Abstract
Recently, the smoking cessation therapeutic varenicline, a nicotinic acetylcholine receptor (nAChR) partial agonist, has been shown to reduce alcohol consumption. However, the mechanism and nAChR subtype(s) involved are unknown. Here we demonstrate that varenicline and alcohol exposure, either alone or in combination, selectively activates dopaminergic (DAergic) neurons within the posterior, but not the anterior, ventral tegmental area (VTA). To gain insight into which nAChR subtypes may be involved in the response to alcohol, we analyzed nAChR subunit gene expression in posterior VTA DAergic neurons. Ethanol-activated DAergic neurons expressed higher levels of α4, α6, and β3 subunit genes compared with nonactivated neurons. To examine the role of nicotinic receptors containing the α4 subunit (α4* nAChRs) in varenicline-induced reduction of alcohol consumption, we examined the effect of the drug in two complementary mouse models, a knock-out line that does not express the α4 subunit (α4 KO) and another line that expresses α4* nAChRs hypersensitive to agonist (Leu9′Ala). While varenicline (0.1–0.3 mg/kg, i.p.) reduced 2% and 20% alcohol consumption in wild-type (WT) mice, the drug did not significantly reduce consumption in α4 KO animals. Conversely, low doses of varenicline (0.0125–0.05 mg/kg, i.p.) that had little effect in WT mice dramatically reduced ethanol intake in Leu9′Ala mice. Infusion of varenicline into the posterior, but not the anterior VTA was sufficient to reduce alcohol consumption. Together, our data indicate that activation of α4* nAChRs is necessary and sufficient for varenicline reduction of alcohol consumption.
Introduction
Complications from alcoholism are responsible for up to 1.8 million deaths per year making it the third largest cause of preventable mortality in the world (World Health Organization, 2004). Despite large costs to society, very few therapeutics that successfully aid in curbing alcohol consumption are available, highlighting the need to identify new molecular targets and treatments for alcoholism. Recently, varenicline, a neuronal nicotinic acetylcholine receptor (nAChR) partial agonist, currently FDA approved as a smoking cessation aid (Coe et al., 2005; Jorenby et al., 2006), was shown to reduce drinking in alcohol preferring rats (Steensland et al., 2007) and in a group of heavy-drinking smokers (McKee et al., 2009), suggesting that nAChRs may represent novel therapeutic targets for reducing alcohol consumption.
Ethanol is reinforcing, at least in part, by its propensity to activate dopaminergic (DAergic) neurons within the ventral tegmental area (VTA) (Brodie and Appel, 1998; Brodie et al., 1999), a key region of the mesocorticolimbic DA system, resulting in DA release in the nucleus accumbens (NAc), a phenomenon widely associated with drug reinforcement (Soderpalm et al., 2009). Several nAChR subunit genes are expressed throughout the mesocorticolimbic DA system particularly in DAergic neurons of the VTA. Neuronal nAChRs are ligand-gated, pentameric cation channels normally activated by the endogenous neurotransmitter, acetylcholine (ACh). Twelve mammalian genes encoding neuronal nAChR subunits have been identified (α2-α10, β2-β4) which form either hetero- or homomeric receptors yielding a vast array of nAChR subtypes with distinct pharmacological and biophysical properties (Albuquerque et al., 2009). Although ethanol is not a direct agonist of nAChRs, alcohol increases ACh release into the VTA (Ericson et al., 2003) and potentiates the response of high affinity nAChR subtypes to ACh (Zuo et al., 2002). Mecamylamine, a nonselective nAChR antagonist, either directly infused into the VTA or delivered systemically, decreases ethanol-mediated DA release in the NAc (Blomqvist et al., 1997; Larsson et al., 2002) and also decreases self-administration in rodents (Ericson et al., 1998; Hendrickson et al., 2009). Thus, nAChR activation modulates alcohol consumption and reinforcement.
While varenicline was designed as a selective α4β2* nAChR partial agonist (Coe et al., 2005; Jorenby et al., 2006), it is also a partial agonist at α3β2* and α6* nAChR subtypes, and a full agonist at α3β4* and α7 nAChR subtypes (Mihalak et al., 2006). Varenicline has also been shown to reduce alcohol consumption in knock-out (KO) mice lacking expression of either α7 or β2* nAChRs (Kamens et al., 2010). Thus, the nAChR subtype(s) that varenicline targets to reduce alcohol consumption is unknown. The goal of the present study was to localize and identify nAChR subtypes expressed in the VTA that may be involved in the response to alcohol and to determine whether they play a role in the molecular mechanism by which varenicline reduces alcohol consumption.
Materials and Methods
Animals.
Adult (8- to 10-week-old), male C57BL/6J mice bred in house were used for immunohistochemistry, gene expression, and brain infusion experiments (n = 46). For consumption experiments, adult, male α4 knock-out (α4 KO) mice and their wild type (WT) litter mates (n = 45), as well as heterozygous Leu9′Ala knock-in mice and their WT litter mates (n = 42), all bred on site, were used. The genetic engineering of both α4 KO and Leu9′Ala mouse lines have been described previously (Ross et al., 2000; Tapper et al., 2004). Both lines have been back-crossed to a C57BL/6J background for at least nine generations. C57BL/6J mice were group housed four mice/cage and given food and water ad libitum. For consumption experiments, mice were individually housed on a reversed 12 h light/dark cycle (lights on 10PM, off 10:00 A.M.) with ad libitum access to food and water (except during experiments as described below). All experiments were conducted in accordance with the guidelines for care and use of laboratory animals provided by the National Research Council (National Research Council, 1996), as well as with an approved animal protocol from the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
Drugs and drinking solutions.
Ethanol drinking solutions were prepared from 190 proof absolute anhydrous ethanol (Pharmco-Aaper) diluted to 2% or 20% ethanol (v/v) using tap water. Sucrose was dissolved in tap water to make a 10% (w/v) concentration. Varenicline tartrate, a gift from Pfizer, and ethanol were dissolved in 0.9% saline and administered via intraperitoneal injection at the indicated doses. For infusion of drug into the brain, varenicline was dissolved in artificial CSF (aCSF containing, in mm: 126 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 25 d-glucose). For immunohistochemistry and behavioral experiments, varenicline doses were chosen based on previous studies of varenicline effects on nicotine self-administration and DA turnover in addition to predicted therapeutic concentrations achieved in smokers' brains (O'Connor et al., 2010; Rollema et al., 2010). Varenicline concentrations are reported as free base.
