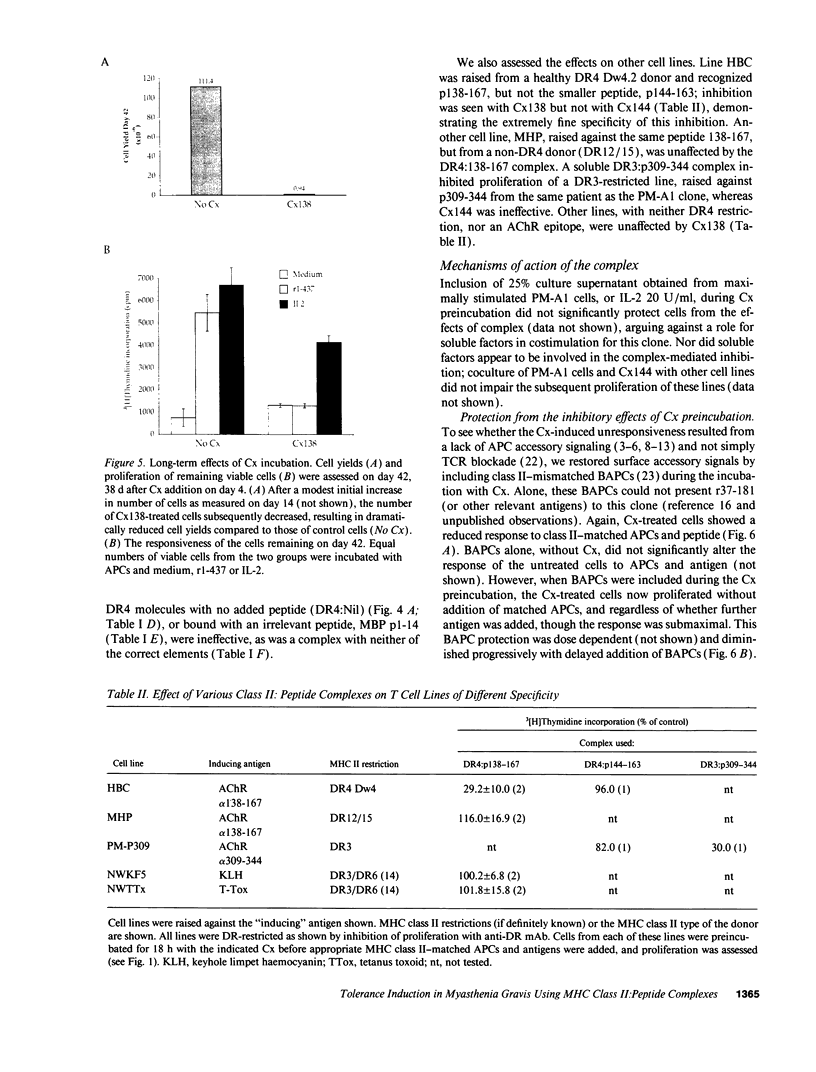
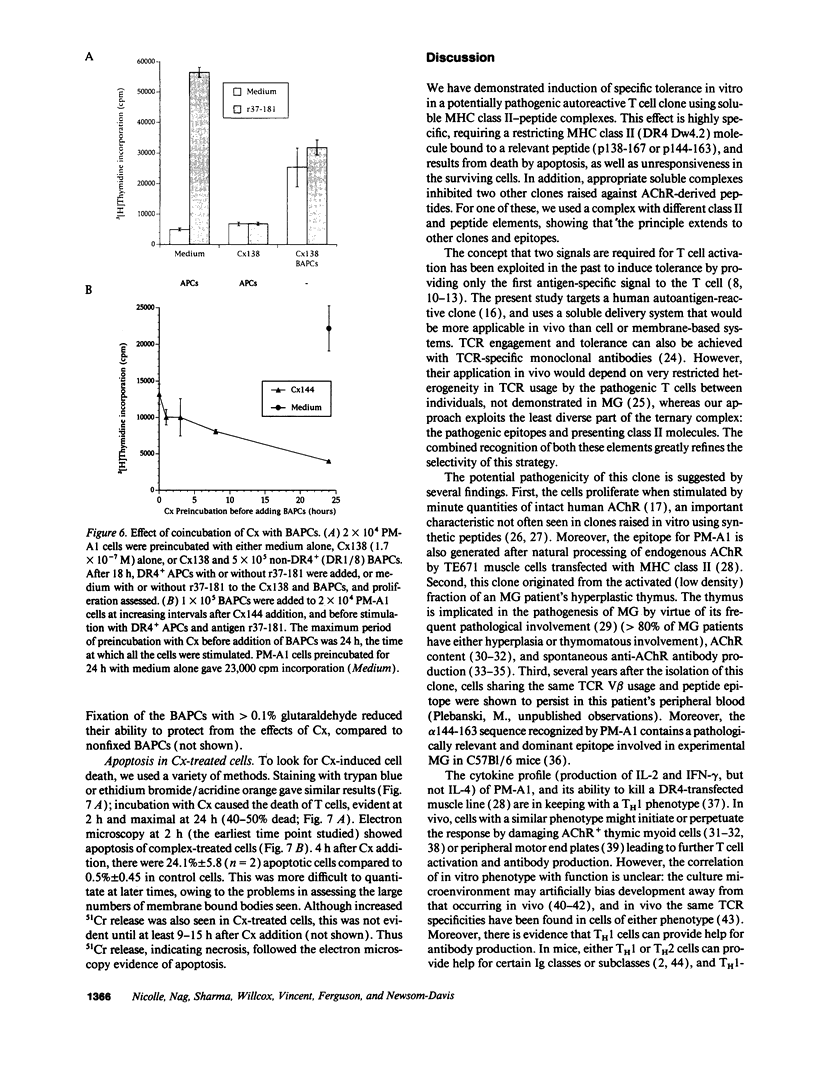
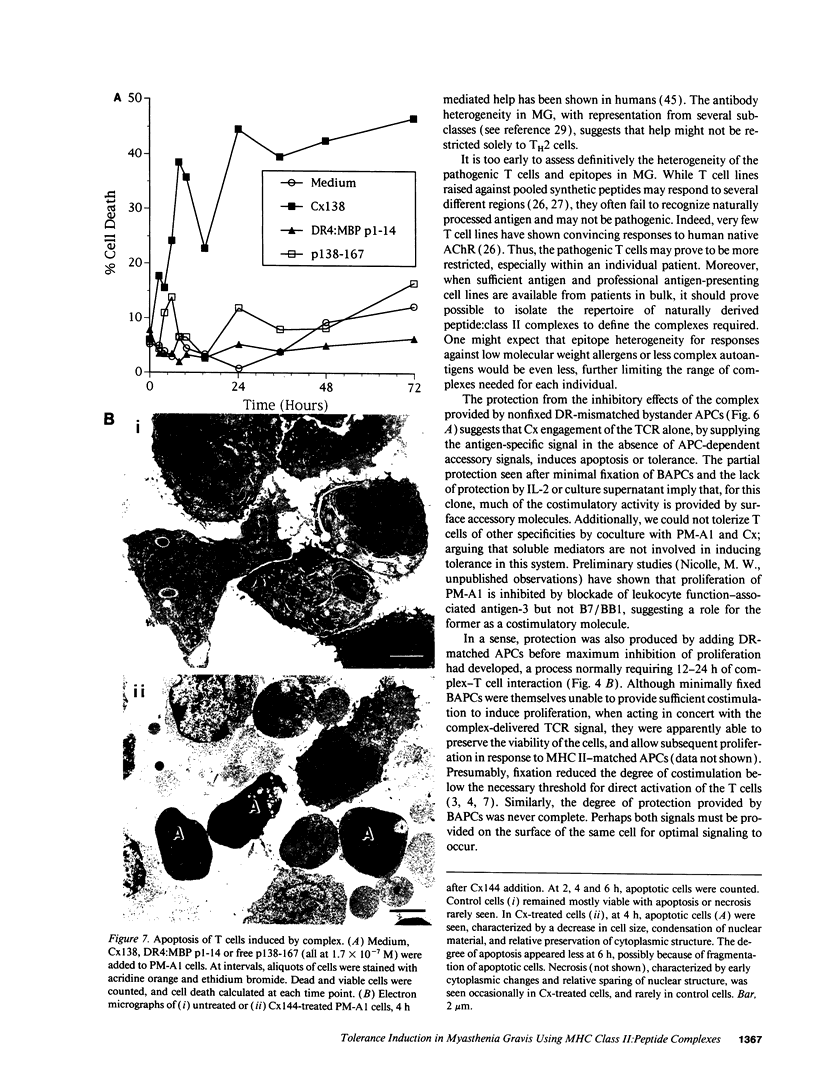
Abstract
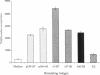
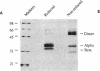
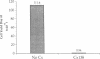
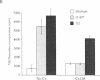
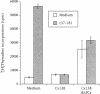
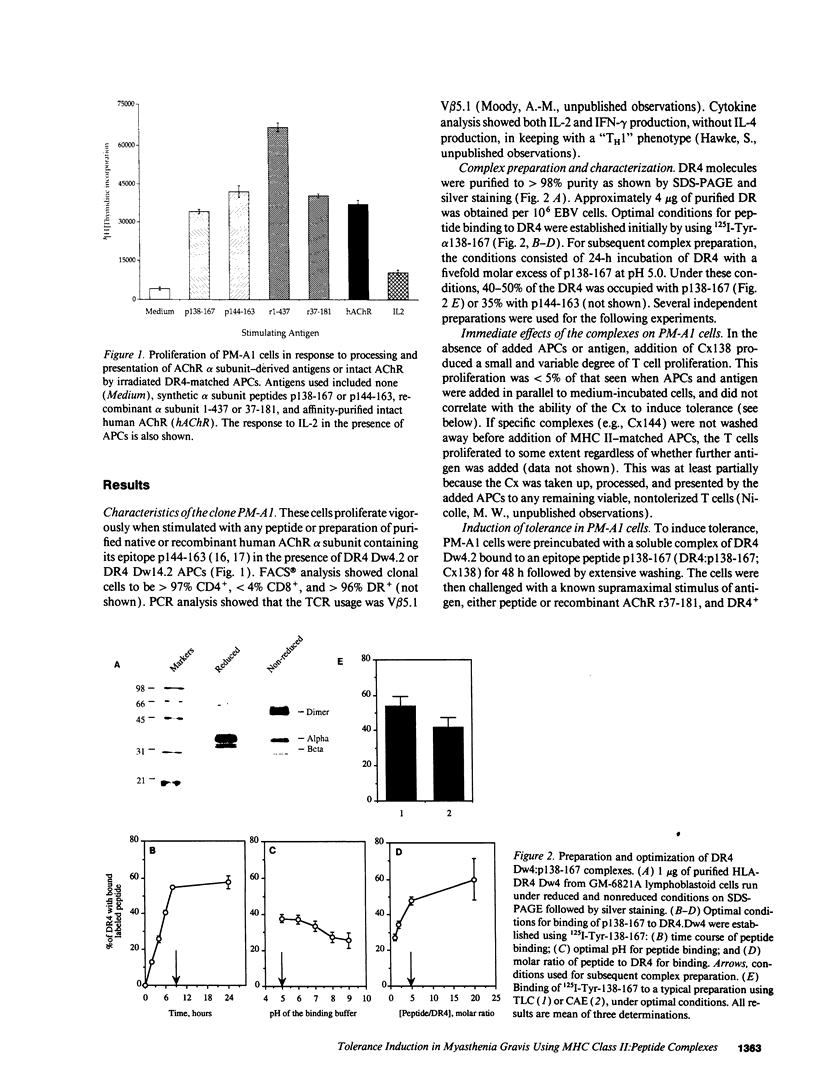
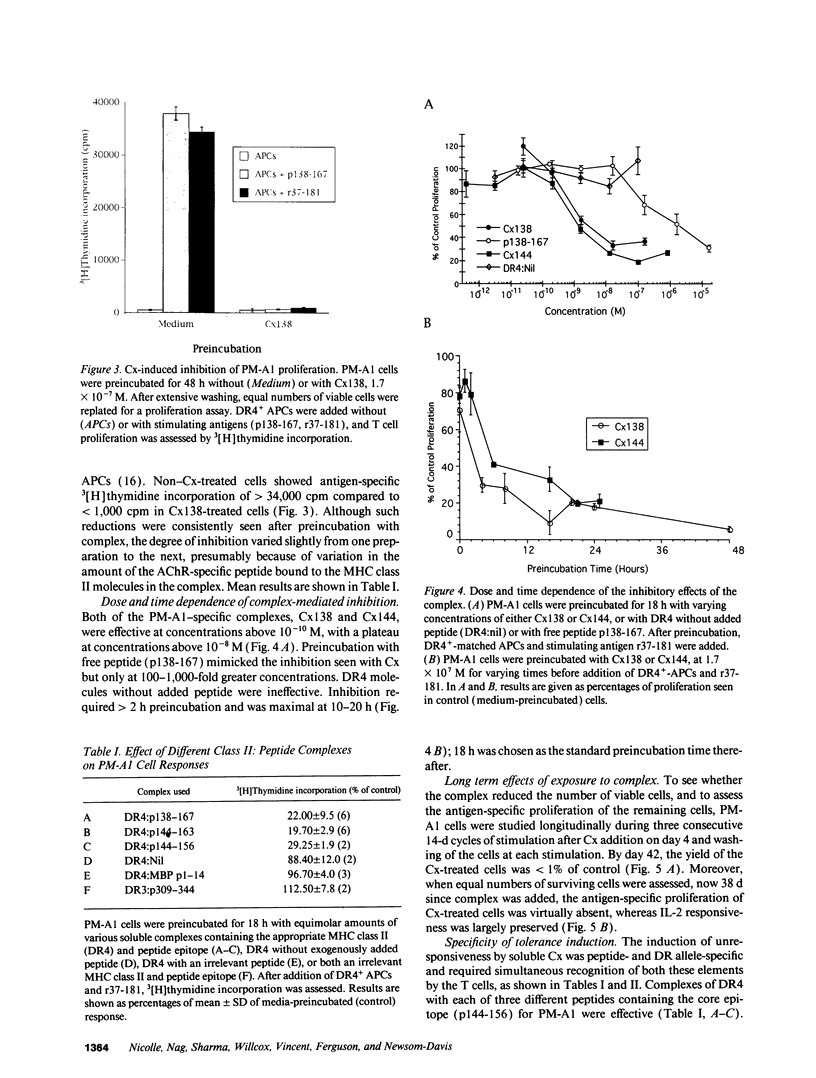
In autoimmune disorders, inactivation of pathogenic antigen-specific T cells, rather than global immunosuppression, would be highly desirable. One way to achieve this would be to deliver the first antigen-specific signal to the T cell in the absence of the second costimulatory signal. Myasthenia gravis (MG) is a well-characterized autoimmune disease in which T cell-dependent autoantibodies are directed against the acetylcholine receptor (A ChR) at the neuromuscular junction. AChR-specific T cells have been cloned from MG patients, and in this study, we have induced long-lasting tolerance in vitro in one particular clone (PM-A1) with a known peptide epitope (alpha 144-163) and MHC class II restriction (DR4 Dw14.2 or 4.2) by using soluble MHC-class II peptide complexes. Preincubation of PM-A1 T cells with such complexes induced death by apoptosis in < or = 40-50% of the AChR-specific cells. Surviving cells remained refractory to stimulation with AChR-derived synthetic peptides or recombinant polypeptides for < or = 38 d after complex treatment. These effects were highly specific, dose-dependent and required > 2 h preincubation. The T cells could be protected from the tolerizing effects of complex by coincubation with DR-matched or -mismatched antigen-presenting cells. This work shows that antigen-specific T cells can be selectively killed or anergized using