (≥ 25 kg/m2) | 3.38 (0.61 to 18.80) | 1.24 (0.43 to 3.55) | 1.00 (REF)2 | 1.26 (0.40 to 4.01) |
| Incident PE | ||||
| Stratifying Variable | ||||
| Nulliparous | 6.16 (0.68 to 55.90) | 2.67 (0.70 to 10.20) | 1.00 (REF)1 | 2.57 (0.76 to 8.72) |
| Multiparous | † | 4.61 (0.28 to 75.70) | 1.00 (REF)1 | † |
| Lean (< 25 kg/m2) | 6.71 (0.72 to 62.80) | 2.73 (0.53 to 14.10) | 1.00 (REF)2 | † |
| Overweight (≥ 25 kg/m2) | 12.70 (1.04 to 154.40) | 2.59 (0.45 to 14.90) | 1.00 (REF)2 | 5.14 (1.20 to 22.10) |
Adjusted for maternal age and pre-pregnancy body mass index;
Adjusted for maternal age and parity;
There were too few subjects in this stratum to estimate adjusted odds ratios.
DISCUSSION
Maternal self-reported nightly short and long sleep durations in early pregnancy are associated with elevated blood pressures, particularly mean third trimester blood pressures. For instance, the differences in mean third-trimester SBP, DBP, and MAP for women who reported short early pregnancy sleep durations (≤ 6 h) compared with those in the referent group (9 h) were 3.72, 3.04, and 3.18 mm Hg, respectively after adjustment for maternal age, race/ethnicity, parity, educational status, and pre-pregnancy body mass index. The corresponding differences in mean third-trimester blood pressures for women who reported long sleep durations (≥ 10 h), compared with those reporting sleeping 9 h nightly, were 4.21, 3.43, and 3.65 mm Hg. Very short early pregnancy sleep duration (< 5 h) was also associated with an increased risk of preeclampsia after adjusting for maternal age, parity, pre-pregnancy body mass index, prenatal vitamin use, and alcohol consumption during pregnancy (OR = 9.52; 95% CI 1.83 to 49.40). To our knowledge there have been no published studies on maternal sleep duration, blood pressure, and preeclampsia. Our findings, however, are generally consistent with reports documenting associations between habitual sleep duration, blood pressure values, and hypertension in men, non-pregnant women, adolescents, and children.5–9,18,19 In a recent study of 1,741 men and non-pregnant women, Vgontzas et al,9 observed that the highest risk of hypertension was in subjects with insomnia who slept < 5 h per night (OR = 5.1; 95% CI 2.2 to 11.8) compared with individuals with normal sleep duration (defined as > 6 h).
Our present study has several important strengths. First, determination of maternal sleep duration was based on reports made early during pregnancy, so reporting was not conditional on pregnancy outcomes or on signs and symptoms of elevated blood pressures. Our results suggest that habitual short and long sleep duration precede elevations in late-pregnancy blood pressure values and precede the clinical diagnosis of hypertensive disorders of pregnancy. Second, the high follow-up rate (> 95%) minimized possible selection bias. However, several limitations merit discussion and consideration. First, we relied on recorded clinical blood pressure measurements that are influenced by circadian rhythms, stress, diet, and other sources of within-person variation.31,32 Errors in these measurements may also arise from misreading, digit preference, and use of an incorrect cuff size in non-research, clinical settings. These sources of error are likely unrelated to maternal reports of sleep duration, thus we speculate that our reported measures of association are likely biased towards the null. Second, maternal habitual nightly sleep duration before and during pregnancy was obtained from self-report, and thus is likely susceptible to misclassification. Reported sleep duration is known to be only moderately correlated with wrist actigraph-measured sleep duration (r = 0.47), and reports are generally longer by approximately 34 minutes for each hour of objectively measured sleep.33 We did not collect information on frequency and duration of naps taken by mothers in this study, thus total sleep durations may be underestimated in this cohort. Future studies will require making objective assessments of maternal nightly sleep duration, number and duration of naps during the day, and sleep quality. Third, we did not have information concerning participants' shift-work or insomnia status thus cannot attribute observed associations of short sleep duration with elevated blood pressures to occupational or medical conditions associated with short sleep duration. For instance, though related, short sleep duration and insomnia are different entities. Insomnia entails dissatisfaction with the quality of sleep and an inability to sleep given adequate opportunity. Insomnia