Immunohistochemistry.
Adult (8- to 10-week-old), male, C57BL/6J mice were injected intraperitoneally with saline for 3 d before the start of the experiment to habituate them to handling and to reduce c-Fos activation due to stress. Four groups of three mice were used in each of the following conditions: an intraperitoneal injection of saline followed by an intraperitoneal injection of saline, an intraperitoneal injection of saline followed by a 2.0 g/kg ethanol injection, an intraperitoneal injection of 0.3 mg/kg varenicline followed by a saline injection, and 0.3 mg/kg varenicline i.p. injection followed by a 2.0 g/kg ethanol injection. The time between the first and second injections was 15 min. Ninety minutes after the second injection, all mice were deeply anesthetized with sodium pentobarbital (200 mg/kg, i.p.) and perfused transcardially with 10 ml of 0.1 m PBS followed by 10 ml of 4% paraformaldehyde in 0.1 m sodium phosphate buffer, pH 7.4. Brains were removed and postfixed for 2 h with the same fixative and cryoprotected in sodium phosphate buffer containing 30% sucrose until brains sank. VTA serial coronal sections (20 μm) were cut on a microtome (Leica Microsystems Inc.) and collected into a 24-well tissue culture plate containing 1× PBS. Slices containing VTA were collected between −2.8 mm and −4.04 mm from bregma. After rinsing sections in PBS twice for 5 min, they were treated with 0.2% Triton X-100 PBS (PBST) for 5 min followed by incubation in 2% BSA/PBS for 30 min. Sections were washed with PBS once and then incubated in a mixture of primary antibodies for tyrosine hydroxylase (TH, monoclonal, 1:250 dilution, Santa Cruz Biotechnology) and c-Fos (polyclonal, 1:400 dilution, Santa Cruz Biotechnology) in 2% BSA/PBS overnight at 4°C. The sections were then washed with PBS three times for 5 min followed by incubation in secondary fluorescent labeled antibodies (goat anti-rabbit Alexa Fluor 488 and goat anti-mouse Alexa Fluor 594, 1:300 dilutions, Invitrogen) at room temperature in the dark for 30 min. After washing with PBS 5 times for 5 min/wash, sections were mounted on slides using Vectashield Mounting Medium (Vector Laboratories). The number of positive neurons was counted under a fluorescence microscope (Carl Zeiss MicroImaging Inc.) at a magnification of 400×. The intensity of fluorescence was quantified using a computer-associated image analyzer (Axiovision Rel. 4.6). Neurons were counted as signal-positive if intensities were at least 2 times higher than that of the average value of background (sections stained without primary antibodies). Experimenters were blind to drug treatment.
Laser capture microdissection.
Adult, male C57BL/6J mice were injected intraperitoneally with saline for 3 d before the start of the experiment to habituate them to handling and to reduce c-Fos activation due to stress. On the experiment day, mice were injected intraperitoneally with 2.0 g/kg ethanol and decapitated 90 min later. The brain was removed, snap-frozen in dry ice-cooled 2-methylbutane (−60°C) and stored at −80°C. Coronal serial sections (10 μm) of the VTA were cut using a cryostat (Leica Microsystems Inc.) and mounted on precleaned glass slides (Fisher Scientific). The sections were immediately placed in a slide box on dry ice until completion of sectioning followed by storage at −80°C.
A quick immunofluorescence double-staining protocol for TH and c-Fos was used to identify TH and c-Fos-immunopositive neurons. First, frozen sections were allowed to thaw for 30 s then immediately fixed in cold acetone for 4 min. Slides were then washed in PBS, incubated with a mixture of primary antibodies for mouse anti-TH and rabbit anti-c-Fos (Santa Cruz Biotechnology, 1:50 dilutions) for 10 min, washed in PBS once followed by incubation in secondary fluorescent-labeled antibodies (Invitrogen, a mixture of goat anti-mouse Alex Fluor 594 and goat anti-rabbit Alex Fluor 488, 1:100 dilution) for 10 min. The slides were washed in PBS once, then subsequently dehydrated in a graded ethanol series (for 30 s each in 70% ethanol, 95% ethanol, 100% ethanol, and once for 5 min in xylene). Slides were allowed to dry for 5 min. All antibodies were diluted in DEPC-treated PBS containing 2% BSA and 0.2% Triton X-100. All ethanol solutions and xylene were prepared fresh to preserve RNA integrity.
The Veritas Microdissection System Model 704 (Arcturus Bioscience) was used for laser capture microdissection (LCM). Approximately 800–1400 TH-immunopositive neurons (including c-Fos-immunopositive and c-Fos-immunonegative) were cut from the VTA in each animal. Five to seven different mice were used per treatment. Neurons were captured on CapSure Macro LCM caps (Arcturus Bioscience) for mRNA isolation.
Real-time PCR.
Total RNA was extracted from individual replicate samples using a Micro Scale RNA Isolation Kit (Ambion). RNA samples extracted from DAergic neurons were reverse-transcribed into cDNA using TaqMan Gene Expression Cells-to-CT Kit (Ambion). PCRs were set up in 10 μl reaction volumes using TaqMan Gene Expression Assays (Applied Biosystems). GAPDH was used as an internal control gene to normalize gene expression levels. PCR was performed using an ABI PRISM 7500 Sequence Detection System (Applied Biosystems). Negative controls with no reverse transcriptase were performed for all Taqman Assays. All reactions were performed in triplicate. Relative amplicon quantification was calculated as the difference between Ct values of GAPDH and that of the gene of interest. Relative gene expression differences between c-Fos-immunopositive neurons and c-Fos-immunonegative neurons were calculated using the 2−ΔΔCt method.
Drinking in the dark.
