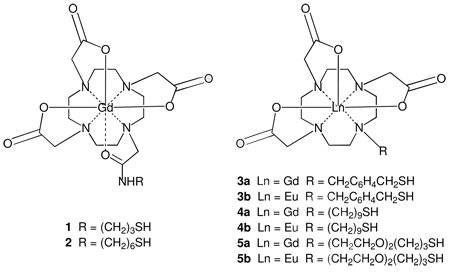
Abstract
The design and synthesis of three 1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid (DO3A) derivatives bearing linkers with terminal thiol groups and a preliminary evaluation of their potential for use in assembling redox-sensitive Magnetic Resonance Imaging (MRI) contrast agents are reported. The linkers were selected based on computational docking with a crystal structure of human serum albumin (HSA). Gd(III)-DO3A and Eu(III)-DO3A complexes were synthesized, and the structure of one complex was established by X-ray crystallographic analysis. The binding to HSA of a Gd(III)-DO3A complex bearing a thiol-terminated 3,6-dioxanonyl chain was competitively inhibited by homocysteine and by the corresponding Eu chelate. Binding to HSA was abolished when the terminal thiol group of this complex was absent. The longitudinal water-proton relaxivities (r1) of the three Gd(III)-DO3A complexes and of two Gd(III)-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) complexes were measured in saline at 7 Tesla. The DO3A complexes exhibited smaller r1 values, in both bound and free states, than the DOTA complexes.
Introduction
Advances in our understanding of the molecular pathogenesis of cancer and many other diseases have lead to the current push toward the development of novel, targeted drugs. This is being accompanied by a transformation of diagnostic radiology from a discipline focused on the imaging of anatomy to one that seeks to interrogate tissue physiology and molecular phenotypes, and provide non-invasive imaging biomarkers of drug targets and drug action. Magnetic Resonance Imaging (MRI)a is a powerful medical diagnostic tool, and while not all MRI examinations require the administration of exogenous contrast material, in clinical practice many MRI examinations employ gadolinium(III)-based contrast agents (CAs).1–3 The efficacy of an MRI contrast agent is dependent on its relaxivity, and in the last two decades much work has been devoted to understanding the parameters that influence the relaxation properties of these CAs.4–6 Clinically approved MRI CAs are suited primarily for highlighting anatomical features on MR images, after intravenous administration and vascular distribution throughout the body. In recent years, the focus has shifted towards the development of MRI CAs that are sensitive to physiochemical changes and biochemical changes in their microenvironment, such as pH, pO2, metal ion concentration, enzymatic activity, and redox state.7–21 Perturbations of tissue redox are linked to the development of hyperglycemia-induced vascular and renal complications in diabetes mellitus.22,23 Evidence also suggests that altered redox homeostasis in cancer cells plays a role in tumor progression and metastasis,24–26 chemoresistance,25 and radioresistance.27 The Nrf2 transcription factor, which is upregulated in many cancers,26 transcriptionally upregulates antioxidant response element (ARE)-containing genes. ARE-containing genes include many that impinge on tissue levels of reduced glutathione, thioredoxin, and cysteine, and code for drug transporters such as the multidrug resistance-associated protein (MRP).25 Reduced thiols such as glutathione and cysteine can directly effect the chemical repair of DNA damage produced by ionizing radiation, thereby providing a degree of radioprotection.28 Nrf2-regulated γ-glutamyltransferase (GGT) can modulate redox equilibria in both the intracellular and extracellular compartments.29,30 GGT can confer a degree of protection to cancer cells from electrophilic drugs such as cisplatin by increasing the extracellular levels of thiols.29,30 Knowledge of tumor redox by itself, and knowledge of tumor redox as a potential downstream biomarker of tumor Nrf2 status, is thus greatly relevant to the management and treatment of cancer. We report the development of thiol-sensitive MRI contrast agents for potential use in the non-invasive interrogation of tumor redox.
Previously we reported the synthesis and characterization of compounds 1 and 2, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) complexes of gadolinium(III) bearing alkyl chains that terminate with a thiol group.31 These compounds bound to human serum albumin (HSA) in a redox-sensitive manner, as evidenced by increased MRI relaxivity and retention in a 30 kDa MWCO filter in the presence of HSA. Loss of both these properties occurred when 1 and 2 were competed with excess homocysteine, which is known to form a disulfide link with cysteine-34 of HSA. More recently, Lacerda, et al., prepared a 1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid (DO3A) macrocycle bearing a terminal β-thioethyl appendage for use as a chelating agent for radionuclides.32 The synthesis of glyconanoparticles capped with a Gd-DO3A chelate bearing pentyl and undecyl appendages with terminal thiol groups has also been reported.33 In a continuation of our work, we have designed and synthesized three DO3A chelators bearing different types of linkers that terminate with thiol groups, prepared the corresponding Gd and Eu complexes 3–5, obtained a crystal structure of Gd-DO3A complex 4a, studied the binding of these complexes to HSA, and have evaluated the relaxivity of these complexes in the presence and absence of HSA as reported herein.
Molecular modeling of the interactions between compounds 1–5 and HSA
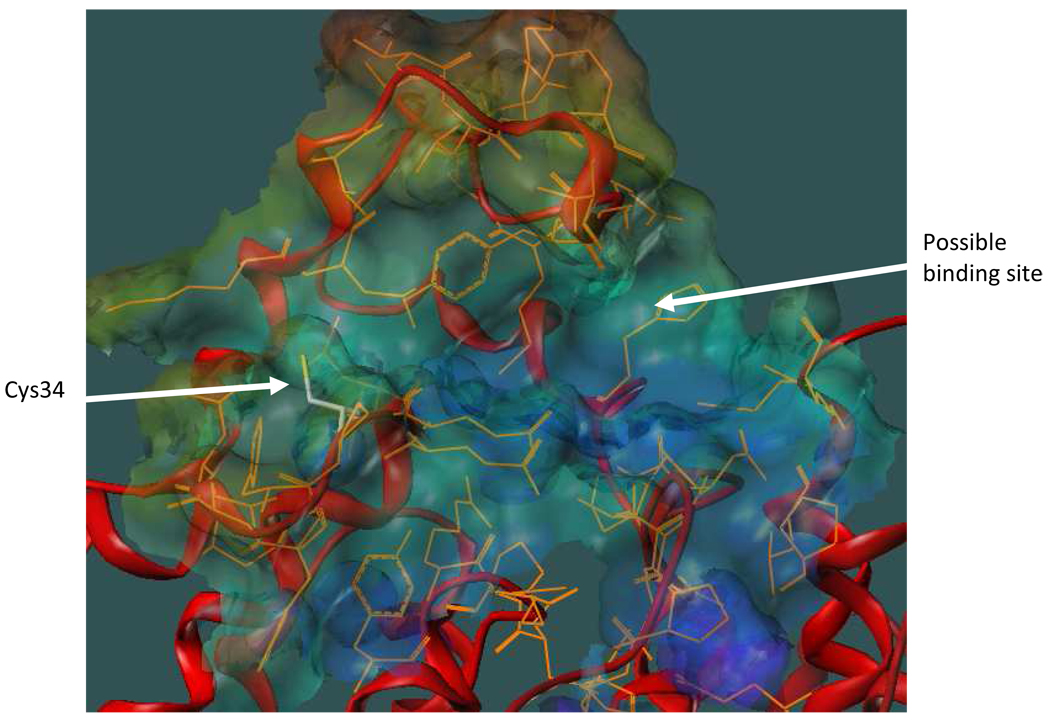
We postulated that compounds such as 1–5 may bind to HSA via one or more of the following: formation of a covalent disulfide bond between the thiol-terminated linker and the Cys34–SH group, linker-protein interactions, and interactions between the chelate moiety and a hydrophobic pocket near the surface of the protein. Our current modeling effort employs a known X-ray crystal structure of HSA34 to design molecules that will maximize binding based on these three key interactions. Figure 1 shows a possible binding site located on the surface of HSA near Cys34. The site consists of a hydrophobic binding pocket and a hydrophobic channel leading from the pocket to Cys34. The pocket is formed by Gly82, Thr83, Tyr84 (backbone), Gly85, Glu86, and Met87 on one side and by Gln104, His105, Lys106, Asp107, Asp108, Glu465, Lys466, and Thr467 on the other side. These residues create a bowl-shaped depression where the chelate moiety might nest. The backbone and side chains of Tyr30, Leu31, Gln32, Gln33, and Tyr84 create a hydrophobic channel where an appropriate linker might bind while permitting covalent attachment of a terminal thiol to the Cys34–SH group.
Figure 1.
HSA with the putative binding region shown as a MOLCAD surface. The protein backbone is depicted as a red ribbon and residues that constitute the binding region are shown in orange. Cys34 is displayed as atom-colored sticks.
Our molecular modeling protocol was designed to simulate a possible two stage binding process.35 In the first stage, non-covalent interactions between compounds 1–5 and the putative binding site described above were computationally examined in the presence of water molecules. During these simulations, movement of the protein side chains to accommodate small molecule docking was allowed. In the second stage, a covalent disulfide linkage between the terminal thiol of the small molecule and the Cys34–SH was established, and the simulations were repeated. At the end of each docking simulation, the interaction energy value was calculated.
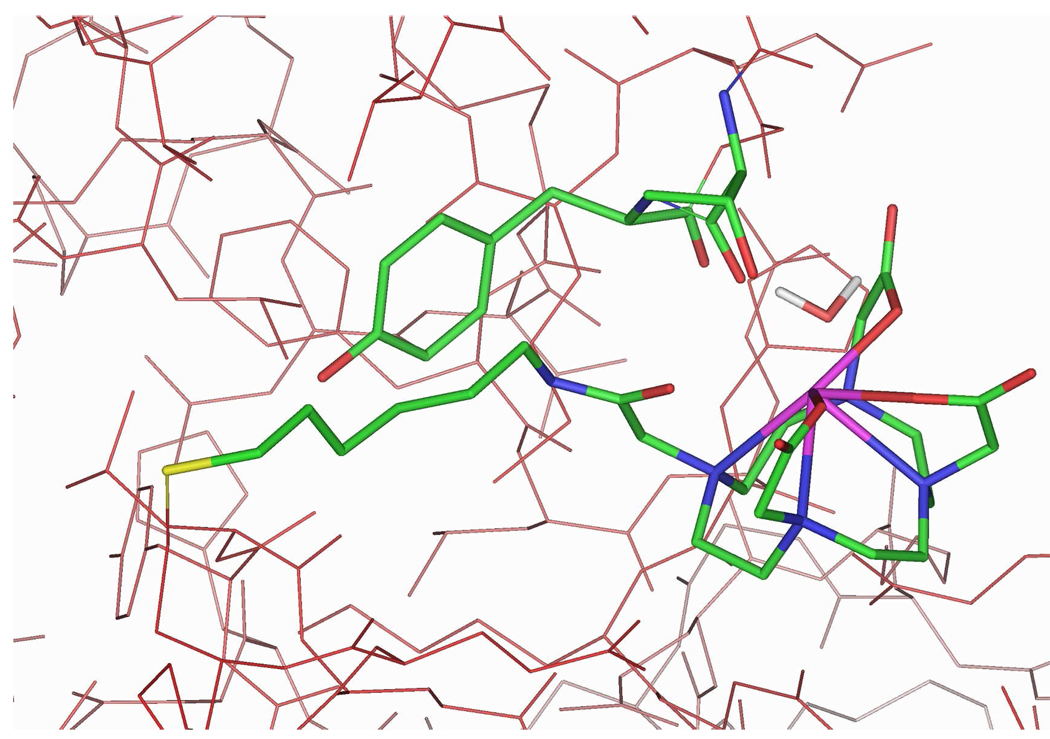
For the DOTA-containing compounds 1 and 2, Gd chelation by the amide group in the linker provides an extra measure of structural rigidity. Covalent binding of 2 to HSA results in additional coordination by the backbone C=O groups of Thr83 or Tyr84 and one water molecule (see Figure 2). It is hypothesized that the system is dynamic, and that Thr83, Tyr84, and water exchange in the coordination sphere of the gadolinium atom. Compound 2 exhibited a greater interaction energy (−510 kcal/mol, see Table 1) than did 1 (−290 kcal/mol), presumably due to increased van der Waals interactions with the channel. These calculations were qualitatively consistent with the measured binding constants of 1 and 2 (see Table 1).
