Abstract
A substantial portion of patients with obstructive sleep apnea (OSA) seek alternatives to positive airway pressure (PAP), the usual first-line treatment for the disorder. One option is upper airway surgery. As an adjunct to the American Academy of Sleep Medicine (AASM) Standards of Practice paper, we conducted a systematic review and meta-analysis of literature reporting outcomes following various upper airway surgeries for the treatment of OSA in adults, including maxillomandibular advancement (MMA), pharyngeal surgeries such as uvulopharyngopalatoplasty (UPPP), laser assisted uvulopalatoplasty (LAUP), and radiofrequency ablation (RFA), as well as multi-level and multi-phased procedures. We found that the published literature is comprised primarily of case series, with few controlled trials and varying approaches to pre-operative evaluation and post-operative follow-up. We include surgical morbidity and adverse events where reported but these were not systematically analyzed. Utilizing the ratio of means method, we used the change in the apnea-hypopnea index (AHI) as the primary measure of efficacy. Substantial and consistent reductions in the AHI were observed following MMA; adverse events were uncommonly reported. Outcomes following pharyngeal surgeries were less consistent; adverse events were reported more commonly. Papers describing positive outcomes associated with newer pharyngeal techniques and multi-level procedures performed in small samples of patients appear promising. Further research is needed to better clarify patient selection, as well as efficacy and safety of upper airway surgery in those with OSA.
Citation:
Caples SM; Rowley JA; Prinsell JR; Pallanch JF; Elamin MB; Katz SG; Harwick JD. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. SLEEP 2010;33(10):1396-1407.
Keywords: Obstructive sleep apnea, surgical modifications, maxillo-mandibular advancement, uvulopalatopharyngoplasty, multi-level surgery
1.0. INTRODUCTION
For patients with obstructive sleep apnea (OSA), positive airway pressure (PAP) therapy is usually prescribed as first-line treatment, a recommendation supported by high-level evidence for efficacy of PAP in preventing upper airway collapse and relieving symptoms such as daytime sleepiness,1 and mounting data suggesting that PAP therapy may favorably impact cardiovascular outcomes.2 However, for varied reasons, some patients find it difficult to adhere to PAP therapy,3 prompting a substantial proportion to seek alternative treatment, including upper airway surgery.
Surgical modifications of the upper airway have been performed for decades as a treatment for OSA.4 Yet, the role of such procedures in the management of OSA remains controversial. Critics point to the lack of high-level, controlled studies in the surgical literature and the absence of standardized criteria to define surgical “success,”5,6 while proponents cite ethical and logistical limitations to controlled surgical studies and contend that the “all or none” principle of eradication of OSA (i.e., an apnea-hypopnea index [AHI] < 5) as the standard of care is flawed and impractical for many patients.7 The end result is a lack of a firm evidence base upon which the sleep medicine clinician can rely to guide management decisions in patients with OSA.
A few systematic reviews of surgical treatments of OSA have been previously published.8 Some reviews limited analysis to a small number of randomized, controlled trials (RCT),9 one excluded papers published before 2001,5 and results were rarely pooled. The last systematic review by the American Academy of Sleep Medicine (AASM) of non-randomized observational studies of the efficacy of surgical modifications of the upper airway for treatment of OSA was performed in 1996.10 A review and practice parameters specifically on laser-assisted uvulopalatoplasty were published in 2001.11
Clinical guidelines on the evaluation, management, and long-term care of OSA in adults were recently published by the AASM.12 These guidelines included several consensus-based statements regarding the surgical treatment of OSA, though the document was not designed to be a systematic literature review.
The AASM convened a task force in 2007 to systematically review the available literature to provide a basis for updated standards of practice recommendations on surgical modifications of the upper airway for treatment of OSA. It was decided a priori to exclude publications of isolated nasal procedures, isolated tonsillectomy, and tracheostomy, in part because these procedures are often performed for indications other than OSA.
2.0. METHODS
2.1. Search Strategy
Study eligibility was predicated upon the reporting of the apnea-hypopnea index (AHI) before and after surgical intervention. Acceptable study methodologies included randomized and non-randomized controlled trials, cohort studies and case series (sample size > 1) published in English. Those papers that only reported surgical “success” without precise outcomes data on individual study subjects were excluded.
2.2. Study Identification
An expert reference librarian (P.J.E.) designed and conducted the electronic search strategy with input from study investigators with expertise in conducting systematic reviews. To identify eligible studies, we searched electronic databases (MEDLINE, EMBASE, Current Contents, and Cochrane CENTRAL through the Ovid interface) from 1966 through June, 2008, cross-referencing the key words sleep, sleep apnea AND surgery with UPPP, uvulopalatopharyngoplasty, hypopharynx, tongue, tongue base, tongue volume reduction, epiglottis, genioglossus, advancement, mortised, genioplasty, glossectomy, tongue radiofrequency, hyoid, suspension, stabilization, maxillomandibular, osteotomy, laser assisted uvuloplasty, LAUP, palatoplasty, pharyngoplasty, palatopharyngoplasty, tracheostomy, maxillary advancement, myotomy, septoplasty, polypectomy, adenoidectomy, tonsillectomy, adenotonsillectomy, soft palate, implant, uvula, positive airway pressure, CPAP, oral appliance, mandibular advancement device, sclerosis, sclerotherapy.
Task force members screened all abstracts and titles for candidate studies. Two reviewers, working independently, screened the full text publications for eligibility. Inter-reviewer agreement was adequate (κ = 0.7); disagreements were resolved by consensus and, if needed, adjudication by a third party (S.M.C).
2.3. Data Collection
Two reviewers, working independently and using a standardized, web-based form, extracted data from all eligible studies, which included:
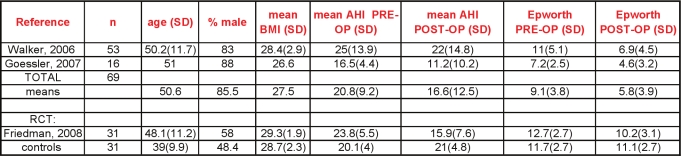
Descriptive data—sample size, mean patient age, % male gender, mean body mass index (BMI), length of follow-up
Methodologic data—method of polysomnographic monitoring (in-lab, unattended) and sleep scoring (i.e., Rechtschaffen and Kales); elements of bias protection, such as allocation concealment, blinding, proportions of patients lost to follow-up
Outcome data—the specific surgical procedure, change in the AHI, assessment of or change in validated secondary outcomes such as daytime sleepiness (Epworth Sleepiness Scale) or quality of life, major complications, or death
The data tables are available online at www.journalsleep.org.
2.4. Statistical Analysis: Meta-analysis
To describe the effect of surgery on AHI, we used a relative measure of effect, the ratio of means (ROM), which describes the extent to which the mean postoperative AHI has changed compared to the mean AHI before surgery. ROM from each study were pooled across studies—rendering a pooled ROM and its associated 95% confidence interval (CI)—using the inverse variance method for random effects meta-analysis as implemented in RevMan 5.0 (Cochrane Collaboration, 2009), as described by Friedrich and colleagues.13 To interpret this measure, readers can subtract the ratio of mean from 1 and multiply the result times 100 to obtain the percentage change in AHI with surgery, a procedure akin to the estimation of relative risk reduction from measures of relative risk. We used the I2 statistic, which estimates the percentage of total variation across studies that is due to heterogeneity rather than chance14 to describe the consistency of estimates of effect across studies. This statistic reflects the proportion of the observed variability that may be due to true differences in these studies, including differences in subject characteristics, their selection by the surgeon, the surgical technique, and sleep study methodology, quality, and scoring.
We felt that pooling of data was most appropriate for outcomes of a specific type of surgical intervention. In general, this was most feasible with studies of solitary procedures, some of which involve a standardized surgical approach, that address a specific anatomic target. On the other hand, we do recognize the growing interest in the performance of multiple interventions which may target various points of airway collapse, either simultaneously or in phased surgeries. However, because the literature on such procedures describes highly varied and heterogeneous surgical approaches, we chose not to pool such data. We would refer the reader to two systematic review papers on multi-level surgeries by Kezirian et al.15 and Lin et al.16 that reported on some outcomes in aggregate.
Because so few papers independently analyzed the influence of subgroups of variables (such as age, gender, BMI, and severity of sleep apnea at baseline) on surgical outcomes, these data were not available to us to conduct pooled subgroup analyses or to detect prognostic variables that would affect the outcomes of the different surgical procedures.