soluble MHC class II: peptide complexes. Such an antigen-specific therapy offers a rational approach to the immunotherapy of autoimmune or allergic disease in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Mitchell D. J., Timmermann L., Wraith D. C., Tausch G. S., Waldor M. K., Zamvil S. S., McDevitt H. O., Steinman L. Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell. 1988 Jul 15;54(2):263–273. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- Beeson D., Brydson M., Betty M., Jeremiah S., Povey S., Vincent A., Newsom-Davis J. Primary structure of the human muscle acetylcholine receptor. cDNA cloning of the gamma and epsilon subunits. Eur J Biochem. 1993 Jul 15;215(2):229–238. doi: 10.1111/j.1432-1033.1993.tb18027.x. [DOI] [PubMed] [Google Scholar]
- Coffman R. L. Mechanisms of helper T-cell regulation of B-cell activity. Ann N Y Acad Sci. 1993 Jun 21;681:25–28. doi: 10.1111/j.1749-6632.1993.tb22865.x. [DOI] [PubMed] [Google Scholar]
- Damle N. K., Klussman K., Aruffo A. Intercellular adhesion molecule-2, a second counter-receptor for CD11a/CD18 (leukocyte function-associated antigen-1), provides a costimulatory signal for T-cell receptor-initiated activation of human T cells. J Immunol. 1992 Feb 1;148(3):665–671. [PubMed] [Google Scholar]
- De Magistris M. T., Alexander J., Coggeshall M., Altman A., Gaeta F. C., Grey H. M., Sette A. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992 Feb 21;68(4):625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- Del Prete G. F., De Carli M., Ricci M., Romagnani S. Helper activity for immunoglobulin synthesis of T helper type 1 (Th1) and Th2 human T cell clones: the help of Th1 clones is limited by their cytolytic capacity. J Exp Med. 1991 Oct 1;174(4):809–813. doi: 10.1084/jem.174.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. R., Bissonnette R. P., Glynn J. M., Shi Y. Activation-induced apoptosis in lymphoid systems. Semin Immunol. 1992 Dec;4(6):379–388. [PubMed] [Google Scholar]