Figure 2.
Docking of 2 with HSA. Compound 2, HSA residues Thr83 and Tyr84, and a bound water molecule (2.71 Å from Gd) are displayed as atom-colored sticks. Hydrogen atoms and remaining water molecules are omitted for clarity.
Table 1.
Binding of Compounds 1, 2, 3a–5a, and 24 to Human Serum Albumin.
| Calculated Interaction Energy (kcal/mol) |
Measured KAc (mM−1) |
Calculated Distance of Water from Gd in the |
|||
|---|---|---|---|---|---|
| Compound | Covalent Complexa |
Non-covalent Complexb |
Presence of HSA |
Absence of HSA |
|
| 1d | −290 | −210 | 5.0 ± 1.5 | 2.76 Å | 2.71 Å |
| 2.75 Å | |||||
| 2d | −510 | −496 | 64 ± 16 | 2.71 Å | 2.65 Å |
| 2.72 Å | |||||
| 3a | −410 | −366 | mbe | 2.54 Å | 2.67 Å |
| 2.89 Å | |||||
| 4a | −471 | −404 | mbe | 2.75 Å | 2.73 Å |
| 2.74 Å | |||||
| 5a | −453 | −413 | 22 ± 1.4 | 2.75 Å | 2.73 Å |
| 2.74 Å | |||||
| 24 | naf | −406 | ~0 | ncg | ncg |
Includes a disulfide bond between the compound thiol and Cys34-SH.
Without a disulfide bond between the compound thiol and Cys34-SH.
Measured at 37°C.
Results taken from reference 31.
Results suggest this compound binds to multiple sites on HSA; see text for explanation.
Not applicable.
Not calculated.
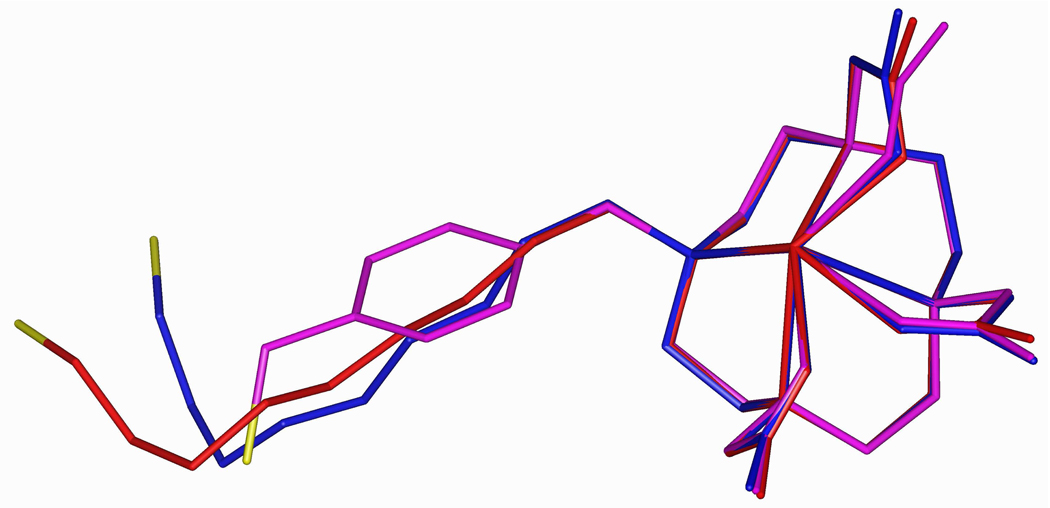
In contrast with Gd-DOTA chelates in which only one water molecule may be coordinated with the Gd (q = 1), Gd-DO3A chelates allow for the possibility of two coordinated water molecules (q = 2) and may exhibit very different binding to HSA and different MRI relaxivity characteristics. In modeling Gd-DO3A chelates, the three linkers shown in compounds 3–5 were selected from a number of possibilities that were initially considered. Included are linkers based on a rigid hydrophobic p-xylyl group, a flexible hydrophobic nonyl chain, and a flexible hydrophilic 3,6-dioxanonyl chain. Figure 3 shows an overlay of energy-minimized structures for compounds 3a–5a.
Figure 3.
Overlay of energy-minimized structures for compounds 3a (magenta), 4a (red), and 5a (blue). Sulfur is colored yellow, and hydrogen atoms are omitted for clarity.
In the computational docking studies, compound 3a possessing the xylyl linker exhibited somewhat weaker binding (−410 kcal/mol, see Table 1) than did compounds 4a and 5a (−471 and −453 kcal/mol, respectively). The xylyl linker has neither the reach nor the flexibility of the nonyl and 3,6-dioxanonyl chains which are able to interact more favorably with the channel connecting Cys34 and the hydrophobic binding pocket on the surface of HSA. Still, docking indicates that all the three compounds show similar profiles with respect to binding of the gadolinium chelate moiety. Backbone amide C=O groups of Thr83 and Tyr84 and one water molecule are in the inner coordination sphere of the gadolinium atom (Table 1). In simulations carried out in water, two water molecules occupy two inner sphere coordination sites for each of the DO3A chelates 3a–5a. Thus, one would expect different relaxivity for protein-bound and unbound chelates, and so these compounds were selected for synthesis and evaluation of binding and relaxivity properties.
Synthesis
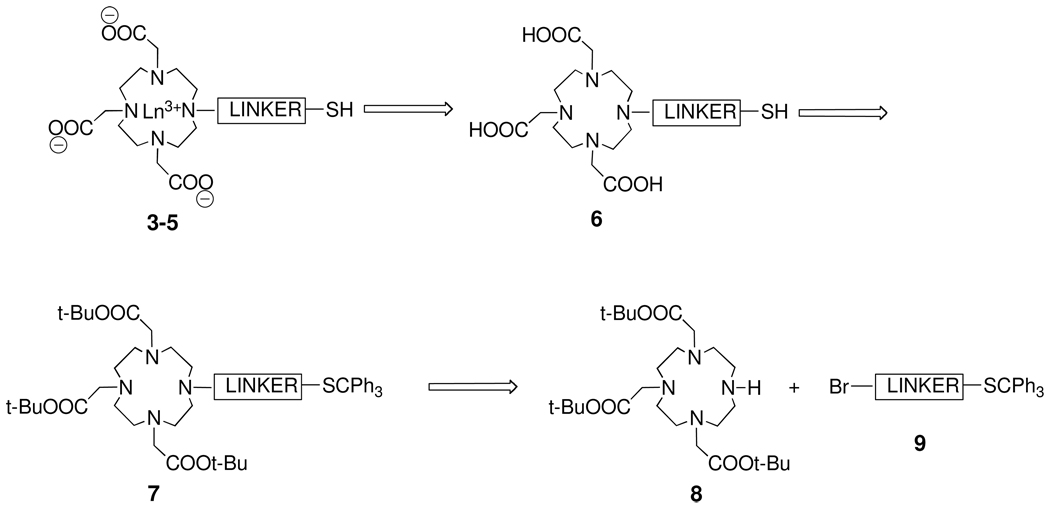
A retrosynthetic analysis for construction of Ln-DO3A conjugates 3–5 is depicted in Scheme 1. A key step would be the simultaneous deprotection of the three tert-butyl esters and the tritylthio group in 7 to afford DO3A derivatives 6, which, upon complexation with the desired lanthanide, would give the complexes 3–5. N-Alkylation of the known DO3A derivative 8 with primary bromides 9 would give access to 7.
Scheme 1.
Retrosynthetic Analysis of Ln-DO3A Conjugates 3–5.
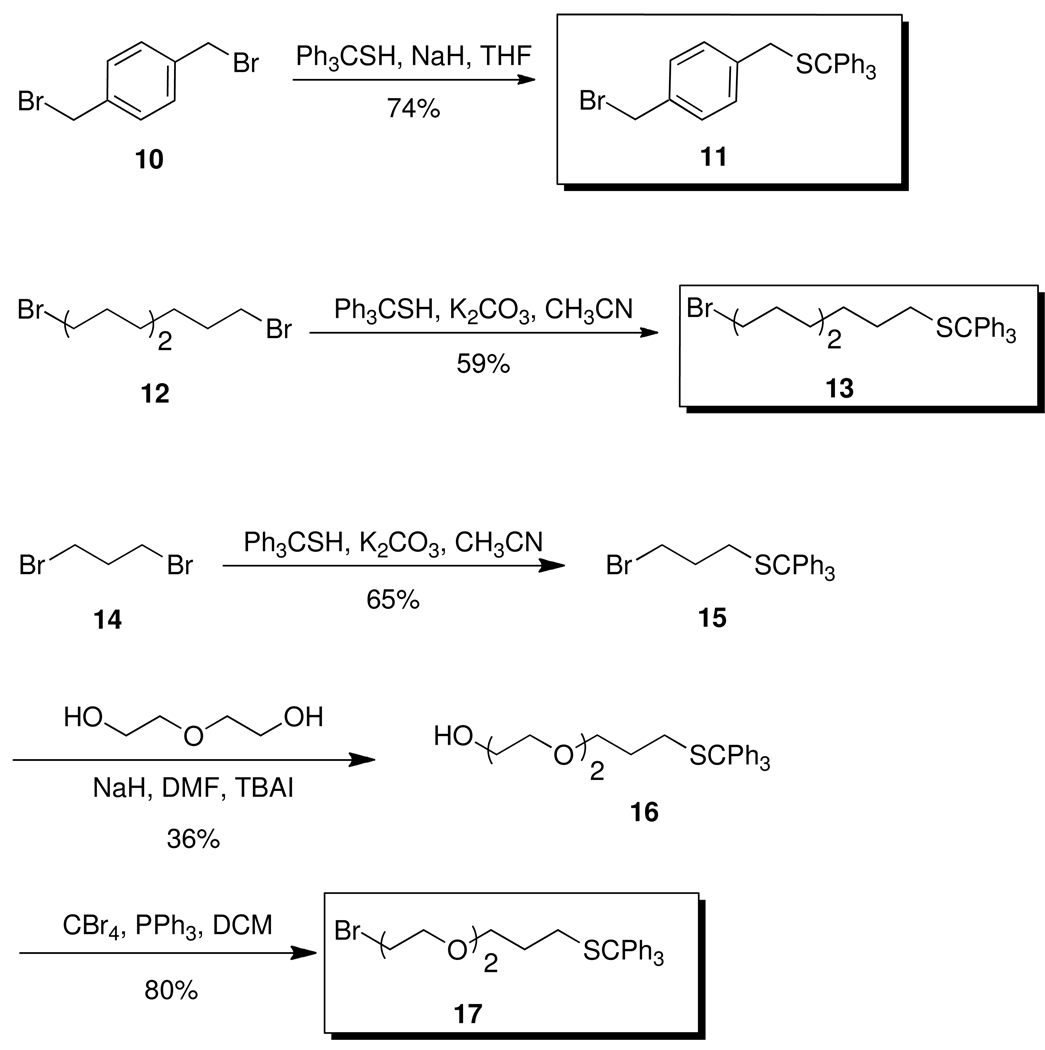
The required bromides were synthesized as shown in Scheme 2. Bromide 1136 was prepared by reacting p-xylene dibromide 10 with trityl mercaptan and sodium hydride in anhydrous THF. Bromide 13 was similarly prepared by reacting 1,9-dibromononane (12) with trityl mercaptan and K2CO3 in acetonitrile. Bromide 17 was prepared in three steps. 1,3-Dibromopropane (14) was reacted with trityl mercaptan and K2CO3 in acetonitrile to give 15. Bromide 15 was converted to 16 by reaction with diethylene glycol, sodium hydride, and TBAI in dry THF.37 Finally, bromination of 16 with CBr4 and PPh3 gave 17.38
Scheme 2.