Ethanol consumption was measured using a Drinking in the Dark (DID) procedure similar to that previously described (Hendrickson et al., 2009). Animals were singly housed in experimental chambers for 1 week before the beginning of the DID sessions. The mice received a 15 ml graduated water bottle fitted with a one-hole rubber stopper with a stainless steel double-ball-bearing sipper tube which was sealed with Parafilm to prevent leakage. For the first three nights, 2 h after the lights were off, mice were injected intraperitoneally with saline immediately before their water bottle was replaced with the ethanol bottle (2% or 20%), and allowed to drink for 2 h. This procedure was used to acclimatize the mice to the experimental conditions and allow them to reach a baseline of ethanol intake before drug administration. On the fourth night, the mice received their first dose of drug just before placement of the ethanol bottle. The amount of ethanol consumed was recorded after each 2 h session and converted to g/kg per each animal's ethanol consumption and body weight. The mice were given 2 d of rest (no injections or ethanol) and then began the saline injection/ethanol consumption assay for 2–3 d or until a stable ethanol intake was reached. Once the baseline returned, a second, higher dose of drug was administered before the ethanol bottle being placed in the cage. In this design, all mice in one group drink a single concentration of ethanol throughout the experiment, but receive two doses of drug, 4–5 d apart, with the lower concentration of drug first. The baseline value immediately before the first drug exposure is shown in all figures. There was no significant difference in baseline ethanol intake between doses in any of the experiments (data not shown). For control experiments, mice received 10% sucrose for 2 h instead of ethanol.
Cannula surgeries.
C57BL/6J mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) (VEDCO). The surgical area was shaved and disinfected. Mice were placed in a stereotaxic frame (Stoelting Co.) with mouse adaptor and a small incision was cut in the scalp to expose the skull. Using bregma and lambda as landmarks, the skull was leveled in the coronal and sagittal planes. For cannula placement, holes were drilled in the skull at the anteroposterior (AP, in reference to bregma) and mediolateral (ML) coordinates that correspond to either the anterior VTA (−2.6 mm AP, ± 0.5 mm ML) or posterior VTA (−3.4 mm AP, ± 0.5 mm ML) based on the work of Paxinos and Franklin (2000). Stainless steel unilateral guide cannulas (−4.0 mm dorsal ventral, Plastics One) with dummy cannulas in place, were inserted into the brain and secured to the skull with Cerebond (Plastics One). Mice were allowed to recover for at least 3 d before behavioral testing.
Intra-VTA infusions and DID.
Two hours after the lights were turned off, mice were anesthetized with 2% isoflurane via a nose cone adaptor at a flow rate of 800 ml/L. Once anesthetized, an infusion cannula designed to reach −4.5 mm below the skull was inserted into the guide cannula and vehicle, 10 pmol, 100 pmol, or 1000 pmol of varenicline was infused at a rate of 1 μl/min for 45 s and a total volume of 0.75 μl. Immediately after infusion, mice were placed back into their home cages and monitored until awake, ∼45 s. During this time, the home cage water bottle was removed and replaced with a bottle containing 20% ethanol, which was left in place for 2 h as previously described for the DID experiments. Before the start of each experiment, mice were infused with vehicle daily until a stable baseline of ethanol intake was reached. After completion of behavioral experiments, mice were culled and brains were removed and frozen on dry ice. Coronal sections (20 μm) were cut with a cryostat (Leica Microsystems Inc.). Sections were mounted and stained with neutral red (Sigma) to visualize cannula placement. A total of 6 mice were excluded from analysis due to incorrect cannula placement.
Data analysis.
Data were analyzed using two-way ANOVA with drug treatment and either genotype or brain region as variables as indicated followed by Bonferroni post hoc tests. Data were analyzed using GraphPad software. Paired t tests were used to analyze fold expression of qRT-PCR data. Results were considered significant at p < 0.05. All data are expressed as means ± SEM.
Results
Varenicline and alcohol activate DAergic neurons within posterior VTA
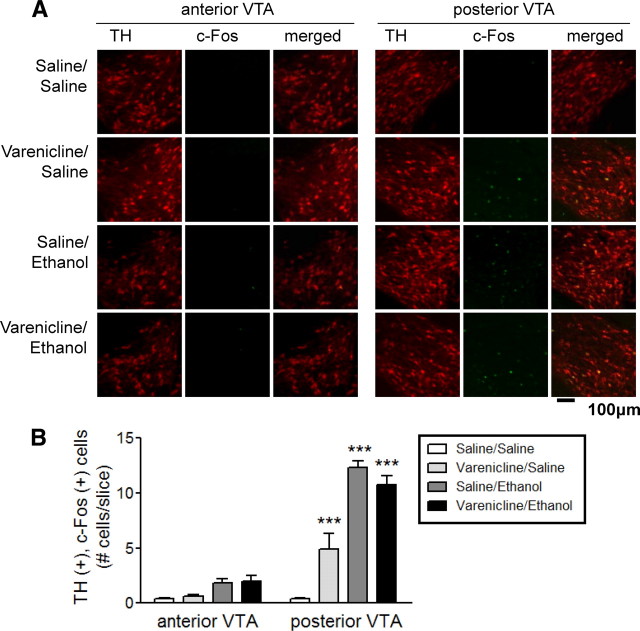
Recent evidence suggests that the VTA is not a homogeneous brain structure but is divided into distinct subregions (Ikemoto, 2007; Shabat-Simon et al., 2008). Thus, using c-Fos expression as a marker of neuronal activation and TH as a marker of DAergic neurons of the VTA, we examined the activating effects of varenicline and ethanol alone and in combination throughout the VTA. The VTA was divided into two distinct regions, anterior and posterior, using known neuroanatomical landmarks and stereotaxic coordinates based on the work of Paxinos and Franklin (2000) and previous publications (Shabat-Simon et al., 2008). C57BL/6J mice were injected intraperitoneally with drug(s) and their brains were examined for c-Fos expression within TH-immunopositive neurons 90 min later. Overall, there was a significant main effect of brain region (F(1,20) = 166.5, p < 0.001), treatment (F(3,20) = 49.21, p < 0.001), and a significant brain region × treatment interaction (F(3,20) = 28.25, p < 0.001). Mice treated with 0.3 mg/kg varenicline followed by a saline injection exhibited an increase in the number of TH-, c-Fos-immunopositive neurons, which were restricted to the posterior VTA (Fig. 1A,B). Mice injected intraperitoneally with saline, followed by 2 g/kg ethanol, also displayed a dramatic increase in the number of TH-, c-Fos-immunopositive neurons within the posterior but not anterior VTA (Fig. 1A,B). Finally, mice injected intraperitoneally with 0.3 mg/kg varenicline followed by 2.0 g/kg ethanol also exhibited a significant increase in the number of TH-, c-Fos-immunopositive neurons restricted to the posterior VTA (Fig. 1A,B). Bonferroni post-tests revealed that each treatment condition was significantly different from control injections (saline/saline) within the posterior VTA (Fig. 1B, p < 0.001). There were no significant effects of treatment on the number of TH-, c-Fos-immunopositive neurons within the anterior VTA when compared with saline injection. These results are consistent with the finding that the VTA can indeed be divided into distinct regions and that ethanol and varenicline predominantly activate DAergic neurons within the posterior VTA.