- Hawke S., Willcox N., Harcourt G., Vincent A., Newsom-Davis J. Stimulation of human T cells by sparse antigens captured on immunomagnetic particles. J Immunol Methods. 1992 Oct 19;155(1):41–48. doi: 10.1016/0022-1759(92)90269-y. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Ashwell J. D., Schwartz R. H. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol. 1988 May 15;140(10):3324–3330. [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987 Feb 1;165(2):302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K. The role of cell division in the induction of clonal anergy. Immunol Today. 1992 Feb;13(2):69–73. doi: 10.1016/0167-5699(92)90137-V. [DOI] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Linsley P. S., Thompson C. B. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990 Jun;11(6):211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- Kabelitz D., Pohl T., Pechhold K. Activation-induced cell death (apoptosis) of mature peripheral T lymphocytes. Immunol Today. 1993 Jul;14(7):338–339. doi: 10.1016/0167-5699(93)90231-9. [DOI] [PubMed] [Google Scholar]
- Kirchner T., Hoppe F., Schalke B., Müller-Hermelink H. K. Microenvironment of thymic myoid cells in myasthenia gravis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;54(5):295–302. doi: 10.1007/BF02899226. [DOI] [PubMed] [Google Scholar]
- Kopf M., Le Gros G., Bachmann M., Lamers M. C., Bluethmann H., Köhler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993 Mar 18;362(6417):245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- LaSalle J. M., Tolentino P. J., Freeman G. J., Nadler L. M., Hafler D. A. Early signaling defects in human T cells anergized by T cell presentation of autoantigen. J Exp Med. 1992 Jul 1;176(1):177–186. doi: 10.1084/jem.176.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maselli R. A., Richman D. P., Wollmann R. L. Inflammation at the neuromuscular junction in myasthenia gravis. Neurology. 1991 Sep;41(9):1497–1504. doi: 10.1212/wnl.41.9.1497. [DOI] [PubMed] [Google Scholar]
- Melms A., Oksenberg J. R., Malcherek G., Schoepfer R., Müller C. A., Lindstrom J., Steinman L. T-cell receptor gene usage of acetylcholine receptor-specific T-helper cells. Ann N Y Acad Sci. 1993 Jun 21;681:313–314. doi: 10.1111/j.1749-6632.1993.tb22904.x. [DOI] [PubMed] [Google Scholar]
- Moiola L., Protti M. P., Manfredi A. A., Yuen M. H., Howard J. F., Jr, Conti-Tronconi B. M. T-helper epitopes on human nicotinic acetylcholine receptor in myasthenia gravis. Ann N Y Acad Sci. 1993 Jun 21;681:198–218. doi: 10.1111/j.1749-6632.1993.tb22887.x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Nag B., Deshpande S. V., Clark B. R. Novel methods to rapidly and sensitively analyze antigenic peptide binding to MHC class II molecules. J Immunol Methods. 1991 Aug 28;142(1):105–111. doi: 10.1016/0022-1759(91)90297-s. [DOI] [PubMed] [Google Scholar]
- Nag B., Passmore D., Deshpande S. V., Clark B. R. In vitro maximum binding of antigenic peptides to murine MHC class II molecules does not always take place at the acidic pH of the in vivo endosomal compartment. J Immunol. 1992 Jan 15;148(2):369–372. [PubMed] [Google Scholar]