Because of the small number of studies in each subgroup, and the substantial heterogeneity in outcomes between studies of each surgical procedure, we considered a funnel plot unreliable to aid in the determination of the presence or absence of publication bias.17
3.0. EVALUATION OF TREATMENTS
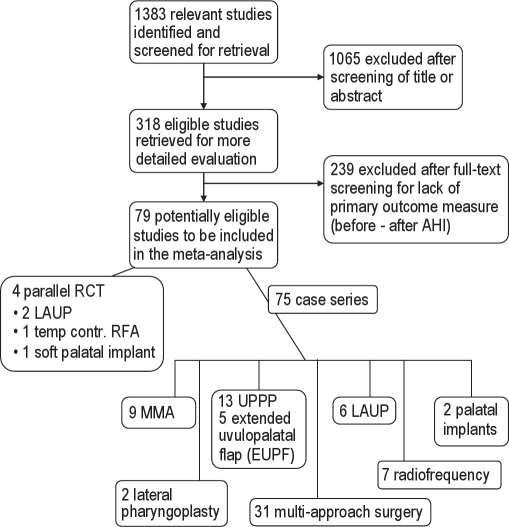
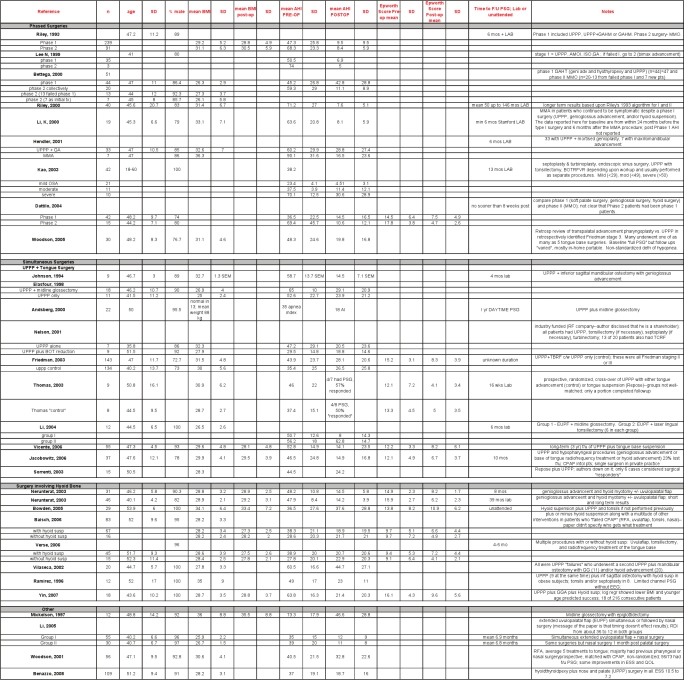
Figure 1 shows the results of our systematic search, with subdivisions by surgical procedure. Forty-seven papers described a single surgical procedure as treatment for OSA, three of which were randomized controlled trials (RCT). Thirty-two involved a multi-level surgical approach, one of which was an RCT. Table 1 summarizes the findings in an evidence table.
Figure 1.
Table 1.
Evidence table
| Quality assessment |
Summary of findings |
||||||
|---|---|---|---|---|---|---|---|
| Type of Surgery | No. of studies | Study Design | Limitations | No of patients |
% reduction in AHI (95% CI) | Quality | |
| Intervention | Control | ||||||
| MMA | 9 | Observational | Serious | 234 | NA | 87% (80 to 92) | ⊗○○○ Very low |
| UPPP | 15 | Observational | Serious | 950 | NA | 33% (23 to 42) | ⊗○○○ Very low |
| LAUPa | 2 | Randomized trial | No serious limitations | 34 | 36 | 18% (35 to -3)b | ⊗⊗○○ Low |
| RFAc | 8 | Observational | Serious | 175 | NA | 34% (19-46) | ⊗○○○ Very low |
| Implantsd | 2 | Observational | Serious | 69 | NA | 26% (9-29) | ⊗○○○ Very low |
Six observational studies not included;
95% confidence interval includes up to a 3% increase in AHI;
A single randomized trial (Woodson et al.71) not included in this table, detailed in text;
A single randomized trial (Freidman et al.72) not included in this table, detailed in text; From GRADEpro. [Computer program]. Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2008.
3.1. Maxillo-Mandibular Advancement (MMA)
Maxillo-mandibular advancement (MMA) is a multilevel skeletal surgery designed to enlarge the velo-orohypopharyngeal airway without direct manipulation of the pharyngeal tissues. It advances the anterior pharyngeal tissues (soft palate, tongue base, and suprahyoid musculature) attached to the maxilla, mandible, and hyoid bone and is accomplished by LeFort I and bilateral sagittal split rami osteotomies that are stabilized with screws, plates, or bone grafts.
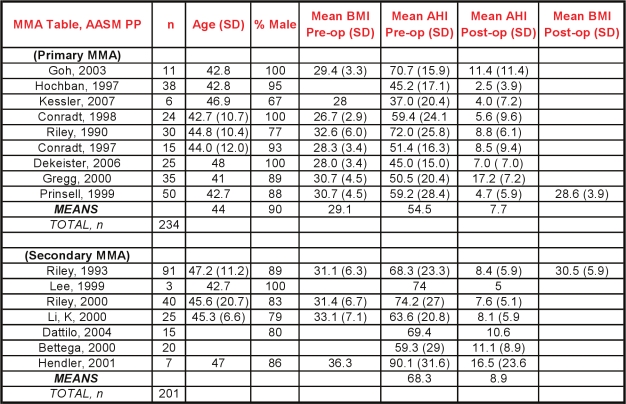
Nine case series describing maxillo-mandibular advancement (MMA) as a sole or primary intervention were included in the analysis.18–26 Two hundred thirty-four subjects were studied, 90% of whom were men, with a mean age of 43.9 years (mean range from 41 to 48). The mean BMI was 29.1 kg/m2 (mean range 26.7 to 32.6). All subjects had severe OSA at baseline, with a mean AHI of 54.4 / hour (mean ranging from 37 to 71/h).
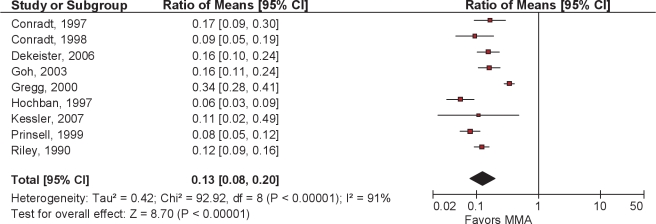
Following primary MMA surgery, there was an overall reduction in AHI of 87% (95% CI 80% to 92%) with a mean postoperative AHI of 7.7 (Figures 2 and 3). Although there was significant heterogeneity across studies with regards to this continuous outcome, as indicated by the I2 value of 91%, every paper reported substantial reductions in AHI; all but two had a residual postoperative AHI of less than 10/h.
Figure 2.
Ratio of means meta-analysis of MMA. CI refers to confidence interval.
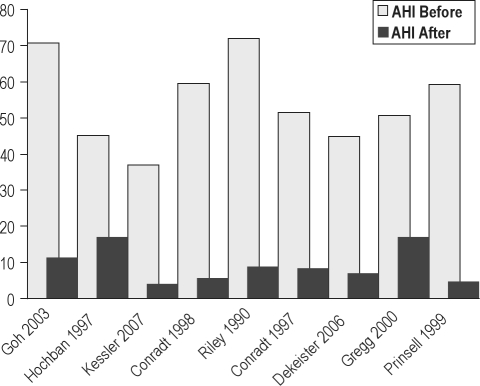
Figure 3.
Before and after mean AHI in 9 studies of MMA
Another seven case series described MMA outcomes as a secondary surgery (so-called phase II, discussed in section 3.6) following another surgery, usually a pharyngeal procedure.27–33 These seven series described a total of 201 subjects, with a mean AHI before MMA of 68.3 and a mean postoperative AHI of 8.9. Notably, in one of these series, 67 of the 91 MMA subjects did not participate in phase I of their two-phase protocol but, rather, proceeded directly to MMA,32 and in another all 15 MMA subjects received no prior surgery.28
Commonly utilized anatomic criteria for MMA are hypopharyngeal and/or velo-orohypopharyngeal narrowing, which usually occur with co-existent skeletal hypoplasia, and may clinically manifest as retrognathia. However, two case series reported efficacy of MMA in the absence of maxillomandibular horizontal deficiency.22,31 Although the preoperative evaluation to select optimal surgical candidates in MMA has yet to be standardized, one paper describing 50 consecutive patients reported universal success in those felt to have radiographic evidence of hypopharyngeal narrowing.22
Secondary measures, such as sleepiness or quality of life were rarely measured in these case series. One reported a reduction in the Epworth score from 17.8 to 4.7,28 another reported improvements in blood pressure 6 months after MMA.22
No serious adverse events were reported in this group of publications, though it is important to note that this review did not systematically explore adverse outcomes and the findings here rely entirely on author self-reporting. MMA is a lengthy and technically challenging procedure and presents inherent risks of dental malocclusion and facial neurosensory deficits.
3.2. Soft Palatal Procedures
Soft palatal procedures serve to reduce or reconstruct the collapsible portions of the soft palate. Acknowledging the difficulties in accounting for subtle differences in technique between surgeons which may or may not be apparent on paper, the task force aimed to differentiate between what was felt to represent traditional pharyngeal surgical approaches (see section 3.2.1 below) and modifications to that approach (section 3.2.2), while striving to avoid oversimplification of such distinctions. The reader is also referred to the sections on multi-level surgeries (3.6 and 3.7), which also often involve soft palatal interventions.
3.2.1. Uvulopalatopharyngoplasty (UPPP)
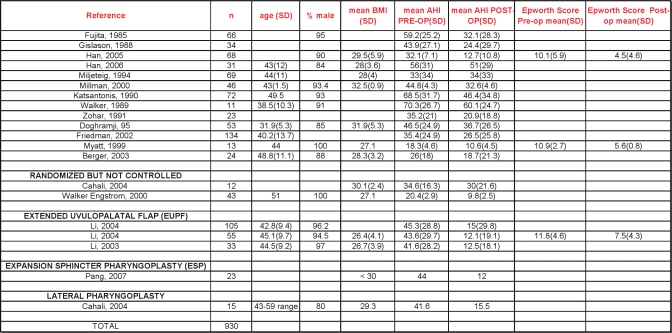
UPPP, as first described by Fujita,4 involves excision of the tonsils and posterior soft palate /uvula, and closure of the tonsillar pillars. Fifteen papers describing outcomes following UPPP were included.34–49 Two were randomized prospective trials that compared UPPP with other active treatments, namely oral appliances47 and another surgical procedure, lateral pharyngoplasty.35 The remaining were either prospective or retrospective observational reports.