Figure 1.
Varenicline activates DAergic neurons within the posterior VTA. A, Representative images of coronal sections from the anterior (left) and posterior (right) VTA. Sections were isolated from C57BL/6J mice injected with saline, 0.3 mg/kg varenicline, 2 g/kg ethanol, or both drugs. Sections were immunolabeled to detect TH expression (red, left columns) and c-Fos expression (green, middle columns). Merged images are represented in the right column. B, Quantification of the number of TH-, c-Fos-immunopositive [TH (+), c-Fos (+)] neurons within each region of the VTA after each drug treatment (34–51 slices analyzed per region, n = 3 mice/treatment). ***p < 0.001.
Differential expression of nAChR subunits in alcohol-activated posterior VTA neurons
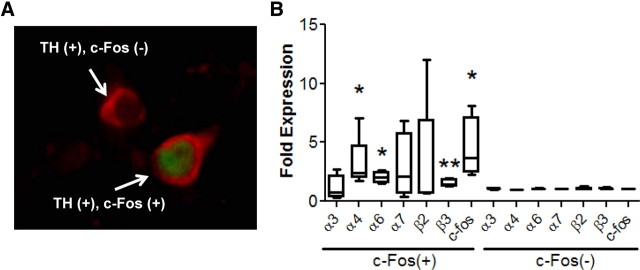
To gain insight into potential nicotinic receptor subunits that may be involved in alcohol activation of posterior VTA DAergic neurons, we challenged C57BL/6J mice with 2.0 g/kg ethanol and used LCM and qRT-PCR to analyze nicotinic receptor gene expression in activated, TH-, c-Fos-immunopositive neurons compared with nonactivated, TH-immunopositive, c-Fos-immunonegative neurons. mRNA from the two groups of neurons (Fig. 2A) only within the posterior VTA was isolated and reverse transcribed. Neuronal nAChR subunit gene expression was then analyzed using qRT-PCR. In TH-, c-Fos-immunopositive neurons, the order of expression for nicotinic receptor subunit genes, from highest to lowest expression, was: α4 > α6 > β3 > β2 > α7 > α3 > α5. For TH-immunopositive, c-Fos-immunonegative neurons, the order of expression was: α4 > β3 > β2 > α6 > α7 > α3 > α5 (Table 1). A paired t test showed significantly higher levels of expression of the α4 (t = 2.24, df = 4, p < 0.05), α6 (t = 4.06, df = 3, p < 0.05), and β3 (t = 4.28, df = 4, p < 0.01) nAChR subunit genes as well as c-Fos (t = 2.69, df = 3, p < 0.05) in the c-Fos-immunopositive neurons compared with c-Fos-immunonegative neurons (Fig. 2B, Table 1). These results indicate specific nAChR subtypes (α4α6β3*) may be important for modulating alcohol activation of posterior VTA DAergic neurons.
Figure 2.
Differential nAChR subunit gene expression in alcohol-activated versus nonactivated DAergic neurons. A, Immunofluorescence image of a coronal midbrain slice from a C57BL/6J mouse challenged with 2.0 g/kg ethanol. Sections were immunolabeled to detect TH (red) expression to visualize DAergic neurons and c-Fos (green) expression to identify neurons activated by ethanol. Arrows point to two different cells, one, a TH-immunopositive c-Fos-immunonegative neuron [TH (+), c-Fos (−)] and the other a TH-, c-Fos-immunopositive neuron [TH (+), c-Fos (+)]. B, Fold change of nAChR subunit gene expression, as measured by qRT-PCR, in TH-, c-Fos-immunopositive (left) neurons compared with TH-immunopositive, c-Fos-immunonegative neurons (right). Neurons were captured via LCM. TH-, c-Fos-immunopositive neurons n = 2298 and TH-immunopositive, c-Fos-immunonegative n = 3645. *p < 0.05, **p < 0.01, paired t test.
Table 1.
Quantitative gene expression of nAChR subunit genes in pVTA DAergic neurons either activated or not activated by 2.0 g/kg ethanol [TH (+), c-Fos (+) or TH (+), c-Fos (−), respectively]
| nAChR subunit | α3 | α4* | α5 | α6* | α7 | β2 | β3** | c-Fos** |
|---|---|---|---|---|---|---|---|---|
| TH (+), c-Fos (+) | −8.12 ± 0.42 | −2.51 ± 0.33 | −9.54 ± 0.46 | −4.42 ± 0.44 | −7.05 ± 0.41 | −4.89 ± 0.42 | −4.84 ± 0.42 | −4.51 ± 0.68 |
| TH (+), c-Fos (−) | −7.97 ± 0.61 | −3.70 ± 0.31 | −9.66 ± 0.55 | −5.71 ± 0.30 | −7.83 ± 0.38 | −5.47 ± 0.37 | −5.43 ± 0.34 | −7.38 ± 0.30 |
Comparison of nAChR subunit gene expression (−ΔCt) of c-Fos-immunopositive and c-Fos-immunonegative DAergic neurons in pVTA after an acute ethanol (2 g/kg) injection. Values represent the negative change in threshold cycle (−ΔCt) compared with GAPDH.