- Nag B., Wada H. G., Deshpande S. V., Passmore D., Kendrick T., Sharma S. D., Clark B. R., McConnell H. M. Stimulation of T cells by antigenic peptide complexed with isolated chains of major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1604–1608. doi: 10.1073/pnas.90.4.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong B., Willcox N., Wordsworth P., Beeson D., Vincent A., Altmann D., Lanchbury J. S., Harcourt G. C., Bell J. I., Newsom-Davis J. Critical role for the Val/Gly86 HLA-DR beta dimorphism in autoantigen presentation to human T cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7343–7347. doi: 10.1073/pnas.88.16.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. R., Bryan V. M., Oi V. T., Herzenberg L. A. Antigen-specific identification and cloning of hybridomas with a fluorescence-activated cell sorter. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1962–1966. doi: 10.1073/pnas.76.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quill H., Schwartz R. H. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987 Jun 1;138(11):3704–3712. [PubMed] [Google Scholar]
- Reim J., McIntosh K., Martin S., Drachman D. B. Specific immunotherapeutic strategy for myasthenia gravis: targeted antigen-presenting cells. J Neuroimmunol. 1992 Nov;41(1):61–70. doi: 10.1016/0165-5728(92)90196-r. [DOI] [PubMed] [Google Scholar]
- Reiner S. L., Wang Z. E., Hatam F., Scott P., Locksley R. M. TH1 and TH2 cell antigen receptors in experimental leishmaniasis. Science. 1993 Mar 5;259(5100):1457–1460. doi: 10.1126/science.8451641. [DOI] [PubMed] [Google Scholar]
- Roska A. K., Lipsky P. E. Dissection of the functions of antigen-presenting cells in the induction of T cell activation. J Immunol. 1985 Nov;135(5):2953–2961. [PubMed] [Google Scholar]
- Scadding G. K., Vincent A., Newsom-Davis J., Henry K. Acetylcholine receptor antibody synthesis by thymic lymphocytes: correlation with thymic histology. Neurology. 1981 Aug;31(8):935–943. doi: 10.1212/wnl.31.8.935. [DOI] [PubMed] [Google Scholar]