Synthesis of Bromides 11, 13, and 17.
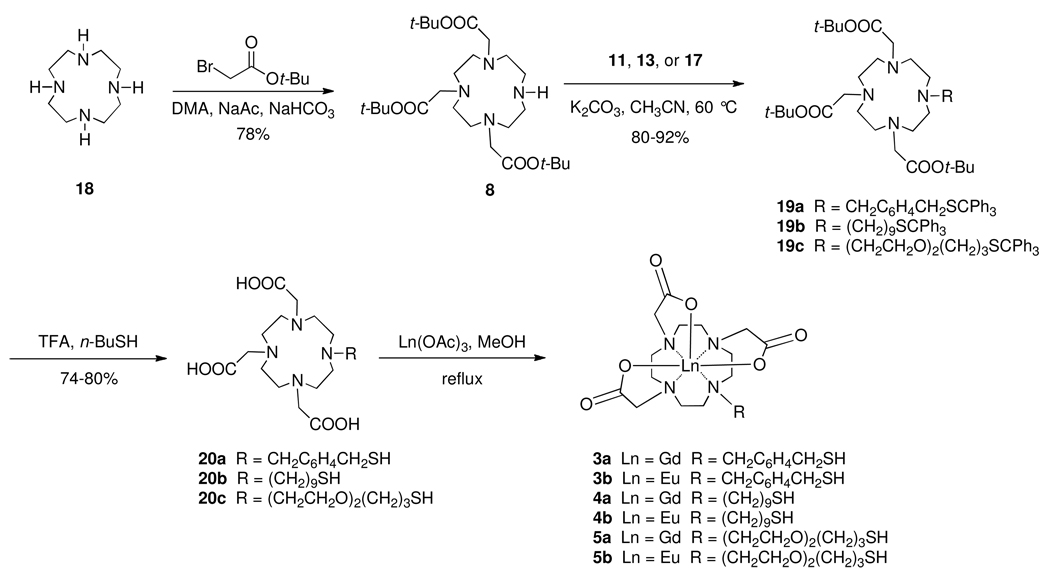
Ester 8 was prepared from commercially available cyclen 18 by treatment with 3 equivalents of tert-butyl bromoacetate and sodium acetate in N,N-dimethylacetamide using a modification of a published procedure (Scheme 3).39 N-Alkylation of 8 with bromide 11 in acetonitrile using K2CO3 as base gave 19a in 80% yield. Simultaneous removal of tert-butyl and trityl groups was attempted by treatment of 19a with triethylsilane and trifluoroacetic acid (1% v/v). Under these conditions, it appeared that the trityl group came off easily, while the ester groups were incompletely hydrolyzed. Longer reaction times and the use of solvents such as dichloromethane and chloroform led to what appeared by mass spectrometry to be the monoester derivative.40 We reasoned that this was not a case of incomplete ester hydrolysis, but rather of capture of a tert-butyl cation by the thiol group after loss of the trityl group. To eliminate this undesired process, an excess of butanethiol was added to the reaction medium to scavenge tert-butyl cations. This led to isolation of the fully deprotected tricarboxylic acid 20a in 80% yield. Acid 20a was metalated with Gd(OAc)3 or Eu(OAc)3 in refluxing methanol to give the corresponding Gd(III) and Eu(III) complexes 3a and 3b in 75% and 71% yields, respectively, following reverse phase silica gel chromatography.
Scheme 3.
Synthesis of Gd-DO3A Complexes 3a–5a and and Eu-DO3A Complexes 3b–5b.
Following a similar synthetic pathway, amine 8 was alkylated with bromide 13 to give 19b in 80% yield. Deprotection of 19b produced 20b in 75% yield, and metalation of 20b with Gd(OAc)3 and Eu(OAc)3 in refluxing methanol gave the complexes 4a and 4b in 57% and 58% yields, respectively, following reverse phase silica gel chromatography. Finally, N-Alkylation of 8 with 17 gave 19c in 92% yield. Deprotection of 19c gave 20c in 75% yield, and metalation of 20c with Gd(OAc)3 and Eu(OAc)3 produced complexes 5a and 5b in 75% and 82% yields, respectively, following reverse phase silica gel chromatography.
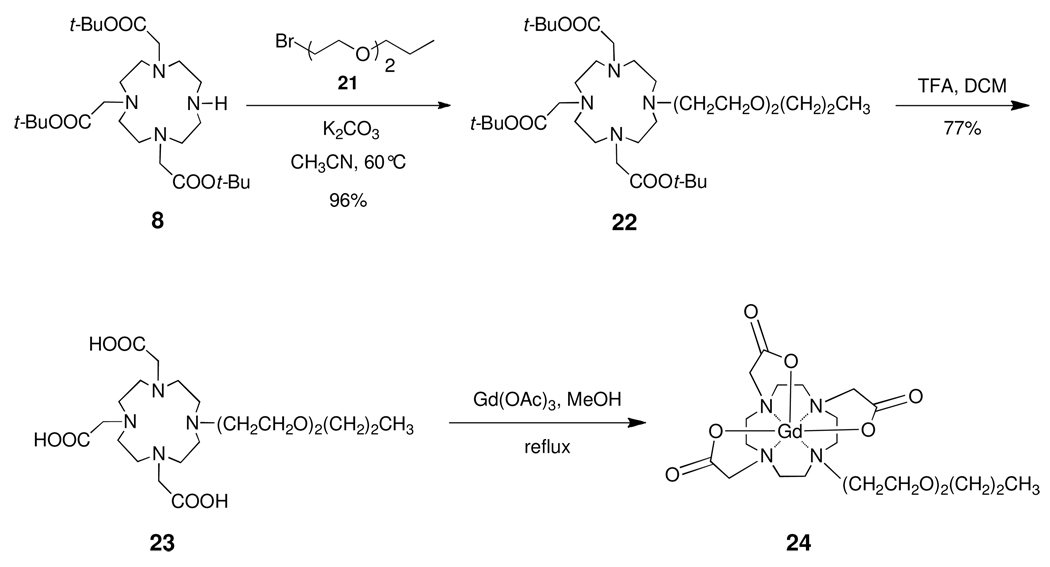
In addition to the syntheses of 3a–5a and 3b–5b, the synthesis of Gd-DO3A complex 24 is depicted in Scheme 4. Bromide 21 was prepared from diethylene glycol monopropyl ether in 90% yield by use of the Appel reaction.38 Alkylation of 8 with 21 in acetonitrile using K2CO3 as base gave 22 in 87% yield. Deprotection of the t-butyl ester groups using TFA in DCM gave tricarboxylic acid 23 in 77% yield. Metalation with Gd(OAc)3 hydrate in refluxing methanol gave complex 24 in 75% yield following reverse phase silica gel chromatography.
Scheme 4.
Synthesis of Gd-DO3A Complex 24.
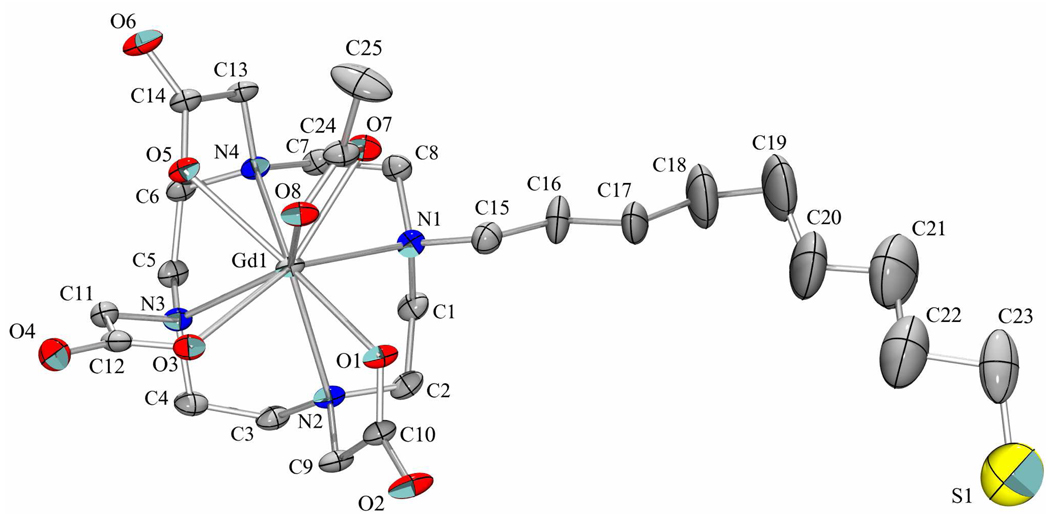
The structures of all compounds, except 3a, 4a, 5a, and 24, were characterized by 1H NMR, 13C NMR, and HRMS. Compounds 3a–5a, 3b–5b, and 24 gave appropriate masses and isotopic patterns in HRMS analysis (see the Supporting Information). Further evidence for the structures of the Gd-DO3A macrocycles was obtained by X-ray crystallographic analysis of 4a. Crystals were grown according to a procedure used previously for growing an In-DO3A–TPP complex.41 An equimolar mixture of 20b and Gd(OAc)3 was heated at 100°C for 30 min in NH4OAc buffer (0.5 M, pH = 6). Slow diffusion of acetone into the reaction mixture produced colorless, flat plates. Compound 4a crystallized in space group P-1 as a dihydrate with an acetate anion coordinated to the gadolinium center (Figure 5) and a charge-balancing ammonium cation present in the asymmetric unit. Water is also present in the asymmetric unit, some of which was removed by SQUEEZE.42 There is some disorder in the ω-thiononyl chain, which adopts a mostly extended conformation.43
Figure 5.
The molecular structure of 4a, with anisotropic displacement parameters at the 30% probability level. The minor disorder component, hydrogen atoms, water molecules, and the ammonium cation have been omitted for clarity.
Binding and Relaxometry
The binding of compounds 1, 2, 3a–5a, and 24 to commercially obtained HSA was measured at 37°C in buffered saline (see Table 1). For commercial samples, some percentage of the HSA present bears homocysteine or another thiol of low molecular weight covalently attached at Cys34.44 Thus, calculation of an apparent equilibrium association constant (KA) was possible from the binding experiments described herein. Compounds 3a and 4a bound to HSA with a stoichiometry >3:1 (data not shown), while compounds 1, 2, and 5a bound to HSA with a stoichiometry ~1:1. For compound 5a, KA = 22 mM−1, a value intermediate between the published values for DOTA complexes 1 and 2.31 The binding of compounds 1, 2, and 5a to HSA could be competitively inhibited by addition of homocysteine (see Table 2). The binding of 5a to HSA was inhibited even more effectively by the structurally related Eu chelate, 5b. Binding to HSA was abolished for 24, a compound similar to 5a but lacking the terminal thiol group.
Table 2.