can result in short sleep duration, but individuals with short sleep duration do not necessarily suffer from insomnia (i.e., participants may sleep less because they choose to do so or because they lack the opportunity to sleep). Future studies that allow for the ascertainment of maternal insomnia, severity of insomnia and sleep quality will be needed to more thoroughly assess the impact sleep duration and sleep disorders on parturition. Fourth, although we adjusted for several potential confounders, we cannot exclude the possibility of residual confounding due to misclassification of adjusted variables (e.g., maternal pre-pregnancy body mass index) or confounding by other unmeasured variables (e.g., medication use that may impact maternal sleep). In consideration of evidence suggesting that adiposity may be a mediating factor along the causal pathway of sleep duration and blood pressure,8 we report results from multivariable models with and without adjustment for maternal pre-pregnancy body mass index. We also report results stratified by maternal lean and overweight status. Lastly, the generalizability of our study may also be limited, as our cohort was primarily comprised of Non-Hispanic White and well-educated women. The concordance of our results with those from other studies that have included racially, ethnically and geographically diverse populations,5–9,18,19 however, serve to attenuate somewhat these concerns
A number of mechanisms have been proposed by which habitual short sleep duration may lead to increased blood pressure and hypertension. Blood pressure is known to dip by an average of 10% to 20% during sleep, hence some investigators speculate that shorter sleep durations increase hemodynamic load by raising average 24-h blood pressure and heart rate, which over time can lead to structural adaptation that gradually resets the entire cardiovascular system to operate at an elevated pressure equilibrium.34,35 Alterations in the hypothalamus-pituitary-adrenal (HPA) system following sleep loss have also been proposed as a mechanism for observed blood pressure elevations after partial sleep loss. Partial sleep restriction has been shown to cause disruptions in nocturnal cortisol secretion and to acutely increase blood pressure.10,16,36,37 The HPA system becomes highly sensitive to negative feedback inhibition as a result of partial sleep deprivation.36 Increased secretion of catecholamines,38 abnormalities in sympathovagal balance10 and abnormal secretion of vasoactive hormones such as endothelin, vasopressin, and aldosterone17 have been some mechanisms thought to link acute and/or chronic sleep duration with hypertension.
Long sleep duration was also associated with elevated mean third-trimester blood pressures and preeclampsia risk. We speculate that unmeasured confounders may lead to both hypertension and increased need for sleep. Sleep disordered breathing, for instance, has been shown to be associated with hypertension39 and preeclampsia,40 and is known to fragment sleep. An alternative hypothesis is that conditions associated with mild chronic inflammation such as depression, insulin resistance, and obesity4 cause longer sleep times and alterations in sleep inducing pro-inflammatory cytokines, including interleukin-141 and tumor necrosis factor-a.42 Notably, chronic systemic inflammation,43 depression,44,45 insulin resistance,46 sleep disordered breathing,40 and obesity47 are risk factors of hypertensive disorders of pregnancy, particularly, preeclampsia.
Whatever the mechanisms, the positive relationship between maternal habitual short and long sleep duration with third-trimester blood pressure and preeclampsia risk was evident in our cohort. Taken together with previously published literature, these results suggest important health benefits of improved sleep hygiene before and during early pregnancy. If confirmed by other studies, our findings may motivate increased efforts aimed at exploring lifestyle approaches, particularly improved sleep habits, to lower preeclampsia risk. Future research with objective measures of sleep duration and sleep quality during pregnancy is needed to confirm our findings and to address whether voluntary short sleep duration and/or insomnia contribute to hypertensive disorders of pregnancy.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTs
This research was supported by awards from the National Institutes of Health (R01HD-055566 and R01HD-32562). The authors are indebted to the staff of the Center for Perinatal Studies for their expert technical assistance.