The group had a mean age of 44 years and was composed of 91.9% males. The average body mass index, where reported, was 29 kg/m2. The apnea-hypopnea index at baseline averaged 40.3/hour. Follow-up sleep studies (some attended, in-lab multi-channel, others portable polygraphy) occurred anywhere from 3 months to one year later.
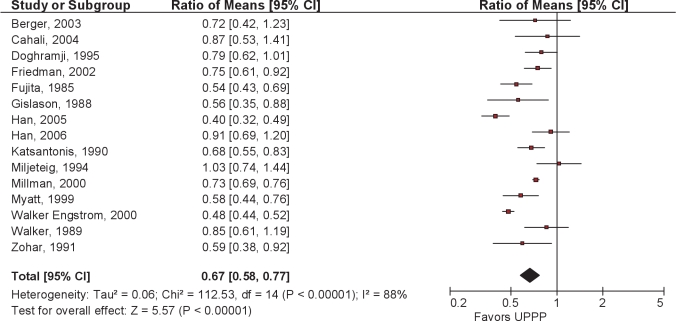
Following UPPP, there was an overall reduction in AHI of 33% (95% CI 23% to 42%). Postoperative residual AHI remained elevated, averaging 29.8/hour (Figures 4 and 5).
Figure 4.
Ratio of means meta-analysis of UPPP. CI refers to confidence interval.
Figure 5.
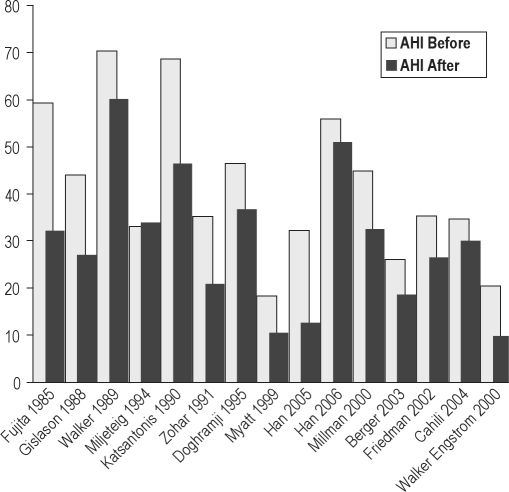
Before and after mean AHI in 15 studies of UPPP
Most papers described varied approaches to the pre-operative evaluation of potential surgical candidates, including endoscopy, cephalometry, and computed tomography (CT) scans (Table 2). The presence or absence of tonsils was not always specified. In general, the relationship of such variables with surgical success was not analyzed. Friedman et al.37 did propose a method of classification of pre-operative tonsil size, tongue-palate position, and BMI that could be used for refining the prediction of success for subgroups of patients who undergo UPPP. Whether standardized clinical measures and/or imaging studies will improve patient selection and lead to improved surgical outcomes requires further research.
Table 2.
Preoperative assessment and reported predictors of success in pharyngeal surgeries
| Study # | Pre-op assessment performed | Predictors of success |
|---|---|---|
| Berger, 2003 (LAUP study) | “Full otolaryngologic examination using flexible scope” but further details not provided | Not analyzed |
| Cahali, 2004 | CT scans | Not analyzed |
| Doghramji, 1995 | Cephalometry Mueller's maneuver at both naso- and oropharynx; scored on scale of 1 (minimal change) to 4 (complete collapse) | No cephalometric variable or the Mueller's maneuver correlated predicted response. Age, BMI, and AHI were not different between the two groups |
| Friedman, 2002 | Friedman palate position and tonsil size were combined into a staging scheme; note all BMI > 40 put into highest stage | Stage 1 had clearly higher success rate (80%) than higher stages (< 40%); also created an equation that could be used to predict success/failure based upon tonsil score and Friedman palate position |
| Fujita, 1985 | Upper airway examination performed but details not provided | Not analyzed |
| Gislason, 1988 | Neck CT and cephalometry Ventilatory response to hyperoxic hypercapnia | Univariate analysis: predictors of success were lower AHI, lower BMI, smaller tongue width. Multivariate analysis: both AHI and BMI were significant predictors |
| Han, 2005 | Oropharyngeal cavity dimensions | Not analyzed |
| Han, 2006 | Not mentioned | Not analyzed |
| Katsantonis, 1990 | Not mentioned | Not analyzed |
| Miljeteig, 1994 | Upper airway examination performed but details not provided | Not analyzed |
| Millman, 2000 | Cephalometry | Only MP-H distance (≤ 20 mm) was significant in univariate and multivariate models; AHI (< 38) and ANB angle (< 3°, c/w retrognathia) included in model but were not significant. |
| Myatt, 1999 | Nasoendoscopy | Not analyzed specifically, though conclusions are presented in the abstract that are not supported by results section |
| Walker, 1989 | Examination by ENT but no details provided | Not analyzed |
| Walker-Engstrom, 2000 | Not mentioned | Not analyzed |
| Wilhelmsson, 1999 | Mueller's maneuver with endoscopy at oropharynx; separated collapse by location: 1: oropharynx, 3: hypopharynx; 2: both | Obstructive type 2 and 3 had lower success rate; higher BMI correlated with a larger improvement in AI (AHI not mentioned) |
| Zohar, 1991 | Nasoendoscopy | Not analyzed |
Dates of publication ranged from 1985 to 2006. The bar graphs in Figure 5, which are chronologically arranged from left to right by decade, show no apparent time-dependent change in outcomes.
The Epworth Sleepiness Scale was measured in 2 studies40,45; the reductions following surgery were without a controlled comparison. Multiple sleep latency testing (MSLT) reported in one paper showed an increase from a mean of 6.9 minutes to 7.7 minutes following the procedure.36
Some papers in these series reported adverse events following UPPP surgery. Two cases of post-operative bleeding were reported. In these papers, no deaths were reported. Other review papers have specifically assessed postoperative morbidity and mortality. Side effects include difficulty swallowing/nasal regurgitation, taste disturbances, and voice changes.9 A recent systematic review of side effects of surgery for snoring and OSA found 7 papers reporting at least one death after UPPP, with an overall prevalence between 0% and 16%.9 The authors found that lower complication rates are reported in more recent publications than those published in the 1980s. A large survey of the VA Administration records, yielding a group of middle-aged men similar to the demographic described in our review, reported a 1% to 2% risk of a life-threatening adverse events and 0.2% risk of death following UPPP.50
These data leave questions about specific sub-groups of patients unanswered. Outcomes in women, some minorities, and those more than 50 years of age are lacking. Whether body weight imparts influence on outcomes is not clearly evident from the current dataset, though the suggestion by Friedman et al. that a BMI > 40 kg/m2 is a marker of poorer outcomes is notable37 and could serve as a model for future research. The effects of UPPP on the cardiovascular and/or systemic sequelae of OSA remain to be fully elucidated. The efficacy of novel variations in pharyngeal surgery, such as EUPF51 and lateral pharyngoplasty35 as reported by papers from single centers (see subsequent section), are promising and warrant further investigation.
3.2.2. Modified UPPP and other pharyngeal procedures
One multidisciplinary group from Taiwan published three papers describing the extended uvulopalatal flap (EUPF), a modified UPPP where additional mucosa and submucosal adipose tissue is removed superior and lateral to the tonsillar fossa and from the posterior soft palate. In some cases nasal surgery was also performed.51–53 Major reductions in AHI /RDI were reported in young, predominantly male Asian populations with severe OSA at baseline. Better response rates were seen in those with lower Mallampati scores. One paper reported significant improvements in quality of life (QOL) based upon the SF-36 form, but these changes did not correlate with RDI.52 The same publication showed an improvement in sleepiness as measured by the Epworth scale.
In a prospective trial utilizing a technique called expansion sphincter pharyngoplasty, Pang and colleagues found substantial reductions in AHI, improving from 44/h to 12/h. Notably, the 23 subjects comprised a highly select group, with baseline characteristics to include a BMI < 30, small tonsils and, using Friedman and Fujita scales, clinical evidence for retropalatal obstruction, and lateral pharyngeal wall collapse.54
Two papers were published by a single group from Brazil describing outcomes after lateral pharyngoplasty, a modified form of uvulopalatoplasty where tissue from the lateral free margin of the soft palate is removed.35,55 One of the papers, as a doctoral thesis, was a prospective, randomized trial compared with conventional UPPP.35 Both papers included a small number of patients who were selected on the basis of bulky lateral oropharyngeal tissues. Mean AHI at baseline was > 40, with follow-up 6 months later showing AHI of approximately 15.
3.3. Laser Assisted Uvulopalatoplasty (LAUP)
This outpatient surgical technique involves a series of laser incisions and vaporizations designed to shorten the uvula and modify and tighten the soft palatal tissue.
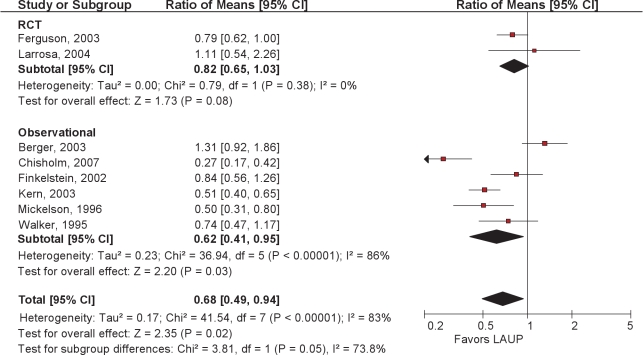
The papers consisted of 2 RCTs and 6 observational studies. LAUP resulted in an average pooled reduction in AHI of 32% (Figure 6).