*p < 0.05,
**p < 0.01, paired t test.
Role of α4* nAChRs in varenicline-induced reduction of alcohol consumption
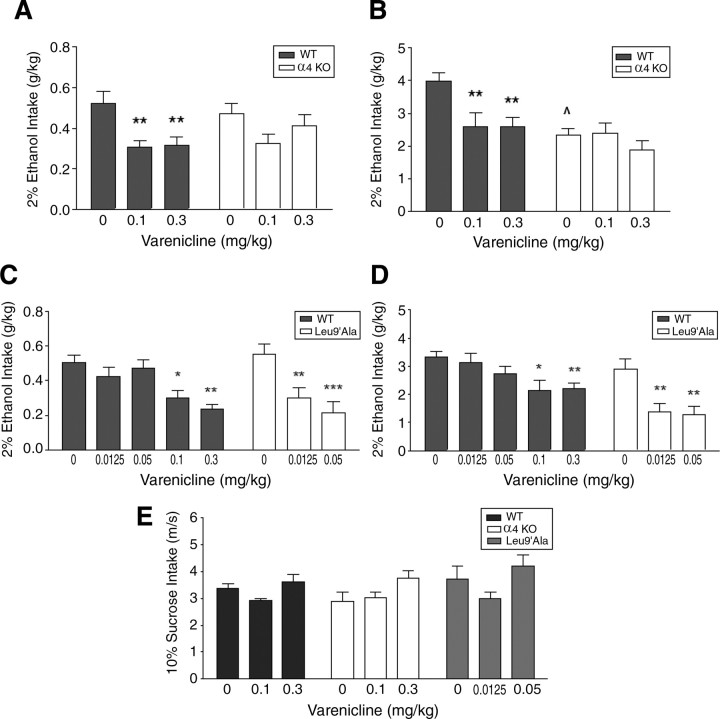
Because (1) the specific role of α4* nAChR in alcohol consumption has not been described and (2) α4 gene expression was higher in DAergic neurons activated by ethanol within posterior VTA, we tested the hypothesis that varenicline may reduce alcohol consumption via these receptors. First, we examined the effects of 0.1 mg/kg and 0.3 mg/kg varenicline on 2% alcohol intake in mice that do not express α4* nAChRs (α4 KO), and their WT litter mates (Fig. 3A). There was a significant main effect of treatment (F(2,45) = 8.0, p < 0.001) but not genotype and there was no significant interaction between these two factors. Both doses of varenicline significantly decreased 2% ethanol consumption in WT mice compared with saline (p < 0.01 for both doses), but did not reduce consumption in α4 KO mice (Fig. 3A). This experiment was repeated in a separate group of animals with the same doses of varenicline, but using a higher concentration of alcohol, 20%. Two-way ANOVA revealed a significant main effect of treatment (F(2,64) = 5.31, p < 0.01), genotype (F(1,64) = 12.11, p < 0.001), and a significant treatment × genotype interaction (F(2,64) = 3.19, p < 0.05). Whereas WT and α4 KO mice consumed similar baseline quantities of 2% alcohol, baseline 20% alcohol consumption after saline injection was significantly less in α4 KO mice compared with WT (p < 0.01, Fig. 3B). Varenicline significantly decreased 20% ethanol consumption in WT animals (Fig. 3B) and post hoc tests indicated that alcohol consumption after each varenicline dose was significantly lower compared with consumption after saline injection (p < 0.01 for both doses). In α4 KO mice, alcohol consumption after either varenicline dose was not significantly different compared with consumption after saline. Thus, these data suggest that expression of α4* nAChRs is necessary for varenicline-induced reduction of ethanol consumption.
Figure 3.
Alcohol intake after varenicline treatment in α4 KO, Leu9′Ala and WT mice. A, Ethanol intake (2%) in WT and α4 KO mice after saline or varenicline treatment (n = 8–9/genotype). B, Ethanol intake (20%) in WT and α4 KO mice after saline or varenicline treatment (n = 10–12/genotype). C, Effect of saline and varenicline on 2% ethanol intake in WT and Leu9′Ala (n = 10–11/genotype). D, Effect of saline and varenicline on 20% ethanol intake in Leu9′Ala and WT mice (n = 6–9/genotype). E, Effect of varenicline treatment on 10% sucrose intake (n = 5–11/genotype) in WT, α4 KO, and Leu9′Ala mice. *p < 0.05, **p < 0.01, ***p < 0.001, ∧p < 0.01 (α4 KO compared with WT after saline injection).
To determine whether activation of α4* nAChRs by varenicline was sufficient to decrease ethanol intake, we analyzed the effect of low doses of drug on alcohol consumption in mice expressing a single point mutation, Leu9′Ala, that renders α4* nAChRs hypersensitive to agonist (Tapper et al., 2004). Leu9′Ala and WT litter mate mice were challenged with intraperitoneal injections of 0.0125 mg/kg and 0.05 mg/kg varenicline before receiving a 2% ethanol bottle. There was a significant main effect of treatment (F(2,55) = 6.86, p < 0.01) and genotype (F(1,55) = 6.14, p < 0.05) and a significant treatment × genotype interaction (F(2,55) = 3.74, p < 0.05). Surprisingly, both low doses of varenicline significantly decreased 2% ethanol intake in Leu9′Ala (0.0125 mg/kg and 0.05 mg/kg compared with saline, p < 0.01 and p < 0.001, respectively) but not WT mice (Fig. 3C). However, higher doses (0.1 mg/kg and 0.3 mg/kg) of varenicline did significantly reduce consumption in WT mice (Fig. 3C, p < 0.05, and p < 0.01 comparing 0.1 mg/kg and 0.3 mg/kg varenicline to saline, respectively). Similar results were obtained when this experiment was repeated and the concentration of alcohol was increased to 20%. There was a main effect of treatment (F(2,40) = 6.73, p < 0.01) and genotype (F(1,40) = 22.65) but no significant interaction. Varenicline significantly decreased ethanol consumption in Leu9′Ala but not WT mice when challenged with low doses of the drug, and each dose of varenicline was significantly different compared with a saline injection (Fig. 3D, p < 0.01 for both doses). Together, these data suggest that selective activation of α4* nAChRs by varenicline is sufficient for reduction of alcohol consumption.
Importantly, varenicline did not significantly reduce 10% sucrose consumption in any of the genotypes, indicating that the effect of varenicline was specific for alcohol intake (Fig. 3E).