Author contributions: MAW had full access to all the data in the study and takes responsibility for the integrity of the data, the accuracy of the data analysis, and the decision to submit for publication. MAW conceived, designed, and obtained funding for the study. RSM and CQ analyzed the data. MAW and RSM drafted the manuscript. All authors interpreted the data, critically revised the draft for important intellectual content, and gave final approval of the manuscript to be published.
REFERENCES
- 1.NHLBI. National Sleep Disorders Research Plan. National Heart, Lung, and Blood Institute. Bethesda, MD: National Institutes of Health; 2003. [Google Scholar]
- 2.National Sleep Foundation. Sleep America. 2009 [Google Scholar]
- 3.IOM. Institute of Medicine, Committee on Sleep Medicine and Research. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, DC: National Academy of Sciences Press; 2006. [Google Scholar]
- 4.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 5.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 6.Cappuccio FP, Stranges S, Kandala NB, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. Erratum in: Hypertension. 2007 Nov;50:e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotani K, Saiga K, Sakane N, Mu H, Kurozawa Y. Sleep status and blood pressure in a healthy normotensive female population. Int J Cardiol. 2008;125:425–7. doi: 10.1016/j.ijcard.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 8.Knutson KL, Van Cauter E, Rathouz PJ, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med. 2009;169:1055–61. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tochikubo O, Ikeda A, Miyajima E, Ishii M. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27:1318–24. doi: 10.1161/01.hyp.27.6.1318. [DOI] [PubMed] [Google Scholar]
- 11.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–70. [PubMed] [Google Scholar]
- 12.Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab. 1999;84:1979–85. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 13.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 14.Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: Sex differences. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 17.Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35:1173–5. doi: 10.1161/01.hyp.35.5.1173. [DOI] [PubMed] [Google Scholar]
- 18.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118:1034–40. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayer O, Neuhauser H, von Kries R. Sleep duration and blood pressure in children: a cross-sectional study. J Hypertens. 2009;27:1789–93. doi: 10.1097/HJH.0b013e32832e49ef. [DOI] [PubMed] [Google Scholar]
- 20.National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Report of the National High Blood pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 21.ADA. Gestational diabetes mellitus. Diabetes Care. 2004;27:88–90. [Google Scholar]
- 22.AAP/ACOG. Guidelines for prenatal care, 3rd ed. Washington, DC: American Academy of Pediatrics/American College of Obstetricians and Gynecologists; 1992. [Google Scholar]
- 23.Thompson ML, Miller RS, Williams MA. Construction and characterisation of a longitudinal clinical blood pressure database for epidemiological studies of hypertension in pregnancy. Paediatr Perinat Epidemiol. 2007;21:477–86. doi: 10.1111/j.1365-3016.2007.00868.x. [DOI] [PubMed] [Google Scholar]
- 24.Darovic GO. Haemodynamic Monitoring: invasive and non-invasive clinical application, 3rd ed. Philadelphia, PA: W.B. Saunders; 2002. [Google Scholar]
- 25.Hedman C, Pohjasvaara T, Tolonen U, Suhonen-Malm AS, Myllyla VV. Effects of pregnancy on mothers' sleep. Sleep Med. 2002;3:37–42. doi: 10.1016/s1389-9457(01)00130-7. [DOI] [PubMed] [Google Scholar]
- 26.Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27:1405–17. doi: 10.1093/sleep/27.7.1405. [DOI] [PubMed] [Google Scholar]
- 27.Chang JJ, Pien GW, Duntley SP, Macones GA. Sleep deprivation during pregnancy and maternal and fetal outcomes: Is there a relationship? Sleep Med Rev. 2009 doi: 10.1016/j.smrv.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diggle P, Liang KY, Zeger S. Analysis of longitudinal data. Oxford: Oxford Science Publications; 1994. [Google Scholar]
- 29.Stata Corp. Stata Statistical Software: Release 8.2 Stata Corporation, College Station, TX. 2004.