Figure 6.
Ratio of means meta-analysis of LAUP. CI refers to confidence interval.
The 2 randomized controlled trials of LAUP were conducted in patients with mild to moderate OSA; Ferguson and colleagues56 compared LAUP with no treatment. Subjects were well matched at baseline, with mean age 47 years and mean BMI 31.6. The AHI went from a mean of 18.6/h at baseline to 14.7/h after LAUP, measured a mean of 15 months after baseline PSG and 7 months after the last LAUP treatment. The AHI increased in the control group from 16.1 to 22.7/h. There were no significant improvements in daytime sleepiness or quality of life associated with LAUP.
Larrosa et al.57 randomized 28 subjects to LAUP or sham surgery, 25 of whom completed follow-up. Mean age was 44 years, mean BMI 27.2. Following LAUP, the mean AHI increased from 13.6 to 15.1. No significant changes were seen in any secondary outcome measures.
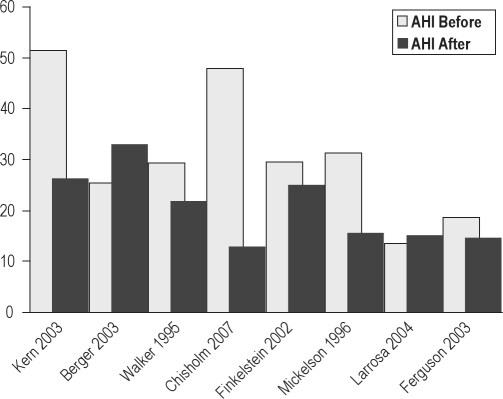
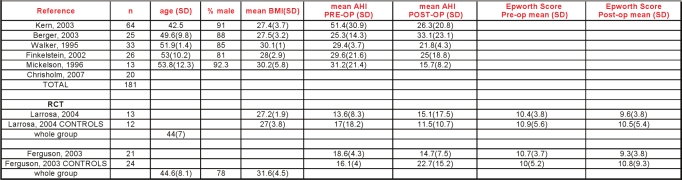
The 6 observational reports34,58–62 studied patients who were slightly older (mean age 50 years) and had more severe OSA, with an average baseline AHI in excess of 30 per hour. In their case series of 20 subjects, Chisholm and Kotecha attributed a 73% reduction in AHI to careful selection based upon sleep nasendoscopy.62 Although 12 of the 20 had concomitant tonsillectomy, this was not a factor in surgical response. Tonsillectomy was performed in another study of LAUP where somewhat larger reductions in AHI were noted.59 One observational study34 showed a significant net increase in AHI after surgery. Sleepiness was measured in a minority of papers. A summary of the LAUP AHI data is shown in Figure 7.
Figure 7.
Before and after mean AHI in 8 studies of LAUP.
3.4. Upper Airway Radiofrequency Treatment
Radiofrequency has been used in the upper airway with and without temperature control of the probe tip. Prior to its use in the setting of OSA, this temperature controlled technique had been utilized in surgical procedures involving other organ systems, and was developed with the intent to precisely apply temperature controlled energy to target tissue while sparing adjacent tissues using a thermocouple on the probe tip.
3.4.1. Upper airway radiofrequency treatment of the soft palate
3.4.2. Upper airway radiofrequency treatment of the base of tongue
3.4.3. Upper airway radiofrequency treatment of multiple levels
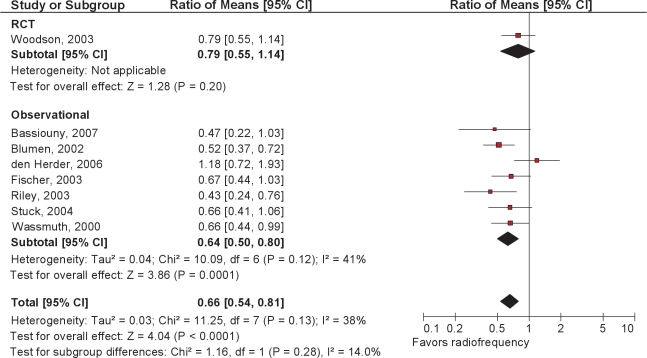
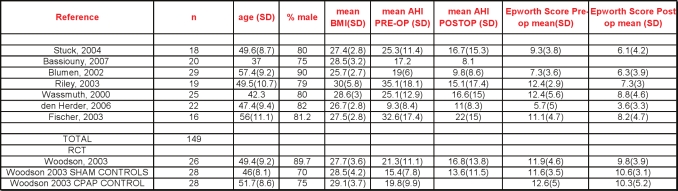
The papers describing RF treatment at multiple levels consisted of 1 RCT and 2 observational studies.69–71
3.4.4. Analysis
The 1 randomized trial compared temperature controlled radiofrequency tissue ablation (TCRFA) of the tongue and soft palate with sham surgery and CPAP in those with mild to moderate OSA.71 The stated primary outcomes were vigilance testing (reaction time) and quality of life. In the active surgical treatment group (90% male, mean BMI 27.7, mean age 49) the AHI was reduced an average of 4.5 /h from a baseline of 21/h (21% reduction). The AHI in the sham surgical group decreased an average of 1.8/h, from a baseline of 15.4/h. Follow-up mean AHI in the CPAP group was not reported. Compared with sham surgery, both the surgical and CPAP groups demonstrated more improvements in measures of quality of life. However, only the TCRFTA group had significantly shorter reaction times than the sham group.
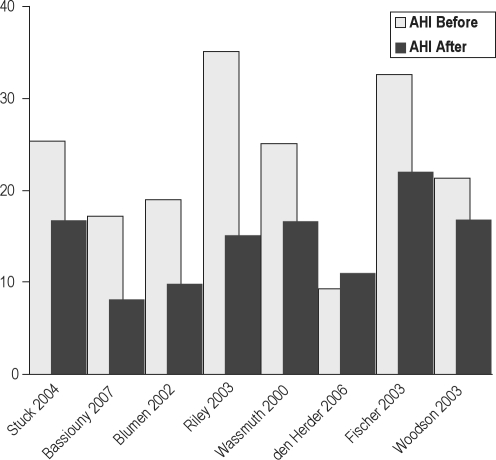
The 7 observational studies63–70 showed a change in mean AHI from 23.4 to 14.2 following RFA. The significance of the reduction in Epworth score from 9.7 to 6.7 in the absence of comparisons controlling for placebo effects is not known (Figures 8 and 9).
Figure 8.
Ratio of means meta-analysis of upper airway radiofrequency ablation. CI refers to confidence interval.
Figure 9.
Before and after mean AHI in 8 studies of RFA.
3.5. Soft Palatal Implants
Soft palatal implants potentially represent a less invasive or lower morbidity procedure for treating the palate for mild to moderate OSA. In this procedure, Dacron rods are inserted into the soft palate under local anesthesia. One randomized, double-blind, sham surgery controlled trial has been published.72 Unbalanced randomization resulted in a significantly older treatment group (48.1 vs. 39 years); males comprised about half of the study subjects. The mean BMI was about 29 kg/m2, and the mean neck circumference was reported at less than 15 inches in both groups. The authors reported statistically significant changes in outcomes. There were modest reductions in AHI with treatment (23.8 before, 15.9 after), and the mean Epworth score was > 10 following intervention.
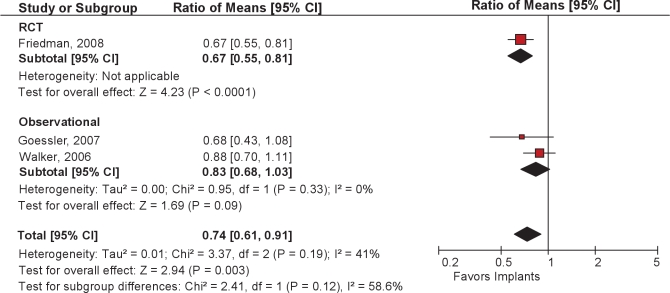
Two case series73,74 studied populations of predominantly non-obese men approximating 50 years of age. For all 3 studies, the pooled reduction in AHI after soft palatal implants placement was 26% (95CI 9% to 39%) (Figures 10 and 11).
Figure 10.
Ratio of means meta-analysis of soft palatal implants. CI refers to confidence interval.
Figure 11.
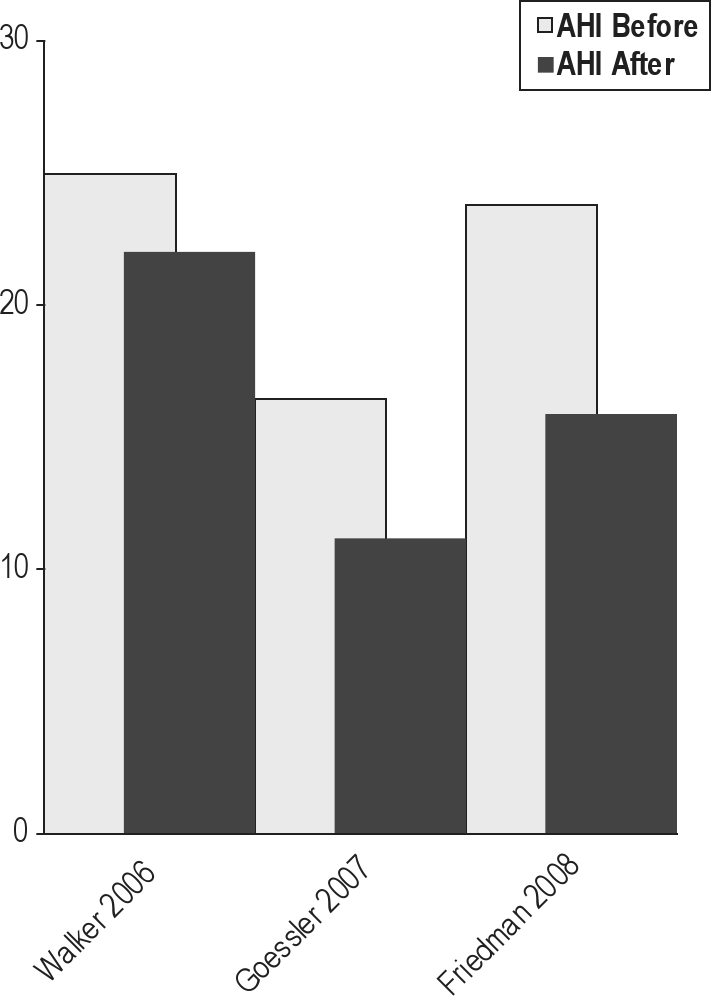
Before and after mean AHI in 3 studies of palatal implants.