Competitive Binding Study for Compounds 1, 2, and 5a.a
| Inhibitor Concentration (mM) | |||||
|---|---|---|---|---|---|
| Compound | Inhibitor | 0.0 | 0.5 | 1.0 | 2.0 |
| KA (mM−1) | |||||
| 1 | homocysteine | 5.0 ± 1.5 | 2.0 ± 0.12 | 1.5 ± 0.2 | 0.87 ± 0.05 |
| 2 | homocysteine | 64 ± 16 | 17 ± 2.2 | 6.1 ± 0.54 | 3.6 ± 0.23 |
| 5a | homocysteine | 22 ± 1.4 | 11 ± 3.4 | 6.6 ± 2.7 | 3.4 ± 0.84 |
| 5a | 5b | 22 ± 1.4 | 6.2 ± 1.2 | 3.0 ± 0.91 | ~ 0 |
Measured at 37°C.
The longitudinal water-proton relaxivities (r1) of compounds 1, 2, and 5a were measured in HEPES-buffered saline. For compounds 3a and 4a, r1 was measured in acetate-buffered saline because these complexes were poorly soluble in the absence of acetate. Results reported include r1(free), determined in the absence of HSA, and r1(bound), determined in the presence of this protein (see Table 3). The DO3A–based complexes had smaller relaxivities, in both bound and free states, than the DOTA-based complexes. For compounds 1, 2, 4a, and 5a, r1(free) was smaller than r1(bound), most likely due to increased times for rotational reorientation of the complexes when bound to HSA. In the case of 3a, r1(free) was comparable to values observed for the other DO3A chelates, 4a and 5a, but r1(bound) was smaller than r1(free), suggesting that other factors limit the relaxivity of 3a when bound to HSA.
Table 3.
Relaxivities of Compounds 1, 2, and 3a–5a.a
| Compound | r1,free (1/mM•s) |
r1,bound (1/mM•s) |
|---|---|---|
| 1 | 3.86 ± 0.44 | 4.54 ± 0.14 |
| 2 | 3.23 ± 0.33 | 4.74 ± 0.32 |
| 3a | 2.37 ± 0.18 | 1.54 ± 0.15 |
| 4a | 2.27 ± 0.37 | 3.28 ± 0.17 |
| 5a | 2.18 ± 0.58 | 3.15 ± 0.51 |
Measured at 37°C and 7 Tesla.
Discussion
Calculated interaction energies for compounds 1, 2, 3a–5a, and 24 with HSA are given in Table 1. The covalent interaction energies, which include a disulfide linkage, are lower (i.e., the covalent complex is more stable) than the corresponding noncovalent interaction energies. The differences in the calculated covalent and noncovalent interaction energies are 80, 14, 44, 67, and 40 kcal/mol for compounds 1, 2, and 3a–5a, respectively, in reasonable agreement with typical disulfide bond strengths (70 kcal/mol).45 In the case of compound 2, the energy gained through disulfide bond formation is presumably counterbalanced by losses of other stabilizing interactions. Consistent with this interpretation, the calculated noncovalent interaction energies for 5a and compound 24, which lacks a terminal thiol group and cannot form a disulfide bond, are very similar. In addition, the calculated covalent interaction energies correlate reasonably well with experimentally determined apparent equilibrium binding constants (KA, Table 1).
Homocysteine is known to bond covalently with Cys34 of HSA.44 Increasing concentrations of homocysteine resulted in corresponding decreases in KA for compounds 1, 2, and 5a (see Table 2), suggesting that these compounds also bond with HSA at Cys34. In contrast, compounds 3a and 4a exhibited essentially complete and irreversible binding to HSA. Due to insolubility, binding assays for these compounds were carried out in acetate-buffered saline. In the centrifugal filtration assay used to quantify binding, essentially no Gd was recovered in the filtrates from incubations of up to 2.0 mM 3a or 4a with 0.66 mM HSA (3:1). These results were unchanged when up to 2.0 mM homocysteine was included, indicating that 3a and 4a bind to HSA at sites other than Cys34. In aqueous solutions 3a and 4a would be expected to possess two water molecules in the inner coordination sphere (see Table 1).46 Displacement of inner sphere water molecules by acetate is a possible reason why the r1(free) values for 3a and 4a are lower than those of the DOTA complexes, 1 and 2. In addition, it has been reported that inner sphere water molecules can be displaced by carboxylate groups on albumin.47 There are 97 aspartate and glutamate residues in HSA. Examination of the X-ray crystal structure suggests that the majority of side chain carboxylate groups are present on the protein surface. Given the low solubility of 3a and 4a in the absence of acetate and the presence of a bifurcated acetate ligand in the crystal structure of 4a, it is conceivable that carboxylate groups on the HSA surface constitute the multiple sites to which these compounds bind. Displacement of inner sphere water molecules would also help explain the surprisingly low value of r1(bound) observed for 3a. Compound 5a might possess only one water molecule in the inner coordination sphere since the oxygen atom in the PEG sidechain proximal to the DO3A moiety can coordinate to gadolinium.48 Thus, 5a might exhibit properties more like those of a DOTA complex. However, r1(free) and r1(bound) for 5a were significantly lower than values for 1 and 2, possibly due to suboptimal coordination geometry or decreased basicity of the macrocyclic nitrogen that bears the pendant arm.49
Conclusion
A general method for the synthesis of DO3A–based lanthanum chelates possessing different nitrogen-attached sidearms that terminate with redox-active thiol groups has been demonstrated. The synthesis is relatively short and efficient. To the best of our knowledge, the first X-ray crystal structure of a gadolinium-DO3A complex has been obtained. Taken together with protein binding and relaxivity data, the crystal structure supports the suggestion that coordination by surface carboxylate groups may be important in protein binding and water relaxivity for DO3A chelates in vivo.47 Direct and competition binding assays indicate that, for DOTA chelates and DOTA-like DO3A chelates bearing terminal thiol groups, covalent attachment through a disulfide linkage with Cys34 is an important mode of HSA binding. Studies to further examine the redox chemistry of gadolinium DO3A and DOTA chelates and the associated MRI properties are underway.
Experimental
Small molecule docking with HSA
An X-ray crystal structure of HSA (PDB code: 1AO6) was refined and used as the basis for docking simulations that were carried out using Insight II 2005L.35 Compounds to be docked were placed in the vicinity of the Cys34–SH group and the protein–small molecule system surrounded with a 7.5 Å layer of water. Charges were assigned using extensible systematic force field (ESFF).50 The gadolinium atom was assigned a +3.0 formal charge. The system was then energy minimized using 5000 steps, followed by a molecular dynamics simulation with a 50 picosecond equilibration and a 100 picosecond simulation at 300 K. Frames were collected after every 100 femtoseconds during the 100 picosecond simulation phase. At the end of the molecular dynamics run, trajectories were analyzed on the basis of potential energy. The frame with the lowest potential energy was further minimized using 5000 steps. During covalent simulations, an S-S linkage was first established between the thiol groups of the small molecule and Cys34. This was then followed by energy minimization and molecular dynamics protocols as described above. Interaction energies were calculated as the difference between the energy of the small molecule-protein complex and the sum of the individual energies of the protein and the small molecule.
Simulations in aqueous solution
The small molecule of interest was surrounded with a 7.5 Å layer of TIP3P water molecules. The system was then minimized using 5000 steps. This was followed by a molecular dynamics simulation with a 50 picosecond equilibration and a 100 picosecond simulation at 300 K. Frames were collected after every 100 femtoseconds during the 100 picosecond simulation phase. At the end of the molecular dynamics run, trajectories were analyzed on the basis of potential energy. The frame with the lowest potential energy was further minimized using 5000 steps. This minimized frame was used to study the coordination of the gadolinium atom by water molecules.
Synthesis51
1-Bromomethyl-4-(triphenylmethylthio)methylbenzene (11).36
To a suspension of NaH (46 mg, 1.89 mmol) in dry THF (10 mL) was added a solution of trityl mercaptan (523 mg, 1.89 mmol) in dry THF (10 mL) with stirring. After 0.5 h, the solution was added dropwise to a solution of 10 (1.0 g, 3.7 mmol) in dry THF (20 mL) and the reaction mixture stirred overnight at room temperature. Ether (20 mL) and water (25 mL) were added to the reaction mixture, the organic layer separated, washed with water (25 mL), brine (25 mL), dried (MgSO4), filtered, and concentrated. Flash chromatography (10:1→5:1, hexanes/DCM) afforded 678 mg (1.4 mmol, 74%) of 11 as a white solid, mp 117–118°C, Rf 0.54 (2:1 hexanes/DCM on silica gel 60 F254). 1H NMR (500 MHz, CDCl3) δ 3.29 (2H, s), 4.41 (2H, s), 7.07 (2H, d, J = 8 Hz), 7.19–7.25 (5H, m), 7.26–7.3 (6H, m), 7.44 (6H, m); 13C NMR (125 MHz, CDCl3) δ 33.3, 36.6, 67.5, 126.7, 127.9, 129.1, 129.4, 129.5, 136.4, 137.4, 144.5. Anal. Calcd for C27H23BrS: C 70.58, H 5.05, S 6.98. Found: C 70.64, H 5.56, S 6.11.
1-Bromo-9-tritylthiononane (13)
To a solution of 1,9-dibromononane (12, 5.72 g, 20 mmol, 4 mL) and trityl mercaptan (2.76 g, 10 mmol) in CH3CN (50 mL) was added anhydrous K2CO3 (10.35 g, 75 mmol) and the mixture stirred overnight at room temperature. The reaction mixture was filtered and the filtrate evaporated under reduced pressure. The residue was subjected to flash chromatography on silica gel eluted with hexanes to give 13 (2.82 g, 5.86 mmol, 59%) as a white solid, mp 58–60°C, Rf 0.48 (3:1 hexanes/DCM on silica gel 60 F254). 1H NMR (500 MHz, CDCl3) δ 1.12–1.28 (8H, m), 1.38 (4H, quintet, J = 7.5 Hz, 7 Hz), 1.82 (2H, quintet, J = 7.5 Hz), 2.12 (2H, t, J = 7.5 Hz), 3.38 (2H, t, J = 7 Hz), 7.17–7.21 (3H, m), 7.24–7.28 (6H, m), 7.39–7.42 (6H, m); 13C NMR (125 MHz, CDCl3) δ 28.1, 28.5, 28.6, 29.0, 29.2, 32.0, 32.8, 33.9, 66.3, 126.4, 127.7, 129.5, 145.0. Anal. Calcd for C28H33BrS: C 69.84, H 6.91, S 6.66. Found: C 70.01, H 6.77, S 6.44.
1-Bromo-3-tritylthiopropane (15)
To a solution of 1,3-dibromopropane 14 (8.0 g, 40 mmol, 4 mL) and trityl mercaptan (5.4 g, 20 mmol) in CH3CN (150 mL) was added anhydrous K2CO3 (20.7 g, 150 mmol) and the mixture stirred overnight at room temperature. The reaction mixture was filtered and the filtrate evaporated under reduced pressure. The residue was subjected to flash chromatography on silica gel eluted with hexanes to give 15 (5.1 g, 12.9 mmol, 65%) as a white solid, mp 90–92°C, Rf 0.45 (3:1 hexanes/DCM on silica gel 60 F254). 1H NMR (500 MHz, CDCl3) δ 1.80 (2H, quintet, J = 7 Hz, 6.5 Hz), 2.31 (2H, t, J = 7 Hz), 3.31 (2H, t, J = 6.5 Hz), 7.18–7.23 (3H, m), 7.25–7.29 (6H, m), 7.40–7.41 (6H, m); 13C NMR (125 MHz, CDCl3) δ 30.2, 31.6, 32.2, 66.7, 126.6, 127.8, 129.5, 144.6. Anal. Calcd for C22H21BrS: C 66.50, H 5.33, S 8.07. Found: C 66.76, H 5.26, S 7.42.