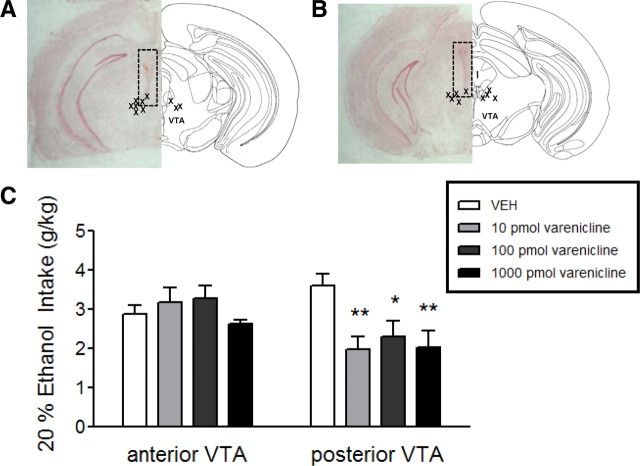
Varenicline infusion into the anterior and posterior VTA
To determine whether varenicline reduction of alcohol consumption was mediated by drug action in the posterior VTA, we selectively infused the drug into either the anterior or posterior VTA of C57BL/6J mice and measured alcohol consumption. Guide cannulas were implanted into either brain region (see methods) and vehicle, 10, 100, or 1000 pmol of varenicline was infused into the VTA before presentation of a 20% alcohol bottle. Figure 4, A and B, depicts the location of the guide cannula within each mouse brain from the two groups of animals. Two-way ANOVA revealed a significant main effect of treatment (F(3,47) = 3.13, p < 0.05) and brain region (F(1,45) = 4.88, p < 0.05) and a significant treatment × brain region interaction (F(3,47) = 4.39, p < 0.01). When infused into the anterior VTA, varenicline did not significantly reduce alcohol consumption compared with vehicle infusion (Fig. 4C). However, 10, 100, and 1000 pmol of varenicline, when infused into the posterior VTA, significantly reduced alcohol consumption (Fig. 4C, p < 0.01, p < 0.05, and p < 0.01 respectively). These data indicate that infusion of varenicline into the posterior VTA is sufficient to reduce alcohol consumption.
Figure 4.
Alcohol intake after varenicline infusion into anterior or posterior VTA. A, Left, representative neutral red stained coronal brain slice from a mouse with a guide cannula placed just dorsal to the anterior VTA (dotted box). Right, schematic diagram of anterior VTA −3.08 mm from bregma (adapted from Paxinos and Franklin, 2000). X's represent guide cannula placements within the anterior VTA. B, Left, representative neutral red stained coronal brain slice from a mouse with a guide cannula placed just dorsal to the posterior VTA (dotted box). Right, schematic diagram of posterior VTA at bregma −3.52. X's represent guide cannula placements within the posterior VTA. C, Ethanol intake (20%) after infusion of aCSF, 10 pmol, 100 pmol, or 1000 pmol of varenicline in either the anterior or posterior VTA (n = 9–10/brain region). *p < 0.05, **p < 0.01.
Discussion
Because alcohol is one of the most commonly abused psychoactive drugs in the world resulting in significant health consequences, it is critical to identify novel therapies and molecular targets to treat alcoholism. The nAChR partial agonist, varenicline, is an FDA approved smoking cessation aid that may hold promise as a treatment for alcoholism (Coe et al., 2005; Gonzales et al., 2006). For example, varenicline reduces alcohol consumption and seeking in rats (Steensland et al., 2007) and also significantly reduces consumption in heavy drinking smokers (McKee et al., 2009). Although much is known about how varenicline may reduce nicotine dependence, much less is known regarding the brain regions and nAChR subtypes that varenicline may target to reduce alcohol consumption.
Varenicline-induced activation of the posterior VTA reduces alcohol consumption
Our data indicate that varenicline and ethanol interact in the VTA. Indeed, much emphasis has been placed on the VTA because of its central importance in the mesocorticolimbic reward pathway (Funk et al., 2006). Alcohol activates DAergic neurons within this region, ultimately increasing DA release in the NAc and driving dependence (Pidoplichko et al., 1997; Brodie and Appel, 1998; Brodie et al., 1999; Mansvelder et al., 2002). Mounting evidence indicates that the VTA is not a homogeneous brain region; rather it can be anatomically and functionally divided into at least two brain regions, the anterior and posterior VTA (Ikemoto, 2007; Shabat-Simon et al., 2008). Although both regions of the VTA contain predominantly two subtypes of neurons, DAergic projection neurons and local GABAergic interneurons, studies have shown that neurons within the anterior and posterior VTA project to distinct regions of striatum and also may respond differently to drugs of abuse (Zangen et al., 2006; Ikemoto, 2007; Shabat-Simon et al., 2008). The predominant VTA subregion that is critical for alcohol's action in the VTA is unclear. Previous studies indicate that the anterior VTA is an important modulator of alcohol intake (Ericson et al., 2008; Moore and Boehm, 2009) while others find a role for the posterior VTA (Linsenbardt and Boehm, 2009). Additionally, local infusion of ethanol into the anterior VTA does not increase NAc DA output (Ericson et al., 2003; Löf et al., 2007a) while local infusion of ethanol to the posterior VTA is sufficient for increased DA release in the NAc (Ding et al., 2009). Our data are in line with previous work highlighting the importance of the posterior VTA in alcohol-mediated activation of DAergic neurons. Injection of 2.0 g/kg alcohol, a dose that conditions a place preference in C57BL/6J mice (i.e., a rewarding dose) activated DAergic neurons predominantly in the posterior VTA. Varenicline also activated DAergic neurons selectively in this region and infusion of varenicline directly into the posterior, but not anterior, VTA reduced alcohol consumption, suggesting that the posterior VTA, specifically, could be a neuroanatomical substrate where both drugs interact. Thus, our data support previous studies indicating that rats will self-administer nicotine or ethanol directly into the posterior, but not anterior, VTA (Rodd-Henricks et al., 2000; Rodd et al., 2004; Ikemoto et al., 2006).
Activation of DAergic neurons by alcohol at least partially mediates the rewarding properties of the drug. Interestingly, varenicline also activates these neurons and this reduces alcohol consumption. Although the precise mechanism of this effect is unknown, one possibility is that activation of DAergic neurons by varenicline precludes further activation by alcohol. At the molecular level, varenicline may serve to occupy or desensitize nAChRs necessary for alcohol activation of DAergic neurons, thereby reducing DA release in the NAc and decreasing consumption. Supporting this idea, varenicline has been shown to reduce alcohol stimulated NAc DA release in rats (Ericson et al., 2009). This would also be consistent with the mechanism by which varenicline is thought to reduce nicotine reinforcement (Rollema et al., 2007).