- 30.MacGillivary I, Rose GA, Rowe B. Blood pressure survey in pregnancy. Clinical Sciences. 1969;37:395–407. [PubMed] [Google Scholar]
- 31.Ayala DE, Hermida RC, Mojon A, et al. Blood pressure variability during gestation in healthy and complicated pregnancies. Hypertension. 1997;30:611–8. doi: 10.1161/01.hyp.30.3.611. [DOI] [PubMed] [Google Scholar]
- 32.Ayala DE, Hermida RC, Mojon A, Fernandez JR, Iglesias M. Circadian blood pressure variability in healthy and complicated pregnancies. Hypertension. 1997;30:603–10. doi: 10.1161/01.hyp.30.3.603. [DOI] [PubMed] [Google Scholar]
- 33.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gangwisch JE, Malaspina D, Posner K, et al. Insomnia and sleep duration as mediators of the relationship between depression and hypertension incidence. Am J Hypertens. 2010;23:62–9. doi: 10.1038/ajh.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folkow B. “Structural factor” in primary and secondary hypertension. Hypertension. 1990;16:89–101. doi: 10.1161/01.hyp.16.1.89. [DOI] [PubMed] [Google Scholar]
- 36.Spath-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol Psychiatry. 1991;29:575–84. doi: 10.1016/0006-3223(91)90093-2. [DOI] [PubMed] [Google Scholar]
- 37.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 38.Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am J Hypertens. 1999;12:63–8. doi: 10.1016/s0895-7061(98)00200-3. [DOI] [PubMed] [Google Scholar]
- 39.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 40.Franklin KA, Holmgren PA, Jonsson F, Poromaa N, Stenlund H, Svanborg E. Snoring, pregnancy-induced hypertension, and growth retardation of the fetus. Chest. 2000;117:137–41. doi: 10.1378/chest.117.1.137. [DOI] [PubMed] [Google Scholar]
- 41.Obal F, Jr, Opp M, Cady AB, et al. Interleukin 1 alpha and an interleukin 1 beta fragment are somnogenic. Am J Physiol. 1990;259:R439–46. doi: 10.1152/ajpregu.1990.259.3.R439. [DOI] [PubMed] [Google Scholar]
- 42.Kapas L, Hong L, Cady AB, et al. Somnogenic, pyrogenic, and anorectic activities of tumor necrosis factor-alpha and TNF-alpha fragments. Am J Physiol. 1992;263:R708–15. doi: 10.1152/ajpregu.1992.263.3.R708. [DOI] [PubMed] [Google Scholar]
- 43.Williams MA, Farrand A, Mittendorf R, et al. Maternal second trimester serum tumor necrosis factor-alpha-soluble receptor p55 (sTNFp55) and subsequent risk of preeclampsia. Am J Epidemiol. 1999;149:323–9. doi: 10.1093/oxfordjournals.aje.a009816. [DOI] [PubMed] [Google Scholar]
- 44.Qiu C, Sanchez SE, Lam N, Garcia P, Williams MA. Associations of depression and depressive symptoms with preeclampsia: results from a Peruvian case-control study. BMC Womens Health. 2007;7:15. doi: 10.1186/1472-6874-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu C, Williams MA, Calderon-Margalit R, Cripe SM, Sorensen TK. Preeclampsia risk in relation to maternal mood and anxiety disorders diagnosed before or during early pregnancy. Am J Hypertens. 2009;22:397–402. doi: 10.1038/ajh.2008.366. [DOI] [PubMed] [Google Scholar]
- 46.Kaaja R, Tikkanen MJ, Viinikka L, Ylikorkala O. Serum lipoproteins, insulin, and urinary prostanoid metabolites in normal and hypertensive pregnant women. Obstet Gynecol. 1995;85:353–6. doi: 10.1016/0029-7844(94)00380-V. [DOI] [PubMed] [Google Scholar]
- 47.Frederick IO, Rudra CB, Miller RS, Foster JC, Williams MA. Adult weight change, weight cycling, and prepregnancy obesity in relation to risk of preeclampsia. Epidemiology. 2006;17:428–34. doi: 10.1097/01.ede.0000221028.33245.0b. [DOI] [PubMed] [Google Scholar]