9-Tritylthio-3,6-dioxanonan-1-ol (16)
To a mixture of NaH (0.25 g, 10.6 mmol) and TBAI (0.19 g, 0.5 mmol) in dry THF (5 mL) was added a solution of diethylene glycol (1.6 g, 15.1 mmol, 1.43 mL) in THF (25 mL) dropwise. After 30 min, a solution of 15 (2.0 g, 5 mmol) in dry THF (30 mL) was added dropwise and the mixture heated at reflux overnight. The reaction mixture was cooled, cautiously quenched with water (50 mL), and the mixture extracted with ether (3 × 50 mL). The combined organic layers were washed with brine, dried (MgSO4), filtered, and concentrated in vacuo. The residue was subjected to flash chromatography on silica gel eluted with hexanes/EtOAc (2:1→1:1) to give 16 (0.76 g, 1.8 mmol, 36%) as a white solid, mp 63–64°C, Rf 0.29 (1:1 hexanes/EtOAc on silica gel 60 F254). 1H NMR (500 MHz, CDCl3) δ 1.64 (2H, quintet, J = 7 Hz, 6.5 Hz), 2.23 (2H, t, J = 7 Hz), 2.33 (1H, br s), 3.40 (2H, t, J = 6.5 Hz), 3.50 (2H, m), 3.55–3.60 (4H, m), 3.68 (2H, m), 7.19 (3H, m), 7.26 (6H, m), 7.38–7.42 (6H, m); 13C NMR (125 MHz, CDCl3) δ 28.6, 61.8, 66.5, 69.9, 70.1, 70.3, 72.4, 126.5, 127.7, 129.5, 144.8; HRMS-ESI calculated for C26H30O3SNa (M+Na)+ 445.1808, found 445.1809. Anal. Calcd for C26H30O3S: C 73.90, H 7.16. Found C 73.52, H 7.19.
1-Bromo-9-tritylthio-3,6-dioxanonane (17)
To a solution of 16 (1.0 g, 2.36 mmol) in DCM (20 mL) was added CBr4 (0.86 g, 2.60 mmol) followed by PPh3 (0.68 g, 2.60 mmol) and the mixture stirred at room temperature. After 4 h, the pale brown solution was concentrated under reduced pressure and the residue subjected to flash chromatography on silica gel. Elution with hexanes/EtOAc (85:15) gave 17 (0.88 g, 1.88 mmol, 80%) as a white solid, mp 68–70°C, Rf 0.53 (4:1 hexanes/EtOAc on silica gel 60 F254). 1H NMR (500 MHz, CDCl3) δ 1.64 (2H, quintet, J = 7 Hz, 6.5 Hz), 2.23 (2H, t, J = 7 Hz), 3.38–3.43 (4H, m), 3.49 (2H, m), 3.59 (2H, m), 3.75 (2H, t, J = 6.5 Hz), 7.19 (3H, m), 7.26 (6H, m), 7.40 (6H, m); 13C NMR (125 MHz, CDCl3) δ 28.7, 30.2, 66.4, 69.9, 70.0, 70.4, 71.2, 126.5, 127.7, 129.5, 144.8; HRMS-ESI calculated for C26H29O2BrSNa (M+Na)+ 507.0964, found 507.0946. Anal. Calcd for C26H29O2BrS: C 64.32, H 6.02. Found: C 64.28, H 6.07.
1,4,7-Tris(tert-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane (8).39
To a suspension of cyclen 18 (2.00 g, 11.62 mmol) and sodium acetate (2.85 g, 34.8 mmol) in N,N-dimethyl acetamide (DMA, 25 mL) at 0°C was added a solution of t-butyl bromoacetate (6.79 g, 34.82 mmol, 5.14 mL) in DMA (10 mL) dropwise. The reaction mixture was stirred at room temperature for 5 d, after which it was poured into water (125 mL) to give a clear yellow solution. Solid NaHCO3 was added portionwise until 8 precipitated as a white solid. The precipitate was collected by filtration and dissolved in CHCl3 (150 mL). The solution was washed with water (75 mL), dried (MgSO4), filtered, and concentrated to about 20 mL. Ether (150 mL) was added, after which 8 crystallized as a white fluffy solid, mp 201–202°C, Rf 0.37 (10:1 DCM/MeOH on silica gel 60 F254). Yield: 4.7 g (9.1 mmol, 78%). 1H NMR (500 MHz, CDCl3) δ 1.38 (27H, s), 2.78–2.88 (12H, m), 3.01 (4H, m), 3.21 (2H, br s), 3.30 (4H, br s), 10.18 (1H, br s); 13C NMR (125 MHz, CDCl3) δ 28.0, 28.1, 47.4, 48.5, 49.1, 51.0, 51.2, 58.1, 81.4, 81.6, 169.4, 170.3; HRMS-ESI calcd for C26H51N4O6 (M+H)+ 515.3809, found 515.3817.
1,4,7-Tris(tert-butoxycarbonylmethyl)-10-[4-(triphenylmethylthio)methyl)phenyl]methyl-1,4,7,10-tetraazacyclododecane (19a)
To a solution of 8 (2.0 g, 3.9 mmol) in dry CH3CN (40 mL) were added 11 (1.9 g, 4.1 mmol) and K2CO3 (4.3 g, 31.1 mmol) and the mixture heated at 60°C. After 2 h, the reaction mixture was filtered and the filtrate evaporated to dryness under reduced pressure. The residue was dissolved in DCM (100 mL) and the resulting solution was washed with 1N HCl (2 × 50 mL), saturated aqueous NaHCO3 (50 mL), and brine. The organic layer was dried (MgSO4), filtered, and concentrated in vacuo. The residue was subjected to flash chromatography on silica gel eluted with DCM/MeOH (25:1) to give 19a (2.8 g, 3.14 mmol, 80%) as a white foam, Rf 0.38 (10:1 DCM/MeOH on silica gel 60 F254). 1H NMR (500 MHz, CDCl3) δ 1.43 (9H, br s), 1.46 (18H, br s), 2.20 (4H, m), 2.35 (6H, m), 2.50–2.90 (6H, m), 3.02 (4H, m), 3.23 (2H, m), 7.10 (2H, d, J = 7.5 Hz), 7.22 (2H, m), 7.29 (9H, m), 7.45 (6H, m); 13C NMR (125 MHz, CDCl3) δ 27.8, 27.9, 28.0, 28.1, 36.5, 53.3, 55.6, 55.8, 59.2, 67.2, 82.2, 82.6, 126.6, 127.8, 129.3, 129.4, 130.0, 136.0, 136.3, 144.4, 172.4, 173.3; HRMS-ESI calcd for C53H73N4O6S (M+H)+ 893.5251, found 893.5216.
1,4,7-Tris(tert-butoxycarbonylmethyl)-10-[9-(triphenylmethylthio)nonyl]-1,4,7,10-tetraaza-cyclododecane (19b)
To a solution of 8 (2.0 g, 3.9 mmol) in dry CH3CN (40 mL) was added 13 (2.0 g, 4.1 mmol) and K2CO3 (4.3 g, 31.1 mmol) and the mixture heated at 60°C. After 4 h, the reaction mixture was filtered and the filtrate evaporated to dryness under reduced pressure. The residue was dissolved in DCM (100 mL) and the resulting solution was washed with 1N HCl (2 × 50 mL), saturated aqueous NaHCO3 (50 mL), and brine. The organic layer was dried (MgSO4), filtered, and concentrated in vacuo. The residue was subjected to flash chromatography on silica gel eluted with DCM/MeOH (25:1) to give 19b (2.84 g, 3.1 mmol, 80%) as a white foam, Rf 0.40 (10:1 DCM/MeOH on silica gel 60 F254). 1H NMR (500 MHz, CDCl3) δ 1.11–1.26 (10H, m), 1.34–1.41 (4H, m), 1.42–1.47 (27H, m), 2.12 (2H, t, J = 7 HZ), 2.25–2.50 (10H, m), 2.79 (4H, m), 3.00–3.22 (6H, m), 3.28 (2H, br s), 3.47 (2H, m), 7.19 (3H, m), 7.23–7.29 (6H, m), 7.36–7.41 (6H, m); 13C NMR (125 MHz,CDCl3) δ 27.7, 27.9, 28.0, 28.1, 28.5, 28.9, 29.0, 29.1, 29.2, 29.3, 29.5, 31.9, 47.7, 50.2, 50.3, 50.5, 52.6, 52.9, 54.4, 55.7, 56.3, 56.8, 66.2, 81.5, 81.6, 82.2, 82.5, 126.3, 127.6, 129.4, 144.8, 169.7, 170.2, 172.4; HRMS-ESI calcd for C54H83N4O6S (M+H)+ 915.6028, found 915.6019.
1,4,7-Tris(tert-butoxycarbonylmethyl)-10-[9-(triphenylmethylthio)-3,6-dioxanonyl]1,4,7,10-tetraazacyclododecane (19c)
To a solution of 8 (1.72 g, 3.37 mmol) in dry CH3CN (40 mL) was added 17 (1.73 g, 3.54 mmol) and K2CO3 (3.9 g, 28.32 mmol) and the mixture heated at 60°C. After 4 h, the reaction mixture was filtered and the filtrate evaporated to dryness under reduced pressure. The residue was dissolved in 1N HCl (50 mL) and brine (50 mL) and the resulting solution was extracted with DCM (3 × 50 mL). The organic extracts were combined, washed with saturated aqueous NaHCO3 (50 mL), brine, dried (MgSO4), filtered, and concentrated in vacuo. The residue was subjected to flash chromatography on silica gel eluted with DCM/MeOH (25:1) to give 19c (2.84 g, 3.09 mmol, 92%) as a white foam, Rf 0.43 (10:1 DCM/MeOH on silica gel 60 F254). 1H NMR (500 MHz, CDCl3) δ 1.44 (27, m), 1.61 (2H, quintet, J = 7 Hz, 6.5 Hz), 2.20 (2H, t, J = 7 Hz), 2.22–2.50 (8H, cluster of m), 2.82 (8H, m), 3.02 (8H, m), 3.33 (2H, t, J = 6.5 Hz), 3.48 (4H, m), 3.60 (2H, m), 7.20 (3H, m), 7.26 (6H, m), 7.37 (6H, m); 13C NMR (125 MHz, CDCl3) δ 27.9, 28.0, 28.1, 28.2, 28.6, 28.7, 49.7, 50.5, 52.2, 53.4, 55.6, 56.3, 66.5, 67.5, 69.5, 69.9, 70.0, 82.0, 82.1, 126.5, 127.7, 129.4, 144.7, 172.4; HRMS-ESI calcd for C52H79N4O8S (M+H)+ 919.5613, found 919.5612.
1,4,7-Tris(carboxymethyl)-10-[4-(thiomethyl)phenyl]methyl-1,4,7,10-tetraazacyclodode-cane (20a)
To a mixture of 19a (2.0 g, 2.2 mmol), Et3SiH (0.28 g, 2.4 mmol, 386 µL), and butanethiol (20 mL) was added TFA (20 mL) dropwise. The mixture was stirred overnight at room temperature. Volatiles were removed in vacuo, a minimum amount of MeOH was added to effect a solution, and ether (30 mL) was added. The precipitated white solid was collected by filtration and purified by flash chromatography on silica gel eluted with CHCl3/MeOH/aq. NH4OH (5:3:1) to give 20a as a waxy solid (0.85 g, 1.76 mmol, 80%), Rf 0.41 (5:3:1 DCM/MeOH/aq. NH4OH on silica gel 60 F254). 1H NMR (600 MHz, TFA-d) δ 3.21 (4H, m), 3.33 (3H, m), 3.42 (3H, m), 3.58 (4H, m), 3.78 (2H, m), 3.72 (2H, m), 3.77 (4H, m), 3.87 (2H, br s), 4.44 (2H, br s), 4.66 (2H, br s); 13C NMR (150 MHz, TFA-d) δ 29.1, 49.9, 50.3, 51.7, 54.1, 54.5, 56.4, 60.7, 127.4, 131.5, 133.1, 146.9, 171.0, 177.4; HRMS-ESI calcd for C20H35N4O6S (M+H)+ 483.2277, found 483.2273.