- Schluep M., Willcox N., Ritter M. A., Newsom-Davis J., Larché M., Brown A. N. Myasthenia gravis thymus: clinical, histological and culture correlations. J Autoimmun. 1988 Oct;1(5):445–467. doi: 10.1016/0896-8411(88)90067-4. [DOI] [PubMed] [Google Scholar]
- Schluep M., Willcox N., Vincent A., Dhoot G. K., Newsom-Davis J. Acetylcholine receptors in human thymic myoid cells in situ: an immunohistological study. Ann Neurol. 1987 Aug;22(2):212–222. doi: 10.1002/ana.410220205. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Schönbeck S., Padberg F., Hohlfeld R., Wekerle H. Transplantation of thymic autoimmune microenvironment to severe combined immunodeficiency mice. A new model of myasthenia gravis. J Clin Invest. 1992 Jul;90(1):245–250. doi: 10.1172/JCI115843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. Selective differentiation of CD4+ T helper cell subsets. Curr Opin Immunol. 1993 Jun;5(3):391–397. doi: 10.1016/0952-7915(93)90058-z. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E., Davis M. M., Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992 Oct 1;176(4):1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. D., Nag B., Su X. M., Green D., Spack E., Clark B. R., Sriram S. Antigen-specific therapy of experimental allergic encephalomyelitis by soluble class II major histocompatibility complex-peptide complexes. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11465–11469. doi: 10.1073/pnas.88.24.11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy M., Oshima M., Atassi M. Z., Christadoss P. Suppression of experimental autoimmune myasthenia gravis by epitope-specific neonatal tolerance to synthetic region alpha 146-162 of acetylcholine receptor. Clin Immunol Immunopathol. 1993 Mar;66(3):230–238. doi: 10.1006/clin.1993.1030. [DOI] [PubMed] [Google Scholar]