The role of nAChRs in alcohol consumption
Reduction of alcohol consumption by varenicline indicates that nAChRs may play a critical role in the reinforcing properties of ethanol. Several studies have used various nicotinic agonists and antagonists to implicate nAChR activation as potentially important for alcohol consumption. The nonspecific nAChR antagonist, mecamylamine, either delivered systemically or selectively into the VTA, reduces alcohol intake and blocks alcohol-mediated DA release in NAc (Blomqvist et al., 1993, 1996, 1997; Hendrickson et al., 2009). However, specific nicotinic receptor subtypes involved in alcohol consumption are unclear. Previous studies have demonstrated that the selective α4β2* nAChR competitive antagonist, DHβE, fails to modulate alcohol consumption suggesting that this subtype may not be involved in the response to alcohol (Lê et al., 2000; Hendrickson et al., 2009). The α7 selective antagonist, methyllycaconitine, also does not reduce alcohol intake (Hendrickson et al., 2009). To gain insight into nAChR subtypes that may influence alcohol consumption, and be targeted by varenicline, we compared nAChR subunit gene expression between posterior VTA DAergic neurons that were activated by alcohol and posterior VTA DAergic neurons not activated by alcohol. We found that DAergic neurons that were activated by alcohol express higher levels of α4, α6, and β3 nAChR subunit transcripts. Although this difference in mRNA expression does not necessarily translate into protein and/or assembled receptor expression, these data suggest that α4α6β3* nAChRs may be involved in alcohol consumption. Our results are consistent with previous studies indicating that the α6/β3* nAChR selective antagonist α-conotoxin MII, when infused into the VTA, can reduce alcohol consumption and block alcohol-mediated DA release in NAc (Larsson et al., 2004; Jerlhag et al., 2006; Löf et al., 2007b). Importantly, a significant portion of α6β3* nAChRs also contain the α4 subunit and these receptors represent one of the highest affinity nAChRs identified in the brain thus far (Salminen et al., 2004, 2007).
Activation of α4* nAChRs are critical for varenicline-induced reduction of alcohol consumption
The role of α4* nAChRs in varenicline reduction of alcohol consumption has not been examined previously. We used two complementary genetic nAChR mouse models, one lacking the gene encoding the α4 subunit, CHRNA4 (α4 KO) (Ross et al., 2000) and another that has a single point mutation, Leu9′Ala, within the endogenous CHRNA4 exon 5 resulting in α4* nAChRs that are hypersensitive to agonist (Leu9′Ala) (Tapper et al., 2004; Fonck et al., 2005). Varenicline reduced consumption of both a low and high dose of alcohol in WT mice but did not significantly reduce consumption in α4 KO mice indicating that expression of α4* nAChRs is necessary for the effects of the drug. In contrast to the effects of varenicline in the KO animals, low doses of varenicline that had little effect on consumption in WT mice dramatically reduced ethanol intake in Leu9′Ala mice suggesting that activation of α4* nAChRs is also sufficient for varenicline effects on alcohol consumption. Importantly, varenicline did not reduce sucrose intake indicating that the effects of the drug were specific for alcohol consumption and did not dampen general reward signaling. Because varenicline may also target α7 as well as other nAChR subtypes in addition to α4β2* nAChRs, the mechanism by which varenicline reduces alcohol consumption is an open question (Mihalak et al., 2006; Papke et al., 2010). A recent study demonstrated that varenicline reduced ethanol consumption in mice lacking either α7 or β2* nAChRs similar to WT (Kamens et al., 2010), indicating that expression of these receptors is not necessary for varenicline's effects. As discussed above, our data suggest that α4α6β3* nAChRs mediate varenicline's effects on alcohol consumption, although it is expected that this subtype should also contain the β2 subunit (Grady et al., 2007). Thus, varenicline reduction of ethanol intake in β2 KO mice may occur because of compensatory mechanisms in nAChR expression or subunit composition. Alternatively, we cannot rule out the possibility that higher doses of varenicline than used in our study may reduce alcohol consumption by a non-α4* nAChR-dependent mechanism. However, we expect that the doses we used would result in concentrations of varenicline more selective for high affinity nAChRs. For example, 0.1 mg/kg varenicline is predicted to yield a brain concentration of 38 nm (Rollema et al., 2009). This concentration is predicted to be within the range experienced by smokers taking therapeutic doses of varenicline (Rollema et al., 2010). Similar doses also reduce nicotine self-administration in rats without impacting food reinforcement unlike higher doses (O'Connor et al., 2010). In addition, this range of varenicline dose also increases DA turnover in rat NAc (Rollema et al., 2010) consistent with our data illustrating that 0.3 mg/kg varenicline activates DAergic neurons.
Together, our results demonstrate that ethanol and varenicline selectively activate DAergic neurons within the posterior VTA and that activation of α4* nAChRs is necessary and sufficient for varenicline-induced reduction of ethanol consumption. Our data combined with recent clinical studies indicate that varenicline could potentially be a therapeutic candidate for the treatment of alcoholism.
Footnotes
This study was supported by National Institute on Alcohol Abuse and Alcoholism Awards R01AA017656 (A.R.T.) and F31AA018915 (L.M.H.) and by National Institute of Neurological Disorders and Stroke Award R01NS030243 (P.D.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Neurological Disorders and Stroke, or the National Institutes of Health. We thank Dr. Hans Rollema for helpful discussions.