[1,4,7-Tris(carboxymethyl)-10-(9-mercaptononyl)-1,4,7,10-tetraazacyclododecane (20b)
To a mixture of 19b (2.16 g, 2.36 mmol), Et3SiH (0.30 g, 2.6 mmol, 415 µL), and butanethiol (20 mL) was added TFA (20 mL) dropwise. The mixture was stirred overnight at room temperature. Volatiles were removed in vacuo, a minimum amount of MeOH was added to effect a solution, and ether (30 mL) was added. The precipitated white solid was collected by filtration and purified by flash chromatography on silica gel eluted with CHCl3/MeOH/aq. NH4OH (5:3:1) to give 20b as a waxy solid (0.88 g, 1.74 mmol, 74%), Rf 0.54 (5:3:1 DCM/MeOH/aq. NH4OH on silica gel 60 F254). 1H NMR (500 MHz, TFA-d) δ 1.43 (12H, m), 1.72 (2H, m), 1.86 (2H, m), 2.67 (1H, m), 3.13–3.22 (4H, m), 3.30–3.45 (6H, m), 3.57 (4H, br s), 3.67–3.84 (8H, m), 4.34 (2H, br s); 13C NMR (125 MHz, CDCl3) δ 25.3, 25.8, 28.1, 29.8, 30.4, 30.6, 30.8, 35.0, 50.3, 50.9, 52.0, 54.5, 54.7, 56.5, 57.6, 171.4, 178.2; HRMS-ESI calcd for C23H45N4O6S (M+H)+ 505.3059, found 505.3052.
1,4,7-Tris(carboxymethyl)-10-(9-mercapto-3,6-dioxanonyl)-1,4,7,10-tetraazacyclododecane (20c)
To a mixture of 19c (2.4 g, 2.6 mmol), Et3SiH (0.34 g, 2.9 mmol, 460 µL), and butanethiol (25 mL) was added TFA (25 mL) dropwise. The mixture was stirred overnight at room temperature. Volatiles were removed in vacuo, a minimum amount of MeOH was added to effect a solution, and ether (30 mL) was added. The precipitated white solid was collected by filtration and purified by flash chromatography on silica gel eluted with CHCl3/MeOH/aq. NH4OH (5:3:1) to afford 20c as a waxy solid (0.98 g, 1.92 mmol, 74%), Rf 0.52 (5:3:1 DCM/MeOH/aq. NH4OH on silica gel 60 F254). 1H NMR (500 MHz, TFA-d) δ 1.89 (2H, m), 2.2 (1H, t, J = 7 Hz), 2.95–3.18 (8H, m), 3.45 (2H, m), 3.54–3.75 (18H, m), 3.85 (3H, m), 4.21 (2H, br s); 13C NMR (125 MHz, CDCl3) δ 21.8, 33.6, 50.1, 50.7, 53.1, 54.4, 54.7, 56.4, 56.6, 66.0, 70.9, 71.6, 71.9, 170.9, 177.4; HRMS-ESI calcd for C21H41N4O6S (M+H)+ 508.2640, found 508.2634.
{1,4,7-Tris(carboxymethyl)-10-[4-(thiomethyl)phenyl]methyl-1,4,7,10-tetraazacyclododec-anato}gadolinium (3a)
To a solution of 20a (100 mg, 0.207 mmol) in MeOH (3 mL) was added Gd(OAc)3 (69.2 mg, 0.207 mmol) and the mixture heated at reflux. After 2 h volatiles were removed in vacuo. The residue was subjected to flash chromatography on reverse-phase silica gel 60 RP-18 eluted with MeOH/H2O (7:3) to give 3a as a white solid (0.1 g, 0.15 mmol, 75%), Rf 0.16 (MeOH/H2O 19:1 on silica gel 60 RP-18 F254); ESI-MS calcd for C20H32GdN4O6S (M+H)+ 638.1287, found 638.1307.
[1,4,7-Tris(carboxymethyl)-10-(9-mercaptononyl)-1,4,7,10-tetraazacyclododecanato] gadolinium (4a)
To a solution of 20b (100 mg, 0.198 mmol) in MeOH (3 mL) was added Gd(OAc)3 (66.2 mg, 0.198 mmol) and the mixture heated at reflux. After 2 h volatiles were removed in vacuo. The residue was subjected to flash chromatography on reverse-phase silica gel 60 RP-18 eluted with MeOH/H2O (7:3) to give 4a as a white solid (75 mg, 0.113 mmol, 57%), Rf 0.17 (MeOH/H2O 19:1 on silica gel 60 RP-18 F254); ESI-MS calcd for C23H42GdN4O6S (M+H)+ 660.2061, found 660.2069. Crystals were grown for X-ray diffraction analysis according to a procedure used previously for growing an In-DO3A-TPP complex.41 An equimolar mixture of 20b and Gd(OAc)3 was heated at 100°C for 30 min in NH4OAc buffer (0.5 M, pH = 6). Slow diffusion of acetone into the reaction mixture produced colorless, flat plates.
{1,4,7-Tris(carboxymethyl)-10-[(9-mercapto-3,6-dioxa)nonyl]-1,4,7,10-tetraazacyclo-dodecanato}gadolinium (5a)
To a solution of 20c (100 mg, 0.196 mmol) in MeOH (3 mL) was added Gd(OAc)3 (65.8 mg, 0.196 mmol) and the mixture heated at reflux. After 2 h volatiles were removed in vacuo. The residue was subjected to flash chromatography on reverse-phase silica gel 60 RP-18 eluted with MeOH/H2O (1:4) to give 5a as a white solid (0.1 g, 0.15 mmol, 75%), Rf 0.23 (MeOH/H2O 1:4 on silica gel 60 RP-18 F254); ESI-MS calcd for C21H38GdN4O8S (M+H)+ 664.1646, found 664.1650.
{1,4,7-Tris(carboxymethyl)-10-[4-(thiomethyl)phenyl]methyl-1,4,7,10-tetraazacyclododec-anato}europium (3b)
To a solution of 20a (100 mg, 0.207 mmol) in MeOH (3 mL) was added Eu(OAc)3 (68.3 mg, 0.207 mmol) and the mixture heated at reflux. After 2 h volatiles were removed in vacuo. The residue was subjected to flash chromatography on reverse-phase silica gel 60 RP-18 eluted with MeOH/H2O (7:3) to give 3b as a white solid (93 mg, 0.147 mmol, 71%), Rf 0.13 (MeOH/H2O 19:1 on silica gel 60 RP-18 F254); ESI-MS calcd for C20H32EuN4O6S (M+H)+ 633.1259, found 633.1243.
[1,4,7-Tris(carboxymethyl)-10-(9-mercaptononyl)-1,4,7,10-tetraazacyclododecanato]-europium (4b)
To a solution of 20b (100 mg, 0.198 mmol) in MeOH (3 mL) was added Eu(OAc)3 (65.3 mg, 0.198 mmol) and the mixture heated at reflux. After 2 h volatiles were removed in vacuo. The residue was subjected to flash chromatography on reverse-phase silica gel 60 RP-18 eluted with MeOH/H2O (7:3) to give 4b as a white solid (75 mg, 0.114 mmol, 58%), Rf 0.10 (MeOH/H2O 19:1 on silica gel 60 RP-18 F254); ESI-MS calcd for C23H42EuN4O6S (M+H)+ 655.2032, found 655.2035.
{1,4,7-Tris(carboxymethyl)-10-[(9-mercapto-3,6-dioxa)nonyl]-1,4,7,10-tetraazacyclo-dodecanato}europium (5b)
To a solution of 20c (100 mg, 0.196 mmol) in MeOH (3 mL) was added Eu(OAc)3 (64.8 mg, 0.196 mmol) and the mixture heated at reflux. After 2 h volatiles were removed in vacuo. The residue was subjected to flash chromatography on reverse-phase silica gel 60 RP-18 eluted with MeOH/H2O (1:4) to give 5b as a white solid (106 mg, 0.161 mmol, 82%), Rf 0.20 (MeOH/H2O 1:4 on silica gel 60 RP-18 F254); ESI-MS calcd for C21H38EuN4O6S (M+H)+ 659.1617, found 659.1617.
1-Bromo-3,6-dioxanonane (21)
To a solution of diethylene glycol monopropyl ether (5.0 g, 30 mmol) in DCM (150 mL) was added CBr4 (12.3 g, 37 mmol) followed by PPh3 (9.7 g, 37 mmol). The mixture stirred at room temperature. After 12 h, the pale brown solution was concentrated in vacuo and the residue subjected to flash chromatography on silica gel. Elution with hexanes/EtOAc (80:20) gave 21 (5.7 g, 27 mmol, 90%) as a colorless oil, Rf 0.54 (4:1 hexanes/EtOAc on silica gel 60 F254). 1H NMR (500 MHz, CDCl3) δ 0.92 (2H, t, J = 6.5 Hz), 1.61 (2H, m), 3.43 (2H, m), 3.47 (2H, m), 3.59 (2H, m), 3.67 (2H, m), 3.82 (2H, t, J = 6.5 Hz); 13C NMR (125 MHz, CDCl3) δ 10.4, 22.8, 30.2, 70.0, 70.5, 71.2.
1,4,7-Tris(tert-butoxycarbonylmethyl)-10-(3,6-dioxanonyl)-1,4,7,10-tetraazacyclododecane (22)
To a solution of 8 (2.0 g, 4 mmol) in dry CH3CN (40 mL) were added 21 (0.88 g, 4.2 mmol) and K2CO3 (4.4 g, 32 mmol) and the mixture heated at 60°C. After 12 h, the reaction mixture was filtered and the filtrate evaporated to dryness in vacuo. The residue was dissolved in 1N HCl (50 mL) and brine (50 mL) and the resulting solution was extracted with DCM (3 × 50 mL). The organic extracts were combined, washed with saturated aqueous NaHCO3 (50 mL), brine, dried (MgSO4), filtered, and concentrated in vacuo. The residue was subjected to flash chromatography on silica gel eluted with DCM/MeOH (25:1) to give 22 (2.47 g, 3.8 mmol, 96%) as a white foam, Rf 0.52 (10:1 DCM/MeOH on silica gel 60 F254). 1H NMR (500 MHz, CDCl3) δ 0.89 (3H, t, J = 7.5 Hz), 1.45 (27, m), 1.55 (2H, m), 2.22–2.60 (8H, cluster of m), 2.70–2.90 (10H, m), 3.05 (4H, m), 3.33 (2H, m), 3.50 (4H, m), 3.70 (4H, m); 13C NMR (125 MHz, CDCl3) δ 10.5, 22.7, 27.9, 28.0, 28.1, 28.2, 49.8, 50.4, 52.2, 55.7, 56.4, 67.5, 69.3, 69.9, 70.1, 82.0, 82.1, 172.4, 172.7; HRMS-ESI calcd for (M+H)+ C33H65N4O8 645.4796, found 645.4799.