3.6. Multi-level Simultaneous Surgery
Because collapse can occur at various sites along the upper airway, some have advocated a multi-level approach to the surgical treatment of OSA. These include MMA and RF treatments (discussed previously), and a variety of other combinations of procedures during the same surgical session (non-phased). The majority of these combine UPPP with a surgical procedure that involves surgery on the tongue, whether radiofrequency ablation, mid-line glossectomy, tongue advancement, or tongue suspension. All of the studies are case series.
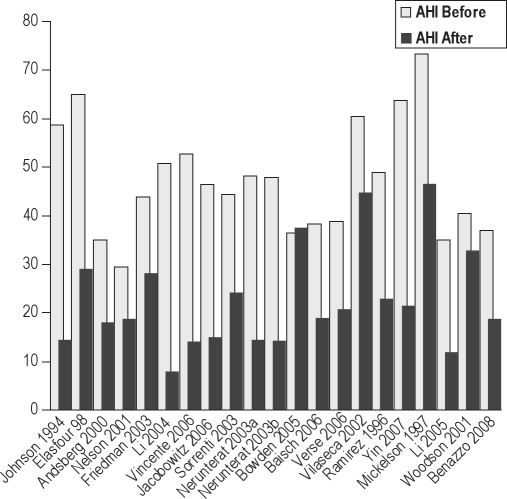
Our search found 31 papers describing such surgery that met criteria for inclusion (Figure 12).27–33,75–98 As noted above, the heterogeneity of the approach to and choice of surgical procedures precluded us from pooling outcomes data, though we refer to other papers that attempted such an approach.15,16 We make some general comments with regard to some papers. It is acknowledged that observational reports of multi-phase surgery are biased by the self-selection of patients who willingly returned for a subsequent surgical procedure.
Figure 12.
Before and after mean AHI in simultaneous surgeries.
Kao82 demonstrated significant improvements in AHI following nasal, sinus and/or palatal surgery in a small group of men with mild to moderate, but not severe OSA. Two papers showed significant short-term improvements in AHI in a small number of Asian patients following various phase I interventions; subjective sleepiness was also reduced.83,87
Two papers compared the results of multi-level surgery to UPPP alone. The largest series was reported by Friedman.79 In this study, the results of UPPP with radiofrequency ablation in 143 patients were compared to UPPP alone, performed in 143 subjects. In the UPPP+RFA group, mean AHI decreased from 44 to 28, with an overall reported success rate of 40% (using a 50% reduction and final AHI < 20 as criteria for success). Postoperative AHI data for the UPPP only group was not reported. However, when the patients were divided by stage (using the Friedman classification), success was significantly higher in the UPPP+RFA group compared to the UPPP group (for Stage II patients, 55.1% v. 37.9%, for Stage III patients, 33.0% v. 8.1%). In a much smaller series, Nelson compared 9 patients who received UPPP+RFA to 7 patients who received UPPP alone. There was no difference in reported surgical success between the 2 groups (50.0% for UPPP+RF and 57.1% for UPPP alone).
Two other larger series have reported results of multilevel surgery combining UPPP with tongue surgery. Vicente91 reported 3 years postoperative results in a group of 55 patients (mostly male) with a mean pre-operative AHI of 53. Mean postoperative AHI was 14.9, and overall success (defined as Epworth < 11, AHI < 20, with a reduction > 50% from baseline) was 78%. Jacobowitz80 reported results in 37 patients who underwent UPPP with genioglossal advancement and/or radiofrequency ablation with a hyoid suspension (not usually performed at same time as the tongue surgery) in a subset of patients. Overall AHI decreased from 46.5 to 14.0, with an overall success rate of 76% (AHI < 20 with 50% reduction). There was no breakdown of results for those with tongue surgery only.
Several studies have reported results in patients who had hyoid surgery in addition to other upper airway or tongue procedures. Baisch76 reported the results of 83 patients, 67 of whom had multi-level surgery including hyoid suspension and 16 who had multi-level surgery without hyoid suspension. AHI significantly decreased in the patients whose surgery included hyoid suspension (38.3 to 18.9) and did not decrease in patients without hyoid suspension (28.6 to 21.7). Verse90 studied 45 patients with uvulopalatal flap and radiofrequency ablation of the tongue with hyoid suspension, and compared the results to 15 patients with the flap and ablation without hyoid suspension. AHI significantly decreased in the group who had a hyoid suspension (38.9 to 20.7), while there was no change in the group who did not have a hyoid suspension (27.8 to 22.9). The success rate in the group with hyoid suspension (51% using AHI < 15 and 50% reduction as criteria for success) was higher than in the no hyoid suspension group (40%). Neruntarat88 reported long-term (mean time to repeat of PSG of 39 months) follow-up of 46 patients who underwent geniohyoid advancement and hyoid myotomy. AHI significantly decreased from 48 to 14, and overall success was 78.3% for AHI < 20 and 50% reduction. In contrast, in a group of 29 patients who had hyoid suspension in conjunction with UPPP (n = 28) without tongue surgery, there was only a 17% success rate with no significant decrease in the AHI.77
3.7. Multi-Level Phased Surgeries
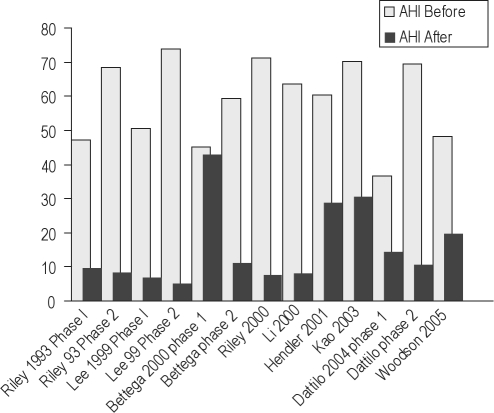
Some have advocated a multi-phase approach to the surgical treatment of OSA. Riley and colleagues32 were among the first to describe observations in 306 of 415 patients who completed a step-wise (multi-phase) surgical protocol. Although a select group of patients, this cohort remains the largest published to date. Choice of surgical procedure was determined by a preoperative evaluation including awake endoscopy and cephalometric measurements. Phase I surgery included UPPP and/or genioglossus advancement and hyoid myotomy (GAHM). Phase II, utilizing maxillary and mandibular advancement osteotomy (MMO), was offered to those who failed to respond to phase I but was also performed as the first procedure on 67 of 91 patients who did not enter the protocol. Overall, the mean RDI was significantly improved after completion of both phases of the surgical protocol. Notably, while the authors reported a 95% long-term success rate upon completion, 61% of patients were deemed successfully treated after phase I surgery, compared with 97% after phase II. The authors found those who were more obese or with more severe OSA were less likely to respond to a phase I intervention.
In a group of 27 patients, Lee et al.30 utilized a similar surgical approach as Riley, reporting a higher success rate in phase I, with only 3 patients advancing to phase II. Bettega and colleagues27 reported little change in the AHI following phase I surgery, but significant improvements after phase II.
Dattilo and Drooger assessed change in AHI and Epworth score in subjects who underwent a combination of phase I procedures compared with MMA (criteria for patient and surgery selection not specified). A higher proportion of patients in the MMA group achieved surgical success (defined as postoperative AHI < 15, reduced by at least 50% from baseline) and had a postoperative Epworth score ≤ 10, compared to patients treated with phase I procedures.28 Figure 13 summarizes the findings.
Figure 13.
Before and after mean AHI in phased surgeries.
4.0. CONCLUSIONS
In this systematic review of surgical modifications of the upper airway, we have found a paucity of high level evidence upon which to base standards of practice recommendations (see accompanying Practice Parameter paper).99 Most of the data are drawn from small case series of selected patients, in whom there were varied preoperative and surgical approaches. Sleep study methodologies were often not standardized. We acknowledge the limitations of relying on the apnea-hypopnea index as the primary outcome of interest. While the AHI is imperfect, at present, there is not a superior objective measure of treatment efficacy.
Data on non-respiratory sleep outcomes, quality of life, and cardiovascular/systemic interactions are sparse. The optimal components of a preoperative evaluation are not certain; the role of clinical exam, imaging studies, and endoscopy need to be clarified.
Limited outcomes following MMA described here are promising. Patients who underwent MMA as a primary procedure were found to have substantial reductions in AHI across studies. Furthermore, it would appear that significant benefit of “phased” surgeries for patients failing phase 1 treatment were derived from MMA (phase II). Further research is needed to confirm promising long-term results reported in one study.33 Finally, since this review did not systematically assess morbidity of MMA, more data are needed on its safety and to determine if it can or should be performed in a wider population of patients.