References
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist O, Engel JA, Nissbrandt H, Söderpalm B. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol. 1993;249:207–213. doi: 10.1016/0014-2999(93)90434-j. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Johnson DH, Engel JA, Söderpalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Engel JA, Söderpalm B. Accumbal dopamine overflow after ethanol: localization of the antagonizing effect of mecamylamine. Eur J Pharmacol. 1997;334:149–156. doi: 10.1016/s0014-2999(97)01220-x. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Appel SB. The effects of ethanol on dopaminergic neurons of the ventral tegmental area studied with intracellular recording in brain slices. Alcohol Clin Exp Res. 1998;22:236–244. [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, McBride WJ. Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol Clin Exp Res. 2009;33:1571–1581. doi: 10.1111/j.1530-0277.2009.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M, Blomqvist O, Engel JA, Söderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol. 1998;358:189–196. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- Ericson M, Molander A, Löf E, Engel JA, Söderpalm B. Ethanol elevates accumbal dopamine levels via indirect activation of ventral tegmental nicotinic acetylcholine receptors. Eur J Pharmacol. 2003;467:85–93. doi: 10.1016/s0014-2999(03)01564-4. [DOI] [PubMed] [Google Scholar]
- Ericson M, Löf E, Stomberg R, Chau P, Söderpalm B. Nicotinic acetylcholine receptors in the anterior, but not posterior, ventral tegmental area mediate ethanol-induced elevation of accumbal dopamine levels. J Pharmacol Exp Ther. 2008;326:76–82. doi: 10.1124/jpet.108.137489. [DOI] [PubMed] [Google Scholar]
- Ericson M, Löf E, Stomberg R, Söderpalm B. The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther. 2009;329:225–230. doi: 10.1124/jpet.108.147058. [DOI] [PubMed] [Google Scholar]
- Fonck C, Cohen BN, Nashmi R, Whiteaker P, Wagenaar DA, Rodrigues-Pinguet N, Deshpande P, McKinney S, Kwoh S, Munoz J, Labarca C, Collins AC, Marks MJ, Lester HA. Novel seizure phenotype and sleep disruptions in knock-in mice with hypersensitive alpha 4* nicotinic receptors. J Neurosci. 2005;25:11396–11411. doi: 10.1523/JNEUROSCI.3597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Marinelli PW, Lê AD. Biological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Res Health. 2006;29:186–192. [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Tapper AR. Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6J mice. Psychopharmacology (Berl) 2009;204:563–572. doi: 10.1007/s00213-009-1488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu ZH. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci. 2006;26:723–730. doi: 10.1523/JNEUROSCI.4542-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Grøtli M, Luthman K, Svensson L, Engel JA. Role of the subunit composition of central nicotinic acetylcholine receptors for the stimulatory and dopamine-enhancing effects of ethanol. Alcohol Alcohol. 2006;41:486–493. doi: 10.1093/alcalc/agl049. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology (Berl) 2010;208:613–626. doi: 10.1007/s00213-009-1759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson A, Svensson L, Söderpalm B, Engel JA. Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol. 2002;28:157–167. doi: 10.1016/s0741-8329(02)00244-6. [DOI] [PubMed] [Google Scholar]
- Larsson A, Jerlhag E, Svensson L, Söderpalm B, Engel JA. Is an alpha-conotoxin MII-sensitive mechanism involved in the neurochemical, stimulatory, and rewarding effects of ethanol? Alcohol. 2004;34:239–250. doi: 10.1016/j.alcohol.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Lê AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL., 2nd Agonism of the endocannabinoid system modulates binge-like alcohol intake in male C57BL/6J mice: involvement of the posterior ventral tegmental area. Neuroscience. 2009;164:424–434. doi: 10.1016/j.neuroscience.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löf E, Ericson M, Stomberg R, Söderpalm B. Characterization of ethanol-induced dopamine elevation in the rat nucleus accumbens. Eur J Pharmacol. 2007a;555:148–155. doi: 10.1016/j.ejphar.2006.10.055. [DOI] [PubMed] [Google Scholar]
- Lof E, Olausson P, deBejczy A, Stomberg R, McIntosh JM, Taylor JR, Soderpalm B. Nicotinic acetylcholine receptors in the ventral tegmental area mediate the dopamine activating and reinforcing properties of ethanol cues. Psychopharmacology (Berl) 2007b;195:333–343. doi: 10.1007/s00213-007-0899-4. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Moore EM, Boehm SL., 2nd Site-specific microinjection of baclofen into the anterior ventral tegmental area reduces binge-like ethanol intake in male C57BL/6J mice. Behav Neurosci. 2009;123:555–563. doi: 10.1037/a0015345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Washington DC: National Academy; 1996. Guide for the care and use of laboratory animals. [Google Scholar]
- O'Connor EC, Parker D, Rollema H, Mead AN. The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology (Berl) 2010;208:365–376. doi: 10.1007/s00213-009-1739-5. [DOI] [PubMed] [Google Scholar]
- Papke RL, Wecker L, Stitzel JA. Activation and inhibition of mouse muscle and neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2010;333:501–518. doi: 10.1124/jpet.109.164566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. Ed 2. San Diego: Academic; 2000. The mouse brain in stereotaxic coordinates. [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004;24:1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 2000;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rollema H, Hajós M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, Horner WE, Chapin DS, Hoffmann WE, Johnson DE, McLean S, Freeman J, Williams KE. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol. 2009;78:813–824. doi: 10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- Rollema H, Shrikhande A, Ward KM, Tingley FD, 3rd, Coe JW, O'Neill BT, Tseng E, Wang EQ, Mather RJ, Hurst RS, Williams KE, de Vries M, Cremers T, Bertrand S, Bertrand D. Pre-clinical properties of the alpha4beta2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. Br J Pharmacol. 2010;160:334–345. doi: 10.1111/j.1476-5381.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, Scheffer IE, Kola I, Waddington JL, Berkovic SF, Drago J. Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci. 2000;20:6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of alpha-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol. 2007;71:1563–1571. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- Shabat-Simon M, Levy D, Amir A, Rehavi M, Zangen A. Dissociation between rewarding and psychomotor effects of opiates: differential roles for glutamate receptors within anterior and posterior portions of the ventral tegmental area. J Neurosci. 2008;28:8406–8416. doi: 10.1523/JNEUROSCI.1958-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderpalm B, Lof E, Ericson M. Mechanistic studies of ethanol's interaction with the mesolimbic dopamine reward system. Pharmacopsychiatry. 2009;42(Suppl 1):S87–S94. doi: 10.1055/s-0029-1220690. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Geneva: World Health Organization; 2004. Global status report on alcohol 2004. [Google Scholar]
- Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA. Two brain sites for cannabinoid reward. J Neurosci. 2006;26:4901–4907. doi: 10.1523/JNEUROSCI.3554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Kuryatov A, Lindstrom JM, Yeh JZ, Narahashi T. Alcohol modulation of neuronal nicotinic acetylcholine receptors is alpha subunit dependent. Alcohol Clin Exp Res. 2002;26:779–784. [PubMed] [Google Scholar]