1,4,7-Tris(carboxymethyl)-10-(3,6-dioxanonyl)-1,4,7,10-tetraazacyclododecane (23)
To a solution of 22 (0.60 g, 0.93 mmol) in DCM (4 mL) was added TFA (4 mL) dropwise. The mixture was stirred overnight at room temperature. Volatiles were removed in vacuo and the residue was subjected to flash chromatography on silica gel eluted with CHCl3/MeOH/aq. NH4OH (4:2:1) to afford 23 as a waxy solid (0.34 g, 0.72 mmol, 77%), Rf 0.54 (4:2:1 DCM/MeOH/aq. NH4OH on silica gel 60 F254). 1H NMR (500 MHz, TFA-d) δ 1.03 (3H, t, J = 7 Hz), 1.79 (2H, m), 3.15–3.29 (4H, m), 3.30–3.42 (4H, m), 3.68 (2H, m), 3.7–3.8 (8H, m), 3.88 (2H, m), 3.93 (2H, m), 4.08 (2H, m), 4.45 (2H, br s); 13C NMR (125 MHz, CDCl3) δ 10.8, 23.9, 50.7, 51.3, 53.9, 55.0, 55.2, 57.1, 57.3, 67.0, 71.2, 72.5, 76.2, 171.9, 178.3; HRMS calcd for (M+H)+ C21H41N4O8 477.2918, found 477.2926.
[1,4,7-Tris(carboxymethyl)-10-(3,6-dioxa)nonyl-1,4,7,10-tetraazacyclododecanato] gadolinium (24)
To a solution of 23 (100 mg, 0.21 mmol) in MeOH (3 mL) was added Gd(OAc)3 (70.2 mg, 0.21 mmol) and the mixture heated at reflux. After 2 h volatiles were removed in vacuo. The residue was subjected to flash chromatography on reverse-phase silica gel 60 RP-18 eluted with MeOH/H2O (1:4) to give 24 as a white solid (120 mg, 0.19 mmol, 75%), Rf 0.25 (MeOH/H2O 3:7 on silica gel 60 RP-18 F254); ESI-MS calcd for C21H39GdN4O6 (M+H)+ 632.1930, found 632.1932.
Binding of Gd-thiols to Human Serum Albumin
Solutions of Gd complexes were made in either phosphate-buffered saline (PBS), acetate-buffered saline, or HEPES-buffered saline, and in corresponding solutions that contained 0.66 mM human serum albumin (HSA, Sigma), as well as 0–2.0 mM homocysteine (Sigma), to final gadolinium concentrations of 0–1.0 mM. All solutions also contained 10 mM sodium azide, and the final pH of all solutions was 7.40 ± 0.05 at room temperature. Solutions were allowed to equilibrate overnight at 37°C prior to measurements. Aliquots (500 µL) of each solution containing HSA were placed in pre-warmed ultrafiltration units (Amicon UItra-4 Centrifugal Filter Units, 30000 MW cutoff, Millipore Corporation) and immediately centrifuged at 7451 g for 10.7 min, inclusive of braking time. It was assumed that gadolinium bound to HSA (Gdbound) would not pass through the membrane, and that gadolinium in the filtrate (Gdfree) accurately represented the unbound gadolinium in each sample. Gadolinium concentrations in the filtrates were determined by MRI relaxometry. The apparent equilibrium binding constant (KA) for each complex was calculated from the Law of Mass Action.52
Relaxivity of Gd complexes in the absence of HSA
Measurements of the longitudinal water-proton relaxivities of Gd complexes in buffered saline were made at 37°C on a 7 T Bruker Biospec MR Instrument (Bruker Biospin, Billerica, MA). 96-Well tissue culture plates (Falcon) were cut down to 6×8 wells, creating a sample tray which fit inside a 72 mm ID birdcage radio-frequency transmitter-receiver coil (Bruker). Aliquots (250 µL) of the gadolinium solutions were loaded into these wells. Spaces between the wells were filled with water in order to reduce air-water susceptibility artifacts in the images. The sample tray was maintained at 37°C during imaging by flowing heated air over the sample tray. Sample temperature was continuously monitored using a fluoroptic temperature probe (Luxtron Corporation, Santa Clara, CA, USA). Spin-echo MR images of cross-sections of the wells were acquired with recycle times (TR) ranging between 35–8000 ms, and an echo time (TE) of 4 ms. Signal intensity S in each well was fitted to Eq. [1] to extract the T1 at each solution condition. The factor c was between 0.98–1.0 in all regressions.
| [1] |
The T1 relaxation times of the solutions of gadolinium in saline containing 0–2.0 mM homocysteine thus calculated were then fit to Eq. [2], and a longitudinal relaxivity r1 obtained for each solution condition.
| [2] |
Here 1/T10 is the relaxation rate in the absence of contrast agent, and [Gd] is the concentration of gadolinium in the solution.
Relaxivity of Gd complexes in the presence of HSA
The relaxivity of gadolinium in HSA-containing solutions was approximated to have only two components: “free” (monomer, homodimer, heterodimer), and “bound” (to HSA). The T1 times of the solutions of both gadolinium complexes in saline + HSA were inserted into Eq. [3] and fitted for the relaxivity of bound gadolinium, r1(bound)
| [3] |
where [Gd(free)] was obtained as described previously31 with KA constrained to equal the binding constant calculated for the respective HSA-containing solution. In these regressions, r1(free) was constrained to equal the relaxivity measured in the corresponding HSA-free solution.
Supplementary Material
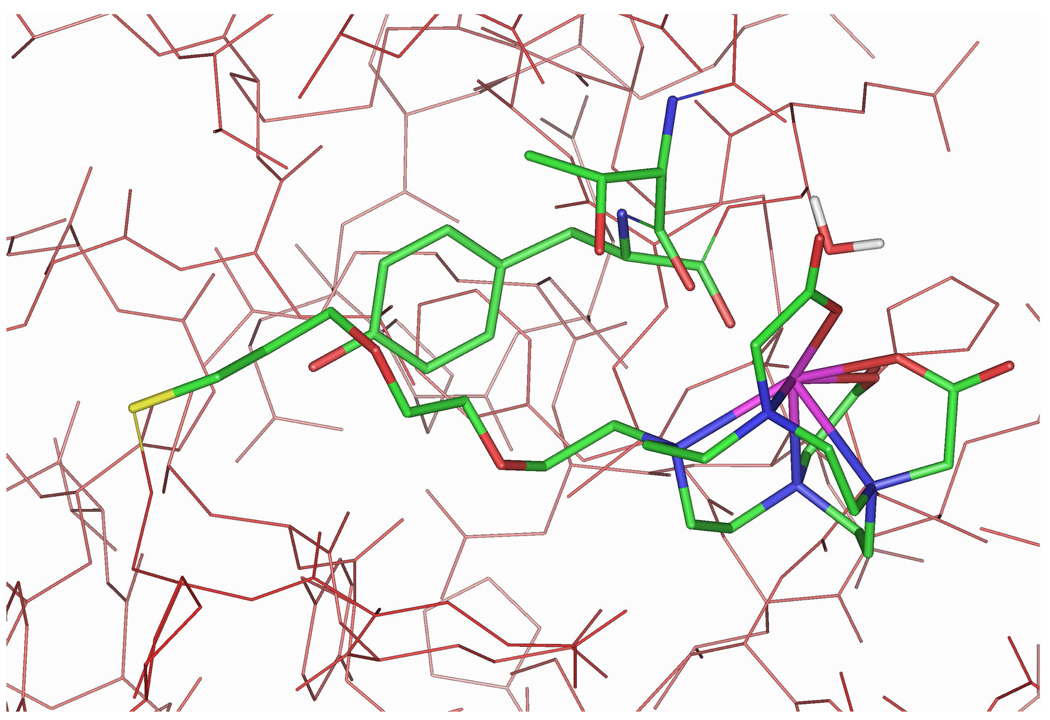
Figure 4.
Docking of 5a with HSA. Compound 5a, HSA residues Thr83 and Tyr84, and a bound water molecule (2.67 Å from Gd) are displayed as atom-colored sticks. Hydrogen atoms and the remaining water molecules are omitted for clarity.
Acknowledgement
This work was supported by grants R01-CA118359 and P30-CA023074 from the National Institutes of Health. The single crystal X-ray diffractometer was purchased with funds from grant CHE-9610347 from the National Science Foundation.
Footnotes
Abbreviations: ARE, antioxidant response element; CAs, contrast agents; DCM, dichloromethane; DO3A, 1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid; DOTA, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; HSA, human serum albumin; GGT, γ-glutamyltransferase; MRI, magnetic resonance imaging; MRP, multidrug resistance-associated protein; PBS, phosphate buffered saline; PEG, polyethylene glycol; TBAI, tetrabutylammonium iodide.