Regarding isolated pharyngeal/soft palatal interventions, these data showed many of these procedures to inconsistently reduce the AHI. A few papers, particularly descriptions of UPPP, reported some morbidity. Many of the isolated pharyngeal procedures resulted in many patients having a significant level of residual sleep apnea post surgery, even in those with mild to moderate OSA at baseline.
Significant improvements in AHI were reported in some small series of multi-level surgeries, the efficacy of which were attributed in part to careful patient selection. Acknowledging these outcomes and the increasing interest in multi-level surgeries, we suggest that further trials focusing on standardization of preoperative approach and surgical targets seem warranted.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Caples has received research support from ResMed and Ventus Medical. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This paper was funded and supported by the American Academy of Sleep Medicine. The contributions of Ms. Pat J. Erwin, Mayo Clinic Librarian in the systematic search process are greatly appreciated. We also acknowledge the systematic review expertise and statistical support of the Mayo Clinic Knowledge and Encounter Research (KER) Unit, especially Melanie Lane and director Victor Montori, MD.
Table S1.
MMA
Table S2.
UPPP
Table S3.
LAUP
Table S4.
Radiofrequency
Table S5.
Soft palatal implants
Table S6.
Multilevel
REFERENCES
- 1.Engleman HM, Douglas NJ. Sleep. 4: Sleepiness, cognitive function, and quality of life in obstuctive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59:618–22. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haentjens P, Van Meerhaeghe A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlld randomized trials. Arch Intern Med. 2007;167:757–64. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 3.McArdle N, Devereux G, et al. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1108–14. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 4.Fujita S, Conway W, et al. Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg. 1981;89:923–34. doi: 10.1177/019459988108900609. [DOI] [PubMed] [Google Scholar]
- 5.Elshaug AG, Moss JR, Southcott AM, Hiller JE. Redefining success in airway surgery for obstructive sleep apnea: a meta analysis and synthesis of the evidence. Sleep. 2007;30:461–7. doi: 10.1093/sleep/30.4.461. [DOI] [PubMed] [Google Scholar]
- 6.Phillips B. Upper airway surgery does not have a major role in the treatment of sleep apnea. Con. J Clin Sleep Med. 2005;1:241–5. [PubMed] [Google Scholar]
- 7.Powell N. Upper airway surgery does have a major role in the treatment of obstructive sleep apnea “the tail end of the dog”. Pro. J Clin Sleep Med. 2005;1:236–40. [PubMed] [Google Scholar]
- 8.Sundaram S, Lim J, Lasserson TJ. Surgery for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2008;(Issue 3) doi: 10.1002/14651858.CD001004.pub2. Art. No: CD001004. [DOI] [PubMed] [Google Scholar]
- 9.Franklin KA, Heighway K, et al. Effects and side-effects of surgery for snoring and obstructive sleep apnea--a systematic review. Sleep. 2009;32:27–36. [PMC free article] [PubMed] [Google Scholar]
- 10.Sher AE, Schechtman KB, et al. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–77. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 11.Littner M, Kushida CA, Hartse K, et al. Practice parameters for the use of laser-assisted uvulopalatoplasty: an update for 2000. Sleep. 2001;24:603–19. doi: 10.1093/sleep/24.5.603. [DOI] [PubMed] [Google Scholar]
- 12.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 13.Friedrich JO, Adhikari NK, Beyene J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: a simulation study. BMC Med Res Methodol. 2008;8:32. doi: 10.1186/1471-2288-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ Clin Res Ed. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kezirian EJ, Goldberg AN. Hypopharyngeal surgery in obstructive sleep apnea: an evidence-based medicine review. Arch Otolaryngol Head Neck Surg. 2006;132:206–13. doi: 10.1001/archotol.132.2.206. [DOI] [PubMed] [Google Scholar]
- 16.Lin H-C, Friedman M, Chang H-W, Gurpinar B. The efficacy of multilevel surgery of the upper airway in adults with obstructive sleep apnea/hypopnea syndrome. Laryngoscope. 2008;118:902–8. doi: 10.1097/MLG.0b013e31816422ea. [DOI] [PubMed] [Google Scholar]
- 17.Lau J, Ioannidis J, Terrin N, et al. Evidence based medicine - The case of the misleading funnel plot. BMJ. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conradt R, Hochban W, Brandenburg U, Heitmann J, Peter JH. Long-term follow-up after surgical treatment of obstructive sleep apnoea by maxillomandibular advancement. Eur Respir J. 1997;10:123–8. doi: 10.1183/09031936.97.10010123. [DOI] [PubMed] [Google Scholar]
- 19.Goh YH, Lim KA. Modified maxillomandibular advancement for the treatment of obstructive sleep apnea: a preliminary report. Laryngoscope. 2003;113:1577–82. doi: 10.1097/00005537-200309000-00031. [DOI] [PubMed] [Google Scholar]
- 20.Gregg JM, Zedalis D, Howard CW, Boyle RP, Prussin AJ. Surgical alternatives for treatment of obstructive sleep apnoea: review and case series. Ann R Australas Coll Dent Surg. 2000;15:181–4. [PubMed] [Google Scholar]
- 21.Kessler P, Ruberg F, Obbarius H, Iro H, Neukam FW. [Surgical management of obstructive sleep apnea] Mund Kiefer Gesichtschir. 2007;11:81–8. doi: 10.1007/s10006-007-0051-y. [DOI] [PubMed] [Google Scholar]
- 22.Prinsell JR. Maxillomandibular advancement surgery in a site-specific treatment approach for obstructive sleep apnea in 50 consecutive patients. Chest. 1999;116:1519–29. doi: 10.1378/chest.116.6.1519. [DOI] [PubMed] [Google Scholar]
- 23.Riley RW, Powell NB, Guilleminault C. Maxillofacial surgery and nasal CPAP. A comparison of treatment for obstructive sleep apnea syndrome. Chest. 1990;98:1421–5. doi: 10.1378/chest.98.6.1421. [DOI] [PubMed] [Google Scholar]
- 24.Conradt R, Hochban W, Heitmann J, et al. Sleep fragmentation and daytime vigilance in patients with OSA treated by surgical maxillomandibular advancement compared to CPAP therapy. J Sleep Res. 1998;7:217–23. doi: 10.1046/j.1365-2869.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- 25.Dekeister C, Lacassagne L, Tiberge M, Montemayor T, Migueres M, Paoli JR. [Mandibular advancement surgery in patients with severe obstructive sleep apnea uncontrolled by continuous positive airway pressure. A retrospective review of 25 patients between 1998 and 2004] Revue des maladies respiratoires. 2006;23(5 Pt 1):430–37. doi: 10.1016/s0761-8425(06)71813-7. [DOI] [PubMed] [Google Scholar]
- 26.Hochban W, Conradt R, Brandenburg U, Heitmann J, Peter JH. Surgical maxillofacial treatment of obstructive sleep apnea. Plast Reconstr Surg. 1997;99:619–26. doi: 10.1097/00006534-199703000-00002. discussion 627-8. [DOI] [PubMed] [Google Scholar]
- 27.Bettega G, Pepin JL, Veale D, Deschaux C, Raphael B, Levy P. Obstructive sleep apnea syndrome. fifty-one consecutive patients treated by maxillofacial surgery. Am J Respir Crit Care Med. 2000;162(2 Pt 1):641–9. doi: 10.1164/ajrccm.162.2.9904058. [DOI] [PubMed] [Google Scholar]
- 28.Dattilo DJ, Drooger SA. Outcome assessment of patients undergoing maxillofacial procedures for the treatment of sleep apnea: comparison of subjective and objective results. J Oral Maxillofac Surg. 2004;62:164–8. doi: 10.1016/j.joms.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Hendler BH, Costello BJ, Silverstein K, Yen D, Goldberg A. A protocol for uvulopalatopharyngoplasty, mortised genioplasty, and maxillomandibular advancement in patients with obstructive sleep apnea: an analysis of 40 cases. J Oral Maxillofac Surg. 2001;59:892–97. doi: 10.1053/joms.2001.25275. discussion 898-9. [DOI] [PubMed] [Google Scholar]
- 30.Lee NR, Givens CD, Jr, Wilson J, Robins RB. Staged surgical treatment of obstructive sleep apnea syndrome: a review of 35 patients. J Oral Maxillofac Surg. 1999;57:382–5. doi: 10.1016/s0278-2391(99)90272-0. [DOI] [PubMed] [Google Scholar]
- 31.Li KK, Riley RW, Powell NB, Guilleminault C. Maxillomandibular advancement for persistent obstructive sleep apnea after phase I surgery in patients without maxillomandibular deficiency. Laryngoscope. 2000;110(10 Pt 1):1684–8. doi: 10.1097/00005537-200010000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Riley RW, Powell NB, Guilleminault C. Obstructive sleep apnea syndrome: a review of 306 consecutively treated surgical patients. Otolaryngol Head Neck Surg. 1993;108:117–25. doi: 10.1177/019459989310800203. [DOI] [PubMed] [Google Scholar]