- Sidhu S., Deacock S., Bal V., Batchelor J. R., Lombardi G., Lechler R. I. Human T cells cannot act as autonomous antigen-presenting cells, but induce tolerance in antigen-specific and alloreactive responder cells. J Exp Med. 1992 Sep 1;176(3):875–880. doi: 10.1084/jem.176.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P., Anasetti C., Hansen J. A., Melrose J., Brunvand M., Bradshaw J., Ledbetter J. A., Linsley P. S. Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1. J Exp Med. 1993 Jan 1;177(1):165–173. doi: 10.1084/jem.177.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A. Immunology of acetylcholine receptors in relation to myasthenia gravis. Physiol Rev. 1980 Jul;60(3):756–824. doi: 10.1152/physrev.1980.60.3.756. [DOI] [PubMed] [Google Scholar]
- Weaver C. T., Unanue E. R. The costimulatory function of antigen-presenting cells. Immunol Today. 1990 Feb;11(2):49–55. doi: 10.1016/0167-5699(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Wekerle H., Ketelsen U. P. Intrathymic pathogenesis and dual genetic control of myasthenia gravis. Lancet. 1977 Mar 26;1(8013):678–680. doi: 10.1016/s0140-6736(77)92118-3. [DOI] [PubMed] [Google Scholar]
- Willcox N., Baggi F., Batocchi A. P., Beeson D., Harcourt G., Hawke S., Jacobson L., Matsuo H., Moody A. M., Nagvekar N. Approaches for studying the pathogenic T cells in autoimmune patients. Ann N Y Acad Sci. 1993 Jun 21;681:219–237. doi: 10.1111/j.1749-6632.1993.tb22888.x. [DOI] [PubMed] [Google Scholar]
- van der Pouw-Kraan T., de Jong R., Aarden L. Development of human Th1 and Th2 cytokine responses: the cytokine production profile of T cells is dictated by the primary in vitro stimulus. Eur J Immunol. 1993 Jan;23(1):1–5. doi: 10.1002/eji.1830230102. [DOI] [PubMed] [Google Scholar]