Supporting Information Available. General experimental procedures, proton and carbon NMR spectra for compounds 3b–5b, 8, 11, 13, 17, 19a–c, 20a–c, and 21–23, mass spectra for compounds 3a–5a, 3b–5b, and 24, and an X-ray crystallographic information file (CIF) for compound 4a. This material is available free of charge via the Internet at http://pubs.acs.org
References and Notes
- 1.Lauffer RB. Paramagnetic Metal Complexes as Water Proton Relaxation Agents for NMR Imaging: Theory and Design. Chem. Rev. 1987;87:901–927. [Google Scholar]
- 2.Tóth É, Helm L, Merbach AE. Relaxivity of MRI Contrast Agents. Top. Curr. Chem. 2002;221:61–101. [Google Scholar]
- 3.Westbrook C, Roth CK, Talbot J. MRI in Practice. 3rd ed. Malden, MA: Blackwell; 2005. [Google Scholar]
- 4.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 5.Caravan P. Strategies for Increasing the Sensitivity of Gadolinium Based MRI Contrast Agents. Chem. Soc. Rev. 2006;35:512–523. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 6.Hermann P, Kotek J, Kubìcek V, Lukes I. Gadolinium(III) Complexes as MRI Contrast Agents: Ligand Design and Properties of the Complexes. Dalton Trans. 2008:3027–3047. doi: 10.1039/b719704g. [DOI] [PubMed] [Google Scholar]
- 7.Aime S, Castelli DD, Terreno E. Novel pH-Reporter MRI Contrast Agents. Angew. Chem., Int. Ed. 2002;41:4334–4336. doi: 10.1002/1521-3773(20021115)41:22<4334::AID-ANIE4334>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Lowe MP, Parker D, Reany O, Aime S, Botta M, Castellano G, Gianolio E, Pagliarin R. pH-Dependent Modulation of Relaxivity and Luminescence in Macrocyclic Gadolinium and Europium Complexes Based on Reversible Intramolecular Sulfonamide Ligation. J. Am. Chem. Soc. 2001;123:7601–7609. doi: 10.1021/ja0103647. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Wu K, Sherry AD. A Novel pH-Sensitive MRI Contrast Agent. Angew. Chem., Int. Ed. 1999;38:3192–3194. [PubMed] [Google Scholar]
- 10.Aime S, Crich SG, Botta M, Glovenzana G, Palmisano G, Sisti MA. Macromolecular Gd(III) Complex as pH-responsive Relaxometric Probe for MRI Applications. Chem. Commun. 1999:1577–1578. [Google Scholar]
- 11.Burai L, Scopelliti R, Tóth ÉE. EuII-cryptate with Optimal Water Exchange and Electronic Relaxation: A Synthon for Potential pO2 Responsive Macromolecular MRI Contrast Agents. Chem. Commun. 2002:2366–2367. doi: 10.1039/b206709a. [DOI] [PubMed] [Google Scholar]
- 12.Aime S, Botta M, Gianolio E, Terreno E. A p(O2)-Responsive MRI Contrast Agent Based on the Redox Switch of Manganese(II/III)-Porphyrin Complexes. Angew. Chem., Int. Ed. 2000;39:747–750. doi: 10.1002/(sici)1521-3773(20000218)39:4<747::aid-anie747>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Que EL, Gianolio E, Baker SL, Wong AP, Aime S, Chang CJ. Copper-Responsive Magnetic Resonance Imaging Contrast Agents. J. Am. Chem. Soc. 2009;131:8527–8536. doi: 10.1021/ja900884j. [DOI] [PubMed] [Google Scholar]
- 14.Mishra A, Fousková P, Angelovski G, Balogh E, Mishra AK, Logothetis NK, Tóth É. Facile Synthesis and Relaxation Properties of Novel Bispolyazamacrocyclic Gd3+ Complexes: An Attempt Towards Calcium-Sensitive MRI Contrast Agents. Inorg. Chem. 2008;47:1370–1381. doi: 10.1021/ic7017456. [DOI] [PubMed] [Google Scholar]
- 15.Hanaoka K, Kikuchi K, Urano Y, Nagano T. Selective Sensing of zinc ions with a Novel Magnetic Resonance Imaging Contrast Agent. J. Chem. Soc., Perkin Trans. 2. 2001:1840–1843. [Google Scholar]
- 16.Li W-H, Fraser SE, Meade TJ. A Calcium-Sensitive Magnetic Resonance Imaging Contrast Agent. J. Am. Chem. Soc. 1999;121:1413–1414. [Google Scholar]
- 17.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. In vivo Visualization of Gene Expression using Magnetic Resonance Imaging. Nat. Biotechnol. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 18.Duimstra JA, Femia FJ, Meade TJ. A Gadolinium Chelate for Detection of β-Glucuronidase: A Self-Immolative Approach. J. Am. Chem. Soc. 2005;127:12847–12855. doi: 10.1021/ja042162r. [DOI] [PubMed] [Google Scholar]
- 19.Moats RA, Fraser SE, Meade TJ. A Smart Magnetic Resonance Imaging Agent that Reports on Specific Enzymatic Activity. Angew Chem., Int. Ed. 1997;36:726–728. [Google Scholar]
- 20.Tu C, Nagao R, Louie AY. Multimodal Magnetic-Resonance/Optical-Imaging Contrast Agent Sensitive to NADH. Angew Chem., Int. Ed. 2009;48:6547–6551. doi: 10.1002/anie.200900984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tu C, Louie AY. Photochromically-Controlled, Reversibly-Activated MRI and Optical Contrast Agent. Chem. Commun. 2007:1331–1333. doi: 10.1039/b616991k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulze PC, Yoshioka J, Takahashi T, He Z, King GL, Lee RT. Hyperglycemia Promotes Oxidative Stress through Inhibition of Thioredoxin Function by Thioredoxin-interacting Protein. J. Biol. Chem. 2004;279:30369–30374. doi: 10.1074/jbc.M400549200. [DOI] [PubMed] [Google Scholar]
- 23.Yoh K, Hirayama A, Ishizaki K, Yamada A, Takeuchi M, Yamagichi S, Morito N, Nakano T, Ojima M, Shimohata H, Itoh K, Takahashi S, Yamamoto M. Hyperglycemia Induces Oxidative and Nitrosative Stress and Increases Renal Functional Impairment in Nrf2-Deficient Mice. Genes to Cells. 2008;13:1159–1170. doi: 10.1111/j.1365-2443.2008.01234.x. [DOI] [PubMed] [Google Scholar]
- 24.Wu WS. The Signaling Mechanism of ROS in Tumor Progression. Cancer Metastasis Rev. 2006;25:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 25.Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual Roles of Nrf2 in Cancer. Pharmacol. Res. 2008;58:262–270. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes JD, McMahon M. NRF2 and KEAP1 Mutations: Permanent Activation of an Adaptive Response in Cancer. TIBS. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Biaglow JE, Varnes ME, Clark EP, Epp ER. The Role of Thiols in Cellular Response to Radiation and Drugs. Radiat. Res. 1983;95:437–455. [PubMed] [Google Scholar]
- 28.Fahey RC, Prise KM, Stratford MRL, Watfa RR, Michael BD. Rates for Repair of pBR 322 DNA Radicals by Thiols as Measured by the Gas Explosion Technique: Evidence that Counter-Ion Condensation and Co-Ion Depletion are Significant at Physiological Ionic Strength. Int. J. Radiat. Biol. 1991;59:901–917. doi: 10.1080/09553009114550801. [DOI] [PubMed] [Google Scholar]
- 29.Pompella A, De Tata V, Paolicchi A, Zunino F. Expression of γ-Glutamyltransferase in Cancer Cells and its Significance in Drug Resistance. Biochem. Pharmacol. 2006;71:231–238. doi: 10.1016/j.bcp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Anderson CL, Iyer SS, Ziegler TR, Jones DP. Control of Extracellular Cysteine/Cystine Redox State by HT-29 Cells is Independent of Cellular Glutathione. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1069–R1075. doi: 10.1152/ajpregu.00195.2007. [DOI] [PubMed] [Google Scholar]
- 31.Raghunand N, Jagadish B, Trouard TP, Galons J–P, Gillies RJ, Mash EA. Redox-sensitive Contrast Agents for MRI Based on Reversible Binding of Thiols to Serum Albumin. Magn. Reson. Med. 2006;55:1272–1280. doi: 10.1002/mrm.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacerda S, Campello MP, Marques F, Gano L, Kubícek V, Fousková P, Tóth É, Santos I. A Novel Tetraazamacrocycle Bearing a Thiol Pendant Arm for Labeling Biomolecules with Radiolanthanides. Dalton Trans. 2009:4509–4518. doi: 10.1039/b820375j. [DOI] [PubMed] [Google Scholar]
- 33.Marradi M, Alcántara D, de la Fuente JM, García-Martín ML, Cerdán S, Penadés S. Paramagnetic Gd-based Gold Glyconanoparticles as Probes for MRI: Tuning Relaxivities with Sugars. Chem. Commun. 2009:3922–3924. doi: 10.1039/b900957d. [DOI] [PubMed] [Google Scholar]
- 34.Sugio S, Kashima A, Mochizuki S, Noda M, Kobayashi K. Crystal Structure of Human Serum Albumin at 2.5 Å Resolution. Protein Eng. 1999;12:439–446. doi: 10.1093/protein/12.6.439. [DOI] [PubMed] [Google Scholar]
- 35.Insight II 2005L modeling software was obtained from Accelrys Inc. San Diego CA.
- 36.Bugaut A, Jantos K, Wietor J-L, Rodriguez R, Sanders JKM, Balasubramanian S. Exploring the Differential Recognition of DNA G-Quadruplex Targets by Small Molecules Using Dynamic Combinatorial Chemistry. Angew. Chem., Int. Ed. 2008;47:2677–2680. doi: 10.1002/anie.200705589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowen ME, Monguchi Y, Sankaranarayanan R, Vagner J, Begay LJ, Xu L, Jagadish B, Hruby VJ, Gillies RJ, Mash EA. Design, Synthesis, and Validation of a Branched Flexible Linker for Bioactive Peptides. J. Org. Chem. 2007;72:1675–1680. doi: 10.1021/jo062276g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Appel R. Tertiary Phosphane/Tetrachloromethane, A Versatile Reagent for Chlorination, Dehydration and P-N Linkage. Angew. Chem. Int. Ed. Engl. 1975;14:801–811. [Google Scholar]
- 39.Axelsson O, Olsson A. Synthesis of Cyclen Derivatives. WO 2006112723 A1; CAN. 145:419189. [Google Scholar]
- 40.Lin Y, Favre-Reguillon A, Pellet-Rostaing S, Lemaire M. Synthesis of Pyridine-Based Polyaminocarboxylic Ligands Bearing a Thioalkyl Anchor. Tetrahedron Lett. 2007;48:3463–3466. [Google Scholar]
- 41.Yang C-T, Li Y, Liu S. Synthesis and Structural Characterization of Complexes of a DO3A–Conjugated Triphenylphosphonium Cation with Diagnostically Important Metal Ions. Inorg. Chem. 2007;46:8988–8997. doi: 10.1021/ic7010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spek AL. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003;36:7–13. [Google Scholar]
- 43.Crystal data for 4a: C25H52GdN5O10S; M = 772.04 g mol−1, T = 223(2) K, Mo Kα, thin colorless plates, triclinic, P-1, a = 8.0364(13) Å, b = 10.4906(17) Å, c = 21.799(4) Å, α = 76.573(3)°, β = 86.307(3)°, γ = 88.816(3)°, V = 1783.8(5) Å3, Z = 2, 6582 reflections, Rint 0.0216, GOF on F2 = 1.074, R1 = 0.0275 and wR2 = 0.0672 ([F2 >2σ]), R1 = 0.0331 and wR2 = 0.0690 (all data).
- 44.Sengupta S, Chen H, Togawa T, DiBello PM, Majors AK, Büdy B, Ketterer ME, Jacobsen DW. Albumin Thiolate Anion is an Intermediate in the Formation of Albumin-S– S-Homocysteine. J. Biol. Chem. 2001;276:30111–30117. doi: 10.1074/jbc.M104324200. [DOI] [PubMed] [Google Scholar]
- 45.Franklin JL, Lumpkin HE. Some C–S, H–S and S–S Bond Strengths by the Electron Impact Method. J. Am. Chem. Soc. 1952;74:1023–1026. [Google Scholar]
- 46.Yerly F, Borel A, Helm L, Merbach AE. MD Simulations of Acyclic and Macrocylic Gd3+-Based MRI Contrast Agents: Influence of the Internal Mobility on Water Proton Relaxivity. Chem. Eur. J. 2003;9:5468–5480. doi: 10.1002/chem.200305175. [DOI] [PubMed] [Google Scholar]
- 47.Aime S, Gianolio E, Terreno E, Giovenzana GB, Pagliarin R, Sisti M, Palmisano G, Botta M, Lowe MP, Parker D. Ternary Gd(III)L-HSA Adducts: Evidence for the Replacement of Inner-Sphere Water Molecules by Coordinating Groups of the Protein. Implications for the Design of Contrast Agents for MRI. J. Biol. Inorg. Chem. 2000;5:488–497. doi: 10.1007/pl00021449. [DOI] [PubMed] [Google Scholar]
- 48.Botta M, Quici S, Pozzi G, Marzanni G, Pagliarin R, Barra S, Crich SG. NMR Relaxometric Study of New GdIII Macrocyclic Complexes and their Interaction with Human Serum Albumin. Org. Biomol. Chem. 2004;2:570–577. doi: 10.1039/b313677a. [DOI] [PubMed] [Google Scholar]
- 49.Terreno E, Boniforte P, Botta M, Fedeli F, Milone L, Mortillaro A, Aime S. The Water-Exchange Rate in Neutral Heptadentate DO3A–Like GdIII Complexes: Effect of the Basicity at the Macrocyclic Nitrogen Site. Eur. J. Inorg. Chem. 2003:3530–3533. [Google Scholar]
- 50.Discover_3 ESFF (extensible systematic force field). Molecular mechanics force fields, Insight II, 2005L. San Diego: Accelrys, Inc.; [Google Scholar]
- 51.The General Experimental Section appears in the Supporting Information that accompanies this article. The purities of synthetic intermediates were estimated to be ≥95% based on elemental analyses and/or thin-layer chromatographic analyses. The purities of compounds 3a–5a, 3b–5b, and 24 were estimated to be ≥95% based on thin-layer chromatographic analyses.
- 52.Motulsky H, Christopoulos A. Fitting Models to Biological Data using Linear and Nonlinear Regression. A Practical Guide to Curve Fitting. San Diego: GraphPad Software, Inc.; 2003. pp. 187–191. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.