- 33.Riley RW, Powell NB, Li KK, Troell RJ, Guilleminault C. Surgery and obstructive sleep apnea: long-term clinical outcomes. Otolaryngol Head Neck Surg. 2000;122:415–21. doi: 10.1016/S0194-5998(00)70058-1. [DOI] [PubMed] [Google Scholar]
- 34.Berger G, Stein G, Ophir D, Finkelstein Y. Is there a better way to do laser-assisted uvulopalatoplasty? Arch Otolaryngol Head Neck Surg. 2003;129:447–53. doi: 10.1001/archotol.129.4.447. [DOI] [PubMed] [Google Scholar]
- 35.Cahali MB, Formigoni GG, Gebrim EM, Miziara ID. Lateral pharyngoplasty versus uvulopalatopharyngoplasty: a clinical, polysomnographic and computed tomography measurement comparison. Sleep. 2004;27:942–50. doi: 10.1093/sleep/27.5.942. [DOI] [PubMed] [Google Scholar]
- 36.Doghramji K, Jabourian ZH, Pilla M, Farole A, Lindholm RN. Predictors of outcome for uvulopalatopharyngoplasty. Laryngoscope. 1995;105(3 Pt 1):311–14. doi: 10.1288/00005537-199503000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg. 2002;127:13–21. doi: 10.1067/mhn.2002.126477. [DOI] [PubMed] [Google Scholar]
- 38.Fujita S, Conway WA, Zorick FJ, et al. Evaluation of the effectiveness of uvulopalatopharyngoplasty. Laryngoscope. 1985;95:70–4. doi: 10.1288/00005537-198501000-00017. [DOI] [PubMed] [Google Scholar]
- 39.Gislason T, Lindholm CE, Almqvist M, et al. Uvulopalatopharyngoplasty in the sleep apnea syndrome. Predictors of results. Arch Otolaryngol Head Neck Surg. 1988;114:45–51. doi: 10.1001/archotol.1988.01860130049013. [DOI] [PubMed] [Google Scholar]
- 40.Han D, Ye J, Lin Z, Wang J, Wang J, Zhang Y. Revised uvulopalatopharyngoplasty with uvula preservation and its clinical study. ORL J Otorhinolaryngol Relat Spec. 2005;67:213–9. doi: 10.1159/000087390. [DOI] [PubMed] [Google Scholar]
- 41.Han F, Song W, Li J, Zhang L, Dong X, He Q. Influence of UPPP surgery on tolerance to subsequent continuous positive airway pressure in patients with OSAHS. Sleep Breath. 2006;10:37–42. doi: 10.1007/s11325-005-0041-y. [DOI] [PubMed] [Google Scholar]
- 42.Katsantonis GP, Miyazaki S, Walsh JK. Effects of uvulopalatopharyngoplasty on sleep architecture and patterns of obstructed breathing. Laryngoscope. 1990;100(10 Pt 1):1068–72. doi: 10.1288/00005537-199010000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Miljeteig H, Mateika S, Haight JS, Cole P, Hoffstein V. Subjective and objective assessment of uvulopalatopharyngoplasty for treatment of snoring and obstructive sleep apnea. Am J Respir Crit Care Med. 1994;150(5 Pt 1):1286–90. doi: 10.1164/ajrccm.150.5.7952554. [DOI] [PubMed] [Google Scholar]
- 44.Millman RP, Carlisle CC, Rosenberg C, Kahn D, McRae R, Kramer NR. Simple predictors of uvulopalatopharyngoplasty outcome in the treatment of obstructive sleep apnea. Chest. 2000;118:1025–30. doi: 10.1378/chest.118.4.1025. [DOI] [PubMed] [Google Scholar]
- 45.Myatt HM, Croft CB, Kotecha BT, Ruddock J, Mackay IS, Simonds AK. A three-centre prospective pilot study to elucidate the effect of uvulopalatopharyngoplasty on patients with mild obstructive sleep apnoea due to velopharyngeal obstruction. Clin Otolaryngol Allied Sci. 1999;24:95–103. doi: 10.1046/j.1365-2273.1999.00215.x. [DOI] [PubMed] [Google Scholar]
- 46.Walker EB, Frith RW, Harding DA, Cant BR. Uvulopalatopharyngoplasty in severe idiopathic obstructive sleep apnoea syndrome. Thorax. 1989;44:205–8. doi: 10.1136/thx.44.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker-Engstrom ML, Wilhelmsson B, Tegelberg A, Dimenas E, Ringqvist I. Quality of life assessment of treatment with dental appliance or UPPP in patients with mild to moderate obstructive sleep apnoea. A prospective randomized 1-year follow-up study. J Sleep Res. 2000;9:303–8. doi: 10.1046/j.1365-2869.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- 48.Wilhelmsson B, Tegelberg A, Walker-Engstrom ML, et al. A prospective randomized study of a dental appliance compared with uvulopalatopharyngoplasty in the treatment of obstructive sleep apnoea. Acta Otolaryngol. 1999;119:503–9. doi: 10.1080/00016489950181071. [DOI] [PubMed] [Google Scholar]
- 49.Zohar Y, Finkelstein Y, Talmi YP, Bar-Ilan Y. Uvulopalatopharyngoplasty: evaluation of postoperative complications, sequelae, and results. Laryngoscope. 1991;101(7 Pt 1):775–9. doi: 10.1288/00005537-199107000-00015. [DOI] [PubMed] [Google Scholar]
- 50.Kezirian EJ, Weaver EM, Yueh B, et al. Incidence of serious complications after uvulopalatopharyngoplasty. Laryngoscope. 2004;114:450–3. doi: 10.1097/00005537-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 51.Li HY, Li KK, Chen NH, Wang PC. Modified uvulopalatopharyngoplasty: The extended uvulopalatal flap. Am J Otolaryngol. 2003;24:311–16. doi: 10.1016/s0196-0709(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 52.Li HY, Chen NH, Shu YH, Wang PC. Changes in quality of life and respiratory disturbance after extended uvulopalatal flap surgery in patients with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2004;130:195–200. doi: 10.1001/archotol.130.2.195. [DOI] [PubMed] [Google Scholar]
- 53.Li HY, Chen NH, Lee LA, Shu YH, Fang TJ, Wang PC. Use of morphological indicators to predict outcomes of palatopharyngeal surgery in patients with obstructive sleep apnea. ORL J Otorhinolaryngol Relat Spec. 2004;66:119–23. doi: 10.1159/000079330. [DOI] [PubMed] [Google Scholar]
- 54.Pang KP, Woodson BT. Expansion sphincter pharyngoplasty: a new technique for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2007;137:110–14. doi: 10.1016/j.otohns.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 55.Cahali MB. Lateral pharyngoplasty: a new treatment for obstructive sleep apnea hypopnea syndrome. Laryngoscope. 2003;113:1961–8. doi: 10.1097/00005537-200311000-00020. [DOI] [PubMed] [Google Scholar]
- 56.Ferguson KA, Heighway K, Ruby RR. A randomized trial of laser-assisted uvulopalatoplasty in the treatment of mild obstructive sleep apnea. Am J Respir Crit Care Med. 2003;167:15–9. doi: 10.1164/rccm.2108050. [DOI] [PubMed] [Google Scholar]
- 57.Larrosa F, Hernandez L, Morello A, Ballester E, Quinto L, Montserrat JM. Laser-assisted uvulopalatoplasty for snoring: does it meet the expectations? Eur Respir J. 2004;24:66–70. doi: 10.1183/09031936.04.00082903. [DOI] [PubMed] [Google Scholar]
- 58.Finkelstein Y, Stein G, Ophir D, Berger R, Berger G. Laser-assisted uvulopalatoplasty for the management of obstructive sleep apnea: myths and facts. Arch Otolaryngol Head Neck Surg. 2002;128:429–34. doi: 10.1001/archotol.128.4.429. [DOI] [PubMed] [Google Scholar]
- 59.Kern RC, Kutler DI, Reid KJ, Conley DB, Herzon GD, Zee P. Laser-assisted uvulopalatoplasty and tonsillectomy for the management of obstructive sleep apnea syndrome. Laryngoscope. 2003;113:1175–81. doi: 10.1097/00005537-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 60.Mickelson SA. Laser-assisted uvulopalatoplasty for obstructive sleep apnea. Laryngoscope. 1996;106(1 Pt 1):10–13. doi: 10.1097/00005537-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Walker RP, Grigg-Damberger MM, Gopalsami C, Totten MC. Laser-assisted uvulopalatoplasty for snoring and obstructive sleep apnea: results in 170 patients. Laryngoscope. 1995;105(9 Pt 1):938–43. doi: 10.1288/00005537-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 62.Chisholm E, Kotecha B. Oropharyngeal surgery for obstructive sleep apnoea in CPAP failures. Eur Arch Otorhinolaryngol. 2007;264:51–5. doi: 10.1007/s00405-006-0139-2. [DOI] [PubMed] [Google Scholar]
- 63.Bassiouny A, El Salamawy A, Abd El-Tawab M, Atef A. Bipolar radiofrequency treatment for snoring with mild to moderate sleep apnea: a comparative study between the radiofrequency assisted uvulopalatoplasty technique and the channeling technique. Eur Arch Otorhinolaryngol. 2007;264:659–67. doi: 10.1007/s00405-007-0244-x. [DOI] [PubMed] [Google Scholar]
- 64.Blumen MB, Dahan S, Fleury B, Hausser-Hauw C, Chabolle F. Radiofrequency ablation for the treatment of mild to moderate obstructive sleep apnea. Laryngoscope. 2002;112:2086–92. doi: 10.1097/00005537-200211000-00033. [DOI] [PubMed] [Google Scholar]
- 65.Wassmuth Z, Mair E, Loube D, Leonard D. Cautery-assisted palatal stiffening operation for the treatment of obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg. 2000;123(1 Pt 1):55–60. doi: 10.1067/mhn.2000.105184. [DOI] [PubMed] [Google Scholar]
- 66.den Herder C, Kox D, van Tinteren H, de Vries N. Bipolar radiofrequency induced thermotherapy of the tongue base: Its complications, acceptance and effectiveness under local anesthesia. Eur Arch Otorhinolaryngol. 2006;263:1031–40. doi: 10.1007/s00405-006-0115-x. [DOI] [PubMed] [Google Scholar]
- 67.Riley RW, Powell NB, Li KK, Weaver EM, Guilleminault C. An adjunctive method of radiofrequency volumetric tissue reduction of the tongue for OSAS. Otolaryngol Head Neck Surg. 2003;129:37–42. doi: 10.1016/S0194-59980300482-0. [DOI] [PubMed] [Google Scholar]
- 68.Stuck BA, Kopke J, Hormann K, et al. Volumetric tissue reduction in radiofrequency surgery of the tongue base. Otolaryngol Head Neck Surg. 2005;132:132–5. doi: 10.1016/j.otohns.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 69.Fischer Y, Khan M, Mann WJ. Multilevel temperature-controlled radiofrequency therapy of soft palate, base of tongue, and tonsils in adults with obstructive sleep apnea. Laryngoscope. 2003;113:1786–91. doi: 10.1097/00005537-200310000-00024. [DOI] [PubMed] [Google Scholar]
- 70.Stuck BA, Starzak K, Hein G, et al. Combined radiofrequency surgery of the tongue base and soft palate in obstructive sleep apnoea. Acta Otolaryngol. 2004;124:827–32. doi: 10.1080/00016480410017378. [DOI] [PubMed] [Google Scholar]
- 71.Woodson BT, Steward DL, Weaver EM, Javaheri S. A randomized trial of temperature-controlled radiofrequency, continuous positive airway pressure, and placebo for obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg. 2003;128:848–61. doi: 10.1016/S0194-59980300461-3. [DOI] [PubMed] [Google Scholar]
- 72.Friedman M, Schalch P, Lin H-C, Kakodkar KA, Joseph NJ, Mazloom N. Palatal implants for the treatment of snoring and obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. 2008;138:209–16. doi: 10.1016/j.otohns.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 73.Goessler UR, Hein G, Verse T, Stuck BA, Hormann K, Maurer JT. Soft palate implants as a minimally invasive treatment for mild to moderate obstructive sleep apnea. Acta Otolaryngol. 2007;127:527–31. doi: 10.1080/00016480600951392. [DOI] [PubMed] [Google Scholar]
- 74.Walker RP, Levine HL, Hopp ML, Greene D, Pang K. Palatal implants: a new approach for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2006;135:549–54. doi: 10.1016/j.otohns.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 75.Andsberg U, Jessen M. Eight years of follow-up--uvulopalatopharyngoplasty combined with midline glossectomy as a treatment for obstructive sleep apnoea syndrome. Acta Otolaryngol Suppl. 2000;543:175–8. doi: 10.1080/000164800454323. [DOI] [PubMed] [Google Scholar]
- 76.Baisch A, Maurer JT, Hormann K. The effect of hyoid suspension in a multilevel surgery concept for obstructive sleep apnea. Otolaryngol Head Neck Surg. 2006;134:856–61. doi: 10.1016/j.otohns.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 77.Bowden MT, Kezirian EJ, Utley D, Goode RL. Outcomes of hyoid suspension for the treatment of obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2005;131:440–5. doi: 10.1001/archotol.131.5.440. [DOI] [PubMed] [Google Scholar]
- 78.Elasfour A, Miyazaki S, Itasaka Y, Yamakawa K, Ishikawa K, Togawa K. Evaluation of uvulopalatopharyngoplasty in treatment of obstructive sleep apnea syndrome. Acta Otolaryngol Suppl. 1998;537:52–6. doi: 10.1080/00016489850182369. [DOI] [PubMed] [Google Scholar]
- 79.Friedman M, Ibrahim H, Lee G, Joseph NJ. Combined uvulopalatopharyngoplasty and radiofrequency tongue base reduction for treatment of obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. 2003;129:611–21. doi: 10.1016/j.otohns.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 80.Jacobowitz O. Palatal and tongue base surgery for surgical treatment of obstructive sleep apnea: a prospective study. Otolaryngol Head Neck Surg. 2006;135:258–64. doi: 10.1016/j.otohns.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 81.Johnson NT, Chinn J. Uvulopalatopharyngoplasty and inferior sagittal mandibular osteotomy with genioglossus advancement for treatment of obstructive sleep apnea. Chest. 1994;105:278–83. doi: 10.1378/chest.105.1.278. [DOI] [PubMed] [Google Scholar]
- 82.Kao YH, Shnayder Y, Lee KC. The efficacy of anatomically based multilevel surgery for obstructive sleep apnea. Otolaryngol Head Neck Surg. 2003;129:327–35. doi: 10.1016/S0194-59980300711-3. [DOI] [PubMed] [Google Scholar]
- 83.Li HY, Wang PC, Hsu CY, Chen NH, Lee LA, Fang TJ. Same-stage palatopharyngeal and hypopharyngeal surgery for severe obstructive sleep apnea. Acta Otolaryngol. 2004;124:820–6. doi: 10.1080/00016480410018034. [DOI] [PubMed] [Google Scholar]
- 84.Li HY, Wang PC, Hsu CY, Lee SW, Chen NH, Liu SA. Combined nasal-palatopharyngeal surgery for obstructive sleep apnea: simultaneous or staged? Acta Otolaryngol. 2005;125:298–303. doi: 10.1080/00016480410022831. [DOI] [PubMed] [Google Scholar]
- 85.Mickelson SA, Rosenthal L. Midline glossectomy and epiglottidectomy for obstructive sleep apnea syndrome. Laryngoscope. 1997;107:614–9. doi: 10.1097/00005537-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 86.Nelson LM. Combined temperature-controlled radiofrequency tongue reduction and UPPP in apnea surgery. Ear Nose Throat J. 2001;80:640–4. [PubMed] [Google Scholar]
- 87.Neruntarat C. Genioglossus advancement and hyoid myotomy under local anesthesia. Otolaryngol Head Neck Surg. 2003;129:85–91. doi: 10.1016/S0194-59980300094-9. [DOI] [PubMed] [Google Scholar]
- 88.Neruntarat C. Genioglossus advancement and hyoid myotomy: short-term and long-term results. J Laryngol Otol. 2003;117:482–6. doi: 10.1258/002221503321892343. [DOI] [PubMed] [Google Scholar]
- 89.Thomas AJ, Chavoya M, Terris DJ. Preliminary findings from a prospective, randomized trial of two tongue-base surgeries for sleep-disordered breathing. Otolaryngol Head Neck Surg. 2003;129:539–46. doi: 10.1016/S0194-59980300728-9. [DOI] [PubMed] [Google Scholar]
- 90.Verse T, Baisch A, Maurer JT, Stuck BA, Hormann K. Multilevel surgery for obstructive sleep apnea: short-term results. Otolaryngol Head Neck Surg. 2006;134:571–7. doi: 10.1016/j.otohns.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 91.Vicente E, Marin JM, Carrizo S, Naya MJ. Tongue-base suspension in conjunction with uvulopalatopharyngoplasty for treatment of severe obstructive sleep apnea: long-term follow-up results. Laryngoscope. 2006;116:1223–7. doi: 10.1097/01.mlg.0000224498.09015.d9. [DOI] [PubMed] [Google Scholar]
- 92.Benazzo M, Pagella F, Matti E, et al. Hyoidthyroidpexia as a treatment in multilevel surgery for obstructive sleep apnea. Acta Otolaryngol. 2008;128:680–4. doi: 10.1080/00016480701636884. [DOI] [PubMed] [Google Scholar]
- 93.Ramirez SG, Ioue DI. Inferior sagittal osteotomy with hyoid bone suspension for obese patients with sleep apnea. Arch Otolaryngol Head Neck Surg. 1996;122:953–57. doi: 10.1001/archotol.1996.01890210031008. [DOI] [PubMed] [Google Scholar]
- 94.Sorrenti G, Piccin O, Latini G, et al. Tongue base suspension technique in obstructive sleep apnea: personal experience. Acta Otorhinolaryngol Ital. 2003;23:274–80. [PubMed] [Google Scholar]
- 95.Vilaseca I, Morello A, Montserrat JM, Santamaria J, Iranzo A. Usefulness of uvulopalatopharyngoplasty with genioglossus and hyoid advancement in the treatment of obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2002;128:435–40. doi: 10.1001/archotol.128.4.435. [DOI] [PubMed] [Google Scholar]
- 96.Woodson BT. A tongue suspension suture for obstructive sleep apnea and snorers. Otolaryngol Head Neck Surg. 2001;124:297–303. doi: 10.1067/mhn.2001.113661. [DOI] [PubMed] [Google Scholar]
- 97.Woodson BT, Robinson S, Lim HJ. Transpalatal advancement pharyngoplasty outcomes compared with uvulopalatopharygoplasty. Otolaryngol Head Neck Surg. 2005;133:211–17. doi: 10.1016/j.otohns.2005.03.061. [DOI] [PubMed] [Google Scholar]
- 98.Yin SK, Yi HL, Lu WY, Guan J, Wu HM, Cao ZY. Genioglossus advancement and hyoid suspension plus uvulopalatopharyngoplasty for severe OSAHS. Otolaryngol Head Neck Surg. 2007;136:626–31. doi: 10.1016/j.otohns.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 99.Aurora RN, Casey KR, Kristo D, Auerbach S, Bista SR, Chowdhuri S, Karippot A, Lamm C, Ramar K, Zak R, Morgenthaler T. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. SLEEP. 2010;33:1408–13. doi: 10.1093/sleep/33.10.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]